Abstract
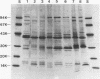
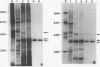
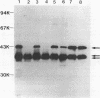
Haemophilus ducreyi contains a major outer membrane protein (MOMP) whose apparent molecular weight is 39,000 to 42,000 for all strains tested. Two monoclonal antibodies (MAbs), designated 9D12 and 2C7, bound to the MOMP for all strains of H. ducreyi tested. As reported previously, MAb 9D12 was H. ducreyi specific (E. J. Hansen and T. A. Loftus, Infect. Immun. 44:196-198, 1984). MAb 2C7 bound to all members of the family Pasteurellaceae tested, suggesting that the MAbs bound to distinct epitopes on the MOMP. The MOMP was purified by extraction of whole cells with Zwittergent and ion-exchange chromatography. A peak eluted from a cation-exchange column contained three bands. All three species bound both MAbs, and the fraction yielded a single N-terminal amino acid sequence, suggesting that the bands represented different conformations of the MOMP. The MOMP was heat modifiable, contained two cysteine residues, and was cationic at pH 8.0, features not usually associated with classical porin proteins. The N-terminal amino acid sequence and total amino acid content of the MOMP were homologous to the OmpA proteins of members of the family Enterobacteriaceae and the OmpA-like protein of Actinobacillus actinomycetemcomitans. An OmpA-specific polyclonal serum bound to the MOMP, and MAb 2C7 bound to Haemophilus influenzae protein 5, an OmpA-like protein, indicating that the MOMP was antigenically related to OmpA. These data indicated that the most abundant protein in the outer membrane of H. ducreyi was not a classical porin and belonged to the OmpA family of proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeck D., Johnson A. P., Taylor-Robinson D. Antigenic analysis of Haemophilus ducreyi by Western blotting. Epidemiol Infect. 1988 Aug;101(1):151–157. doi: 10.1017/s0950268800029319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Dudas K. C., Campagnari A., Rice P., Mylotte J. M., Murphy T. F. Antigenic heterogeneity of lipid A of Haemophilus influenzae. Infect Immun. 1985 Oct;50(1):9–14. doi: 10.1128/iai.50.1.9-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Stierhof Y. D., Gamon K., Hindennach I., Henning U. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem. 1986 Aug 25;261(24):11355–11361. [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M. E., Blake M. S., Koomey M. Porin protein of Neisseria gonorrhoeae: cloning and gene structure. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8135–8139. doi: 10.1073/pnas.84.22.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M., Blake M. S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. Homology of protein III with enterobacterial OmpA proteins. J Exp Med. 1987 Feb 1;165(2):471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Loftus T. A. Monoclonal antibodies reactive with all strains of Haemophilus ducreyi. Infect Immun. 1984 Apr;44(1):196–198. doi: 10.1128/iai.44.1.196-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Sonntag I., Hindennach I. Mutants (ompA) affecting a major outer membrane protein of Escherichia coli K12. Eur J Biochem. 1978 Dec;92(2):491–498. doi: 10.1111/j.1432-1033.1978.tb12771.x. [DOI] [PubMed] [Google Scholar]
- Jessamine P. G., Ronald A. R. Chancroid and the role of genital ulcer disease in the spread of human retroviruses. Med Clin North Am. 1990 Nov;74(6):1417–1431. doi: 10.1016/s0025-7125(16)30488-6. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Coombs R., Plummer F., Holmes K. K., Nikora B., Cameron W., Ngugi E., Ndinya Achola J. O., Corey L. Isolation of human immunodeficiency virus from genital ulcers in Nairobi prostitutes. J Infect Dis. 1989 Sep;160(3):380–384. doi: 10.1093/infdis/160.3.380. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lytton E. J., Blake M. S. Isolation and partial characterization of the reduction-modifiable protein of Neisseria gonorrhoeae. J Exp Med. 1986 Nov 1;164(5):1749–1759. doi: 10.1084/jem.164.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Morse S. A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989 Apr;2(2):137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Tolan R. W., Jr Molecular cloning, expression, and primary sequence of outer membrane protein P2 of Haemophilus influenzae type b. Infect Immun. 1989 Jan;57(1):88–94. doi: 10.1128/iai.57.1.88-94.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988 Oct;56(10):2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Purification and analysis with monoclonal antibodies of P2, the major outer membrane protein of nontypable Haemophilus influenzae. Infect Immun. 1988 May;56(5):1084–1089. doi: 10.1128/iai.56.5.1084-1089.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Harayama S. Identification and characterization of porins in Pseudomonas aeruginosa. J Biol Chem. 1991 Jan 15;266(2):770–779. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989 Nov;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Ronald A. R., Albritton W. L. Characterization of cell proteins of Haemophilus ducreyi by polyacrylamide gel electrophoresis. J Infect Dis. 1983 Oct;148(4):710–714. doi: 10.1093/infdis/148.4.710. [DOI] [PubMed] [Google Scholar]
- Odumeru J. A., Wiseman G. M., Ronald A. R. Virulence factors of Haemophilus ducreyi. Infect Immun. 1984 Feb;43(2):607–611. doi: 10.1128/iai.43.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Cameron D. W., Ndinya-Achola J. O., Kreiss J. K., Gakinya M. N., Waiyaki P., Cheang M., Piot P., Ronald A. R. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991 Feb;163(2):233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggen E. L., De Breucker S., van Dyck E., Piot P. Antigenic diversity in Haemophilus ducreyi as shown by western blot (immunoblot) analysis. Infect Immun. 1992 Feb;60(2):590–595. doi: 10.1128/iai.60.2.590-595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Folds J. D. Immunoblot analysis of antigens associated with Haemophilus ducreyi using serum from immunised rabbits. Genitourin Med. 1986 Oct;62(5):321–328. doi: 10.1136/sti.62.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalla W. O., Sanders L. L., Schmid G. P., Tam M. R., Morse S. A. Use of dot-immunobinding and immunofluorescence assays to investigate clinically suspected cases of chancroid. J Infect Dis. 1986 May;153(5):879–887. doi: 10.1093/infdis/153.5.879. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Hindennach I., Garten W., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Interaction of protein II with lipopolysaccharide. Eur J Biochem. 1978 Jan 2;82(1):211–217. doi: 10.1111/j.1432-1033.1978.tb12013.x. [DOI] [PubMed] [Google Scholar]
- Simonsen J. N., Cameron D. W., Gakinya M. N., Ndinya-Achola J. O., D'Costa L. J., Karasira P., Cheang M., Ronald A. R., Piot P., Plummer F. A. Human immunodeficiency virus infection among men with sexually transmitted diseases. Experience from a center in Africa. N Engl J Med. 1988 Aug 4;319(5):274–278. doi: 10.1056/NEJM198808043190504. [DOI] [PubMed] [Google Scholar]
- Spinola S. M., Griffiths G. E., Bogdan J., Menegus M. A. Characterization of an 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect Immun. 1992 Feb;60(2):385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Kwaik Y. A., Lesse A. J., Campagnari A. A., Apicella M. A. Cloning and expression in Escherichia coli of a Haemophilus influenzae type b lipooligosaccharide synthesis gene(s) that encodes a 2-keto-3-deoxyoctulosonic acid epitope. Infect Immun. 1990 Jun;58(6):1558–1564. doi: 10.1128/iai.58.6.1558-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinola S. M., Peacock J., Denny F. W., Smith D. L., Cannon J. G. Epidemiology of colonization by nontypable Haemophilus influenzae in children: a longitudinal study. J Infect Dis. 1986 Jul;154(1):100–109. doi: 10.1093/infdis/154.1.100. [DOI] [PubMed] [Google Scholar]
- Sugawara E., Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J Biol Chem. 1992 Feb 5;267(4):2507–2511. [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Hanchalay S., Pitarangsi C., Slootmans L., Piot P. Antimicrobial susceptibility and characterization of outer membrane proteins of Haemophilus ducreyi isolated in Thailand. J Clin Microbiol. 1985 Mar;21(3):442–444. doi: 10.1128/jcm.21.3.442-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J. N., Gotschlich E. C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991 Jul;59(7):2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. E. The heat-modifiable outer membrane protein of Actinobacillus actinomycetemcomitans: relationship to OmpA proteins. Infect Immun. 1991 Jul;59(7):2505–2507. doi: 10.1128/iai.59.7.2505-2507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E., Nakae T. Identification of porins in the outer membrane of Pseudomonas aeruginosa that form small diffusion pores. J Biol Chem. 1989 Apr 15;264(11):6297–6301. [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]