Abstract
Objective
We retrospectively evaluated long-term oncological outcomes in patients with germ cell tumors (GCTs) primarily treated at our institution and assessed late recurrence and second primary malignancies.
Methods
This study included a total of 139 males with newly diagnosed GCTs of the testis or extragonadal origin who received treatment, including surgery, chemotherapy and radiation therapy, at our hospital between 1980 and 2005. We reviewed late recurrence that occurred at least 2 years after the initial disease-free status and secondary malignancies as well as oncological outcomes.
Results
In patients with seminoma, 5-year progression-free survival and cause-specific survival rates were 87.2% and 100% for Stage I, 88.9% and 100% for Stage II, and 50.0% and 50.0% for Stage III, respectively, whereas in those with non-seminomatous GCTs, they were 79.1% and 96.3% for Stage I, 89.5% and 89.4% for Stage II, and 85.7% and 78.4% for Stage III, respectively. Late recurrence was found in five (3.6%) patients and all of them responded to salvage treatment and achieved disease-free status. Second primary hematological neoplasms occurred in three (2.2%), although they had a long-term free of the primary disease. All died of the second primary disease.
Conclusions
Late recurrence was successfully managed with appropriate treatments, although its incidence was not negligible. Periodic follow-up may be necessary for >5 years in patients with GCTs for early detection of late recurrence. In addition, care should be taken to watch for the development of life-threatening second primary malignant disease during long-term follow-up.
Keywords: urology, urologic-med, urologic-radOncol
INTRODUCTION
Germ cell tumors (GCTs), mainly presenting as testicular cancer, are relatively rare neoplasms that account for 0.8% of all cancers in males (1). However, GCTs are the most common malignancy among men aged 15–44 years old (2). GCTs have been considered to be curable malignancies, even in the advanced stage, since the introduction of cisplatin (3). Because GCTs occur in young patients, improvement of the prognosis has caused a new clinical problem. Disease recurrence is often found after even more than 5 years (4), and there is no consensus on how long we should follow up patients with periodic examinations. The occurrence of a second primary malignancy is also clinically significant, because chemotherapy and radiotherapy may increase the risk for such an onset after a long-term duration (5). Little is known about second malignant neoplasms in Japanese men who become free of testicular cancer. This is partly because Japanese men have a much lower incidence of the disease than Caucasian men (6), and thus each institute has a limited number of patients.
In this study, we reviewed long-term oncological outcomes in patients with GCT receiving treatment at our hospital and assessed the occurrence of late recurrence and second malignant neoplasms.
PATIENTS AND METHODS
This retrospective study included 139 males with newly diagnosed GCTs of the testis or extragonadal origin treated at our hospital between 1980 and 2005. These patients underwent single or combined treatments including surgery, radiation and chemotherapy according to the extent of the disease. All patients were diagnosed by histological examination of the primary or metastatic site, except for one patient with a disease of mediastinal origin, in whom non-seminomatous GCT (NSGCT) was presumed to be due to elevated serum α-fetoprotein and human choriogonadotropin. Clinical stage was determined based on the 1997 version of the UICC tumor stage classification (7). All of the patients with metastatic disease received platinum-agent-based chemotherapy and, if indicated, subsequent surgical resection of the residual mass. The exception was patients with pure seminoma having a solitary metastatic mass in an abdominal lymph node <5 cm in diameter who underwent radiation therapy or chemotherapy for the metastasis. Selected patients with organ-confined disease in the early 1980s received prophylactic treatment such as chemotherapy, retroperitoneal lymph node dissection (RPLND) or radiation therapy.
Although follow-up intervals depended on the stage of the disease, the evaluation was basically done as follows. In the first 2 years, the follow-up consisted of measurement of tumor markers such as AFP, HCG, and HCG-β every 1–2 months. A complete blood count, blood chemistry and computed tomography (CT) of lung and abdomen were done every 3 months. In the next 3–5 years, tumor markers, complete blood counts and blood chemistry were evaluated every 3–6 months. CT was done every 3–12 months, depending on the stage of the disease and follow-up period. After 6–10 years, we followed patients semi-annually or annually with tumor markers, complete blood counts and blood chemistry evaluations. CT was done if clinically indicated. After 10 years, patients were followed up annually with the clinical examinations described above if needed.
Late recurrence and the occurrence of second malignancy were reviewed. The recurrence was defined as that with an initial histological diagnosis of testicular or extragonadal GCT and recurrence of disease >2 years after the initial successful treatment. Metachronous GCTs that arose from the contralateral testis were excluded from late recurrence. Progression-free survival (PFS) and cause-specific survival (CSS) were estimated by the Kaplan–Meier method.
RESULTS
The patients' characteristics are shown in Table 1. The mean age was 32.5 years and they were followed up for a median period of nearly 6 years. The median follow-up period of current survivors was 7.4 years, with a mean period of 8.6 years. Extragonadal GCTs were found in four patients, three with retroperitoneal disease and one with a mediastinal one. Histological examination revealed pure seminoma in 38.1% of the patients and tumors including a non-seminomatous component in 61.9%. For metastatic lesions, 2 patients received radiation therapy alone and 66 had chemotherapy with or without surgical treatment. Both radiation and chemotherapy were given to three patients. As the first-line regimen, PVB (cisplatin + vinblastine + bleomycin) was used from 1980 to 1990, whereas BEP (bleomycin + etoposide + cisplatin) was used after 1990.
Table 1.
Patients' characteristics
| No. of patients | 139 |
| Mean age: years (range) | 32.5 (15–56) |
| Median follow-up period: months (range) | 75.5 (2.4–304) |
| Primary site (n) | |
| Testis (right/left) | 135 (68/67) (97.1%) |
| Retroperitoneal | 3 (2.2%) |
| Mediastinum | 1 (0.7%) |
| Histology (n) | |
| Seminoma | 53 (38.1%) |
| Clinical stage | |
| I | 41 |
| II | 9 |
| III | 3 |
| Non-seminomatous germ cell tumor | 86 (61.9%) |
| Clinical stage | |
| I | 30 |
| II | 20 |
| III | 36 |
| Treatment added to orchiectomy (n) | |
| None | 25 |
| Radiation only | 38 |
| Chemotherapy only | 16 |
| Radiation + chemotherapy | 2 |
| Chemotherapy + surgical resection of metastatic site | 54 |
| RPLND only | 4 |
| Chemotherapy regimen (n) | |
| PVB | 27 |
| BEP/EP | 63 |
| VIP | 19 |
| VeIP | 2 |
| High-dose ICE + autologous HCT | 11 |
| TIP/TIN | 6 |
| Irinotecan + nedaplatin | 5 |
RPLND, retroperitoneal lymph node dissection; PVB, cisplatin + vinblastine + bleomycin; BEP, bleomycin + etoposide + cisplatin; VIP, etoposide + ifosphamide + cisplatin; VeIP, vinblastine + ifosphamide + cisplatin; ICE, ifosphamide + carboplatin + etoposide; HCT, hematopoietic cell transplantation; TIP, paclitaxel + ifosphamide + cisplatin; TIN, paclitaxel + ifosphamide + nedaplatin.
In the early 1980s, some patients received combination chemotherapy with cisplatin, vinblastine, peplomycin and adriamycin as the first-line treatment. Various chemotherapeutic regimens were used as the second- or third-line treatment as shown in Table 1. A combination of paclitaxel and irinotecan was introduced in 2003. Details of management for advanced disease will be reported elsewhere.
Disease-free status was achieved in 92.1% of the patients by the initially planned treatment. Of them, however, 12.2% developed recurrent disease and a GCT appeared in the contralateral testis in 1.4%. On the other hand, 11 (7.9%) patients never became free from the disease. Finally, 11 (7.9%) patients died of the primary disease. Four patients died of chemotherapy-related complications. Life-threatening second malignant disease developed in three patients who had undergone chemotherapy for GCTs (Table 2). Details of their clinical courses are discussed below.
Table 2.
Outcome of patients
| Total no. | 139 |
| Disease-free after the initially planned treatment | 128 (92.1%) |
| Disease recurrence | 17 (12.2%) |
| Median time to recurrence: months (range) | 8 (1.1–84) |
| Metachronous appearance in contralateral testis | 2 (1.4%) |
| Disease remaining after the initially planned treatment | 11 (7.9%) |
| Alive without disease | 117 (84.2%) |
| Alive with disease | 3 (2.2%) |
| Died of the primary disease | 11 (7.9%) |
| Died of chemotherapy-related complications | 4 (2.9%) |
| Died of second malignant disease | 3 (2.2%) |
| Died of disease other than germ cell tumor | 4 (2.9%) |
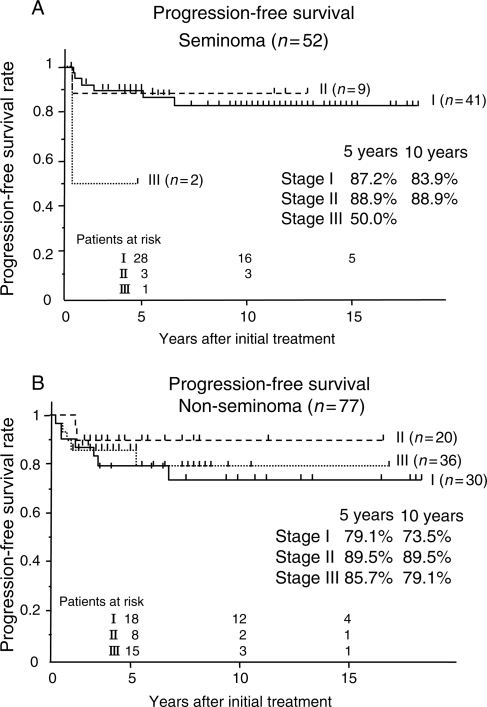
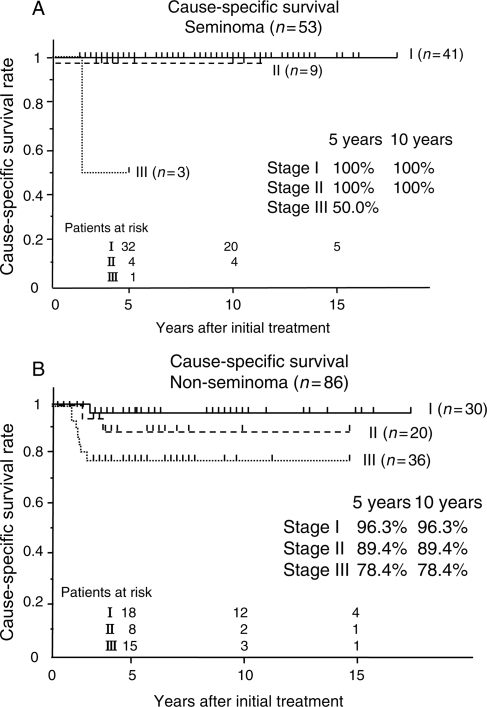
Figure 1 presents the PFS of patients with seminoma and that of those with NSGCTs. One patient with seminoma and 9 with NSGCTs were never free of the disease after the initially planned treatments and all of these 10 were excluded from the analysis of PFS. Stage I disease had worse 5- and 10-year PFS than Stage II disease due to the difference in the recurrence rate. However, CSS was excellent in Stage I disease for both types of histology (Fig. 2).
Figure 1.
(A) Progression-free survival (PFS) of 52 patients with seminoma. (B) PFS of 77 patients with non-seminomatous germ cell tumors (NSGCTs).
Figure 2.
(A) Cause-specific survival (CSS) of 53 patients with seminoma. (B) CSS of 86 patients with NSGCTs.
Late recurrence was seen in five patients (3.6%), including three with Stage I NSGCT, one with Stage I seminoma and one with Stage III NSGCT (Table 3). Interestingly, one patient with Stage I NSGCT developed abdominal lymph node metastasis 7 years after the primary tumor resection. Treatments consisting of chemotherapy and mass resection were successful for the recurrent disease in all patients.
Table 3.
Patients with late recurrence after initial treatment
| Stage | Histology | Initial treatment added to radical orchiectomy | Time to relapse (months) | Site of relapse | Tumor markers at relapse (AFP/HCG-β) | Outcome | Period after the treatment for recurrent disease (months) |
|---|---|---|---|---|---|---|---|
| I | E + T + S | None | 26 | RPLN, lung | 24.9/1.5 | NED | 65 |
| I | S | None | 46 | Dura mater | 1.2/0.1 | NED | 105 |
| I | T + S | None | 84 | RPLN | 465/0.2 | NED | 5 |
| I | E + T | RPLND | 25 | RPLN | 69.0/5.0 | NED | 196 |
| IIIB2 | E + Y + C + T | Chemotherapy + tumor resection | 59 | Mediastinum | 1085/69.0 | NED | 115 |
AFP, α-fetoprotein; HCG-β, human chorionic gonadotropin-β subunit; E, embryonal carcinoma; T, teratoma; S, seminoma; RPLN, retroperitoneal lymph nodes; NED, no evidence of disease; Y, yolk sac tumor; C, choriocarcinoma. HCG was not examined at the time of relapse in these cases.
Patients who developed a second primary malignant disease after the treatment for GCTs are shown in Table 4. They received chemotherapy before 1985. In three patients who developed hematologic neoplasms, two had seminoma in the primary lesion and had undergone prophylactic or therapeutic radiation therapy. All three patients had a history of chemotherapy with a regimen containing cisplatin for the original and recurrent disease, whereas etoposide was administered to one patient. There was another patient who developed renal cell carcinoma (RCC) 20 years after the initial treatment for testicular cancer. He received orchiectomy and RPLND for Stage I disease. After the initial treatment, lung metastasis was found 1.5 years later. He received combination chemotherapy consisting of cisplatin, vinblastine, peplomycin and adriamycin. He underwent a nephrectomy for RCC and is now the only survivor among those who had a second primary malignancy.
Table 4.
Patients developing secondary primary malignant neoplasms
| Stage | Histology | Prior treatment for GCT | Chemotherapeutic regimen | Time to onset (months) | Second neoplasms |
|---|---|---|---|---|---|
| Hematological neoplasm | |||||
| I | S | RPLND + chemotherapy + radiation | CDDP/VBL/PEP/ADM | 186 | Leukemia |
| IIA | S | RPLND + chemotherapy + radiation | CDDP/VBL/PEP/ADM | 62 | MDS |
| IIA | E, T | Chemotherapy | CDDP/etoposide/BLM | 47 | MDS |
| Non-hematological neoplasm | |||||
| I | E | RPLND,a chemotherapyb | CDDP/VBL/PEP/ADM | 240 | RCC |
GCT, germ cell tumor; CDDP, cisplatin; VBL, vinblastine; PEP, peplomycin; ADM, adriamycin; MDS; myelodysplastic syndrome; BLM, bleomycin; RCC; renal cell carcinoma.
aDone the initial treatment together with radical orchiectomy.
bDone for recurrence disease in the lung.
DISCUSSION
In this study, we reviewed the outcomes of patients with GCTs since the introduction of cisplatin. Multimodal treatments consisting of surgical resection, radiation and chemotherapy provided excellent oncological outcomes in patients with clinical stage I and II diseases, as other investigators reported (8). However, the result of treatment for the systemic disseminated disease is still unsatisfactory (9), although details of our results were not fully reported in this manuscript. Only a few years have passed since the newer anti-cancer agents such as paclitaxel and irinotecan began to be employed. Therefore, further observation is needed to evaluate the long-term efficacy of these drugs.
The incidence of late recurrence was 3.6%, which was similar to those in other reports (10). Most patients who developed late recurrence had Stage I disease with surveillance after tumor resection, and relapse >5 years after the diagnosis of GCT may be rare in patients with advanced disease who receive chemotherapy and become free of the disease. These results suggest the efficacy of adjuvant chemotherapy to prevent disease recurrence in some patients with Stage I disease. However, the use of chemotherapeutic agents entails a risk of de novo primary malignancy, as discussed below (11). In addition, all of our patients with late recurrence successfully obtained freedom from disease by chemotherapy with residual mass resection. Taking these findings and the incidence of late recurrence into consideration, adjuvant chemotherapy may not always be necessary for Stage I GCT patients, except those with a high risk for disease recurrence (12), as long as they receive close follow-up (13,14). Oldenburg et al. (15) reported that RPLND might be effective to prevent late relapse in Stage I GCT. However, because of its invasiveness and low incidence of relapse, it is controversial whether RPLND should be performed. Similar to adjuvant chemotherapy, if close follow-up can be done, immediate RPLND may not be necessary in Stage I disease.
Some anti-cancer agents have potential oncogenicity. Topoisomerase II inhibitors increase the risk for secondary leukemia arising in 2 or 3 years (16), with a relative risk of 15–25% (5). However, our leukemic patient had not been administrated etoposide. Moreover, the onset was too late for topoisomerase II-specific leukemia. Travis et al. (17) reported that both radiation therapy and chemotherapy promoted the risk for leukemia in long-term survivors. Even in our cases, the combined therapy might affect the development of leukemia after a long duration. On the other hand, alkylating agent-related myelodysplastic syndrome and acute myeloid leukemia often occur 5–7 years after the administration (18). Our two cases presented its typical course. In this study, one patient developed secondary solid organ malignancy. Its frequency was extremely rare, although if the follow-up period were to be prolonged, solid organ malignancy might increase. Treatment-related solid organ cancers have developed 20 years after radiation therapy and chemotherapy (19). Thus, further follow-up might detect secondary solid organ malignancies in our series.
There is no consensus on the duration of periodic follow-up for GCT patients. As shown in this study, some patients probably develop relapse of the disease >5 years after the initial diagnosis (20). We successfully detected and managed all patients with late relapse in the follow-up periods. Patients who are diagnosed as having a late recurrence after presentation of the recurrence-related symptoms have a tendency to have poorer prognoses (21,22). When we take this into consideration, follow-up for at least 10 years may be preferable (15).
Since long-term survivors who undergo chemotherapy and/or radiation therapy have a high risk for second malignancy, we should carefully follow them to detect the disease. Follow-up >10 years may be needed. However, periodical examinations cannot always detect a second malignant disease. In addition, the clinical efficacy of early detection of second malignancy is still unknown, because the disease tends to be refractory to treatment. Further investigation is needed to establish the management procedure for a second malignant disease.
Conflict of interest statement
None declared.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Verhoeven RH, Coebergh JW, Kiemenney LA, Koldewijn EL, Houterman S. Testicular cancer: trends in mortality are well explained by changes in treatment and survival in the southern Netherlands since 1970. Eur J Cancer. 2007;43:2553–8. doi: 10.1016/j.ejca.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–40. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 4.Dieckmann KP, Albers P, Classen J, Wit M, Pichlmeier U, Rick O, et al. Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol. 2005;173:824–9. doi: 10.1097/01.ju.0000154013.96349.36. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyey C, Schmoll HJ. Treatment of testicular cancer and the development of secondary malignancies. J Clin Oncol. 1995;13:283–92. doi: 10.1200/JCO.1995.13.1.283. [DOI] [PubMed] [Google Scholar]
- 6.Mostofi FK, Sesterhenn IA. Chapter 4. Tumours of the testis and paratesticular tissue. In: Eble JN, Suter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours. Pathology and Genetics. Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 217–78. [Google Scholar]
- 7.Sobin LH, Wittekind CH, editors. International Union Against Cancer. TNM Classification of Malignant Tumors. 5th edn. New York: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 8.Horwich A, Shipley J, Huddart R. Testicular germ-cell cancer. Lancet. 2006;367:754–65. doi: 10.1016/S0140-6736(06)68305-0. [DOI] [PubMed] [Google Scholar]
- 9.Shintaku I, Satoh M, Okajima E, Fujimoto H, Kamoto T, Ogawa O, et al. Survival of metastatic germ cell cancer patients assessed by international germ cell consensus classification in Japan. Jpn J Clin Oncol. 2008;38:281–7. doi: 10.1093/jjco/hyn009. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi M, Norman AR, Dearnaley DP, Nicholls J, Horwich A, Huddart RA. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer. 2002;95:520–30. doi: 10.1002/cncr.10691. [DOI] [PubMed] [Google Scholar]
- 11.Van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–8. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 12.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Horwich A, et al. Guidelines on testicular cancer. Eur Urol. 2005;48:885–94. doi: 10.1016/j.eururo.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Patel MI, Motzer RJ, Sheinfeld J. Management of recurrence and follow-up strategies for patients with seminoma and selected high-risk groups. Urol Clin North Am. 2003;30:803–17. doi: 10.1016/s0094-0143(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 14.Sharir S, Jewett MA, Sturgeon JF, Moore M, Warde PR, Catton CN, et al. Progression detection of stage I nonseminomatous testis cancer on surveillance: implications for the followup protocol. J Urol. 1999;161:472–5. [PubMed] [Google Scholar]
- 15.Oldenburg J, Martin JM, Fossa SD. Late relapse of germ cell malignancies: incidence, management, and prognosis. J Clin Oncol. 2006;24:5503–11. doi: 10.1200/JCO.2006.08.1836. [DOI] [PubMed] [Google Scholar]
- 16.Kollmannsberger C, Beyer J, Droz JP, Harstrick A, Hartmann JT, Biron P, et al. Secondary leukemia following high cumulative doses of etoposide in patients treated for advanced germ cell tumors. J Clin Oncol. 1998;16:3386–91. doi: 10.1200/JCO.1998.16.10.3386. [DOI] [PubMed] [Google Scholar]
- 17.Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429–39. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen-Bjergaard J, Daugaard G, Hansen SW, Philip P, Larsen SO, Rorth M. Increased risk of myelodysplasia and leukemia after etoposide, cisplatin, and bleomycin for germ-cell tumours. Lancet. 1991;338:359–63. doi: 10.1016/0140-6736(91)90490-g. [DOI] [PubMed] [Google Scholar]
- 19.Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 20.Baniel J, Foster RS, Gonin R, Messemer JE, Donohue JP, Einhorn LH. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170–6. doi: 10.1200/JCO.1995.13.5.1170. [DOI] [PubMed] [Google Scholar]
- 21.George DW, Foster RS, Hromas RA, Robertson KA, Vance GH, Ulbright TM, et al. Update on late relapse of germ cell tumor: a clinical and molecular analysis. J Clin Oncol. 2003;21:113–22. doi: 10.1200/JCO.2003.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Sharp DS, Carver BS, Eggener SE, Kondagunta GV, Motzer RJ, Bosl GJ, et al. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol. 2008;26:5524–9. doi: 10.1200/JCO.2007.15.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]