Introduction
Collagen is a fibrous protein comprising a right-handed, triple-helical bundle of three parallel, left-handed polyproline II-type helices. Each strand consists of approximately 300 repeats of the trimer (Xaa–Yaa–Gly), where Xaa is often (2S)-proline (Pro) and Yaa is often (2S,4R)-4-hydroxyproline (Hyp) [1]. The most abundant protein in vertebrates, collagen is of fundamental importance to the three-dimensional architecture of such animals. Understanding the chemical determinants of the structure and stability of collagen is essential for both curing collagen-related diseases and creating collagen-based biomaterials.
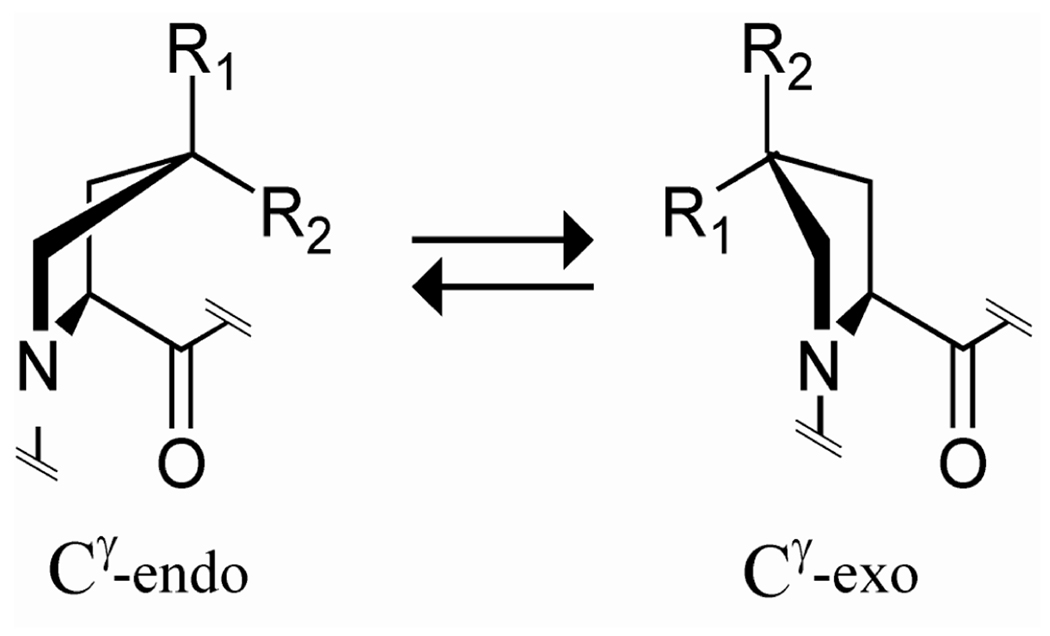
We have previously demonstrated that pyrrolidine ring pucker and thus triple-helical stability can be manipulated by functionalizing the γ-position of the proline ring with moieties that control the ring pucker via either steric or stereoelectronic effects (Figure 1). The presence of Hyp in the Yaa position of natural collagen greatly increases the stability of the triple helix [2]. This enhanced stability is presumably due to a stereoelectronic effect that defines the pyrrolidine ring pucker of Hyp residues as the Cγ-exo pucker. The Cγ-exo ring pucker fixes the ϕ and ψ angles of Hyp residues to those required for triple-helical assembly [3]. Substitution of non-natural proline derivatives that prefer the Cγ-exo ring pucker, such as (2S,4R)-4-fluoroproline (Flp) [4] or (2S,4S)-4-methylproline (Mep) [5], for proline in the Yaa position also stabilizes triple helices. In contrast, the Cγ-endo ring pucker is favorable for triple-helix stability in the Xaa position. Consequently, substitution of (2S,4S)-4-fluoroproline (flp) and (2S,4R)-4-methylproline (mep) for proline in the Xaa position stabilizes triple helices, because flp and mep prefer the Cγ-endo ring pucker [5,6].
Figure 1.
Ring conformations of 4-substituted prolines. The Cγ -endo conformation is favored strongly by stereoelectronic effects when R1 = H, R2 = F (flp) or Cl (clp) and by steric effects when R1 = Me (mep), R2 = H. The Cγ-exo conformation is favored strongly by stereoelectronic effects when R1 = OH (Hyp), F (Flp) or Cl (Clp), R2 = H and by steric effects when R1 = H, R2 = Me (Mep).
Here, we introduce additional proline derivatives that can be utilized to endow conformational stability and unique properties on collagen triple helices, namely the 4-chloroprolines. Additionally, we summarize the ability to modulate the thermal stability of collagen over a wide range of temperatures by modifying the γ-carbon of proline residues.
Results and Discussion
We hypothesized that (2S,4R)-4-chloroproline (Clp) would prefer the Cγ-exo pyrrolidine ring pucker and that (2S,4S)-4-chloroproline (clp) would prefer the Cγ-endo ring pucker (Figure 1). Thus, Clp should stabilize triple helices in the Yaa position, while clp should stabilize triple helices in the Xaa position. To confirm this hypothesis, collagenous peptides containing clp and Clp synthesized by a route similar to that of Berger and coworkers [7] were prepared by segment condensation on the solid phase. We tested the triple-helical propensity of clp and Clp and found that they enable triple helix formation when placed in the Xaa or Yaa position, respectively (Table 1), confirming our hypothesis. (Clp–Pro–Gly)10 and (clp–Clp–Gly)10 do not form triple helices, as expected from prior results for flp and Flp in collagen mimics [6,8].
Table 1.
Effect of clp and Clp on collagen triple-helix stability.
| Peptide | Tm |
|---|---|
| (Pro–Clp–Gly)10 | 52 |
| (Pro–Pro–Gly)10 | 41 |
| (clp–Pro–Gly)10 | 33 |
| (Pro–Clp–Gly)7 | 23 |
| (clp–Pro–Gly)7 | <10 |
| (Pro–Pro–Gly)7 | <10 |
| (clp–Clp–Gly)10 | no helix |
| (Clp–Pro–Gly)10 | no helix |
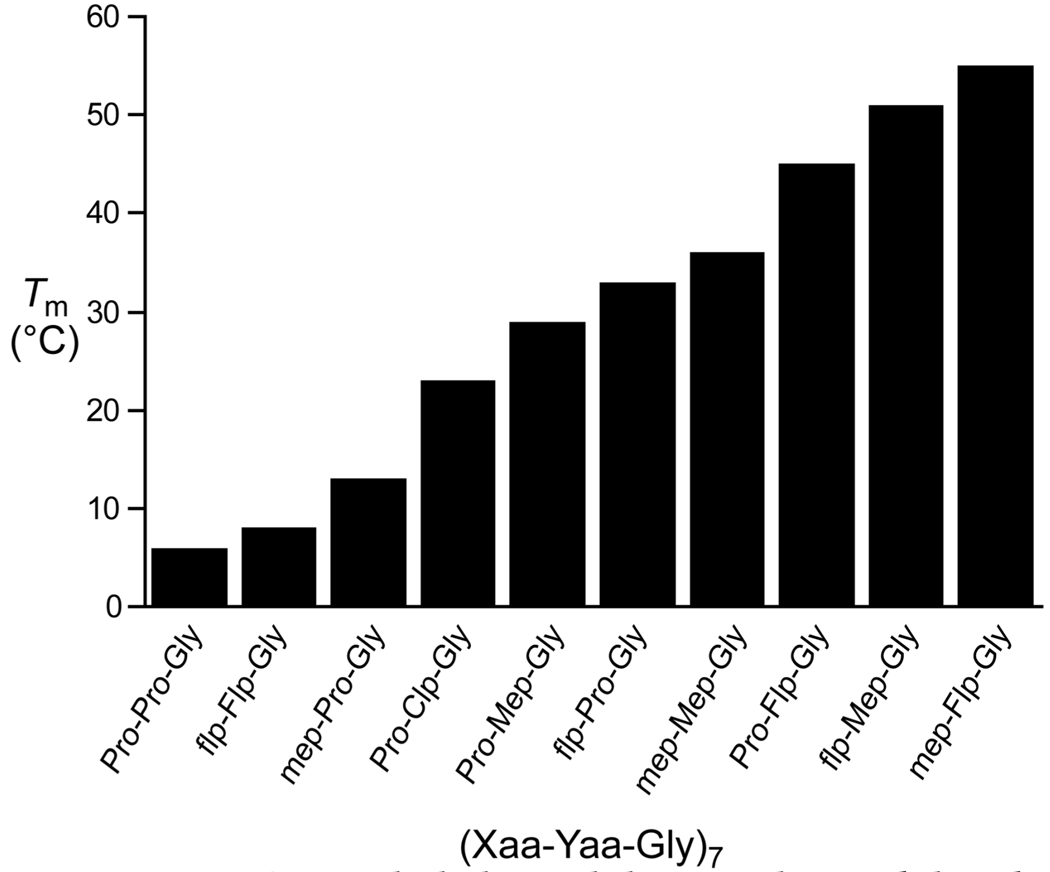
Figure 2 illustrates our ability to fine-tune the thermal stability of triple helices of the same length using the results detailed herein and in previous results from our laboratory [3,5,6,8]. Utilizing 4-chloroprolines, 4-methylprolines, 4-fluoroprolines, and combinations of these residues, we have synthesized 21-residue, blunt-ended collagen triple helices with Tm values ranging from 0–53 °C. This ability to modulate triple-helical stability by simply modifying the γ-position of proline rings portends the development of synthetic collagen mimetics for applications requiring triple-helical stability over a wide range of temperatures.
Figure 2.
Triple-helix stability can be modulated over a wide range of temperatures by controlling the identity and configuration of functional groups at the γ-position of proline residues [3,5,6,8].
Acknowledgments
This work was supported by grant AR44276 (NIH). M.D.S. was supported by a graduate fellowship from the Department of Homeland Security.
References
- 1.Jenkins CL, Raines RT. Nat. Prod. Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]
- 2.Sakakibara S, et al. Biochim. et Biophys. Acta. 1973;303:198–202. doi: 10.1016/0005-2795(73)90164-5. [DOI] [PubMed] [Google Scholar]
- 3.DeRider ML, et al. J. Am. Chem. Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 4.Holmgren SK, et al. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 5.Shoulders MD, Hodges JA, Raines RT. J. Am. Chem. Soc. 2006;128:8112–8113. doi: 10.1021/ja061793d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodges JA, Raines RT. J. Am. Chem. Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]
- 7.Berger Y, et al. J. Med. Chem. 2005;48:483–498. doi: 10.1021/jm040857x. [DOI] [PubMed] [Google Scholar]
- 8.Hodges JA, Raines RT. J. Am. Chem. Soc. 2005;127:15923–15932. doi: 10.1021/ja054674r. [DOI] [PubMed] [Google Scholar]