Abstract
This phase I study was designed to determine the maximum tolerated dose (MTD) and toxicity profile of the combination of gefitinib, capecitabine, and celecoxib in patients with advanced solid tumors. Patients were treated with escalating doses of gefitinib once daily, capecitabine twice daily (14 of 28 days), and celecoxib twice daily. Plasma samples for biomarkers were obtained at baseline and weekly for the first 2 cycles. Pharmacokinetic variables were correlated with toxicity and presence of biological effect. Tumor biopsies from 5 patients were analyzed for changes in tumor metabolic activity by nuclear magnetic resonance spectroscopy. [18F]fluororodeoxyglucose positron emission tomography was done as a correlate in 6 patients at the MTD. Thirty-nine patients received 168 cycles of therapy. The dose-limiting toxicities observed included nausea, dehydration and nausea, diarrhea, and stomatitis. The MTD was 250 mg/d gefitinib (days 1–14) and 2,000 mg/m2/d capecitabine divided twice daily (days 8–21) every 28 days. Celecoxib was eliminated due to concerns of increased risk for cardiovascular toxicity, although no patients in this study had cardiac events. One patient with cholangiocarcinoma had a confirmed partial response. Fourteen of 39 (36%) patients maintained prolonged stable disease for a median of 4 months (range, 3–24 months). [18F]fluorodeoxyglulucose positron emission tomography scan and metabolomic analyses revealed differences in metabolic response to gefitinib versus capecitabine. The combination of gefitinib and capecitabine is well tolerated and appears to have activity against certain advanced solid tumors, providing a rationale for further evaluation in advanced solid malignancies.
Introduction
In recent years, there has been a rapid increase in the treatment options available to patients with advanced malignancies. Better understanding of the signal transduction pathways involved in oncogenesis has allowed therapeutic targeting of cellular processes. Many tumor types overexpress the epidermal growth factor receptor (EGFR) and its ligands (1). Up-regulation of these factors presumably facilitates the sustained uncontrolled growth, metastases, and angiogenic properties of cancer cells, as persistent activation of the EGFR tyrosine kinase activity turns on pro-cell division pathways. For example, colon cancer cells that have metastasized to the liver have more intense EGFR staining compared with colon cancer cells at the primary tumor site (2). EGFR activation in NIH 3T3 cells has been shown to activate ras and raf, stimulating the expression of vascular endothelial growth factor (3).
Gefitinib (Iressa, formerly ZD1839) is a potent EGFR tyrosine kinase inhibitor with a manageable toxicity profile and shows antitumor activity in heavily pretreated cancer patients (4, 5). In initial phase II studies, patients with non-small cell lung cancer (NSCLC) treated with gefitinib at doses of 250 or 500 mg/d showed significant responses and symptomatic improvement. However, several phase III trials failed to confirm overall survival advantage (6). In the ISEL study of 1,692 patients with previously treated NSCLC, there were significant improvements in response rates and time to progression but no significant survival benefit overall. However, preplanned subgroup analysis revealed significant survival advantage for gefitinib in females, patients of Asian ethnicity, and never-smokers (7). As a result, in 2005, the Food and Drug Administration called for new labeling of gefitinib, limiting its indication to cancer patients who are currently benefiting or have benefited previously from gefitinib treatment. New patients are currently only allowed access to gefitinib in the setting of a clinical trial (8). Patients with responses to gefitinib tend to have somatic mutations in the kinase domain of the EGFR, accounting for their apparent disproportionate benefit with this agent (9, 10).
We conducted a phase I clinical trial to define the toxicities and recommended phase II dose (RP2D) of a triplet regimen of gefitinib, capecitabine, and celecoxib. Capecitabine is an orally administered fluoropyrimidine carbamate approved for the treatment of metastatic breast cancer refractory to paclitaxel or anthracyclines (11). It has shown equal efficacy to single agent i.v. 5-fluorouracil (5-FU) in untreated metastatic colorectal cancer patients (12). It also has activity as second-line therapy in patients who have failed bolus 5-FU (13). Celecoxib was included in this regimen because cycloxygenase-2 up-regulation appears to be an important early event in colon carcinogenesis. Because of this, cycloxygenase-2 inhibitors may have a role in chemo-prevention. Celecoxib has been shown in a randomized trial to cause statistically significant regression of colonic polyps in patients with familial adenomatosis polyposis (14). Cycloxygenase-2 may play an equally important role in the development of metastatic disease (15) and appears to be an activator of angiogenesis necessary for tumor growth beyond 2 mm (16, 17). In vitro and in vivo data show that prostaglandins such as PGE2 up-regulate vascular endothelial growth factor, a potent proangiogenic growth factor (18, 19). Moreover, data suggesting that nonspecific, nonsteroidal anti-inflammatory drugs and cycloxygenase-2 inhibitors can inhibit angiogenesis through down-regulation of vascular endothelial growth factor (20, 21). We hypothesized that the use of gefitinib, capecitabine, and celecoxib was more likely to be effective than single-agent treatment and would not only confer complementary antitumor activity by targeting angiogenesis but would be attractive as an all-oral regimen with an acceptable toxicity profile. In addition, these drugs theoretically have no known pharmacokinetic interactions and should not interfere with the metabolism of one another. Gefitinib and celecoxib are metabolized by different P450 isoenzymes, and P450 enzymes do not play a significant role in capecitabine metabolism.
There is increasing evidence that early metabolic changes may complement or in some cases be superior to conventional imaging techniques in the ability to evaluate the effectiveness of targeted treatment regimens early during therapy (22, 23). Alterations in uptake of [18F]fluouorodeoxyglucose (FDG) by positron emission tomography (PET) may help predict therapy response early in treatment. On the other hand, the most recent application of nuclear magnetic resonance (NMR)-based metabolomics, which provides a global biochemical profile of tissue biopsies or body fluids, allows for longitudinal, temporal analyses of additional metabolic changes, such as those seen in glycolysis and fatty acid and phospholipid metabolism (24, 25). To explore these concepts in this study, we evaluated metabolic response by 18FDG-PET and magnetic resonance spectroscopy in selected patients during the first cycle of treatment.
Materials and Methods
Patient Selection
Eligible patients had a confirmed pathologic diagnosis of a nonhematologic malignancy refractory to standard treatment or for which there was no standard therapy. Additional inclusion criteria included age ≥18 years; life expectancy ≥12 weeks; Eastern Cooperative Oncology Group performance status of 0 to 2; discontinuation of previous chemotherapy for at least 4 weeks (6 weeks for nitrosureas or mitomycin C), recovery from any toxic effects of prior treatment; and adequate bone marrow, liver, and renal function. Women of childbearing potential must have had a negative urine pregnancy test before study entry, and men and women agreed to use adequate contraception. The study was approved by the local institutional review board and conducted in accordance with the guidelines of the Declaration of Helsinki. Each patient was informed of the investigational nature of this study and signed a written institutional review board-approved consent before registration and enrollment into the study.
Patients were excluded from this study for active infection, any serious concomitant systemic disorder (including other malignancy), uncontrolled brain metastases, history of hypersensitivity to 5-FU, or documented dihydropyrimidine dehydrogenase deficiency. Patients who were taking cytochrome P450 3A4 inducers, including rifampin, phenytoin, carbamazapine, and barbiturates, were required to discontinue these agents before entering the trial.
Drug Administration
Under the initial protocol, gefitinib was given continuously at a starting dose of 250 mg/d, with subsequent cohorts receiving either 250 or 500 mg/d, in combination with increasing doses of capecitabine (range, 1,000–2,000 mg/m2/d, given days 1–14) and a constant dose of celecoxib 400 mg b.i.d.
Dose Escalation Plan
Three patients were enrolled per dose level in a standard 3 + 3 design. If ≥2 of 3 to 6 patients experienced dose-limiting toxicity (DLT) not related to palmar plantar erythrodysesthesia (PPE), dose escalation ceased. The National Cancer Institute Common Toxicity Criteria version 2.0 was used to grade adverse events. DLT was defined as grade ≥3 nonhematologic toxicity excluding alopecia, skin toxicity, diarrhea, nausea, or vomiting; grade 4 thrombocytopenia; grade 4 neutropenia lasting >5 days or complicated by fever; grade ≥3 skin rash occurring after the second exposure to gefitinib; and treatment delay due to toxicity lasting >2 weeks. PPE was counted independently in assessing DLT because it is an established toxicity of capecitabine. For example, if 1 of 3 patients experienced grade 3 PPE, and up to 6 patients would be enrolled at that dose level. Dose escalation would cease if ≥2 patients developed grade ≥3 PPE or if ≥2 patients developed non-PPE DLTs. The maximum tolerated dose (MTD) for this study was defined as the dose level below which resulted in DLTs in ≥2 of 6 patients. When the MTD was defined, an additional 6 patients were enrolled to further assess the RP2D and to perform further correlative studies.
Courses were repeated every 28 days if counts were adequate, and any grade 2 to 4 treatment-related side effects had resolved. If a treatment-related side effect persisted, treatment was delayed for up to 2 weeks until recovery. Delays of therapy for significant toxicity lasting >2 weeks mandated discontinuation from the study.
Dose Reduction
For grade ≥3 nonhematologic toxicity, except skin toxicity or diarrhea, the gefitinib dose was decreased by one dose level and held at the reduced dose or eliminated if patients were already at the lowest dose level. For patients with grade 3 or 4 pustular rash, secondarily infected rash, or a rash intolerable due to pruritis or aesthetics, gefitinib was held until the rash resolved or improved to grade 1 and then restarted at either the same or a reduced dose. Patients who were already on 250 mg/d gefitinib were not allowed a dose reduction, and gefitinib was discontinued. In the event of grade 3 or 4 diarrhea, gefitinib was held until the diarrhea resolved or improved to ≤grade 2, then restarted without dose reduction if the investigator felt there was another explanation for the diarrhea, or dose-reduced or discontinued if the diarrhea was related to gefitinib. Dose reductions of 25% to 50% capecitabine were made for grade ≥2 PPE and diarrhea based on the grade and the number of recurrences of these events. In all dose cohorts, celecoxib was held indefinitely for an increase in creatinine of ≥20% from baseline, dehydration, or gastrointestinal bleeding. For other grade ≥3 toxicity, celecoxib was held until the toxicity became grade ≤1, then restarted at a dose of 200 mg b.i.d., and/or discontinued for persistent grade 3 toxicity.
On-Study Evaluations
Complete medical histories, physical examinations, assessment of performance status, and routine laboratory studies were done before study initiation and weekly during treatment for the first 2 cycles and then monthly for subsequent cycles. Routine laboratory studies included electrolytes, blood urea nitrogen, serum creatinine, glucose, alkaline phosphatase, total bilirubin, and serum transaminases. In patients taking warfarin, a protime was also obtained at these time points, as both gefitinib and warfarin can interfere with warfarin metabolism. Additional pre-treatment studies also included a urinalysis, serum pregnancy test (as appropriate), electrocardiogram, relevant radiographic studies to evaluate sites of disease, and measurement of any relevant tumor markers. Patient adherence was monitored by pill counts and dosing diaries. Radiographic evaluations for disease status assessment were repeated every 2 cycles. Tumor responses were classified by the Response Evaluation Criteria in Solid Tumors (26). All complete or partial responses were confirmed by repeat assessments done ≥4 and ≥6 weeks after the criteria for response were met.
Biological and Correlative Studies
Pharmacokinetics
To study the pharmacokinetics of gefitinib and capecitabine, whole-blood samples were obtained. Under the initial study protocol, these samples were obtained during a run-in period before treatment (after a predose of gefitinib given 7 days before cycle 1 day 1 and after a predose of capecitabine given 5 days before cycle 1 day 1) and on days 1, 8, and 15 of cycles 1 and 2. For patients enrolled under the modified schedule, pharmacokinetic sampling was obtained on days 8, 14, and 21 of cycle 1 only. Plasma concentrations of gefitinib and capecitabine and its metabolites were measured by liquid chromatography-tandem mass spectrometry. Celecoxib plasma levels were determined by reverse-phase high-performance liquid chromatography with fluorometric detection.
Pharmacodynamics
In consenting patients, changes in metabolic activity were measured in punch biopsy specimens by magnetic resonance spectroscopy pretreatment and within 24 h of cycle 1 day 14. Tumor tissue (~30 mg) was removed by 4 mm punch biopsy under local anesthesia and immediately frozen in liquid nitrogen. The samples were processed using an acid extraction method (27). Tissue endogenous metabolites, including glucose, lactate, choline intermediates, phospholipids, lipids, and amino acids, were quantitatively assessed by 1H NMR at 500 MHz. The quantitative metabolomic data were imported into the R 2.00 Package software and analyzed using principal component analysis (PCA) and partial least-squares discriminant analysis. Both statistical approaches allow for pattern recognition and group classification among complex metabolic data sets (28).
Four patients who were treated at the RP2D underwent whole-body 18FDG-PET scans from the base of skull through the proximal femurs with or without computed tomography coregistration. The standardized uptake values (SUV) were calculated for hyperactive areas at baseline and within 7 days of cycle 1 day 28. PET scans were done in 3 patients who also underwent tumor biopsy to assess the aggregate relative and comparative utility of these noninvasive measurements of response.
Results
Patient Characteristics
Between April 2002 and November 2006, a total of 39 patients were enrolled. Thirty-seven (95%) had prior cytotoxic therapy, 33 (85%) had previous surgery, and 25 (64%) had prior radiation therapy. Table 1 summarizes the patient characteristics. Four patients were not evaluable for full DLT assessment: 2 patients at dose level −1 due to early death from disease progression, 1 patient at dose level 4 due to incorrect dosing of gefitinib in cycle 1, and 1 patient in the expanded MTD cohort due to hospitalization for small bowel obstruction and inability to complete full dosing. Three patients withdrew from the study: 1 patient after >7 months of stable disease, 1 patient to get radiation therapy, and 1 patient for personal preference.
Table 1.
Patient characteristics
| n (%) | |
|---|---|
| Eligible patients | 39 |
| Males | 21 |
| Females | 18 |
| Median (range) age (y) | 60 (25–80) |
| Eastern Cooperative Oncology Group performance status | |
| 0 | 15 (38) |
| 1 | 25 (64) |
| Primary tumor site | |
| Colorectal | 12 |
| Breast | 6 |
| Pancreatic | 4 |
| Head and neck | 2 |
| Cholangiocarcinoma | 2 |
| Bladder | 2 |
| Ovarian | 2 |
| Adenocarcinoma, unknown primary | 2 |
| NSCLC | 2 |
| Other* | 4 |
| Prior cytotoxic therapy | 37 (95) |
| 1 regimen | 3 |
| 2 regimens | 13 |
| 3 regimens | 8 |
| ≥4 regimens | 13 |
| Prior surgery | 33 (85) |
| Prior radiation therapy | 25 (64) |
Other tumor types included 1 patient each with hepatocellular, renal cell carcinoma, endometrial, and melanoma.
Dose Escalation and Toxicity
In all, 168 cycles were administered (median, 2; range, 0–24). Table 2 summarizes the dosing regimens for all evaluated cohorts along with the number of patients and cycles completed. Of 11 patients who were enrolled on the initial treatment schema, 2 of 3 patients treated at dose level 1 experienced DLT. Eight additional patients were enrolled at dose level −1, with 2 patients who did not complete a full treatment cycle and were not evaluable. The remaining 6 patients at dose level −1 did not experience DLT. On the modified dosing schedule, there were no DLTs at dose level 2. At dose level 3, 1 of 6 patients experienced DLT (grade 3 dehydration and nausea). At dose level 4, 1 of 7 patients experienced grade 3 PPE and 1 of 7 patients experienced a non-PPE-related DLT (grade 3 diarrhea). At dose level 5, 2 of 6 evaluable patients developed DLT: 1 patient with grade 3 nausea and vomiting and 1 patient with grade 3 stomatitis. Based on these data, the MTD was determined to be 250 mg/d gefitinib (days 1–14) and 2,000 mg/m2/d capecitabine divided twice daily (days 8–21) in a 28-day cycle. Once the MTD was declared, 5 additional patients were enrolled at the RP2D, and no further DLTs were noted.
Table 2.
Dose escalation scheme
| Dose level | Gefitinib dose (mg/d) | Capecitabine dose (mg/m2/d) | Celecoxib dose (mg b.i.d.) | No. patients enrolled | No. patients with DLT | No. cycles |
|---|---|---|---|---|---|---|
| −1* | 250 (days 1–21) | 1,000 (days 1–14) | 400 (days 1–21) | 8 | 0 | 21 |
| 1* | 250 (days 1–21) | 1,500 (days 1–14) | 400 (days 1–21) | 3 | 2 | 5 |
| 2† | 250 (days 1–14) | 1,000 (days 8–21) | 400 (days 1–28) | 3 | 0 | 22 |
| 3† | 250 (days 1–14) | 1,500 (days 8–21) | 400 (days 1–28) | 6 | 1 | 23 |
| 4† | 250 (days 1–14) | 2,000 (days 8–21) | 400 (days 1–28) | 7 | 1 | 45 |
| 5† | 500 (days 1–14) | 2,000 (days 8–21) | 400 (days 1–28) | 7 | 2 | 38 |
| RP2D | 250 (days 1–14) | 2,000 (days 8–21) | — | 5 | 0 | 14 |
Initial dose escalation schedule.
Modified dosing protocol.
Overall, only 1 of 168 courses of capecitabine was held for 1 week due to toxicity, but 9 patients required dose reduction of one dose level for capecitabine-related toxicity, and 1 patient required two dose reductions for PPE. Five patients required holding of celecoxib in 39 cycles for increased serum creatinine above retreatment variables. Only 1 patient required dose reduction of gefitinib in cycle 2, from 500 to 250 mg, for skin toxicity. This patient remained on study for only 2 cycles due to disease progression but tolerated dose reduction without further difficulty.
Table 3 summarizes the relevant and frequent nonhematologic toxicities observed. In addition to those listed, other toxicities occurring in ≥10% of patients included dry skin (14.9%), grade 1 to 2 elevation of creatinine (12.5%), fatigue (21.4%), anorexia (11.9%), and abdominal pain (11.3%). Overall, the incidence of specific toxicities was consistent with, and in most cases lower than, the previously reported toxicities associated with the individual agents used (29, 30). The majority of the adverse effects were grade 1 to 2 and did not worsen with subsequent cycles. Most patients with PPE had either resolution or reduced toxicity in later cycles. Sequential dosing of gefitinib and capecitabine, when compared with concomitant dosing, did not result in a higher incidence of adverse events. Overall, <10% of 168 cycles had any hematologic toxicity, and only 2 episodes of grade 2 absolute neutrophil count were noted (at dose level −1 and at the RP2D). No other grade ≥3 hematologic toxicity was observed.
Table 3.
Treatment-related, nonhematologic toxicities occurring in ≥10% of patients, by dose level
| Dose level | n | Cycles | G1/G2/G3/G4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Diarrhea | Stomatitis | Nausea | Vomiting | Rash | HFS | |||
| −1 | 8 | 21 | 2/3/0/0 | 4/0/0/0 | 6/0/1/0 | 1/1/2/0 | 9/1/0/0 | 3/0/0/0 |
| 1 | 3 | 5 | 2/1/1/0 | 0/0/0/0 | 0/0/0/0 | 0/1/0/0 | 3/0/0/0 | 0/1/0/0 |
| 2 | 3 | 22 | 3/3/0/0 | 3/3/0/0 | 1/1/1/0 | 2/0/0/0 | 4/2/0/0 | 1/1/0/0 |
| 3 | 6 | 23 | 4/2/0/0 | 0/0/0/0 | 4/1/1/0 | 0/2/0/0 | 8/2/0/0 | 2/0/2/0 |
| 4 | 7 | 45 | 9/3/2/0 | 13/6/0/0 | 4/1/3/0 | 2/0/1/0 | 3/0/1/0 | 10/4/0/0 |
| 5 | 7 | 38 | 12/4/0/0 | 8/4/0/0 | 7/1/2/0 | 1/1/2/0 | 4/1/0/0 | 2/2/0/0 |
| RP2D | 5 | 14 | 4/1/0/0 | 1/2/0/0 | 4/3/0/0 | 3/1/0/0 | 2/0/0/0 | 2/1/0/0 |
| Total | 39 | 168 | 36/17/3/0 | 29/15/0/0 | 26/7/8/0 | 9/6/5/0 | 33/6/1/0 | 20/9/2/0 |
| G1/G2 | 53/168 | 44/168 | 33/168 | 15/168 | 39/168 | 29/168 | ||
| G3/G4 | 3/168 | 0 | 8/168 | 5/168 | 1/168 | 2/168 | ||
| G1/G4 (%) | 56/168 (33.3) | 40/168 (26.2) | 41/168 (24.4) | 20/168 (11.9) | 40/168 (23.8) | 31/168 (18.5) | ||
NOTE: All toxicities were measured according to the National Cancer Institute Common Toxicity Criteria version 2.0.
Study Modifications
The protocol was amended to address concerns of DLT in treatment cohort 1. At dose level 1 (250 mg/d gefitinib, 1,500 mg/m2/d capecitabine, and 400 mg b.i.d. celecoxib), 2 of 3 patients developed grade 3 gastrointestinal toxicity (grade 3 diarrhea in 1 patient with history of metastatic colon cancer with concomitant type 2 diabetes mellitus and ulcerative colitis and grade 3 fatigue and nausea in 1 patient with metastatic pancreatic cancer). No DLT was observed among 6 patients who were evaluable for toxicity at dose level −1 (250 mg/d gefitinib, 1,000 mg/m2/d capecitabine, and 400 mg b.i.d. celecoxib).
We questioned whether the observed MTD represented the true MTD of this combination, because significant comorbid disease could have played a significant role in the adverse events observed in both patients with DLT. The first DLT, grade 3 diarrhea, occurred in a patient who may have been at increased risk for developing diarrhea due to a history of ulcerative colitis (although not clinically active at the time of enrollment). The second DLT, grade 3 nausea and fatigue, occurred in a patient with advanced pancreatic cancer and rapid disease progression. In addition, at this level, we felt the capecitabine dose (1,000 mg/m2/d) was too low to merit further evaluation in phase II clinical trials. We therefore amended the protocol to reevaluate the dose and schedule of this combination. Since the initial protocol development, there were additional preclinical data suggesting that the efficacy of gefitinib in combination with fluoropyrimidines may be improved when gefitinib was administered before a fluoropyrimidine (31). In addition, less overlap of gefitinib and capecitabine dosing facilitated discrimination between single-agent and combination-induced toxicities. Under the amended protocol, gefitinib was given once daily on days 1 to 14 (250 mg/d in cohorts 1–3 and 500 mg/d in cohort 4), capecitabine was given twice daily in equally divided doses on days 8 to 21 (1,000 mg/m2/d in cohort 1, 1,500 mg/m2/d in cohort 2, and 2,000 mg/m2/d in cohorts 3 and 4), and celecoxib was given as 400 mg b.i.d. on days 1 to 28. Once the MTD was established, additional patients were enrolled to further assess toxicities, the recommended dose, and to perform additional biologic correlative studies.
An additional modification to the study occurred in December 2004, when findings of the Cardiovascular Safety Committee evaluating the Adenoma Prevention with Celecoxib Trial showed that patients randomized to celecoxib had a statistically significant 2.5-fold increase in their risk of major fatal and nonfatal cardiovascular events compared with those taking placebo. Specifically, 6 of 679 (0.9%) subjects randomized to the placebo arm experienced cardiovascular events compared with 35 of 1,356 (2.6%) of subjects who received celecoxib (32). Although the cardiovascular risks associated with celecoxib were low, they were serious and sometimes life-threatening. As such, we felt that the potential cardiovascular risks of celecoxib limited the therapeutic ratio of the combination proposed and opted to remove celecoxib from the protocol for subsequent patients.
Antitumor Activity
Thirty-three patients were evaluable for disease response. One patient had a confirmed partial response and 14 maintained stable disease for >2 cycles (median, 4 months; range, 3–24 months). The patient with confirmed partial response was a 60-year-old male with recurrent cholangiocarcinoma who had prior surgical resection and irradiation (5,400 cGy in 25 fractions) with adjuvant capecitabine on days of radiation. He remained stable for ~7 months before progression in the liver and lymph nodes. His radiologic evaluation at study entry was notable for substantial uptake on PET scan as well as para-aortic lymph node enlargement on computed tomography scan. On early follow-up scans, the area of soft tissue noted in the gallbladder fossa remained stable. By the 8th cycle and beyond, there was no detectable evidence of disease recurrence or metastasis by either computed tomography or PET scan. After completing 12 cycles, the patient opted to discontinue therapy, because he no longer had any evidence of discernable disease. He tolerated protocol therapy well, with only mild and reversible adverse effects. He was followed closely off therapy and maintained stable disease for another 15 months before recurrence was again noted. Another patient, a 72-year-old female with metastatic NSCLC who had progressed previously on single-agent gefitinib, maintained stable disease for 23 cycles. Her EGFR mutation status was not known.
Pharmacokinetic and Pharmacodynamic Evaluations
Pharmacokinetics
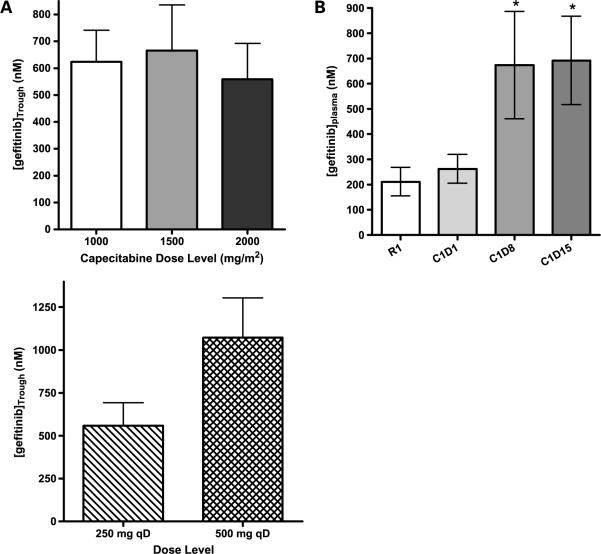
As shown in Table 4B, there was a dose-dependent increase in the area under the curve (AUC) of capecitabine and of 5-fluoro-deoxyuridine, the active metabolite of capecitabine. Although there was no significant difference in Cmax of capecitabine between 1,000 and 2,000 mg/m2, there was a dose-dependent, linear increase in 5-fluoro-deoxyuridine Cmax. Overall, there were no significant effects of gefitinib on the AUC, Cmax, t1/2, and clearance of capecitabine when administered alone (run-in day) or in combination with gefitinib (cycle 1 day 1 as shown in Table 4A). Capecitabine and 5-fluoro-deoxyuridine AUC and Cmax levels were consistent between doses (days 8, 15, and 21) at the 1,500 mg/m2 dose level, showing no difference between these variables throughout the cycle (data not shown). Similarly, capecitabine did not affect the trough levels of gefitinib across dose levels. Gefitinib accumulated in a dose- and time-dependent manner irrespective of capecitabine dosing (Fig. 1). Neither capecitabine nor gefitinib affected steady-state plasma trough levels of celecoxib across any of the doses tested (data not shown).
Table 4.
| (A) Effect of gefitinib on the pharmacokinetics of capecitabine and its active metabolite 5-fluorouridine* | ||||
|---|---|---|---|---|
| Pharmacokinetic variable | Capecitabine |
5-Fluorouridine |
||
| Alone | Combination | Alone | Combination | |
| AUC0→∞ (μmol/L h) | 12.3 ± 4.9 | 13.2 ± 8.1 | 35.7 ± 12.6 | 25.5 ± 14.5 |
| Cmax (μmol/L) | 12.1 ± 10.2 | 10.9 ± 9.9 | 19.4 ± 9.6 | 13.0 ± 9.2 |
| t1/2λ (h) | 0.93 ± 0.92 | 1.09 ± 0.80 | 0.80 ± 0.19 | 0.71 ± 0.19 |
| Vz/F (L) | 363 ± 364 | 411 ± 295 | — | — |
| CL/F (L/h) | 272 ± 120 | 300 ± 204 | — | — |
| (B) Capecitabine and 5-fluorouridine AUC and Cmax values on cycle 1 day 8 at capecitabine 1,000, 1,500, and 2,000 mg/m2 dose levels | |||
|---|---|---|---|
| Dose level |
|||
| 1,000 mg/m2 | 1,500 mg/m2 | 2,000 mg/m2 | |
| Capecitabine | |||
| AUC0→4 h (μmol/L h) | 6.7 ± 8.5 | 8.7 ± 5.1 | 14.8 ± 9.8 |
| Cmax (μmol/L) | 6.4 ± 8.6 | 6.0 ± 2.8 | 11.2 ± 10.9 |
| 5-Fluorouridine | |||
| AUC0→4 h (μmol/L h) | 19.8 ± 15.6 | 33.2 ± 10.5 | 44.9 ± 22.5 |
| Cmax (μmol/L) | 11.2 ± 9.6 | 20.3 ± 9.9 | 24.3 ± 13.9 |
Mean ± SD in 8 patients treated at 1,000 mg/m2 capecitabine for the run-in pharmacokinetic study (Pre) and in combination on C1D1 when also dosed with 250 mg gefitinib.
Figure 1.
A, plasma pharmacokinetic interactions between capecitabine and gefitinib. Top, no significant effect of capecitabine dose on gefitinib plasma trough levels when gefitinib is administered at 250 mg/d; bottom, dose-proportional increase of the plasma trough levels of gefitinib given at 250 and 500 mg/d when administered in combination with the recommended dose of capecitabine at 2,000 mg/m2/d. B, plasma gefitinib trough levels in the absence (run-in day 1) and presence [cycle 1, day 1 (C1D1), day 8 (C1D8), and day 15 (C1D15)] of capecitabine therapy (1,000 mg/m2). Gefitinib dose was 250 mg/d and plasma trough levels on cycle 1 days 8 and 15 are significantly elevated when compared with the run-in period and cycle 1 day 1 levels (P < 0.05).
Magnetic Resonance Spectroscopy Analysis
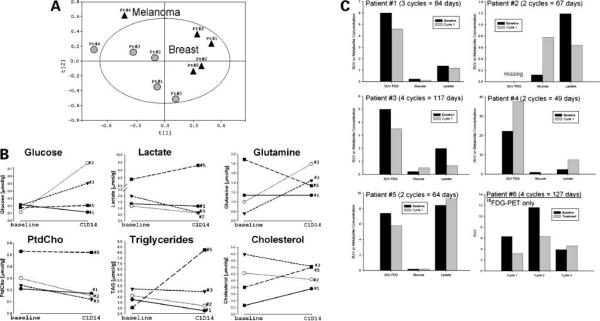
An exploratory study was done to evaluate NMR-based metabolomics on 10 samples from 5 patients with easily accessible tissue amenable to punch biopsy: 4 women with breast cancer and 1 man with melanoma. By PCA, the changes in metabolic profile allowed for group clustering visualization among patients (Fig. 2A).
Figure 2.
A, PCA scores on metabolomic data sets from 5 patients before (black triangles; baseline) and 14 d after starting treatment (gray circles; cycle 1, day 14). Each point was derived from a metabolic data set of 37 endogenous metabolites. B, time-sensitive changes in metabolites from treated patients with breast cancer. Six metabolites were distinguished by the partial least-squares discriminant analysis to differ among patients 1 to 3 (prolonged stable disease) and patient 5 (progressive disease immediately after cycle 2). All values are represented as normalized metabolite concentrations and expressed as micromole per gram biopsy. Patient 4 (melanoma) was excluded from the partial least-squares discriminant analysis due to the entirely different metabolic profile observed. C, metabolic data on 18FDG uptake from PET scans (patients 1 and 3–6) in conjunction with intratumoral concentrations of glucose and lactate (patients 1–5) from 1H NMR metabolomics studies. For patients 1 to 5, the SUV was reported for left axillary nodes to compare with NMR data on biopsies from same anatomic regions. For patient 6, the mean SUV on left lower neck (cycle 1), left axillary lymph nodes (cycle 2), and mediastinal lesions (cycle 4) are reported.
The metabolic profile in patients with breast cancer (1–3 and 5) were different from the melanoma patient (4) at baseline (black triangles) as assessed by PCA. The profile from the patient with melanoma differed, with an increased lipid profile compared with the patients with breast cancer. There was also some group separation after cycle 1 (gray circles) in the breast cancer patients despite a small number of samples analyzed. Patients 1 to 3 are clustered in the middle of the PCA score and showed a metabolic response that mimics gefitinib-induced changes seen in EGFR-positive cell lines in vitro. These 3 patients remained on the study for 3, 2, and 4 treatment cycles, respectively. Patient 5 showed less metabolic response and is clearly separated in the PCA score (Fig. 2A); this patient had clear computed tomography-proven disease progression immediately after cycle 2. From 37 metabolites analyzed, partial least-squares discriminant analysis discriminated six metabolites in patients 1 to 3 compared with patient 5 over time. The strongest predictors for metabolic response were lactate, glutamine, phosphatidylcholine, and triglycerides followed by glucose and cholesterol (Fig. 2B). In general, decreased levels of lactate, phosphatidylcholine, and triglycerides and increased levels of glucose and glutamine after 2 weeks of treatment may suggest predictors for metabolic response and more prolonged stable disease, although the small sample size limits significant conclusions or validation in this population.
18FDG-PET Scanning
Six patients underwent 18FDG-PET scanning pretreatment and post-treatment in an attempt to explore the potential relationship between imaging and 1H NMR metabolomics by spectroscopy. Figure 2C shows that, in 5 patients, SUV of the axillary lymph nodes on PET scan is matched with NMR data on biopsies from the same anatomic region. For patient 6, who was able to undergo serial PET scanning, the mean SUV from the lower neck (cycle 1), the left axillary lymph nodes (cycle 2), and mediastinal nodules (cycle 4) are reported. In this patient, the mean SUV of involved regions decreased pretreatment and post-treatment in cycles 1 and 2, but increased following cycle 4, corresponding to progression of disease. This patient did not have disease amenable to simple biopsy, so only FDG-PET results are reported. In 4 of 5 PET-imaged patients, the mean SUV decreased from baseline to the end of cycle 1. Intratumoral glucose concentrations increased in 2 of these patients and decreased in the other 2 patients, whereas lactate concentrations decreased in 3 patients and increased in 2 patients.
Discussion
First-line chemotherapy for metastatic colorectal cancer commonly involves 5-FU in combination with either oxaliplatin or irinotecan. Currently, the efficacy of second-line therapy with either oxaliplatin or irinotecan is limited. For example, the response rate for second-line single-agent oxaliplatin is only 10% (33). Clearly, there is a need for more effective chemotherapy for patients who relapse after or have poor response to first-line treatment. There are a variety published and ongoing clinical trials studying gefitinib as monotherapy (34) or in combination with anthracyclines, taxanes, or platinum-based therapies (35, 36). There are also a significant number of recent studies evaluating capecitabine in combination with cyclophosphamide and methotrexate (37), oxaliplatin (38, 39), oxaliplatin and erlotinib (40), paclitaxel (41), gemcitabine (42, 43), gemcitabine and vinorelbine (44), and vinorelbine (45). The utility of this regimen was explored in an initial attempt to develop new treatment approaches for patients with colorectal cancer who have progressed after combination therapy with 5-FU and either oxaliplatin or irinotecan.
The rational basis of the combination of gefitinib and capecitabine has been confirmed in preclinical studies (46). In a phase I study of gefitinib and capecitabine in conjunction with radiation therapy for patients with pancreatic and rectal cancer, increased toxicity was observed. The magnitude of effect from radiation therapy in this study was unclear, although our findings suggest that radiation played a prominent role in the toxicity noted (47).
This phase I study showed that the combination of gefitinib and capecitabine is well tolerated and can be administered easily in the outpatient setting as an all-oral regimen. DLTs were readily reversible and consistent with each component of therapy, primarily gastrointestinal effects (nausea, diarrhea, stomatitis, and dehydration) and skin toxicity, all of which are well-described effects of both gefitinib and capecitabine. The recommended doses for further studies for this 28-day regimen are 250 mg/d gefitinib on days 1 to 14 and 2,000 mg/m2/d capecitabine divided twice daily on days 8 to 21.
As predicted, there were no significant pharmacokinetic interactions identified between the agents used in this trial. Dose-dependent, predictable linear increases in capecitabine and 5-fluoro-deoxyuridine, the active metabolite of capecitabine, were observed, and dose-proportional increases in gefitinib were consistent across dose levels. Although celecoxib was eliminated from the regimen due to safety concerns, we observed no interaction between gefitinib and celecoxib at steady state as evidenced by the lack of change in celecoxib levels when the gefitinib dose was doubled from 1,000 to 2,000 mg/m2. As newer-generation cycloxygenase-2 inhibitors are developed, additional studies will be important in evaluating their utility in combination regimens such as this.
A unique application of metabolomics technology was conducted in 5 patients in this study and showed potential to use metabolic response to monitor responsiveness to targeted treatment and to measure the relative contribution of each component of a combination treatment regimen for future studies. Several potential metabolic markers of response identified were decreased lactate (a marker of tumor glycolysis), decreased phosphatidylcholine (a marker of membrane synthesis), and decreased triglycerides and cholesterol (measuring tumor lipogenesis), with increased glucose and glutamine concentrations in biopsies of breast cancer patients. These findings were consistent with 18FDG-PET findings done on the same patients and suggest that a combination of metabolic analyses may be useful in assessing therapeutic response; however, there did not appear to be a relationship between the metabolic markers and 18FDG-PET findings in the patient with melanoma who progressed after 2 cycles. These metabolites may provide mechanistic insights of gefitinib effects on cellular metabolic activity and may serve to guide future therapeutic trials to better assess the relative contributions of each component of therapy. As recently shown by 18FDG-PET studies on glucose uptake, targeted drug regimens may provide metabolic restriction and up-regulation and do not act as cytotoxics (24, 48). It will be valuable to further validate this metabolomics approach in tissues and body fluids in a larger patient population to better understand and establish the clinical utility of these applications.
Although further studies are needed to assess clinical activity, this therapy showed some suggestion of activity against a variety of advanced solid tumors, including colorectal, breast, NSCLC, pancreatic, and cholangiocarcinoma. In this study, 4 of 12 (33.3%) patients with colorectal cancer had stable disease (duration of 4 months). Both (100%) patients with cholangiocarcinoma had either stable disease (duration of 12 months) or a confirmed partial response lasting over 12 months. Another patient with NSCLC who had progression on single-agent gefitinib experienced prolonged stable disease for 23 cycles (21 months), suggesting that lack of response to single-agent gefitinib may not preclude response when this agent is used in combination. Although this regimen was initially conceived as a novel approach for treatment of refractory colorectal cancers, our results suggest that these other tumor types may be excellent targets for disease-directed trials as well.
Acknowledgments
Grant support: National Cancer Institute, NIH grants U01 CA099176, R21 CA108624, and P30 CA046934 and contract N01-C0-12400 (22XS047A-P3358). Gefitinib was provided by the Division of Cancer Treatment and Diagnosis at the National Cancer Institute under a Cooperative Research and Development Agreement between AstraZeneca and the Division of Cancer Treatment and Diagnosis.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 2.Radinsky R, Risin S, Fan D, et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 3.Grugel S, Finkenzellar G, Weindel K, et al. Both v-Ha-Ras and v-Raf stimulate expression of vascular endothelial growth factor in NIH 3T3 cells. J Biol Chem. 1995;270:25915–9. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Herbst R, LoRusso P, et al. Continuous administration of ZD1839 (Iressa), a novel oral epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), in patients with five selected tumor types: evidence of activity and good tolerability [abstract 686] Proc Am Soc Clin Oncol. 2000;19 [Google Scholar]
- 5.Negoro S, Nakagawa K, Fukuoka M, et al. Final results of a phase 1 intermittent dose-escalation trial of ZD1839 (`Iressa') in Japanese patients with various solid tumors [abstract 1292] Proc Am Soc Clin Oncol. 2001;20 [Google Scholar]
- 6.Cappuzzo F, Giovanna F, Giulio M, et al. Clinical experience with gefitinib: an update. Crit Rev Oncol Hematol. 2006;58:31–45. doi: 10.1016/j.critrevonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher N, Chang A, Parikh P, et al. ISEL: a phase III survival study comparing gefitinib (IRESSA) plus best supportive care (BSC) with placebo plus BSC, in patients with advanced non-small-cell lung cancer (NSCLC) who had received one or two prior chemotherapy regimens. Lung Cancer. 2005;49:s4. [Google Scholar]
- 8.Jun., 2005. FDA Alert for Healthcare Professionals: gefitinib (marketed as Iressa)
- 9.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 11.Roche Laboratories . Xeloda (capecitabine) prescribing information. 1998. updated 2000, 2004. [Google Scholar]
- 12.Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. 2001;19:2282–92. doi: 10.1200/JCO.2001.19.8.2282. [DOI] [PubMed] [Google Scholar]
- 13.Hoff P, Abbruzzese JL, Medgyesy D, Thomas M, Carter S. Phase II study of Xeloda (capecitabine) in patients with metastatic colorectal cancer demonstrating progression on 5-FU therapy [abstract 993] Proc Am Soc Clin Oncol. 2000;19 [Google Scholar]
- 14.Steinbach G, Lynch PM, Phillips RKS, et al. The effect of celexocib, a cycloxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 15.Liu XH, Rose DP. Differential expression and regulation of cyclooxygenase-1 and -2 in two human breast cancer cell lines. Cancer Res. 1996;56:5125–7. [PubMed] [Google Scholar]
- 16.Form DM, Auerbach R. PGE2 and angiogenesis. Proc Soc Exp Biol Med. 1983;172:214–8. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 18.Cheng T, Cao W, Wen R, Steinberg RH, LaVail MM. Prostaglandin E2 induces vascular endothelial growth factor mRNA expression in cultured rat Muller cells. Invest Ophthalmol Vis Sci. 1998;39:581–91. [PubMed] [Google Scholar]
- 19.Hoper MM, Voelkel NF, Bates TO, et al. Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am J Respir Cell Mol Biol. 1997;17:748–56. doi: 10.1165/ajrcmb.17.6.2888. [DOI] [PubMed] [Google Scholar]
- 20.Jones MK, Wang H, Peskar BM, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999;5:1418–23. doi: 10.1038/70995. [DOI] [PubMed] [Google Scholar]
- 21.Uefuji K, Ichikura T, Sninomiya N, Mochizuki H. Inhibition of gastric cancer cell growth by JTE-522, a specific cyclooxygenase-2 inhibitor: effects on apoptosis-related oncoproteins and vascular endothelial growth factor [abstract 959] Proc Am Soc Clin Oncol. 2000;19 [Google Scholar]
- 22.Kostakoglu L, Agress H, Jr., Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–40. doi: 10.1148/rg.232025705. [DOI] [PubMed] [Google Scholar]
- 23.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Glunde K, Serkova NJ. Therapeutic targets and biomarkers identified in cancer choline phospholipid metabolism. Pharmacogenomics. 2006;7:1109–23. doi: 10.2217/14622416.7.7.1109. [DOI] [PubMed] [Google Scholar]
- 25.Morvan D, Demidem A. Metabolomics by proton nuclear magnetic resonance spectroscopy of the response to chloroethylnitrosourea reveals drug efficacy and tumor adaptive metabolic pathways. Cancer Res. 2007;67:150–9. doi: 10.1158/0008-5472.CAN-06-2346. [DOI] [PubMed] [Google Scholar]
- 26.Affe CC. Measures of response: RECIST, WHO, and new alternatives. J Clin Oncol. 2006;24:3245–51. doi: 10.1200/JCO.2006.06.5599. [DOI] [PubMed] [Google Scholar]
- 27.Serkova N, Fuller TF, Klawitter J, Freise CE, Niemann CU. H-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney Int. 2005;67:1142–51. doi: 10.1111/j.1523-1755.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson L, Johansson E, Kettaneh-Wold N, Wold S. multi- and megavariate data analysis. Umetrics Academy Press; Umea: 2001. PCA and PLS. [Google Scholar]
- 29.Product Information: Iressa®, gefitinib tablets. AstraZeneca Pharmaceuticals; Wilmington (DE): 2005. [Google Scholar]
- 30.Product Information: Xeloda®, capecitabine tablets. Roche Laboratories; Nutley (NJ): 2005. [Google Scholar]
- 31.Dubreil A, Magne N, Fischel JL, et al. Cell cycle arrest induced by the selective epidermal growth factor receptor tyrosine kinase ZD1839 modifies the activity of the key enzyme linked to fluoropyrimidine activity. Proc Am Assoc Cancer Res. 2002;43 [Google Scholar]
- 32.Solomon S, McMurray J, Pfeffer M, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 33.Machover D, Diaz-Rubio E, deGramont A, et al. Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous treatment with fluoropyrimidines. Ann Oncol. 1996;7:95–8. doi: 10.1093/oxfordjournals.annonc.a010489. [DOI] [PubMed] [Google Scholar]
- 34.Cufer T, Vrdoljak E, Gaafar R, et al. Phase II, open-label, randomized study (SIGN) of single gefitinib (IRESSA) or docetaxel as second-line therapy in patients with advanced (stage IIIb or IV) non-small-cell-lung cancer. Anticancer Drugs. 2006;17:401–9. doi: 10.1097/01.cad.0000203381.99490.ab. [DOI] [PubMed] [Google Scholar]
- 35.Iressa® (gefitinib) Clinical Trial Report Summaries. AstraZeneca Pharmaceuticals; Wilmington (DE): 2006. [Google Scholar]
- 36.Wilding G, Soulie P, Trump D, et al. Results from a pilot phase I trial of gefitinib combined with docetaxel and estramustine in patients with hormone-refractory prostate cancer. Cancer. 2006;106:1917–24. doi: 10.1002/cncr.21831. [DOI] [PubMed] [Google Scholar]
- 37.Mariani G, Petrelli F, Zambetti M, et al. Capecitabine/cyclophosphamide/methotrexate for patients with metastatic breast cancer: a dose-finding, feasibility, and efficacy study. Clin Breast Cancer. 2006;7:321–5. doi: 10.3816/CBC.2006.n.044. [DOI] [PubMed] [Google Scholar]
- 38.Martoni A, Pinto C, Fabio F, et al. Capecitabine plus oxaliplatin (Xelox) versus protracted 5-fluorouracil venous infusion plus oxaliplatin (Pvifox) as first-line treatment in advanced colorectal cancer: a GOAM phase II randomized study (FOCA trial) Eur J Cancer. 10 doi: 10.1016/j.ejca.2006.08.034. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 39.Souglakos J, Kalykaki A, Vamvakas L, et al. Phase II trial of capecitabine and oxaliplatin (CAPOX) plus cetuximab in patients with metastatic colorectal cancer who progressed after oxaliplatin-based chemotherapy. Ann Oncol. 1 doi: 10.1093/annonc/mdl392. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 40.Meyerhardt J, Zhu A, Enzinger P, et al. Phase II study of capecitabine, oxaliplatin, erlotinib in previously treated patients with metastatic colorectal cancer. J Clin Oncol. 2006;24:1892–7. doi: 10.1200/JCO.2005.05.3728. [DOI] [PubMed] [Google Scholar]
- 41.Bentzen J, Hansen H. Phase II analysis of paclitaxel and capecitabine in the treatment of recurrent or disseminated squamous cell carcinoma of the head and neck region. Head Neck. doi: 10.1002/hed.20462. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 42.Schoppmeyer K, Miethe S, Wiedmann M, et al. Radiochemotherapy followed by gemcitabine and capecitabine in extrahepatic bile duct cancer: a phase I/II trial. Am J Clin Oncol. 2006;29:576–82. doi: 10.1097/01.coc.0000239167.17922.82. [DOI] [PubMed] [Google Scholar]
- 43.Rodney A, Dieringer P, Mathew P, et al. Phase II study of capecitabine combined with gemcitabine in the treatment of androgen-independent prostate cancer previously treated with taxanes. Cancer. doi: 10.1002/cncr.21894. Epub 2006. [DOI] [PubMed] [Google Scholar]
- 44.Lee D, Han J, Yoon S, et al. A pilot trial of gemcitabine and vinorelbine plus capecitabine in locally advanced or metastatic non-small-cell lung cancer. Am J Clin Oncol. 2006;29:143–7. doi: 10.1097/01.coc.0000203743.32845.40. [DOI] [PubMed] [Google Scholar]
- 45.Nole F, Catania C, Munzone E, et al. Capecitabine/vinorelbine: an effective and well-tolerated regimen for women with pretreated advanced-stage breast cancer. Clin Breast Cancer. 2006;6:518–24. doi: 10.3816/CBC.2006.n.005. [DOI] [PubMed] [Google Scholar]
- 46.Magne N, Fischel J, Dubreuil A, et al. ZD1839 (Iressa®) modifies the activity of key enzymes linked to fluoropyrimidine activity: rational basis for a new combination therapy with capecitabine. Clin Cancer Res. 2003;9:4735–42. [PubMed] [Google Scholar]
- 47.Czito B, Willett C, Bendell J, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol. 2006;24:656–62. doi: 10.1200/JCO.2005.04.1749. [DOI] [PubMed] [Google Scholar]
- 48.Su H, Bodenstein C, Dumont RA, et al. Monitoring tumor glucose utilization by positron emission tomography for the prediction of treatment response to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2006;12:5659–67. doi: 10.1158/1078-0432.CCR-06-0368. [DOI] [PubMed] [Google Scholar]