Abstract
Recurrence or metastasis of cancer has been considered to occur in the last stage of the patient's life. However, the new notions of oligometastases and oligo-recurrence have been proposed and the paradigm shift in the conceptualization of cancer metastasis or cancer recurrence. Oligometastases is the state in which the patient shows distant relapse in only a limited number of regions. Local therapy such as surgery, radiotherapy and radiofrequency ablation for the relapsed sites could thus improve patient's survival. On the other hand, oligo-recurrence is a notion similar to oligometastases. However, the conditions of oligo-recurrence has a primary site of the cancer controlled, meaning that all gross recurrent or metastatic sites could be treated using local therapy.
Keywords: oligometastases, oligo-recurrence, local therapy, systemic therapy, paradigm shift
Recurrence or metastasis of cancer has usually been considered to occur in the last stage of the patient's life. From this perspective, even if only one site of recurrence or metastasis is present, the cancer can be seeded throughout the body hematogenously, meaning that local therapy cannot eradicate all cancer cells. Systemic chemotherapy can then only prolong life, rather than achieving cure. However, Hellman and Weichselbaum proposed an alternative notion in 1995, bringing about a paradigm shift in the conceptualization of cancer metastasis or cancer recurrence. This new notion is that of oligometastases (1).
OLIGOMETASTASES
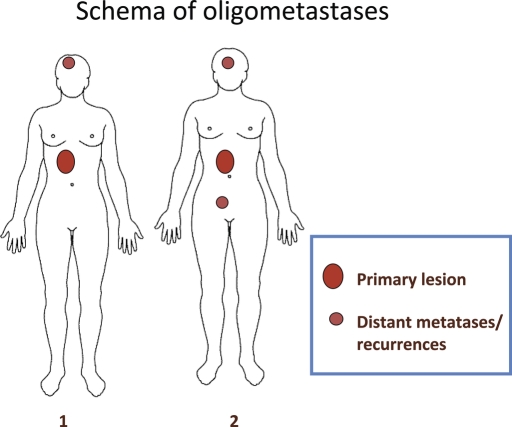
Oligometastases is the state in which the patient shows distant relapse in only a limited number of regions. Local therapy such as surgery, radiotherapy and radiofrequency ablation for the relapsed sites could thus improve patient's survival. The state of oligometastases (Fig. 1) represents an important concept, but one important problem remains to be solved. Oligometastases did not eliminate the uncontrolled primary site with several distant metastases. Then, all metastatic sites were thoroughly treated with local therapy, which did not lead to disappearance of all gross tumors and not might have achieved cure. As the primary site was not or could not be treated with local therapy, the primary site would exacerbate sooner.
Figure 1.
This is a schema of oligometastases. Schema 1 shows one distant metastasis/recurrence with a primary lesion. Schema 2 shows two distant metastases/recurrences with a primary lesion.
OLIGO-RECURRENCE
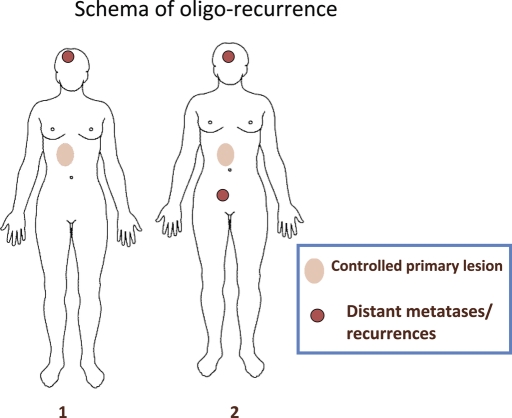
Niibe et al. (2–4) proposed the new notion of oligo-recurrence to overcome these problems. Oligo-recurrence is a notion similar to oligometastases. However, the conditions of oligo-recurrence are: (i) one to several distant metastases/recurrences (usually one) in one to several organs (usually one); (ii) primary site of the cancer controlled; (iii) one to several distant metastases/recurrences can be treated with local therapy; and (iv) no other distant metastases/recurrences other than those in (iii). This state of oligo-recurrence is shown in Fig. 2 and the differences between oligometastasis and oligo-recurrence are listed in Table 1. In the state of oligo-recurrence, recurrent or metastatic sites with a controlled primary lesion were treated with local therapy, meaning that all gross recurrent or metastatic sites could be treated using local therapy.
Figure 2.
This is a schema of oligo-recurrence. Schema 1 shows one distant metastasis/recurrence with a controlled primary lesion. Schema 2 shows two distant metastases/recurrences with a controlled primary lesion. The biggest difference between oligometastases and oligo-recurrences lies in the uncontrolled or controlled primary lesion. Oligo-recurrence requires a controlled primary lesion.
Table 1.
Oligometastases and oligo-recurrence
SYSTEMIC THERAPY AND LOCAL THERAPY
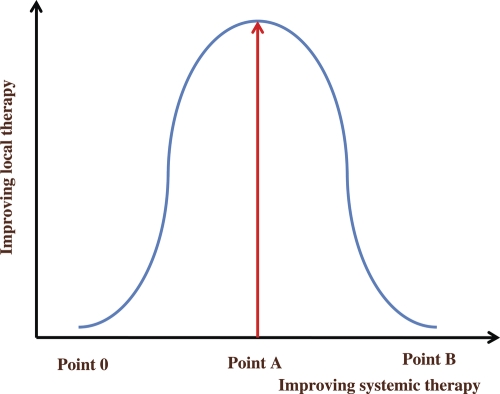
Improvement of systemic chemotherapy including molecular-targeted therapy has allowed micrometastases to be almost completely absent clinically. Theoretically, if several gross metastatic or recurrent sites could be eradiated by local therapy, these patients could be cured with concomitant systemic chemotherapy. Punglia et al. (5) reported that if systemic therapy improved, the role of local therapy would improve and proposed a figure for this correlation. Here, a new figure of the correlation between local therapy and systemic therapy is proposed (Fig. 3), showing that the role of local therapy is initially increasingly important as systemic therapy improves, depending on the sigmoid curve. The current status of cancer therapy lies in the range between 0 and A. However, in the future, extreme improvements in systemic therapy will decrease the importance of local therapy, because cancers will be diminished by systemic therapy alone such as intravenous anti-cancer drug infusion or oral anti-cancer drugs. All cancerous lesions including gross tumors and microinvasive tumors could be eradicated with systemic therapy alone. This desirable state is shown as B in Fig. 3. In the present status (range: 0–A in Fig. 3), systemic therapy is not yet powerful enough that local therapy is not required for eradication, particularly for gross tumor.
Figure 3.
This shows correlations between systemic and local therapies. Until point A, the role of local therapy increases as systemic therapy improves. However, after point A, the role of local therapy decreases as systemic therapy improves, as all cancerous lesions can be cured by systemic therapy at point B.
BRAIN TUMOR
This section and the following four sections focus on organ-specific oligometastases and oligo-recurrence. First, oligometastases and oligo-recurrence of brain metastatic tumors are described.
Classification of metastatic brain tumors such as oligo-recurrence in recursive partitioning analysis (RPA) class I is widely recognized and accepted (6). This class I contains patients with: KPS ≥70; age <65 years; controlled primary; and no extracranial metastases. All RPA class I patients thus show oligo-recurrence. However, RPA class I requires age <65 years, so if age is ≥65 years and even KPS 100, the patient is classified as RPA class II. Rapid progress has recently been made in reducing the invasiveness of surgery and radiotherapy. The age of 65 years is thus no longer the limit of aggressive therapy. The RPA classification was developed in 1997, and more than a decade has passed since the proposal of this classification. Given recent advances in modern medicine, oligo-recurrence is considered to be more appropriate.
Kocher et al. (7) compared 117 patients with one to three previously untreated cerebral metastases who underwent stereotactic radiosurgery (SRS) between 1991 and 1998 with 138 patients with one to three lesions treated using whole-brain radiotherapy (WBRT) between 1978 and 1991. The first modality represents a more powerful treatment of metastatic brain tumors. Of these patients, 32 were classified as RPA class I (SRS, n = 23; WBRT, n = 9). Median survival was 25.4 months with SRS, compared with 4.7 months with WBRT (P < 0.0001). Furthermore, Andrews et al. (8) reported a Phase III trial comparing WBRT to WBRT plus SRS, in which multivariate analysis indicated that patients with WBRT plus SRS survived longer than those with WBRT alone in RPA class I (P < 0.0001). These findings suggest that more powerful local treatment was efficacious for RPA class I. As for oligo-recurrence involving the brain, Niibe et al. (9) reported 17 metastatic brain tumors in 10 patients treated with SRS and surgery achieved 3-year local control in 90% and 3-year overall survival in 51.9%.
LUNG TUMOR
Survival benefits were being reported for complete resection of metastatic lung tumors even in the 1990s. The International Registry of Lung Metastases (IRLM) reported that 5-year overall survival for patients with complete resection of metastatic lung tumors was 36%, compared with 13% for patients without (10). However, clinical outcomes with stereotactic body radiotherapy (SBRT) for Stage I primary lung tumors are reportedly almost the same as with surgery. Onihsi et al. (11) reported a 5-year overall survival of 70.8% for operable Stage I patients, equivalent to that with surgery. This indicates that oligo-recurrent patients, who have no extrathoracic lesions, could receive survival benefit from SBRT. In fact, Bloomgren et al. (12) first reported that 14 metastatic lung tumors in 10 patients treated with SBRT achieved 92% local control. Uematsu et al. (13) reported that 43 metastatic lung tumors in 22 patients treated with SBRT achieved 98% local control. Nagata et al. (14) using SBRT with 48 Gy in four fractions to the isocenter reported that nine metastatic lung tumors in nine patients achieved 67% local control. From the same institution as Nagata, Norihisa et al. (15) using SBRT at 48–60 Gy in four to five fractions to the isocenter reported that 43 metastatic lung tumors in 34 patients achieved a 2-year local control rate of 90% and a 2-year overall survival rate of 84.3%. These are excellent outcomes. However, all these analyses were retrospective. In 2009, Rusthoven et al. (16) reported a Phase I/II prospective study of SBRT for metastatic lung tumors. Thirty-eight metastatic lung tumors in 63 patients treated with SBRT achieved a 2-year local control rate of 96% and a 2-year overall survival of 39%. This result was inferior to that of surgery according to the IRLM. One of the important reasons of poor prognosis in SBRT is that the prospective study included patients with extrapulmonary lesions, meaning oligometastases and no oligo-recurrence. Limited to oligo-recurrence and the small numbers of lung metastases, overall survival may be better and might be almost equivalent to that of the IRLM (16).
LIVER TUMOR
SBRT has also been applied to metastatic liver tumors. In 1998, Blomgren et al. reported that a pilot study using 20–40 Gy in one or two fractions to the periphery of the planning target volume (PTV) achieved 95% local control (17). Several prospective studies have recently been reported. Herfarth et al. (18) reported 56 metastatic liver tumors in 33 patients treated with SBRT using 14–26 Gy per fraction (prescribed to 80%), achieving 78% local control. Kavanagh et al. (19) reported 28 metastatic liver tumors in 21 patients treated with SBRT using 12–20 Gy in three fractions to the periphery of the PTV, achieving 93% local control. Mendez Romero et al. (20) reported 34 metastatic liver tumors in 14 patients treated with SBRT using 37.5 Gy in three fractions (prescribed to 65%), achieving 94% local control. In 2009, Rusthoven et al. (21) reported 63 metastatic liver tumors in 57 patients treated with SBRT using 36–60 Gy in three fractions, achieving a 3-year local control rate of 92% and a 2-year overall survival rate of 30%.
BONE
Oligo-recurrence and oligometastases of bone have been reported in breast cancer. The summary is that high-dose radiotherapy relieves pain for a long time and can even improve overall survival.
Niibe et al. (4) reported on solitary bone metastases in seven patients treated with conventional radiotherapy. Six of seven patients achieved complete remission of pain, which was prolonged at the last follow-up. Only one patient showed relapse of pain. This patient received 30 Gy in 10 fractions (BED10, 39 Gy), representing the smallest dose in that series (other patients received 40–50 Gy in 20–25 fractions; BED10 ≥ 48 Gy). In 2009, Milano et al. (22) reported 85 metastatic lesions in 40 breast cancer patients treated with SBRT, achieving a 2-year overall survival rate of 76% and a 4-year overall survival of 59%. Among these, the most favorable prognostic factor for breast oligometastatic patients was metastases only involving bone. This indicated high-dose radiotherapy using SBRT for bone metastases could contribute to patient survival.
LYMPH NODES
Oligometastases and oligo-recurrence of distant lymph node metastases have been reported for uterine cervical carcinoma. Uterine cervical carcinoma spreads through the lymphatic route rather than hematogenously (2,23). The first site of distant metastasis of uterine cervical carcinoma is the para-aortic lymph node. This has been confirmed in a large population-based study (2).
Hong et al. (24) reported 35 patients with isolated para-aortic lymph node recurrence treated with concurrent chemoradiotherapy achieving a 5-year overall survival rate of 34%. Kim et al. (25) reported 12 patients treated with hyperfractionated radiotherapy totaling 60 Gy combined with concurrent chemoradiotherapy, achieving a 3-year overall survival rate of 19%. To date, the largest study has been reported by Niibe et al. (3) in Japan. They reported 84 patients treated with conventional radiotherapy with or without chemotherapy achieved a 5-year overall survival rate of 31.3%, similar to the 38% for 5-year overall survival rate in a previous, small, population-based study in Japan (26). Recently, Choi et al. (27) reported that 30 uterine cervical and corpus cancer patients with isolated para-aortic lymph node recurrence treated by SBRT using a cyberknife achieved a 4-year overall survival of 50.1%.
CONCLUSIONS
Curative local therapy for oligometastases and oligo-recurrence represents a brilliant opening to the era of cancer therapy. Several decades ago, most metastatic and recurrent cancer patients died within a year. However, we cope with metastases or recurrences considering whether the state is oligometastases or oligo-recurrence. In the state of oligo-recurrence, all the gross tumors could be treated with local therapy, meaning curative treatment. However, in the state of oligometastases, clinicians should judge a primary site to be controlled or not before treatment. If the primary site is controlled, meaning oligo-recurrence, they should pursue to cure the patients. However, if the primary site is uncontrolled or extra-target metastases lesions exist, they intend to prolong survival not to pursue cure.
More appropriate target cancers, treatment modalities and schedules should be established for oligometastases and oligo-recurrence. Moreover, adjuvant chemotherapy will improve dramatically because of molecular-targeted drugs. Further clinical studies are required in this field.
Funding
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Ministry of Health, Labour and Welfare of Japan and Foundation for Promotion of Cancer Research of Japan.
Conflict of interest statement
None declared.
References
- 1.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Niibe Y, Kazumoto T, Toita T, Yamazaki H, Higuchi K, Ii N, et al. Frequency and characteristics of isolated para-aortic lymph node recurrence in patients with uterine cervical carcinoma in Japan: a multi-institutional study. Gynecol Oncol. 2006;103:435–8. doi: 10.1016/j.ygyno.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Niibe Y, Kenjo M, Kazumoto T, Michimoto K, Takayama M, Yamauchi C, et al. Multi-institutional study of radiation therapy for isolated para-aortic lymph node recurrence in uterine cervical carcinoma: 84 subjects of a population of more than 5000. Int J Radiat Oncol Biol Phys. 2006;66:1366–9. doi: 10.1016/j.ijrobp.2006.07.1384. [DOI] [PubMed] [Google Scholar]
- 4.Niibe Y, Kuranami M, Matsunaga K, Takaya M, Kakita S, Hara T, et al. Value of high-dose radiation therapy for isolated osseous metastasis in breast cancer in terms of oligo-recurrence. Anticancer Res. 2008;28:3929–31. [PubMed] [Google Scholar]
- 5.Punglia RS, Morrow M, Winer EP, Harris JR. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 6.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–51. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M, Maarouf M, Bendel M, Voges J, Muller RP, Sturm V. Linac radiosurgery versus whole brain radiotherapy for brain metastases. A survival comparison based on the RTOG recursive partitioning analysis. Strahlenther Onkol. 2004;180:263–7. doi: 10.1007/s00066-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 8.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomized trial. Lancet. 2004;363:1665–72. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 9.Niibe Y, Karasawa K, Nankamura O, Shinoura N, Okamoto K, Ymada R, et al. Survival benefit of stereotactic radiosurgery for metastatic brain tumors in patients with controlled primary lesions and no other distant metastases. Anticancer Res. 2003;23:4157–60. [PubMed] [Google Scholar]
- 10.Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 11.Onihsi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 12.Bloomgren H, Lax I, Naslund I, Vanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–70. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 13.Uematsu M, Shioda A, Tahara K, Fukui T, Yamamoto F, Tsumatori G, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998;82:1062–70. doi: 10.1002/(sici)1097-0142(19980315)82:6<1062::aid-cncr8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Nagata Y, Negoro Y, Aoki T, Mizowaki T, Takayama K, Kokubo M, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2002;15:1041–6. doi: 10.1016/s0360-3016(01)02731-6. [DOI] [PubMed] [Google Scholar]
- 15.Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–84. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 17.Blomgren H, Lax I, Goranson H, Kræpelien T, Nilsson B, Näslund I, et al. Radiosurgery for tumors in the body: clinical experience using a new method. J Radiosurg. 1998;1:63–74. [Google Scholar]
- 18.Herfarthe KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:170. doi: 10.1200/JCO.2001.19.1.164. 164. [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh BD, Shefter TE, Cardenes HR, Stieber VW, Raben D, Timmerman RB, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–55. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 20.Mendez Romero A, Wuderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I–II study. Acta Oncol. 2006;45:831–7. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 21.Rusthoven KE, Kavanagh BD, Candenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–8. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 22.Milano MT, Zhang H, Metcalfe SK, Muhs AG, Okunieff P. Oligometastatic breast cancer treated with curative-intent stereotactic body radiation therapy. Breast Cancer Res Treat. 2009;115:601–8. doi: 10.1007/s10549-008-0157-4. [DOI] [PubMed] [Google Scholar]
- 23.Robert JK, Henry JN, Edward W. Tumors of the cervix. In: Bethesda MD, editor. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology; 1992. pp. 37–139. [Google Scholar]
- 24.Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of the cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:249–57. doi: 10.1016/j.ijrobp.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Kim JS, Kim SY, Kim KH, Cho MJ. Hyperfractionated radiotherapy with concurrent chemotherapy for para-aortic lymph node recurrence in carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2003;55:1247–53. doi: 10.1016/s0360-3016(02)04401-2. [DOI] [PubMed] [Google Scholar]
- 26.Niibe Y, Nakano T, Ohno T, Suzuki Y, Oka K, Tsujii H. Prognostic significance of c-erbB-2/HER2 expression in advanced uterine cervical carcinoma with para-aortic lymph node metastasis treated with radiation therapy. Int J Gynecol Cancer. 2003;13:849–55. doi: 10.1111/j.1525-1438.2003.13397.x. [DOI] [PubMed] [Google Scholar]
- 27.Choi CW, Cho CK, Yoo SY, Kim MS, Yang KM, Yoo HJ, et al. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147–53. doi: 10.1016/j.ijrobp.2008.07.020. [DOI] [PubMed] [Google Scholar]