Abstract
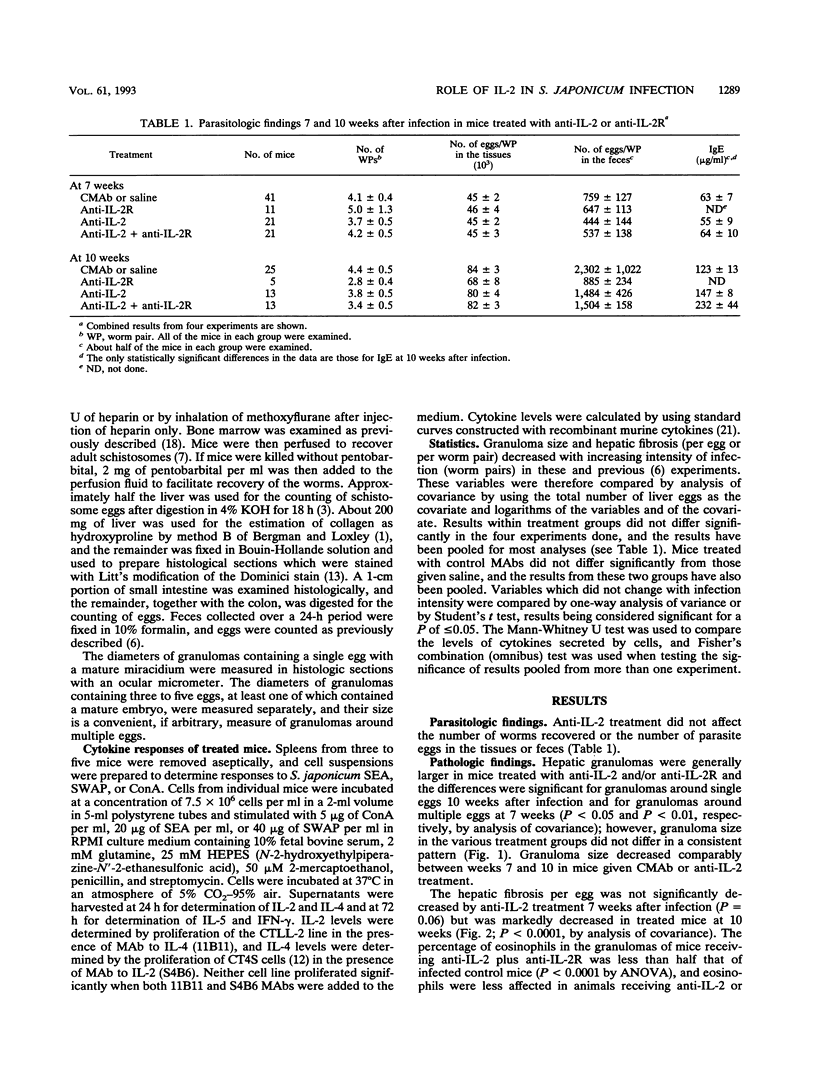
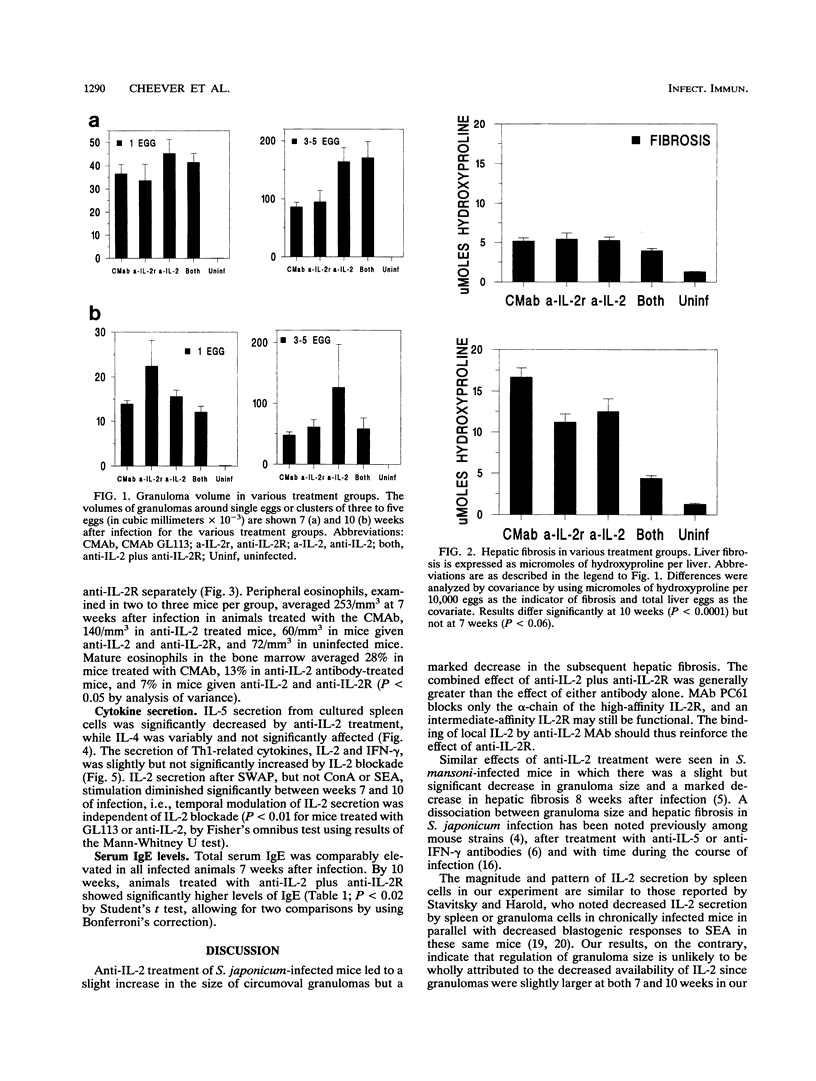
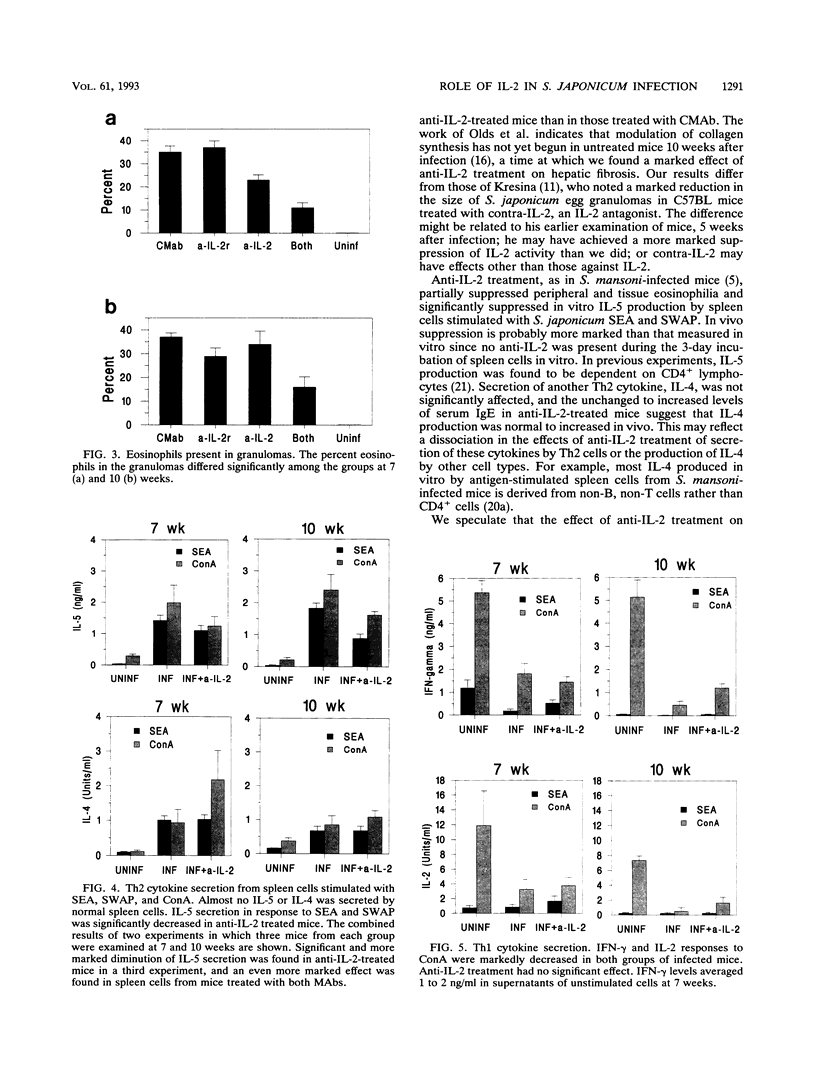
Schistosoma japonicum-infected mice were injected with antibodies to interleukin-2 (IL-2) and/or IL-2 receptor to clarify the role of IL-2 on the granulomatous reaction around schistosome eggs in the liver. Granulomas were of normal or slightly increased size in animals subjected to IL-2 blockade, but hepatic fibrosis was markedly decreased in treated animals 10 weeks after infection. Anti-IL-2 treatment significantly decreased the in vitro secretion of IL-5 by antigen-stimulated spleen cells, and peripheral eosinophilia and tissue eosinophilia were diminished. Secretion of IL-2, IL-4, and gamma interferon was unaffected. Our results indicate that IL-2 is not an essential determinant of granuloma size in S. japonicum-infected mice but that, as in Schistosoma mansoni infection, the development of hepatic fibrosis is critically dependent on IL-2 levels and granuloma size and hepatic fibrosis are differentially regulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Duvall R. H., Hallack T. A., Jr Differences in hepatic fibrosis and granuloma size in several strains of mice infected with Schistosoma japonicum. Am J Trop Med Hyg. 1984 Jul;33(4):602–607. doi: 10.4269/ajtmh.1984.33.602. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Finkelman F. D., Caspar P., Heiny S., Macedonia J. G., Sher A. Treatment with anti-IL-2 antibodies reduces hepatic pathology and eosinophilia in Schistosoma mansoni-infected mice while selectively inhibiting T cell IL-5 production. J Immunol. 1992 May 15;148(10):3244–3248. [PubMed] [Google Scholar]
- Cheever A. W. Relative resistance of the eggs of human schistosomes to digestion in potassium hydroxide. Bull World Health Organ. 1970;43(4):601–603. [PMC free article] [PubMed] [Google Scholar]
- Cheever A. W., Xu Y. H., Sher A., Macedonia J. G. Analysis of egg granuloma formation in Schistosoma japonicum-infected mice treated with antibodies to interleukin-5 and gamma interferon. Infect Immun. 1991 Nov;59(11):4071–4074. doi: 10.1128/iai.59.11.4071-4074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall R. H., DeWitt W. B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967 Jul;16(4):483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Snapper C. M., Mountz J. D., Katona I. M. Polyclonal activation of the murine immune system by a goat antibody to mouse IgD. IX. Induction of a polyclonal IgE response. J Immunol. 1987 May 1;138(9):2826–2830. [PubMed] [Google Scholar]
- Henderson G. S., Conary J. T., Summar M., McCurley T. L., Colley D. G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. I. IL-4 mRNA, not IL-2 mRNA, is abundant in the granulomatous livers, mesenteric lymph nodes, and spleens of infected mice. J Immunol. 1991 Aug 1;147(3):992–997. [PubMed] [Google Scholar]
- Houssiau F. A., Renauld J. C., Fibbe W. E., Van Snick J. IL-2 dependence of IL-9 expression in human T lymphocytes. J Immunol. 1992 May 15;148(10):3147–3151. [PubMed] [Google Scholar]
- Kresina T. F. In vivo regulation of S. japonica granuloma formation by an IL-2 antagonist. Parasitology. 1991 Apr;102(Pt 2):243–249. doi: 10.1017/s0031182000062557. [DOI] [PubMed] [Google Scholar]
- LITT M. Studies in experimental eosinophilia. V. Eosinophils in lynph nodes of guinea pigs following primary antigenic stimulation. Am J Pathol. 1963 May;42:529–549. [PMC free article] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Seder R., Finkelman F. D., Paul W. E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990 Sep 1;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Griffin A., Kresina T. F. Dynamics of collagen accumulation and polymorphism in murine Schistosoma japonicum. Gastroenterology. 1985 Sep;89(3):617–624. doi: 10.1016/0016-5085(85)90459-7. [DOI] [PubMed] [Google Scholar]
- Perrin P. J., Phillips S. M. The molecular basis of granuloma formation in schistosomiasis. III. In vivo effects of a T cell-derived suppressor effector factor and IL-2 on granuloma formation. J Immunol. 1989 Jul 15;143(2):649–654. [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Harold W. W. Deficiency of interleukin-2 activity upon addition of soluble egg antigen to cultures of spleen cells from mice infected with Schistosoma japonicum. Infect Immun. 1988 Jul;56(7):1778–1784. doi: 10.1128/iai.56.7.1778-1784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Harold W. W. Deficiency of interleukin-2 production upon addition of soluble egg antigen to cultures of isolated hepatic granulomas or hepatic granuloma cells from mice infected with Schistosoma japonicum. Infect Immun. 1989 Aug;57(8):2339–2344. doi: 10.1128/iai.57.8.2339-2344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. H., Macedonia J., Sher A., Pearce E., Cheever A. W. Dynamic analysis of splenic Th1 and Th2 lymphocyte functions in mice infected with Schistosoma japonicum. Infect Immun. 1991 Sep;59(9):2934–2940. doi: 10.1128/iai.59.9.2934-2940.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Boros D. L. Changing patterns of lymphocyte proliferation, IL-2 production and utilization, and IL-2 receptor expression in mice infected with Schistosoma mansoni. J Immunol. 1990 Jul 15;145(2):724–731. [PubMed] [Google Scholar]



