Abstract
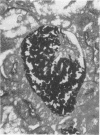
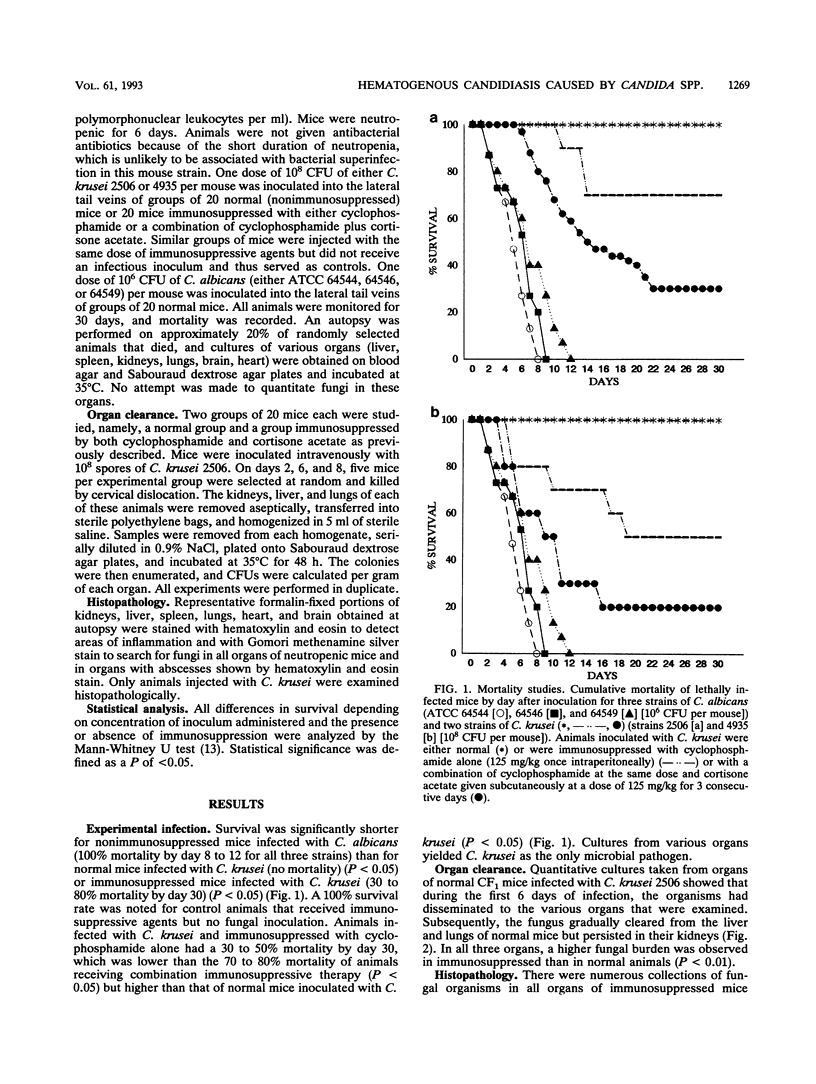
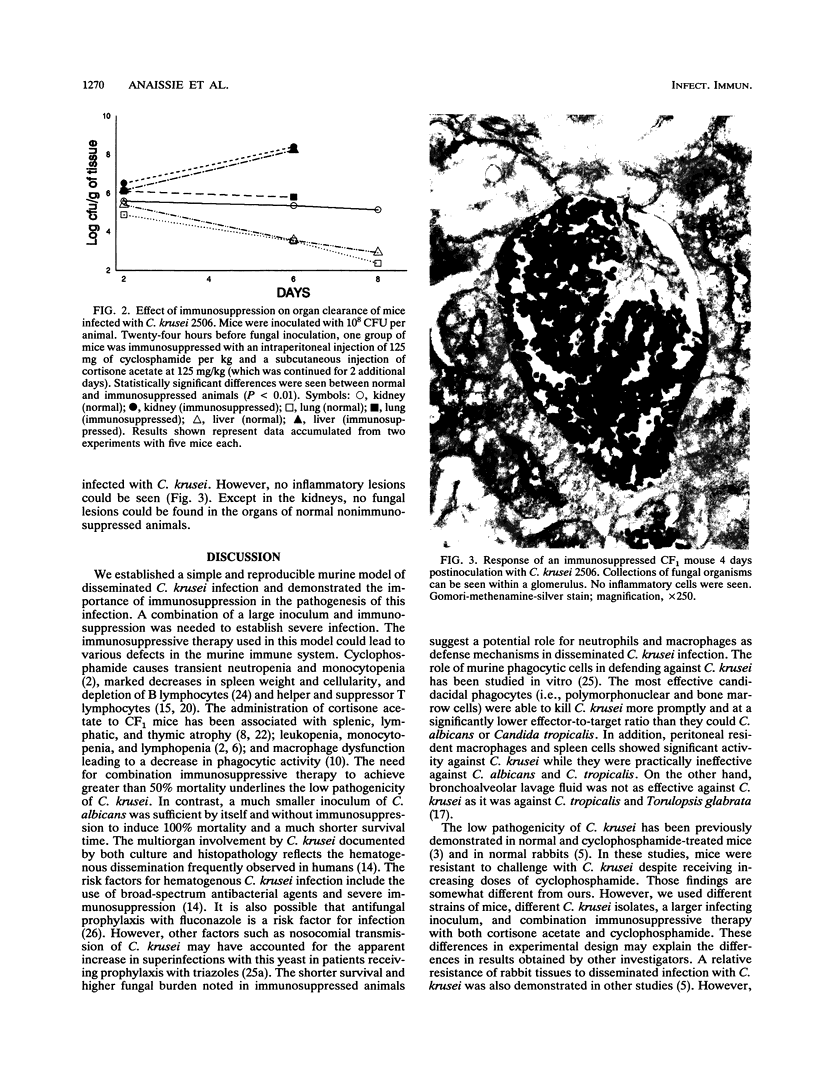
Hematogenous infections caused by Candida krusei have been noted with increasing frequency, particularly in cancer patients receiving prophylaxis with antifungal triazoles. Progress in understanding the pathogenesis of this emerging infection has been limited by the lack of an animal model. We developed a CF1 mouse intravenous inoculation model of candidiasis to evaluate the pathogenicity of C. krusei in normal and immunosuppressed mice and to compare it with that of Candida albicans. Several inocula (10(6) to 10(8) CFU per animal) of two clinical strains of C. krusei and three American Type Culture Collection strains of C. albicans were tested. Groups of 20 mice each were injected with a single intravenous dose of one inoculum. Animals randomized to receive C. krusei were immunosuppressed by intraperitoneal injection of cyclophosphamide or the combination of cyclophosphamide plus cortisone acetate or they did not receive immunosuppressive agents (normal mice). One hundred percent mortality was observed in normal mice injected with 10(6) CFU of C. albicans per mouse compared with no mortality in normal mice that received 10(8) CFU of C. krusei per mouse (P < 0.01). Resistance to C. krusei infection was markedly lowered by immunosuppression, particularly by the combination of cyclophosphamide plus cortisone acetate, with a significantly shorter survival and a higher organ fungal burden in immunosuppressed than in normal animals (P < 0.01). Tissue infection was documented by culture and histopathologic findings in all examined organs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anaissie E. Opportunistic mycoses in the immunocompromised host: experience at a cancer center and review. Clin Infect Dis. 1992 Mar;14 (Suppl 1):S43–S53. doi: 10.1093/clinids/14.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Baccarini M., Blasi E., Marconi P., Puccetti P., Garaci E. Correlation between in vivo and in vitro studies of modulation of resistance to experimental Candida albicans infection by cyclophosphamide in mice. Infect Immun. 1983 Apr;40(1):46–55. doi: 10.1128/iai.40.1.46-55.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Sbaraglia G., Perito S., Cassone A. A comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia. 1984;22(5):409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Montgomerie J. Z., Ishida K., Morrison J. O., Guze L. B. Experimental hematogenous endophthalmitis due to Candida: species variation in ocular pathogenicity. J Infect Dis. 1977 Feb;135(2):294–297. doi: 10.1093/infdis/135.2.294. [DOI] [PubMed] [Google Scholar]
- FRENKEL J. K., HAVENHILL M. A. THE CORTICOID SENSITIVITY OF GOLDEN HAMSTERS, RATS, AND MICE. EFFECTS OF DOSE, TIME, AND ROUTE OF ADMINISTRATION. Lab Invest. 1963 Dec;12:1204–1220. [PubMed] [Google Scholar]
- Filice G. A., Niewoehner D. E. Contribution of neutrophils and cell-mediated immunity to control of Nocardia asteroides in murine lungs. J Infect Dis. 1987 Jul;156(1):113–121. doi: 10.1093/infdis/156.1.113. [DOI] [PubMed] [Google Scholar]
- Fisher M. A., Shen S. H., Haddad J., Tarry W. F. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 1989 Sep;33(9):1443–1446. doi: 10.1128/aac.33.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. L., Winston D. J., Greenfield R. A., Chandrasekar P. H., Fox B., Kaizer H., Shadduck R. K., Shea T. C., Stiff P., Friedman D. J. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992 Mar 26;326(13):845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- Gotjamanos T. Alterations in reticuloendothelial organ structure and function following cortisone administration to mice. J Reticuloendothel Soc. 1970 Nov;8(5):421–433. [PubMed] [Google Scholar]
- Graybill J. R. Systemic fungal infections: diagnosis and treatment. I. Therapeutic agents. Infect Dis Clin North Am. 1988 Dec;2(4):805–825. [PubMed] [Google Scholar]
- LONG D. A., SHEWELL J. A species difference with regard to the effect of cortisone acetate on body weight, gamma-globulin and circulating antitoxin levels. J Hyg (Lond) 1956 Dec;54(4):452–460. doi: 10.1017/s0022172400044739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- McQuillen D. P., Zingman B. S., Meunier F., Levitz S. M. Invasive infections due to Candida krusei: report of ten cases of fungemia that include three cases of endophthalmitis. Clin Infect Dis. 1992 Feb;14(2):472–478. doi: 10.1093/clinids/14.2.472. [DOI] [PubMed] [Google Scholar]
- Merz W. G., Karp J. E., Schron D., Saral R. Increased incidence of fungemia caused by Candida krusei. J Clin Microbiol. 1986 Oct;24(4):581–584. doi: 10.1128/jcm.24.4.581-584.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Morace G., Manzara S., Dettori G. In vitro susceptibility of 119 yeast isolates to fluconazole, 5-fluorocytosine, amphotericin B and ketoconazole. Chemotherapy. 1991;37(1):23–31. doi: 10.1159/000238828. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Fick R. B., Jr Candidacidal factors in murine bronchoalveolar lavage fluid. Infect Immun. 1987 Mar;55(3):541–546. doi: 10.1128/iai.55.3.541-546.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Activity of cilofungin (LY121019) against Candida species in vitro. J Antimicrob Chemother. 1988 Dec;22(6):891–897. doi: 10.1093/jac/22.6.891. [DOI] [PubMed] [Google Scholar]
- Ohkawa M., Tokunaga S., Takashima M., Nishikawa T., Hisazumi H., Fujita S., Wheat R. W. In vitro susceptibility testing of Candida isolates from clinical specimens to four antifungal agents. Chemotherapy. 1990;36(6):396–402. doi: 10.1159/000238795. [DOI] [PubMed] [Google Scholar]
- Röllinghoff M., Starzinski-Powitz A., Pfizenmaier K., Wagner H. Cyclophosphamide-sensitive T lymphocytes suppress the in vivo generation of antigen-specific cytotoxic T lymphocytes. J Exp Med. 1977 Feb 1;145(2):455–459. doi: 10.1084/jem.145.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag M. S., Powderly W. G., Cloud G. A., Robinson P., Grieco M. H., Sharkey P. K., Thompson S. E., Sugar A. M., Tuazon C. U., Fisher J. F. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992 Jan 9;326(2):83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- Thomalla J. V., Steidle C. P., Leapman S. B., Filo R. S. Ureteral obstruction of a renal allograft secondary to Candida krusei. Transplant Proc. 1988 Jun;20(3):551–554. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A., Bistoni F., Cenci E., Perito S., Cassone A. In-vitro killing of Candida species by murine immunoeffectors and its relationship to the experimental pathogenicity. Sabouraudia. 1985 Oct;23(5):377–387. doi: 10.1080/00362178585380541. [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Merz W. G., Rinaldi M. G., Johnson T. R., Karp J. E., Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991 Oct 31;325(18):1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]