Abstract
Objectives
Familial combined hyperlipidemia (FCH) is a common familial lipid disorder characterized by increases in plasma total cholesterol, triglyceride and apolipoprotein B-100 levels. In light of prior metabolic and genetic research, our purpose was to ascertain whether FCH cases had significant abnormalities of plasma markers of cholesterol synthesis and absorption as compared to unaffected kindred members.
Methods and Results
Plasma levels of squalene, desmosterol and lathosterol (cholesterol synthesis markers) and campesterol, sitosterol and cholestanol (cholesterol absorption markers) were measured by gas-liquid chromatography in 103 FCH patients and 240 normolipidemic relatives (NLR). Squalene, desmosterol, and lathosterol levels were 6% (0.078), 31%, (p<0.001) and 51% (p<0.001) higher in FCH as compared to NLR, and these differences were especially pronounced in women. An interaction with obesity was also noted for a subset of these markers. We did not observe any apparent differences for the cholesterol absorption markers among FCH patients and NLR.
Conclusions
Our data indicate that both men and women with FCH have alterations in the cholesterol synthesis pathway, resulting in 51% higher levels of lathosterol (and additionally desmosterol in women). Plasma levels of the cholesterol precursor sterol squalene were only slightly increased (6%), suggesting enhanced conversion of squalene to lathosterol in this disorder.
Keywords: Cholesterol, Lipids, Sterols, Familial Combined Hyperlipidemia
Introduction
Familial combined hyperlipidemia (FCH) is a common genetic lipid disorder. Affected subjects characteristically have elevated levels of plasma total cholesterol, triglycerides and apolipoprotein (apo) B, and are more prone to develop premature cardiovascular disease (CVD) (1, 2). The prevalence of FCH in the general population has been reported to be up to about 6% (3), while in families with premature coronary heart disease prevalence rates of 15 – 20% have been reported (1, 2, 4, 5).
The pathophysiological mechanism underlying FCH is believed to be hepatic overproduction of apoB-100 containing lipoprotein particles, i.e. VLDL and LDL, resulting in increased plasma total cholesterol, triglycerides and apoB levels (6–8). In addition, lower levels of HDL cholesterol and increased amounts of small dense LDL (sdLDL) and remnant lipoprotein particles have been observed in patients with FCH (2, 9–14).
The heterogeneous lipid profile indicates that FCH is a multifactorial disease in which several genes affect the lipid and lipoprotein metabolism. To date, the most important genetic data has been generated by two genome wide scan studies in Finnish and Dutch populations, respectively, showing associations of the FCH phenotype with regions on chromosome 1 and 11 (15, 16). When the same diagnostic criteria were used, both studies linked FCH to the 1q21–23 genomic region which codes for the upstream transcription factor 1 (USF1) gene (17). USF1, however, transcribes a wide array of genes which play a role in the lipid and glucose metabolism (17), making it a complex task to identify the exact genes involved in FCH.
Previously it has been postulated that the apoB secretory rate is related to cholesterol and cholesterol ester mass (18) and studies show that there is a clear link between fatty acid flux to the liver and increased cholesterol synthesis (19, 20). The purpose of the current study was to investigate the underlying mechanisms leading to the lipid elevations seen in FCH patients by measuring plasma markers of cholesterol synthesis and absorption. Total body cholesterol pools represent a balance between endogenous synthesis of cholesterol on the one hand and dietary absorption of cholesterol on the other (21). It has been documented using formal cholesterol balance studies, that plasma level of the cholesterol precursors: squalene, lathosterol and desmosterol serve as markers of cholesterol synthesis (22, 23), while the plant sterols: campesterol and sitosterol (both derived from the diet) and the bile acid residue cholestanol, are all absorbed in very low quantities in the intestine and their plasma levels have been demonstrated to serve as markers of fractional cholesterol absorption (23). In support, treatment with cholesterol synthesis and absorption inhibiters (i.e. statins and ezetemibe) not only lower total cholesterol levels, they also specifically lower plasma levels of these synthesis and absorption markers, respectively (24, 25).
For the current study we measured squalene, desmosterol, lathosterol (markers of synthesis) and campesterol, sitosterol and cholestanol (markers of absorption) in a population of FCH patients and their normolipidemic relatives (NLR). In addition, we also performed an extensive characterization of the lipid profiles in these FCH families, measuring total cholesterol, triglycerides, LDL-C, HDL-C and VLDL-C, sdLDL-C, total apoB levels, apoB48 particles and remnant lipoprotein cholesterol (RLPC), of which apoB-48 is a specific marker of intestinal lipid transport.
Methods
Study population
The study population consisted of 343 subjects, 103 FCH patients and 240 NLR, from 32 well-defined FCH families (26). The diagnosis of FCH was based on a previously established nomogram (27). Briefly, plasma triglycerides and total cholesterol levels, adjusted for age and gender, and absolute apoB levels were included in a nomogram to calculate the likelihood of having FCH. A subject was defined as being affected with FCH if the probability was above 60%, provided that the diagnostic phenotype was also present in at least one first-degree relative and premature CVD (i.e. before the age of 60) was present in at least one individual in the family. Families were excluded when a secondary cause of the hyperlipidemia was diagnosed in the proband (i.e., diabetes mellitus, hypothyroidism, and hepatic or renal impairment). Prior to the start of the study all participants withdrew from using lipid-lowering medication for at least 4 weeks. Blood was drawn after an overnight fast for laboratory analyses and the isolation of DNA. The ethics committee of the Radboud University Nijmegen Medical Center approved the study protocol and all subjects gave informed consent.
Laboratory Measurements
Plasma total cholesterol, triglycerides, LDL-C, VLDL-C, HDL-C, apoB, and glucose concentrations in all subjects were determined using routine laboratory procedures as previously described (28). Insulin was measured directly by an immunoassay obtained from the Otsuka Corporation (Tokyo, Japan) (29). Small dense (sd) LDL-C was measured using kits obtained from the Denka Seiken Company (Nigata, Japan) (30). RLPC was measured using kits obtained from the Kyowa Medex Corporation (Tokyo, Japan) (31), and plasma apoB48 was measured by an enzyme linked immunoassay obtained from the Shibayagi Company (Maebashi, Japan) as previously described (32). All assays had within and between run coefficients of variation of less than 5%, and the non-routine assays were only performed on a subset of subjects because of limited sample availability (see foot note Table 1).
Table 1.
Study characteristics of the normolipidemic relatives (NLR) and patients with familial combined hyperlipidemia (FCH) in the population at large and by gender.
| All Subjects | Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NLR | FCH | % difference |
NLR | FCH | % difference |
NLR | FCH | % difference |
|
| N | 240 | 103 | - | 117 | 45 | - | 123 | 58 | - |
| Age, years | 44 (15) | 51 (15) | 16* | 44 (14) | 52 (12) | 18* | 44 (15) | 50 (17) | 14‡ |
| BMI, kg/m2 | 25 (3) | 29 (4) | 16* | 25.5 (3.3) | 28.8 (3.5) | 13* | 24.8 (3.5) | 28.5 (4.6) | 15* |
| WHR | 0.87 (0.08) | 0.91 (0.08) | 5† | 0.92 (0.07) | 0.97 (0.04) | 5* | 0.83 (0.06) | 0.86 (0.06) | 4‡ |
| Glucose, mmol/L | 4.8 [4.5, 5.2] | 5.1 [4.6, 5.6] | 6† | 4.9 [4.6, 5.3] | 5.4 [5.0, 5.9] | 10‡ | 4.6 [4.4, 5.0] | 4.9 [4.4, 5.3] | 7‡ |
| Insulin, µU/mL | 5.9 [4.1, 8.1] | 8.8 [6.8, 12.4] | 49* | 6.3 [3.9, 8.3] | 8.7 [6.1, 9.5] | 38† | 5.8 [4.2, 7.8] | 8.9 [7.4, 13.0] | 53* |
| Triglycerides, mmol/L | 1.2 [0.9, 1.6] | 2.9 [2.0, 4.1] | 142* | 1.4 [1.0, 1.8] | 3.4 [2.7, 4.8] | 143* | 1.1 [0.8, 1.3] | 2.5 [1.7, 3.2] | 127* |
| Total cholesterol | 5.2 (1.0) | 6.9 (1.2) | 33* | 5.4 (0.9) | 7.0 (1.2) | 30* | 5.1 (0.9) | 6.8 (1.2) | 33* |
| HDL-C, mmol/L | 1.3 (0.3) | 1.1 (0.3) | −15* | 1.2 (0.3) | 1.0 (0.2) | −17* | 1.4 (0.3) | 1.3 (0.2) | −7* |
| LDL-C, mmol/L | 3.5 (0.9) | 4.2 (1.2) | 20* | 3.7 (0.9) | 3.9 (1.3) | 5 | 3.3 (0.9) | 4.4 (1.1) | 33* |
| Non HDL-C, mmol/L | 3.9 (0.9) | 5.7 (1.2) | 46* | 4.2 (0.9) | 6.0 (1.1) | 43* | 3.7 (0.9) | 5.5 (1.2) | 49* |
| VLDL-C, mmol/L | 0.4 [0.2, 0.6] | 1.1 [0.7, 1.9] | 175* | 0.5 [0.3, 0.7] | 1.5 [1.1, 2.5] | 200* | 0.3 [0.2, 0.5] | 0.9 [0.6, 1.7] | 200* |
| VLDL-TG, mmol/L | 0.7 (0.4, 1.0) | 2.2 (1.3, 3.2) | 214* | 0.9 [0.6, 1.2] | 2.7 [2.1, 4.2] | 200* | 0.6 [0.3, 0.8] | 1.6 [1.0, 2.6] | 167* |
| sdLDL-C, mg/dL | 37 (19) | 57 (27) | 54* | 48 (19) | 50 (24) | 4 | 30 (14) | 60 (27) | 100* |
| RLPC, mmol/L | 6 (4, 10) | 20 (12, 35) | 233* | 8 [5, 12] | 29 [19, 53] | 263* | 5 [4, 8] | 16 [9, 26] | 220* |
| ApoB, mg/L | 960 (221) | 1371 (256) | 43* | 1026 (214) | 1372 (198) | 34* | 898 (210) | 1371 (294) | 53* |
| ApoB48, mg/dL | 0.4 [0.2, 0.6] | 0.8 [0.5, 1.6] | 100* | 0.4 [0.3, 0.6] | 1.4 [1.0, 1.8] | 250* | 0.3 [0.2, 0.5] | 0.7 [0.4, 1.1] | 133* |
Values are mean (sd) or median [inter quartile range].
p<0.001,
p<0.01,
p<0.05.
P values were adjusted for age and family structure. Due to limited sample availability Insulin, sdLDL-C, RLPC and ApoB48 were measured in 269 subjects.
Measurements of Plasma Sterols
Plasma concentrations of squalene, lathosterol, desmosterol, campesterol, sitosterol and cholestanol were measured using gas-liquid chromatography according to methods previously described in all subjects and were expressed as µmol/L as well as normalized to 102 mmol/mol total cholesterol (33).
Statistical Analyses
All continuous variables were checked for their distribution and expressed as means ± standard deviation, or in the case of non-linear distributions, as medians and inter quartile ranges. Spearman correlation coefficients were calculated for BMI, WHR, glucose and insulin levels and the cholesterol synthesis and absorption markers. For the comparisons among the NLR and FCH group, p-values were adjusted for age, BMI, family structure by logistic regression, using FCH status as dependent variable and age, BMI, and the family identification number and the variable under investigation as independent variables. A p-value less than 0.05 was considered statistically significant and all calculations were performed using the STATA software package, version 10.
Results
The characteristics of the 103 patients with FCH and the 240 NLR are presented in the study population at large, as well as stratified by gender (Table 1). There was no difference in the gender distribution among FCH patients and their NLR, although the FCH group was significantly older and had higher BMIs and WHRs (p<0.05 for all comparisons). FCH patients had higher glucose (p<0.01) and insulin (p<0.001) levels and as expected, higher levels of total cholesterol, triglycerides, non HDL-C, VLDL-C and VLDL-TG (p<0.001, for all parameters) and HDL-C levels were significantly lower. In addition, RLPC, apoB and apoB48 (the intestinal apoB), were significantly higher (p<0.001, for all comparisons) in FCH as compared to the NLR. There were interesting gender differences among FCH patients and the NLR. In women, FCH was associated with 33% higher LDL-C levels and 100% higher sdLDL-C levels (p<0.001, for both comparisons), while these difference were not observed in the men. As mentioned above, apoB48 levels were higher in the FCH group when compared to the NLR, however, the percentile difference observed in men was almost twice as high as the difference seen in women (250% higher versus +133%).
Individual correlations between markers cholesterol synthesis and absorption in relation to markers of obesity and insulin resistance are presented in Table 2. In the combined study groups, lathosterol and desmosterol levels correlated (all at p<0.001) with BMI and WHR and with glucose and insulin levels. In addition, there was a small inverse correlation for sitosterol with BMI and insulin levels (both p<0.05, Table 2). Supplementary Table I provides data showing a better correlation for lathosterol and desmosterol with glucose levels and a less pronounced correlation with insulin levels in the FCH patients compared to the NLR. The nature of these correlations remained the same in gender subgroups (data not shown).
Table 2.
Correlations of the cholesterol synthesis and absorption markers with BMI, WHR, Glucose and Insulin in all subjects (n=343)
| BMI | WHR | Glucose | Insulin | |
|---|---|---|---|---|
| Squalene | 0.062 | 0.059 | −0.013 | −0.011 |
| Lathosterol | 0.482* | 0.471* | 0.389* | 0.336* |
| Desmosterol | 0.337* | 0.320* | 0.360* | 0.304* |
| Campesterol | −0.082 | 0.081 | 0.076 | −0.074 |
| Sitosterol | −0.128† | 0.030 | 0.074 | −0.148† |
| Cholestanol | −0.063 | 0.032 | −0.013 | −0.110 |
Values represent Spearman Rank correlation coefficients.
p<0.001
p<0.05
We observed a strong correlation between both lathosterol and desmosterol in relation to apoB, triglycerides and LDL-C (p<0.001 for all), which appeared to be stronger in women than in men (Supplementary Table II). In addition, apoB and LDL-C correlated with campesterol and sitosterol in both men and women and with cholestanol in men only (Supplementary Table II).
The FCH phenotype was associated with increased markers of cholesterol synthesis, Table 3. Lathosterol levels were elevated by 51% (p<0.001) in FCH, squalene tended to be higher in FCH (+6%, p=0.125), however, this effect was observed in women only (+15%, p<0.001) and not in men. In addition, desmosterol levels were 54% higher in female FCH patients (p<0.001) and only 11% higher in male FCH patients (p=0.069) when compared to their NLR counterparts. To account for the fact that plasma sterols are mainly transported in the LDL and HDL particles (34), values were also presented as ratio to total cholesterol (/C). Although squalene concentrations were increased in female FCH patients 15% (p=0.001), squalene/C levels were actually reduced in both male and female FCH patients by −21% (p=0.010) and −14% (p=0.050) respectively, independent of BMI. Desmosterol/C and lathosterol/C overall remained comparable to the unadjusted values, desmosterol/C being elevated in women by 24% (p=0.002) and lathosterol/C in both men and women by 17% (p=0.013) and 22% (p<0.001), respectively. Adjusting the lathosterol and desmosterol associations for BMI did not affect the level of significance of the sterol markers when expressed in absolute terms, however, the associations were no longer significant when using the sterol values normalized to total cholesterol.
Table 3.
Cholesterol synthesis markers, expressed in absolute concentrations as well as normalized to total cholesterol, in the normolipidemic relatives (NLR) and patients with familial combined hyperlipidemia (FCH)
| NLR | FCH | % difference FCH vs. NLR |
p value† | BMI adj. p value‡ |
|
|---|---|---|---|---|---|
| Sterols expressed in µmol/L | |||||
| Squalene | |||||
| Total | 1.26 (0.37) | 1.33 (0.43) | 6 | 0.078 | 0.433 |
| Men | 1.36 (0.44) | 1.31 (0.40) | −4 | 0.706 | 0.355 |
| Women | 1.17 (0.27)* | 1.35 (0.44) | 15 | 0.001 | 0.011 |
| Desmosterol | |||||
| Total | 1.6 [1.1, 2.4] | 2.1 [1.6, 3.1] | 31 | <0.001 | 0.018 |
| Men | 1.9 [1.3, 2.8] | 2.1 [1.6, 3.3] | 11 | 0.069 | 0.444 |
| Women | 1.3 [1.0, 1.9]* | 2.0 [1.5, 3.1] | 54 | <0.001 | 0.001 |
| Lathosterol | |||||
| Total | 7.4 (3.1) | 11.2 (4.0) | 51 | <0.001 | <0.001 |
| Men | 8.3 (3.6) | 11.9 (3.7) | 43 | <0.001 | 0.006 |
| Women | 6.6 (2.4)* | 10.7 (4.1) | 62 | <0.001 | <0.001 |
| Sterols expressed as 102 mmol/mol cholesterol | |||||
| Squalene/C | |||||
| Total | 26.5 (8.9) | 22.0 (7.4) | −17 | 0.001 | 0.001 |
| Men | 27.1 (9.6) | 21.4 (7.3) | −21 | 0.010 | 0.007 |
| Women | 25.9 (8.1) | 22.4 (7.6) | −14 | 0.050 | 0.021 |
| Desmosterol/C | |||||
| Total | 34 [24, 46] | 36 [27, 52] | 6 | 0.267 | 0.714 |
| Men | 39 [28, 54] | 34 [25, 52] | −13 | 0.821 | 0.319 |
| Women | 29 [22, 42]* | 36 [28, 53] | 24 | 0.029 | 0.290 |
| Lathosterol/C | |||||
| Total | 150 (55) | 179 (61) | 19 | <0.001 | 0.155 |
| Men | 160 (59) | 187 (64) | 17 | 0.010 | 0.369 |
| Women | 142 (49)* | 173 (59) | 22 | 0.001 | 0.098 |
Significantly different from men at p < 0.05 within the subgroup.
P values adjusted for age and family structure.
Markers of cholesterol absorption in FCH patients and NLR are presented in Table 4. With regard to these markers there was evidence for a gender interaction. In men with FCH, absolute concentrations of campesterol and sitosterol tended to be elevated by 21% (p=0.065), while remaining constant in women. After adjusting for BMI, the differences were significant (p=0.012 and p=0.027). Normalized to total cholesterol, campesterol/C and sitosterol/C were no longer elevated in men and significantly lower in women with FCH (−26%, p=0.003 and −25%, p=0.004 respectively). Absolute cholestanol concentrations were not different in FCH, but cholestanol/C was 16% lower (p<0.001) in both men and women with FCH and independent of BMI.
Table 4.
Cholesterol absorption markers, expressed in absolute concentrations as well as normalized to total cholesterol, in the normolipidemic relatives (NLR) and patients with familial combined hyperlipidemia (FCH)
| NLR | FCH | % difference FCH vs. NLR |
p value† | BMI adj. p value‡ |
|
|---|---|---|---|---|---|
| Sterols expressed in µmol/L | |||||
| Campesterol | |||||
| Total | 9.7 [7.1, 13.2] | 11.0 [7.8, 15.3] | 13 | 0.191 | 0.022 |
| Men | 10.2 [7.7, 14.2] | 12.3 [9.7, 17.3] | 21 | 0.065 | 0.012 |
| Women | 9.1 [6.8, 12.3]* | 9.5 [7.1, 13.0]* | 4 | 0.726 | 0.243 |
| Sitosterol | |||||
| Total | 6.2 [4.7, 8.1] | 7.1 [4.7, 10.1] | 15 | 0.167 | 0.006 |
| Men | 6.2 [4.9, 8.3] | 7.5 [5.5, 10.4] | 21 | 0.131 | 0.027 |
| Women | 6.2 [4.6, 8.0] | 6.1 [4.5, 9.5] | −2 | 0.571 | 0.068 |
| Cholestanol | |||||
| Total | 7.8 (2.8) | 8.1 (3.0) | 4 | 0.387 | 0.303 |
| Men | 8.3 (3.1) | 8.3 (2.3) | 0 | 0.912 | 0.887 |
| Women | 7.3 (2.4)* | 7.9 (3.4) | 8 | 0.180 | 0.125 |
| Sterols expressed as 102 mmol/mol cholesterol | |||||
| Campesterol/C | |||||
| Total | 203 [155, 264] | 169 [135, 241] | −17 | 0.011 | 0.302 |
| Men | 200 [152, 286] | 181 [158, 273] | −10 | 0.839 | 0.369 |
| Women | 207 [158, 259] | 154 [113, 211]† | −26 | 0.003 | 0.052 |
| Sitosterol/C | |||||
| Total | 128 [98, 165] | 109 [82, 152] | −15 | 0.004 | 0.396 |
| Men | 121 [96, 157] | 119 [86, 163] | −2 | 0.347 | 0.710 |
| Women | 135 [100, 170] | 101 [78, 148] | −25 | 0.004 | 0.164 |
| Cholestanol/C | |||||
| Total | 160 (53) | 134 (48) | −16 | 0.001 | 0.010 |
| Men | 162 (56) | 135 (40) | −17 | 0.030 | 0.067 |
| Women | 158 (50) | 133 (53) | −16 | 0.015 | 0.059 |
Significantly different from men at p < 0.05 within the subgroup.
P values adjusted for age and family structure.
P values adjusted for age and family structure and BMI.
Although we didn’t observe correlations between the markers of synthesis and absorption when expressed in absolute terms (data not show), there was a negative correlation between lathosterol/C and campesterol/C in the NLR (r=−0.271, p<0.001) not observed in the FCH patients (r=−0.156, p=0.120). Furthermore, the lathosterol/campesterol ratio was 37% higher in FCH patients than in NLR and this was predominantly due to the elevations observed in women (66%) rather than in men (19%), Table 5.
Table 5.
Ratios of lathosterol over campesterol in the normolipidemic relatives (NLR) and patients with familial combined hyperlipidemia (FCH)
| NLR | FCH | % difference FCH vs. NLR |
p value† | BMI adj. p value‡ |
|
|---|---|---|---|---|---|
| Lathosterol/campesterol ratio | |||||
| Total | 0.71 [0.43, 1.11] | 0.97 [0.67, 1.42] | 37 | <0.001 | 0.194 |
| Men | 0.77 [0.39, 1.27] | 0.92 [0.71, 1.18] | 19 | 0.097 | 0.305 |
| Women | 0.62 [0.44, 1.01] | 1.03 [0.66, 1.71] | 66 | <0.001 | 0.018 |
Significantly different from men at p < 0.05 within the subgroup.
P values adjusted for age and family structure.
P values adjusted for age and family structure and BMI.
Discussion
Since the description and characterization of the FCH phenotype more than 30 years ago (1), many studies have been undertaken to uncover the mechanisms causing FCH. Both the results of biochemical investigations as well as the results from candidate gene studies and genome wide scan studies have been of great interest, but the actual cause of the elevated total cholesterol, triglycerides and apoB levels as well as the underlying genetic defect has not yet been elucidated. This study shows that both men and women with FCH have higher levels of the cholesterol precursor lathosterol when compared to their NLR. Based on these findings we hypothesize that patients with FCH have an underlying defect in sterol metabolism resulting in overproduction of lathosterol and cholesterol.
The higher sterol levels observed in the FCH may suggest that this is the result of impaired plasma disposal of these sterols or an impaired conversion to cholesterol. Alternatively, lathosterol and desmosterol are both formed from squalene, each via a different pathway (see Figure). Although we found marginally higher levels of plasma squalene (6%), this effect was predominantly due to increased squalene levels in women (15%). When the squalene levels were expressed as a ratio over total cholesterol, we found them to be lower in both men and women. The cholesterol precursor lathosterol was elevated in men and women with FCH (both in absolute terms as well as adjusted for total cholesterol), suggesting that there is either enhanced conversion of squalene to lathosterol or increased direct production of lathosterol in these patients. Although a limitation of our study is that the design is cross-sectional and does not establish causation, in FCH we find increased levels of lathosterol which correlate with apoB, triglycerides and LDL-C, suggesting that FCH is (partly) the result of an imbalance in the cholesterol synthesis pathway and future research should focus on regulators within this pathway.
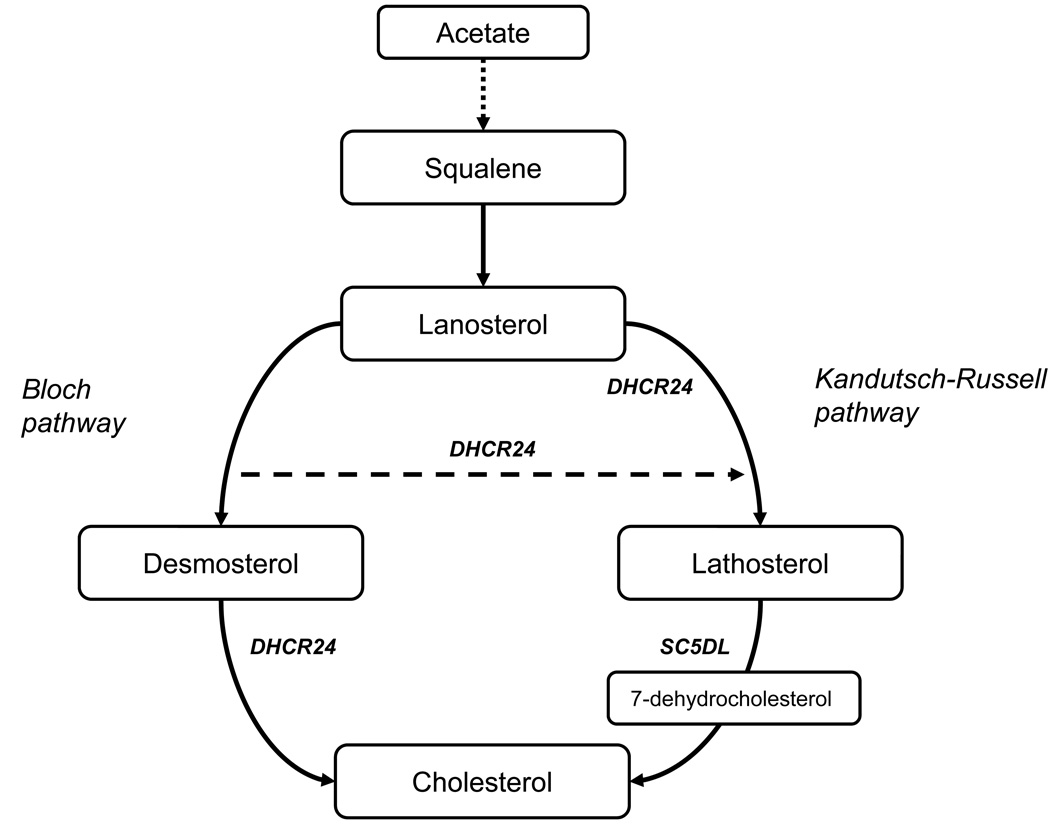
Figure 1.
An overview of the cholesterol synthesis from squalene. Intermediate reactions from lanosterol to desmosterol and lathosterol in the Bloch pathway and Kandutsch-Russell pathway respectively are not shown. Sterol-C5-desaturase-like (SC5DL) converts lathosterol into 7-dehydrocholesterol. The 24-dehydrocholesterol reductase (DHCR24) catalyzes the reduction of the delta-24 double bond of sterol intermediates in the Bloch pathway forming a sterol which is part of the Kandutsch-Russell pathway. DHCR24 also catalyzes the formation of cholesterol from desmosterol.
In contrast to the markers of cholesterol synthesis, the differences among FCH and NLR regarding the markers of cholesterol absorption are less clear. Both campesterol and sitosterol tended to be higher in FCH patients, but these findings were restricted to men and were only significant when taking into account BMI. When the absorption markers were expressed relative to total cholesterol, we found significantly lower levels in women for campesterol/C and sitosterol/C. Cholestanol/C was lower in both men and women. The question remains however if the association of FCH with lower values for the absorption markers over cholesterol is a true effect, i.e. the result of lower cholesterol absorption, or whether it is the result of the dividing a constant concentration of absorption markers by a higher total cholesterol concentration caused by increased cholesterol synthesis. Furthermore, it is known that dietary intake of plant sterols can affect plasma concentrations of campesterol and sitosterol (35). Since no dietary information was collected, we cannot rule out that FCH patients were consuming greater amounts of dietary plant sterol margarines and other sterols as compared to controls. Finally, even though we noted elevated levels of apoB48 in FCH patients, these elevations are likely a reflection of delayed clearance of intestinal remnant lipoproteins as previously described in FCH (36), rather than due to increased absorption, and overall, these findings suggest that FCH is not associated with altered cholesterol absorption.
Previous studies suggest a central role for fat mass in the onset of FCH (37). In agreement with this concept, we found associations indicating that there is an interaction between plasma sterols and BMI which may play a central role in the onset of FCH. The relationship between obesity and cholesterol production is not straight forward since more fat mass does not always cause excess cholesterol production. This concept was illustrated by a recent study from our laboratory in 19 severely obese women undergoing bypass surgery, showing a markedly reduction in fat mass without a significant reduction in total cholesterol levels (38). A possible mechanism linking plasma sterols to fat metabolism may come from the demonstration that sterol intermediates from the cholesterol synthesis pathway, especially desmosterol, act as liver x receptor (LXR) ligands (39). LXR stimulates hepatic triglyceride synthesis and fat storage and has been described as a master regulator of fat metabolism (40). Further studies are necessary to determine a possible interaction between LXR, desmosterol and the other plasma sterols in cases with FCH versus controls.
We have performed extensive characterization of the lipid and apolipoprotein profiles in FCH patients and their NLR, as well as in subgroups of men and women, and found that within the FCH group LDL-C and sdLDL-C were elevated in women only. This may suggest that in female FCH patients there is even greater cholesterol synthesis then in male FCH patients. In agreement, the cholesterol synthesis data also suggests that there are gender specific mechanisms associated with FCH. Although lathosterol was elevated in both male and female FCH patients, the effect seemed to be more prominent in women than in men (62% versus 42%). The other cholesterol precursors, squalene and desmosterol, were significantly higher (15% and 54%, respectively) in FCH women while remaining constant in men. These increases observed in FCH women were due to the fact that in the NLR group the women had lower sterol levels than the men. This was especially true for desmosterol. In women, desmosterol levels were higher in the FCH compared to the NLR group, but similar to the levels seen in the FCH men. To speculate on a possible underlying mechanism, recent studies demonstrate that the enzyme responsible for the conversion of desmosterol to cholesterol, i.e. 24-dehydrocholesterol reductase (DHCR24 also known as Seladin-1), is activated by estrogens (41). In theory the activation of the DHCR24 by estrogens may be reduced in women with FCH, resulting in elevated desmosterol levels. In support of this concept, a post hoc analysis in this study population revealed that desmosterol levels (but not squalene levels) were significantly higher in postmenopausal women when compared to premenopausal women (57% higher, p<0.001).
To our knowledge this is the second study to measure synthesis and absorption markers in FCH patients. Previously, Garcia-Otin and colleagues measured cholesterol synthesis and absorption markers among autosomal dominant hypercholesterolemia (ADH) patients, including thirty-one patients with familial hypercholesterolemia and thirty-eight patients with FCH (42). In contrast to our study, which was designed to gain insight into the mechanism underlying FCH, the primary objective of the Garcia-Otin group was to use these markers to differentiate between the different forms of ADH. They noted that the campesterol/C and sitosterol/C ratios were lower in the FCH patients when compared to controls, which is in line with our cholesterol adjusted data, but they observed no differences for the lathosterol/C ratio, suggesting that alterations in absorption markers were associated with FCH. Their study, however, was conducted in a FCH group consisting of predominantly men (27 versus 11 women), and limited numbers prevented them from analyzing subgroups. Since this is the second study to measure synthesis and absorption markers in a FCH population, replication of these findings are necessary in different study populations, preferably using the same standardized diagnostic criteria for FCH (27).
Finally, we would like to speculate on possible novel candidate genes, taking into account our findings that the FCH phenotype was associated with elevated cholesterol synthesis markers. USF1 has been suggested to be the most important candidate gene for FCH (17). However, in these families USF1 was not a major gene for FCH, but rather a modifier gene contributing to related lipid traits (43). Recently, a whole genome study mapped all the target regions of USF1 and identified multiple regions involved in the lipid metabolism (44), including a number of vital genes in the synthesis of cholesterol summarized in Supplementary Table III. Of these genes, sterol-C5-desaturase-like (SC5DL) plays a crucial role in the formation of cholesterol from lathosterol and the (earlier mentioned) DHCR24 is involved in many steps of the synthesis of cholesterol, one of which is the conversion of desmosterol to cholesterol. Our findings of elevated lathosterol in both men and women with FCH, and the association of lathosterol with LDL-C, triglycerides and apoB in our entire study population, raises the possibility that the SC5DL gene is a candidate gene for FCH. In addition, when the chromosome 1 data was reanalyzed in the Dutch population, it revealed that 1p31 was strongly linked to elevated apoB levels (45). The authors hypothesized that the nearby leptin receptor (LEPR) gene was involved, but the data proved to be negative (45). Although speculative, the DHCR24 gene is also located in this region (Table 4) and based on our findings of increased desmosterol in women and the association of desmosterol with apoB levels, we hypothesize that alterations in this gene may cause elevated apoB rather than the LEPR gene. Future studies, sequencing the SC5DL as well as the DHCR24 genes in FCH patients, appear to be warranted.
In conclusion, our study indicates that FCH patients have alterations in the cholesterol synthesis pathway, resulting in higher levels of lathosterol (and additionally desmosterol in women). In the cholesterol synthesis pathway there are two enzymes which warrant further investigation: SC5DL and DHCR24. The genes for these enzymes are located in exactly those chromosomal regions previous linked to FCH in genome wide scans, and code for the key enzymes involved in the metabolism of lathosterol and desmosterol, the sterols we found to be elevated in FCH patients. Our data indicate that FCH patients appear to have enhanced conversion of squalene to lathosterol, and further examination of the SC5DL and DHCR24 genes and their gene products appear to be justified, and may ultimately lead us to a precise understanding of the defects underlying FCH.
Supplementary Material
Acknowledgments
Sources of Funding
T.M. van Himbergen was supported by the Ruth L. Kirschstein National Research Service Award, training grant no. DK07651 and a research grant from Unilever Food and Health Research Institute, Unilever R & D, Vlaardingen, The Netherland. S. Otokozawa and M. Ai were supported by research fellowships from Kyowa Medex Co, Tokyo Japan and Denka Seiken Co, Tokyo Japan, respectively. E.J. Schaefer was supported by grants R01 HL-60935, HL 74753 and PO50HL083813 from the National Institutes of Health and contract 53-3K – 06 from the United Department of Agriculture Research Service.
Footnotes
Disclosures
There are no financial or other relationships that may lead to a conflict of interest.
References
- 1.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia in coronary heart disease. II. genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genest JJ, Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH, Silberman SR, Wilson PW, Salem DN, Schaefer EJ. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: A case-control comparison from the national heart, lung, and blood institute family heart study. Circulation. 2003;108:519–523. doi: 10.1161/01.CIR.0000081777.17879.85. [DOI] [PubMed] [Google Scholar]
- 4.Brunzell JD, Schrott HG, Motulsky AG, Bierman EL. Myocardial infarction in the familial forms of hypertriglyceridemia. Metabolism. 1976;25:313–320. doi: 10.1016/0026-0495(76)90089-5. [DOI] [PubMed] [Google Scholar]
- 5.Wiesbauer F, Blessberger H, Azar D, Goliasch G, Wagner O, Gerhold L, Huber K, Widhalm K, Abdolvahab F, Sodeck G, Maurer G, Schillinger M. Familial-combined hyperlipidaemia in very young myocardial infarction survivors (<=40 years of age) Eur Heart J. 2009;30:1073–1079. doi: 10.1093/eurheartj/ehp051. [DOI] [PubMed] [Google Scholar]
- 6.Chait A, Albers JJ, Brunzell JD. Very low density lipoprotein overproduction in genetic forms of hypertriglyceridaemia. Eur J Clin Invest. 1980;10:17–22. doi: 10.1111/j.1365-2362.1980.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 7.Kissebah AH, Alfarsi S, Evans DJ. Low density lipoprotein metabolism in familial combined hyperlipidemia. mechanism of the multiple lipoprotein phenotypic expression. Arteriosclerosis. 1984;4:614–624. doi: 10.1161/01.atv.4.6.614. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesan S, Cullen P, Pacy P, Halliday D, Scott J. Stable isotopes show a direct relation between VLDL apoB overproduction and serum triglyceride levels and indicate a metabolically and biochemically coherent basis for familial combined hyperlipidemia. Arterioscler Thromb. 1993;13:1110–1118. doi: 10.1161/01.atv.13.7.1110. [DOI] [PubMed] [Google Scholar]
- 9.Castro Cabezas M, de Bruin TW, de Valk HW, Shoulders CC, Jansen H, Willem Erkelens D. Impaired fatty acid metabolism in familial combined hyperlipidemia. A mechanism associating hepatic apolipoprotein B overproduction and insulin resistance. J Clin Invest. 1993;92:160–168. doi: 10.1172/JCI116544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayyobi AF, McGladdery SH, McNeely MJ, Austin MA, Motulsky AG, Brunzell JD. Small, dense LDL and elevated apolipoprotein B are the common characteristics for the three major lipid phenotypes of familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2003;23:1289–1294. doi: 10.1161/01.ATV.0000077220.44620.9B. [DOI] [PubMed] [Google Scholar]
- 11.Bredie SJ, Kiemeney LA, de Haan AF, Demacker PN, Stalenhoef AF. Inherited susceptibility determines the distribution of dense low-density lipoprotein subfraction profiles in familial combined hyperlipidemia. Am J Hum Genet. 1996;58:812–822. [PMC free article] [PubMed] [Google Scholar]
- 12.Hokanson JE, Krauss RM, Albers JJ, Austin MA, Brunzell JD. LDL physical and chemical properties in familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 1995;15:452–459. doi: 10.1161/01.atv.15.4.452. [DOI] [PubMed] [Google Scholar]
- 13.Vakkilainen J, Jauhiainen M, Ylitalo K, Nuotio IO, Viikari JS, Ehnholm C, Taskinen MR. LDL particle size in familial combined hyperlipidemia: Effects of serum lipids, lipoprotein-modifying enzymes, and lipid transfer proteins. J Lipid Res. 2002;43:598–603. [PubMed] [Google Scholar]
- 14.de Graaf J, van der Vleuten GM, ter Avest E, Dallinga-Thie GM, Stalenhoef AF. High plasma level of remnant-like particles cholesterol in familial combined hyperlipidemia. J Clin Endocrinol Metab. 2007;92:1269–1275. doi: 10.1210/jc.2006-1973. [DOI] [PubMed] [Google Scholar]
- 15.Aouizerat BE, Allayee H, Cantor RM, Davis RC, Lanning CD, Wen PZ, Dallinga-Thie GM, de Bruin TW, Rotter JI, Lusis AJ. A genome scan for familial combined hyperlipidemia reveals evidence of linkage with a locus on chromosome 11. Am J Hum Genet. 1999;65:397–412. doi: 10.1086/302490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajukanta P, Terwilliger JD, Perola M, Hiekkalinna T, Nuotio I, Ellonen P, Parkkonen M, Hartiala J, Ylitalo K, Pihlajamaki J, Porkka K, Laakso M, Viikari J, Ehnholm C, Taskinen MR, Peltonen L. Genomewide scan for familial combined hyperlipidemia genes in finnish families, suggesting multiple susceptibility loci influencing triglyceride, cholesterol, and apolipoprotein B levels. Am J Hum Genet. 1999;64:1453–1463. doi: 10.1086/302365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naukkarinen J, Ehnholm C, Peltonen L. Genetics of familial combined hyperlipidemia. Curr Opin Lipidol. 2006;17:285–290. doi: 10.1097/01.mol.0000226121.27931.3f. [DOI] [PubMed] [Google Scholar]
- 18.Sniderman A, Williams K, Haffner S, Sattar N. Insights from apoB: From better diagnosis & therapy to the medusa hypothesis. Atheroscler Suppl. 2004;5:19–24. doi: 10.1016/j.atherosclerosissup.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Sniderman AD, Zhang XJ, Cianflone K. Governance of the concentration of plasma LDL: A reevaluation of the LDL receptor paradigm. Atherosclerosis. 2000;148:215–229. doi: 10.1016/s0021-9150(99)00282-8. [DOI] [PubMed] [Google Scholar]
- 20.Thompson GR, Naoumova RP, Watts GF. Role of cholesterol in regulating apolipoprotein B secretion by the liver. J Lipid Res. 1996;37:439–447. [PubMed] [Google Scholar]
- 21.Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 2004;174:197–205. doi: 10.1016/S0021-9150(03)00248-X. [DOI] [PubMed] [Google Scholar]
- 22.Matthan NR, Raeini-Sarjaz M, Lichtenstein AH, Ausman LM, Jones PJ. Deuterium uptake and plasma cholesterol precursor levels correspond as methods for measurement of endogenous cholesterol synthesis in hypercholesterolemic women. Lipids. 2000;35:1037–1044. doi: 10.1007/s11745-000-0616-9. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 24.van Himbergen TM, Matthan NR, Resteghini NA, Otokozawa S, Ai M, Stein EA, Jones PH, Schaefer EJ. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J Lipid Res. 2009;50:730–739. doi: 10.1194/jlr.P800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 26.Veerkamp MJ, de Graaf J, Bredie SJ, Hendriks JC, Demacker PN, Stalenhoef AF. Diagnosis of familial combined hyperlipidemia based on lipid phenotype expression in 32 families: Results of a 5-year follow-up study. Arterioscler Thromb Vasc Biol. 2002;22:274–282. doi: 10.1161/hq0202.104059. [DOI] [PubMed] [Google Scholar]
- 27.Veerkamp MJ, de Graaf J, Hendriks JC, Demacker PN, Stalenhoef AF. Nomogram to diagnose familial combined hyperlipidemia on the basis of results of a 5-year follow-up study. Circulation. 2004;109:2980–2985. doi: 10.1161/01.CIR.0000130646.93255.86. [DOI] [PubMed] [Google Scholar]
- 28.van Himbergen TM, van Tits LJ, Ter Avest E, Roest M, Voorbij HA, de Graaf J, Stalenhoef AF. Paraoxonase (PON1) is associated with familial combined hyperlipidemia. Atherosclerosis. 2008;199:87–94. doi: 10.1016/j.atherosclerosis.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H. Immunoassay with stable polystyrene latex particles. J Immunol Methods. 1980;38:353–360. doi: 10.1016/0022-1759(80)90283-5. [DOI] [PubMed] [Google Scholar]
- 30.Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, Schaefer EJ. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318. doi: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi K, Kayahara N, Ishigami M, Kuwata H, Mori H, Sugiuchi H, Irie T, Tanaka A, Yamashita S, Yamamura T. Development of a homogeneous assay to measure remnant lipoprotein cholesterol. Clin Chem. 2007;53:2128–2135. doi: 10.1373/clinchem.2007.092296. [DOI] [PubMed] [Google Scholar]
- 32.Otokozawa S, Ai M, Van Himbergen T, Asztalos BF, Tanaka A, Stein EA, Jones PH, Schaefer EJ. Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B-48 and remnant lipoprotein cholesterol levels. Atherosclerosis. 2009;205:197–201. doi: 10.1016/j.atherosclerosis.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthan NR, Giovanni A, Schaefer EJ, Brown BG, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33:976–982. doi: 10.1046/j.1365-2362.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 35.Chan YM, Varady KA, Lin Y, Trautwein E, Mensink RP, Plat J, Jones PJ. Plasma concentrations of plant sterols: Physiology and relationship with coronary heart disease. Nutr Rev. 2006;64:385–402. doi: 10.1111/j.1753-4887.2006.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 36.Cabezas MC, de Bruin TW, Jansen H, Kock LA, Kortlandt W, Erkelens DW. Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler Thromb. 1993;13:804–814. doi: 10.1161/01.atv.13.6.804. [DOI] [PubMed] [Google Scholar]
- 37.de Graaf J, Veerkamp MJ, Stalenhoef AF. Metabolic pathogenesis of familial combined hyperlipidaemia with emphasis on insulin resistance, adipose tissue metabolism and free fatty acids. J R Soc Med. 2002;95 Suppl 42:46–53. [PMC free article] [PubMed] [Google Scholar]
- 38.Asztalos BF, Swarbrick MM, Schaefer EJ, Dallal GE, Horvath KV, Ai M, Stanhope KL, Austrheim-Smith I, Wolfe BM, Ali M, Havel PJ. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J Lipid Res. 2009 May 14; doi: 10.1194/jlr.P900015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang C, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F, Westover EJ, Covey DF, Mangelsdorf DJ, Cohen JC, Hobbs HH. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem. 2006;281:27816–27826. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 40.Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Peri A, Serio M. Neuroprotective effects of the alzheimer's disease-related gene seladin-1. J Mol Endocrinol. 2008;41:251–261. doi: 10.1677/JME-08-0071. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Otin AL, Cofan M, Junyent M, Recalde D, Cenarro A, Pocovi M, Ros E, Civeira F. Increased intestinal cholesterol absorption in autosomal dominant hypercholesterolemia and no mutations in the low-density lipoprotein receptor or apolipoprotein B genes. J Clin Endocrinol Metab. 2007;92:3667–3673. doi: 10.1210/jc.2006-2567. [DOI] [PubMed] [Google Scholar]
- 43.van der Vleuten GM, Isaacs A, Hijmans A, van Duijn CM, Stalenhoef AF, de Graaf J. The involvement of upstream stimulatory factor 1 in dutch patients with familial combined hyperlipidemia. J Lipid Res. 2007;48:193–200. doi: 10.1194/jlr.M600184-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Rada-Iglesias A, Ameur A, Kapranov P, Enroth S, Komorowski J, Gingeras TR, Wadelius C. Whole-genome maps of USF1 and USF2 binding and histone H3 acetylation reveal new aspects of promoter structure and candidate genes for common human disorders. Genome Res. 2008;18:380–392. doi: 10.1101/gr.6880908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allayee H, Krass KL, Pajukanta P, Cantor RM, van der Kallen CJ, Mar R, Rotter JI, de Bruin TW, Peltonen L, Lusis AJ. Locus for elevated apolipoprotein B levels on chromosome 1p31 in families with familial combined hyperlipidemia. Circ Res. 2002;90:926–931. doi: 10.1161/01.res.0000015885.27134.f0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.