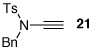Abstract
Rh(II)-catalyzed cyclopropenations of ynamides are described. Although an actual amido-cylopropene intermediate may not be involved, these reactions provide a facile entry to highly substituted 2-amido-fuans, thereby formerly constituting a [3 + 2] cycloaddition. An application of these de novo 2-amido-furans in N-tethered intramolecular [4 + 2] cycloadditions is also illustrated, leading to dihydroindoles and tetrahydroquinolines.
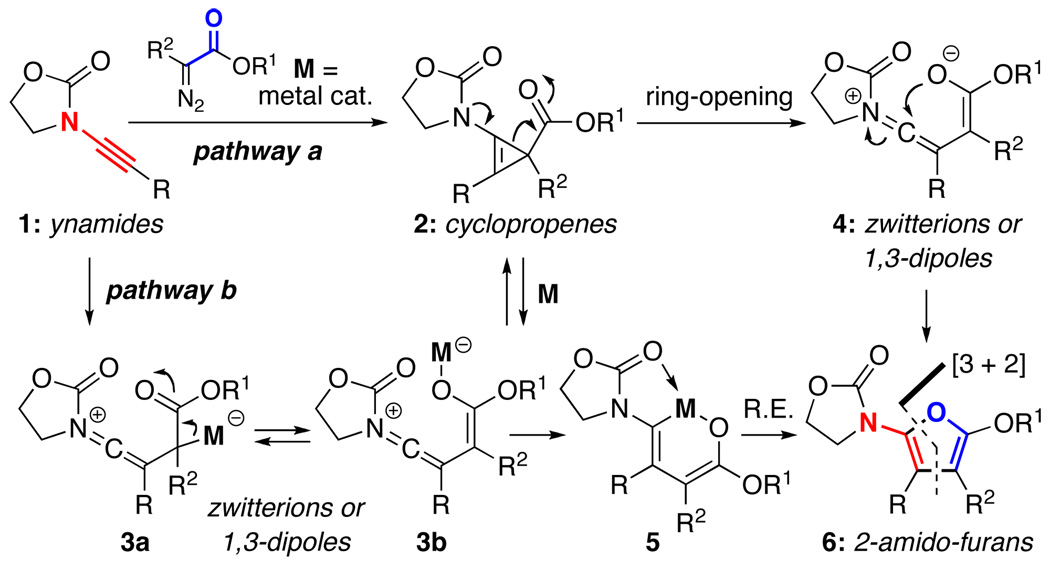
Our involvement with the chemistry of ynamides1,2 and recent interest in cyclopropanation reactions3,4 of various enamides5–7 converged and provoked us to investigate a possible ynamide-cyclopropenation manifold8 that could be useful in synthesis. As shown in Scheme 1, cyclopropenations of ynamides 1 could take place via metal-decomposition of α-diazoacetates4,9,10 to provide cyclopropenes 2 [pathway-a] or metal-bound zwitterionic intermediates or 1,3-dipoles 3a and 3b [pathway-b]. The former can ring-open to give zwitterion 4 [or leading to 3b with the metal assistance], while the latter can in fact serve as intermediates en route to cyclopropenes 2, or providing metallo-oxocyclohexadiene 5 without actually proceeding through a cyclopropenation process.
Scheme 1.
Possible Cyclopropenations of Ynamides.
While it is difficult to precisely distinguish the two pathways, we were interested with the possibility of observing the actual cyclopropenes 2, which can be synthetically useful as demonstrated by an array of elegant work that has appeared in the recent literature.8,11–13 Although cyclopropenations of alkynes have already been beautifully demonstrated as a practical entry to cyclopropenes,8,11–13 accessing 2 could be challenging because with the amide substitution, the ring-opening pathway leading to 1,3-dipoles 4 would be expedited even without the metal assistance.
On the other hand, we were equally intrigued by either metal bound zwitterionic intermediates 3a and 3b or nonmetal bound 4, as both could afford synthetically useful 2-amido-furans 6, respectively, via 5-dig-cyclization and reductive elimination [via 5],14,15 thereby formerly constituting a [3 + 2] cycloaddition. Given that there have been no studies on cyclopropenations of ynamides,1,16 we explored this process and report here our success in the synthesis of highly substituted 2-amido-furans via a Rh(II)-catalyzed cyclopropenation of ynamides.
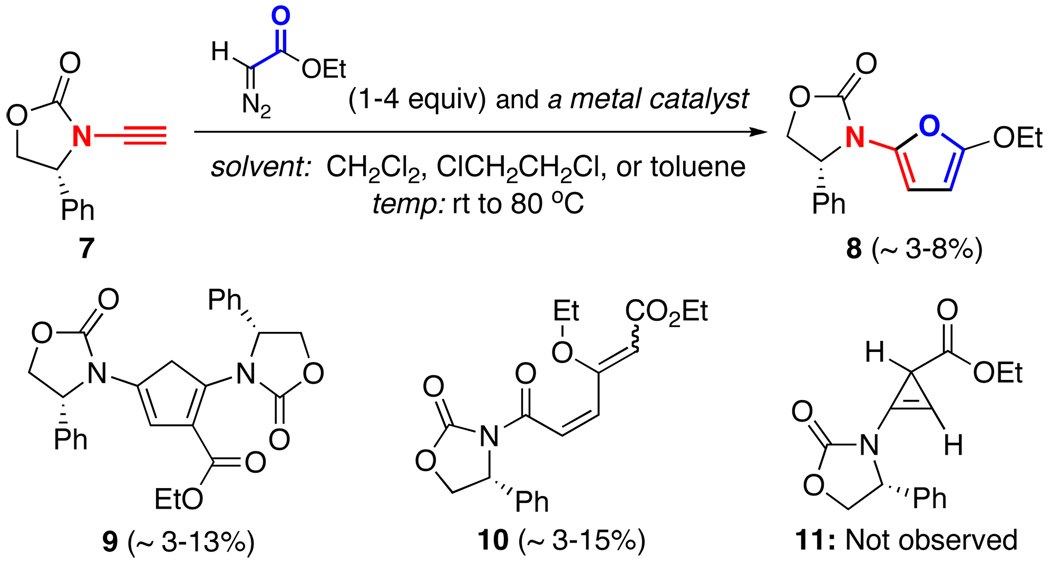
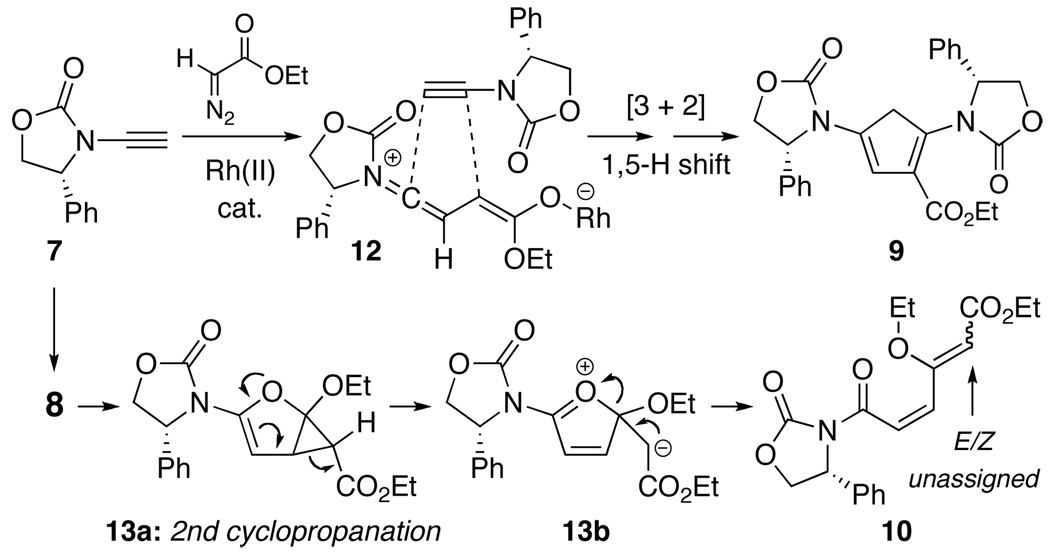
Initial cyclopropenation attempts were carried out employing ethyl α-diazoacetate with either well known metal catalysts8–13 such as Rh2(OAc)4 and Cu(OTf)2, or newer Rh(II) catalysts such as Rh2(capy)417 and Dubois’ catalyst18 [Scheme 2]. These attempts led to a range of low yielding products, which included 2-amido-furan 8, cyclopentadiene 9, and diene 10.19 However, amido-cyclopropene 11 was not one of them.20 The formation of cyclopentadiene 9 could be readily rationalized through a [3 + 2] cycloaddition of zwitterion 12 along with an ensuing 1,5-H-shift to rearrange the conjugation [Scheme 3].21 On the other hand, diene 10 could be derived from 2-amido-furan 8 through a second cyclopropanation process followed by cyclopropyl ring-opening [see 13a22]. The stereochemistry of the vinylogous carbonate double bond in 10 was unassigned.
Scheme 2.
Initial Attempts with Ethyl α-Diazoacetate.
Scheme 3.
Pathways to Cyclopentadiene 10 and Diene 11.
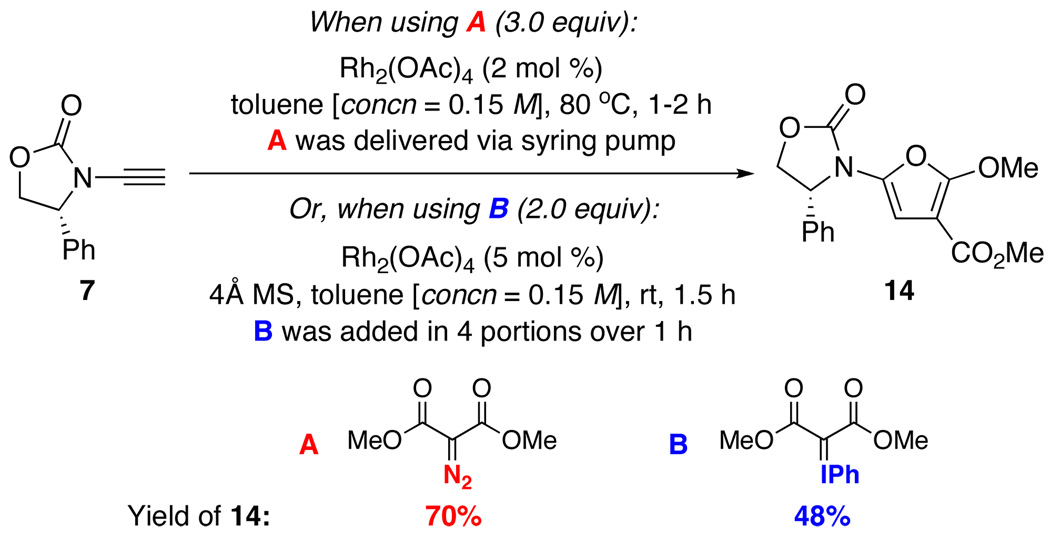
While cyclopentadi ene 9 and diene 10 can be useful synthetically, 2-amido-furan 8 represents the most attractive building block in addition to being an emerging pharmacophore with a range of important biological activities.23 Consequently, we focused on identifying an effective catalytic protocol for the furan formation, which constitutes a [3 + 2] cycloaddition. As shown in Scheme 4, we elected to use diazo dimethyl malonate A as well as phenyl iodonium ylide B15e,24 as the cyclopropanating agent. While the corresponding cyclopropene product remained elusive, after optimizations, we were able to isolate the desired 2-amido-furan 14 in 70% and 48% yield, respectively, from employing A and B. It is noteworthy that the optimized conditions involved delivering diazo malonate A as a toluene solution via syringe pump over 1–2 h, and addition of ylide B as solids in four separate portions over 1 h.
Scheme 4.
Success with Diazo Malonate A and Ylide B.
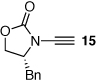
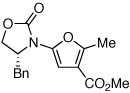
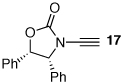
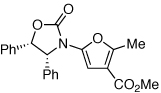
These protocols turned out to be general for constructing a diverse array of de novo 2-amido-furans as summarized in Table 1 and Table 2. In Table 1, a clear trend is that diazo malonate A is a better cyclopropanating agent than phenyl iodonium ylide B, consistently providing higher yields in all entries. In addition, sulfonyl-substituted ynamides 21–24 were quite feasible [entries 4-9], and so were terminally substituted ynamides 29a and 29b to give tetra-substituted furans 30a and 30b, albeit reactions were slower and yields are lower as a consequence [entries 10 and 11].
Table 1.
A General Synthesis of 2-Amido-Furans.
| entry | ynamides | 2-amido-furans | yield [%]:a | using Ab | Bc |
|---|---|---|---|---|---|
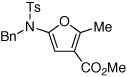
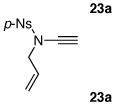
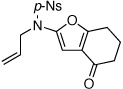
| 1 |  |
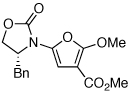 |
18 | 82 | 44 |
| 2 | 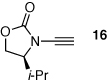 |
 |
19 | 65 | 48 |
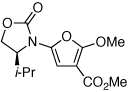
| 3 | 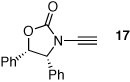 |
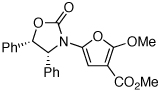 |
20 | 77 | 48 |
| 4 | 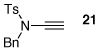 |
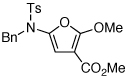 |
25 | 50 | 24 |
| 5 | 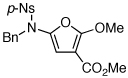 |
26 | 47 | 29 | |
| 6 | 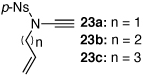 |
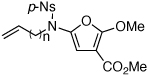 |
27a | 49 | 32 |
| 7 | 27b | 58 | 35 | ||
| 8 | 27c | 61 | 47 | ||
| 9 | 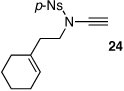 |
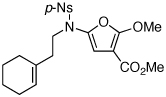 |
28 | 52 | 39 |
| 10 | 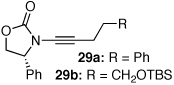 |
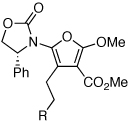 |
30a | 45d | 37e |
| 11 | 30b | 24d | 22e |
Isolated yields.
When using A, all reactions were run in toluene at 80 °C with 2 mol % Rh2(OAc)4, and ynamide concn = 0.15 M. Reagent A [3.0 equiv] was delivered over 1–2 h as a solution in toluene [concn = 0.30 M] via a syringe pump.
When using B, all reactions were run in CH2Cl2 at rt for 1.5 h with 5 mol % Rh2(OAc)4 and 4Å MS, and ynamide concn = 0.15 M. Reagent B [2.0 equiv] was delivered as solid in 0.5 equiv portions over 1 h.
4.0 equiv of A and 5 mol % Rh2(OAc)4 were used, and temp was 110 °C.
Reactions were carried out in ClCH2CH2Cl [concn = 0.15 M] at 50 °C, and a total of 4.0 equiv of reagent B was used.
Table 2.
Other Diazo Compounds and Iodonium Ylides.
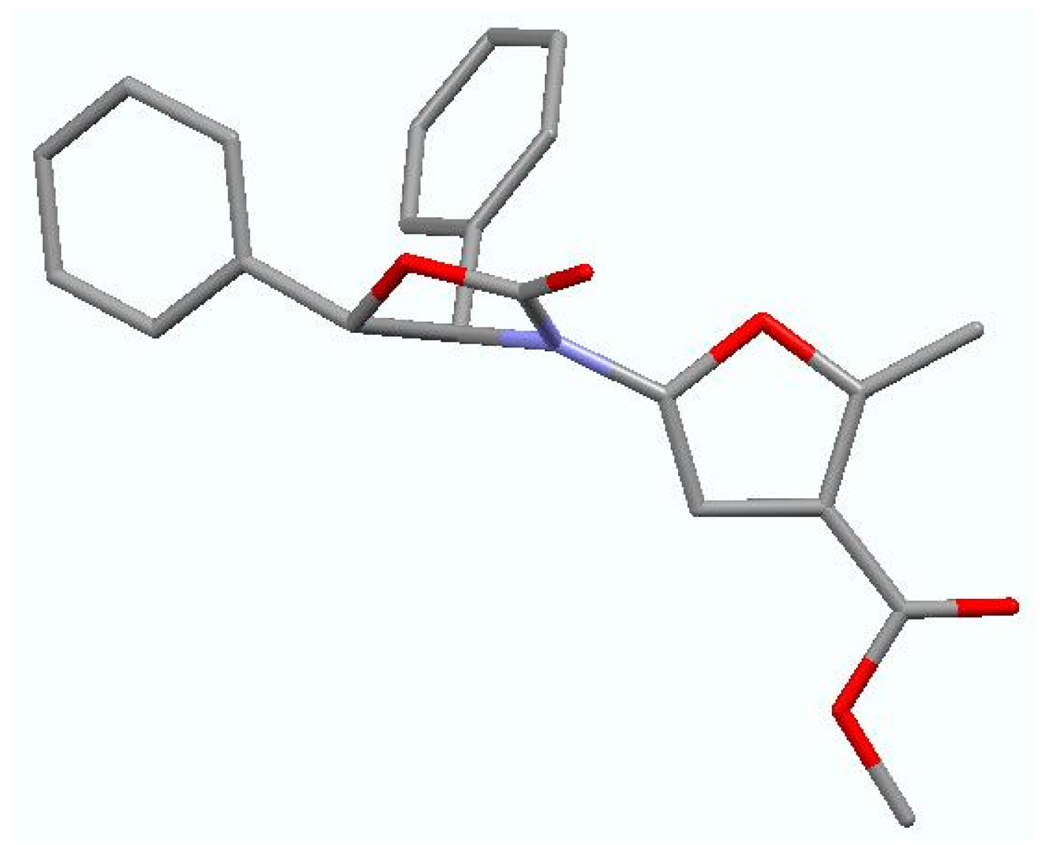
In Table 2, we were able to examine other diazo compounds as well as iodonium ylides. While yields are again better with diazo compounds in comparison to the respective ylides, when using either diazo compound E or ylide F, the furan formation was highly regioselective [entries 2–5 and 7]. The regiochemistry was unambiguously assigned via X-ray single-crystal structure of 2-amido-furan 34 [Figure 1].
Figure 1.
X-Ray Structure of 2-Amido-Furan 34.
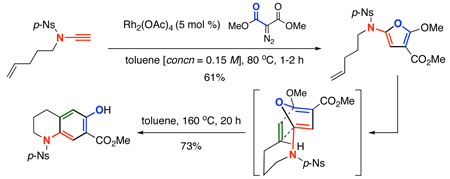
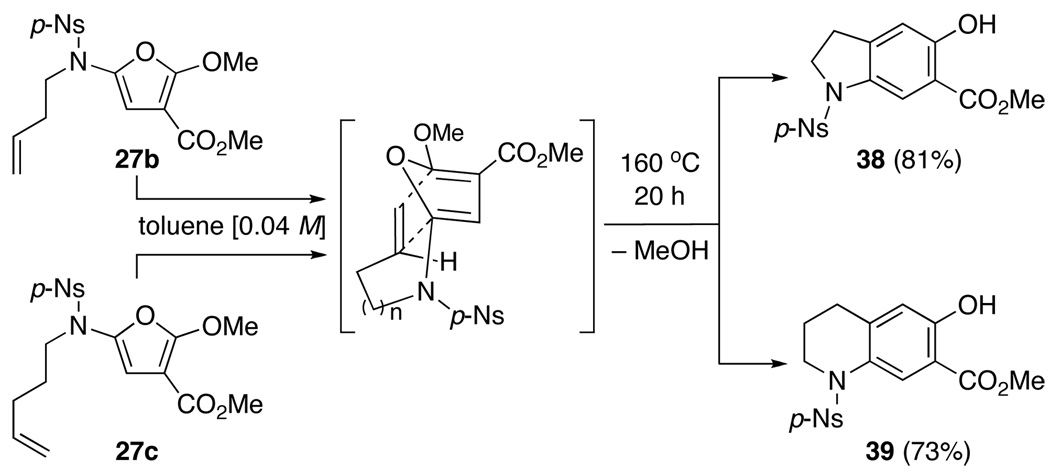
Lastly, we engaged in an immediate application of these 2-amido-furans given their power in serving as a platform for intramolecular Diels-Alder cycloadditions.25 As shown in Scheme 5, after heating 2-amido-furans 27b and 27c at 160 °C in toluene in a sealed tube for 20 h, respective products dihydroindole 38 and tetrahydroquinoline 39 were isolated in high yields. These final products are a result of loss of MeOH from the initial cycloadducts. It is noteworthy that the ability to carry out these N-tethered intramolecular Diels-Alder cycloadditions demonstrates a distinct advantage of furan synthesis from ynamides through the cyclopropenation process.
Scheme 5.
An Application of 2-Amido-Furans in Diels-Alder.
We have described here a process of Rh2(OAc)4 catalyzed cyclopropenations of ynamides. Although an actual amido-cylopropene intermediate may not be involved, these reactions provide a facile entry to highly substituted de novo 2-amido-fuans, which formerly constitutes a [3 + 2] cycloaddition. Developing useful applications of this furan formation are underway.
Supplementary Material
Acknowledgement
Authors thank NIH [GM066055] for financial support. We thank Dr. Victor G. Young, Jr. of University of Minnesota for solving X-ray structure. We also thank Professor Huw Davies for invaluable discussions and suggestions.
Footnotes
Supporting Information Available: Experimental procedures as well as characterizations, X-ray structural data, and NMR spectra are available for all new compounds and free of charge via Internet http://pubs.acs.org.
References
- 1.For reviews on ynamides, see: Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575. Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379. Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455.
- 2.For chemistry of ynamides just in the past 8 months from the literature, see: Cockburn N, Karimi E, Tam W. J. Org. Chem. 2009;74:5762. doi: 10.1021/jo9010206. Yao B, Liang Z, Niu T, Zhang Y. J. Org. Chem. 2009;74:4630. doi: 10.1021/jo900595c. Coste A, Karthikeyan G, Couty F, Evano G. Angew. Chem. Int. Ed. 2009;48:4381. doi: 10.1002/anie.200901099. Gourdet B, Lam HW. J. Am. Chem. Soc. 2009;131:3802. doi: 10.1021/ja900946h. Couty S, Liegault B, Meyer C, Cossy J. Tetrahedron. 2009;65:3882. Deweerdt K, Birkedal H, Ruhland T, Skrydstrup T. Org. Lett. 2009;11:221. doi: 10.1021/ol802477d. Alayrac C, Schollmeyer D, Witulski B. Chem. Commun. 2009:1464. doi: 10.1039/b820291e. Garcia P, Moulin S, Miclo Y, Leboeuf D, Gandon V, Aubert C, Malacria M. Chem. Eur. J. 2009;15:2129. doi: 10.1002/chem.200802301. Sato A, Yorimitsu H, Oshima K. Synlett. 2009:28. Oppenheimer J, Johnson WL, Figueroa R, Hayashi R, Hsung RP. Tetrahedron. 2009;64:5001. doi: 10.1016/j.tet.2009.03.078. Zhang Y, DeKorver KA, Lohse AG, Zhang Y-S, Hsung RP. Org. Lett. 2009;11:899. doi: 10.1021/ol802844z.
- 3.For a leading review on cyclopropanations, see: Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem. Rev. 2003;103:977. doi: 10.1021/cr010007e.
- 4.For other recent reviews on cyclopropanations, see: Brackmann F, de Meijere A. Chem. Rev. 2007;107:4493. doi: 10.1021/cr078376j. Pellissier H. Tetrahedron. 2008;64:7041. Gnad F, Reiser O. Chem. Rev. 2003;103:1603. doi: 10.1021/cr010015v. Brandi A, Cicchi S, Cordero FM, Goti A. Chem. Rev. 2003;103:1213. doi: 10.1021/cr010005u. de Meijere A, Kozhushkov SI. Chem. Rev. 2000;100:93. doi: 10.1021/cr960153y.
- 5.Song Z, Lu T, Hsung RP, Al-Rashid ZF, Ko C, Tang Y. Angew. Chem. Int. Ed. 2007;46:4069. doi: 10.1002/anie.200700681. [DOI] [PubMed] [Google Scholar]
- 6.Lu T, Song Z, Hsung RP. Org. Lett. 2008;10:541. doi: 10.1021/ol702824s. [DOI] [PubMed] [Google Scholar]
- 7.Lu T, Hayashi R, Hsung RP, DeKorver KA, Lohse AG, Song Z, Tang Y. Org. Biomol. Chem. 2009;9:3331. doi: 10.1039/b908205k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For reviews on cyclopropenations of alkynes. Padwa A. Molecules. 2000;6:1. Padwa A. J. Organomet. Chem. 2001;617–618:3. Doyle MP, Hu W. Synlett. 2001:1364.
- 9.For reviews on cyclopropanations via metal-catalyzed decompositions of diazo-esters, see: Doyle MP. Chem. Rev. 1986;86:919. Padwa A, Krumpe KE. Tetrahedron. 1992;48:5385.. (c) 15. Calter MA. Curr. Org. Chem. 1997;1:37. Doyle MP, Forbe DC. Chem. Rev. 1998;98:911. doi: 10.1021/cr940066a. Davies HML, Autoulinakis E. Org. React. 2003;57:1. Maas G. Chem. Soc. Rev. 2004;33:183. doi: 10.1039/b309046a. Doyle MP. J. Org. Chem. 2006;71:9253. doi: 10.1021/jo061411m.
- 10.Also see: Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis With Diazo Compounds. John Wiley and Sons, Inc.; 1998. Chapter 4. and references therein.
- 11.For recent informative reviews on cyclopropene synthesis and its chemistry: Marek I, Simaan S, Masarwa A. Angew. Chem. Int. Ed. 2007;46:7364. doi: 10.1002/anie.200604774. Rubin M, Rubina M, Gevorgyan V. Chem. Rev. 2007;107:3117. doi: 10.1021/cr050988l. Rubin M, Rubina M, Gevorgyan V. Synthesis. 2006:1221. Fox JM, Yan N. Curr. Org. Chem. 2005;9:719. Baird MS. Chem. Rev. 2003;103:1271. doi: 10.1021/cr010021r. Walsh R. Chem. Soc. Rev. 2005;34:714. doi: 10.1039/b310975p. Dolbier WR, Jr, Battiste MA. Chem. Rev. 2003;103:1071. doi: 10.1021/cr010023b.
- 12.For earlier reviews, see: Deem ML. Synthesis. 1972:675. Billups WE, Haley MM, Lee G-A. Chem. Rev. 1989;89:1147. Padwa A, Fryxell GE. Adv. Strain in Org. Chem. 1991;1:117.
- 13.For leading examples on cyclopropenations of alkynes and recent chemistry of cyclopropenes, see: Panne P, Fox JM. J. Am. Chem. Soc. 2007;129:22. doi: 10.1021/ja0660195. Chuprakov S, Gevorgyan V. Org. Lett. 2007;9:4463. doi: 10.1021/ol702084f. Chuprakov S, Hwang FW, Gevorgyan V. Angew. Chem. Int. Ed. 2007;46:4757. doi: 10.1002/anie.200700804. Yang Z, Xie X, Fox JM. Angew. Chem. Int. Ed. 2006;45:3960. doi: 10.1002/anie.200600531. Rubin M, Gevorgyan V. Synthesis. 2004:796. Davis HM, Lee GH. Org. Lett. 2004;6:1233. doi: 10.1021/ol049928c. Doyle MP, Hu W. Tetrahedron Lett. 2000;41:6265. Doyle MP, Ene DG, Forbes DC, Pillow TH. Chem. Commun. 1999:1691. Müller P, Imogai H. Tetrahedron: Asymmetry. 1998;9:4419. Padwa A, Kassir JM, Xu SL. J. Org. Chem. 1997;62:1642.
- 14.For earlier documentations of furan formation from cycloproenation processes, see: Cho SK, Liebeskind LS. J. Org. Chem. 1987;52:2631. Davies HML, Romines KR. Tetrahedron. 1988;44:3343. Müller P, Pautx N, Doyle MP, Baheri V. Helv. Chim. Acta. 1990;73:1233. Hoye TR, Dinsmore CJ, Johnson DS, Korkowski PF. J. Org. Chem. 1990;55:4518. Padwa A, Kassir JM, Xu SL. J. Org. Chem. 1991;56:6971. Fairfax David J, Austin David J, Xu Simon L, Padwa Albert. J. Chem. Soc., Perkin Trans 1. 1992:2837.
- 15.For recent examples of synthesizing furans from cyclopropenations, see: Zhao L-B, Guan Z-H, Han Y, Xie Y-X, He S, Liang Y-M. J. Org. Chem. 2007;72:10276. doi: 10.1021/jo7019465. Ma S, Lu L, Lu P. J. Org. Chem. 2005;70:1063. doi: 10.1021/jo048430l. Rubin M, Gevorgyan V. Synthesis. 2004:796. Padwa A, Straub CS. J. Org. Chem. 2003;68:227. doi: 10.1021/jo020413d.. (e) For an example of using iodonium ylide C see: Lee YR, Yoon SH. Synth. Commun. 2006;36:1941.
- 16.For a lone example of pyrrole-substituted ynamine-cyclopropenation, see: Pirrung MC, Zhang J, Morehead AT., Jr Tetrahedron Lett. 1994;35:6229.
- 17.Rh2(capy)4: dirhodium(II) tetrakis(caprolactam). For leading references, see: Doyle MP, Peterson CS, Protopopova MN, Marnett AB, Parker DL, Jr, Ene DG, Lynch V. J. Am. Chem. Soc. 1997;119:8826. Padwa A, Austin DJ, Hornbuckle SF, Semones MA, Doyle MP, Protopopova MN. J. Am. Chem. Soc. 1992;114:1874.
- 18.Dubois' catalyst: Bis[rhodium(α,α,α’,α’-tetramethyl-1,3-benzenedipropionic acid)]. For a leading reference, see: Espino CG, Fiori KW, Kim M, Du Bois J. J. Am. Chem. Soc. 2004;126:15378. doi: 10.1021/ja0446294.
- 19.See Supporting Information.
- 20.As suggested by a referee, we are currently attempting to detect possible formation of cyclopropenes at low temp using NMR.
- 21.For a beautiful precedent, see: Hoye TR, Dinsmore CJ. Tetrahedron Lett. 1991;32:3755.
- 22.For reports on isolable products related to intermediate 13a, see: Böhm C, Schinnerl M, Bubert C, Zabel M, Labahn T, Parisini E, Reiser O. Eur. J. Org. Chem. 2000:2955. Schinnerl M, Böhm C, Seitz M, Reiser O. Tetrahedron: Asymmetry. 2003;14:765.
- 23.For recent references on biological activities of amino-furans, see: Patch RJ, Brandt BM, Asgari D, Baindur N, Chadha NK, Georgiadis T, Cheung WS, Petrounia IP, Donatelli RR, Chaikin MA, Player MR. Bioorg. Med. Chem. Lett. 2007;17:6070. doi: 10.1016/j.bmcl.2007.09.057. Hall A, Billinton A, Brown SH, Chowdhury A, Giblin GMP, Goldsmith P, Hurst DN, Naylor A, Patel S, Scoccitti T, Theobald PJ. Bioorg. Med. Chem. Lett. 2008;18:2684. doi: 10.1016/j.bmcl.2008.03.018. Isabel, Lopez C, Garcia-Mera X, Stefanachi A, Nicolotti O, Isabel Loza M, Brea J, Esteve C, Segarra V, Vidal B, Carotti A. Bioorg. Med. Chem. 2009;17:3618. doi: 10.1016/j.bmc.2009.03.062.
- 24.For some examples of using iodonium ylides, see: Batsila C, Kostakis G, Hadjiarapoglou LP. Tetrahedron Lett. 2002;43:5997. Muller P, Allenbach YF, Bernardinelli G. Helv. Chim. Acta. 2003;86:3164. Huang X-C, Liu Y-L, Liang Y, Pi S-F, Wang F, Li J-H. Org. Lett. 2008;10:1525. doi: 10.1021/ol800051k.. (d) Also see Reference 15e.
- 25.For leading examples of intramolecular Diels-Alder Reactions of 1-amido-furans, see: Boonsombat J, Zhang H, Chughtai MJ, Hartung J, Padwa A. J. Org. Chem. 2008;73:3539. doi: 10.1021/jo8003716. Zhang H, Padwa A. Org. Lett. 2006;8:247. doi: 10.1021/ol052524f. Padwa A, Brodney MA, Dimitroff M. J. Org. Chem. 1998;63:5304. doi: 10.1021/jo010020z.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.