Abstract
Bone morphogenetic proteins (BMP) provide critical signals for determining cell fate, specifying gastrulation, embryonic patterning, organogenesis, and the remodeling of diverse tissues. Recent work has suggested that in addition to coordinating pivotal events in development, BMPs may also regulate certain homeostatic physiological processes independently of effects on cell growth or differentiation. We recently described the identification of dorsomorphin, a small molecule inhibitor of BMP type I receptors which inhibits BMP signaling in preference to TGF-β, Activin, and other ligands of the TGF-β family. We describe a number of strategies using dorsomorphin and its derivatives as probes to assess the physiologic roles of BMP signaling. We also discuss several potential applications for small molecule BMP inhibitors, including stem cell manipulation, and the therapeutic modification of bone remodeling, heterotopic ossification, and iron homeostasis.
Introduction
Bone morphogenetic proteins, having key roles in embryogenesis, appear to dictate the balance between differentiation and expansion in a number of progenitor cell populations, including embryonic stem cells, hematopoietic stem cells, vascular endothelial progenitors, and cardiac myocyte and skeletal myogenic precursor cells1–6. It is likely that nearly all terminally differentiated or specialized cells encounter functionally critical bone morphogenetic protein (BMP) signals during at least one, if not several steps of maturation as they undergo specification from multipotent progenitors.
BMPs are structurally diverse set of ligands which include more than 20 distinct BMPs subunits which together constitute a sizable component of the larger TGF-β ligand family7–9. MP ligands frequently exist as disulfide-linked homodimers of identical BMP subunits, however, heterodimers consisting of distinct BMP subunits have essential signaling functions in developmental patterning10–12. BMP signals are transduced by heterotetrameric complexes of BMP type II and type I receptors assembled in the context of ligand13. These ligands are selectively recognized by a structurally diverse set of target receptors, with specificity being determined by the cognate pairings of BMP type II receptor (BMPRII) or Activin type II receptor (ActRIIa and ActRIIb) with various BMP type I receptors (ALK1, ALK2, ALK3, and ALK6)7. While BMP ligand homodimers are generally recognized by receptor heterotetramers consisting of two identical type II and two identical type I receptors, heterodimeric ligands composed of structurally distinct subunits may be recognized by heteromers of non-identical type II and/or type I receptors12. Surface coreceptors such as the repulsive guidance molecule (RGM) family and endoglin act to further refine ligand-receptor specificity14–18. Extracellular antagonists such as noggin, follistatin, and chordin function to sequester ligands, inhibiting signaling or forming signaling gradients by their diffusion8, 19. When engaged by ligand, constitutively-active intracellular serine-threonine kinase domains of type II receptors phosphorylate conditionally-active serine-threonine kinases of type I receptors, which in turn phosphorylate intracellular effector proteins, the BMP receptor (BR-) associated SMADs 1, 5, and 8. Activated BR-SMADs, which bind co-SMAD4, are selectively retained in the nucleus to broadly affect gene transcription, activating and repressing broad suites of genes with importance in cell growth and differentiation, including the early BMP transcription target Inhibitor of differentiation (Id) genes9. While converging upon shared effector molecules and transcriptional programs, this highly diverse and modular system achieves specificity in signaling and function by virtue of spatiotemporally coordinated expression of ligands, their inhibitors, and the complement of BMP receptors and coreceptors which specifically recognize these ligands in particular tissues.
Experimental strategies for modifying BMP signaling have previously employed recombinant ligands in order to initiate BMP signaling, and recombinant endogenous antagonists, soluble co-receptors, or neutralizing antibodies in order to block signaling. Alternatively, gene transfer of ligands, antagonists, or receptors have also been successfully applied to modulate signaling, particularly for in vivo settings. As a complementary strategy to these recombinant and genetic approaches, and in order to modulate BMP signaling in vivo with greater flexibility and decreased cost, we actively sought to identify small molecules with the ability to perturb the BMP signaling pathway, using high throughput screening methodologies.
Discovery of dorsomorphin using an embryonic zebrafish screening assay
In the recent years, zebrafish have proven to be a valuable model organism for small molecule discovery20–22. Given their external development, transparency, and rapid maturation, zebrafish embryos offer an ideal platform for observing perturbations in developmental programs. Moreover, phenotypic screening of thousands of embryos on a daily basis is possible given the high fecundity of zebrafish. These features, which were essential for the success of forward genetic screens in this organism, also make zebrafish a uniquely valuable vertebrate model for performing high-throughput phenotype-based screens to identify bioactive small molecules (Figure 1).
Figure 1. Schema for chemical screening using zebrafish embryos.
With the advances and widespread use of high-throughput screening (HTS) technologies, it is not difficult to identify compounds that target a particular protein or a pathway. A greater challenge lies in identifying selective modulators. Traditionally, this involves retesting of selected candidates against an extensive set of related and unrelated targets. Even then, determining which “off target” effects are tolerable or relevant in vivo can be very difficult. Such challenges are crucial for the successful application of small molecules as tools for manipulating inherently complex systems such as whole animals. In this regard, the main advantage of zebrafish-based chemical screening over traditional HTS platforms is the built-in means to assess specificity, efficacy and toxicity of small molecules in the context of whole live animals. In principle, a zebrafish based phenotype-based screen takes advantage of the embryonic cell’s intrinsic capability to distinguish and integrate multiple signaling pathways and to trigger precise developmental outputs. At the same time, nonspecific perturbations lead to nonspecific events like rapid death or developmental arrest. Thus, like some other organism-based high-throughput screening approaches, an embryonic zebrafish chemical screen has the potential to be an optimal “high-content” screen, containing the means to assess the activity of small molecules against many pathways simultaneously in whole organisms, identifying compounds whose effects on phenotype suggest selectivity versus those which are non-selective or toxic.
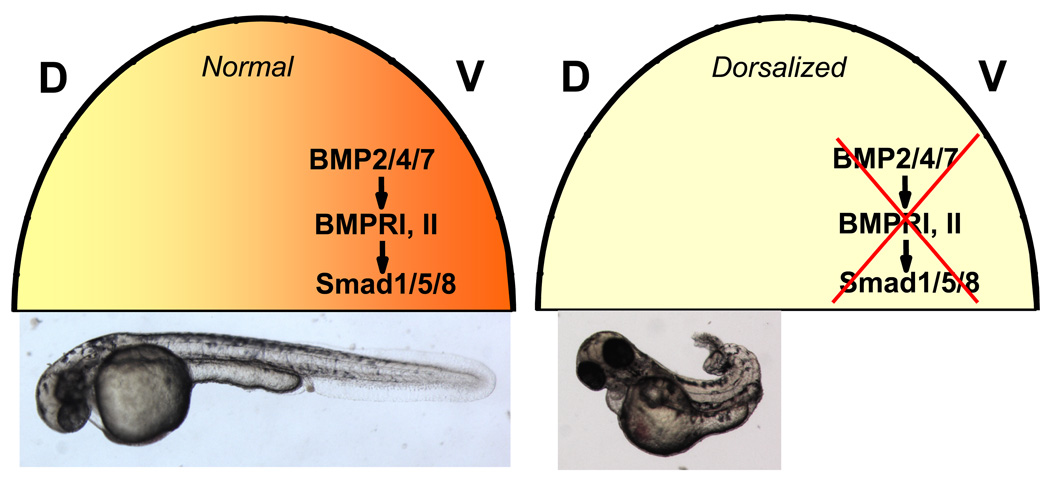
In vertebrates, the BMP signaling serves a crucial role in establishing of embryonic dorso-ventral (DV) axis by inducing the ventral fates (Figure 2)23. In the early zebrafish embryo, a BMP activity gradient is formed which results in the highest levels of signaling in the future ventral pole. The engagement of BMP ligands and receptors activates Smad1/5/8 to induce BMP transcriptional targets, which in this context specifies the ventral development program7. Genetic mutants of the BMP signaling pathway exhibit the dorsalized axial pattern, characterized by an expansion of structures derived from the dorsal blastoderm margin and a reduction of ventrally derived structures (Figure 2, Figure 3)24. Based on the well-characterized and essential role of BMP signaling in DV axis determination, we hypothesized that small molecules which specifically inhibit BMP signaling might recapitulate the dorsalized axial pattern seen in the BMP pathway mutants, or conversely, that BMP sensitizing compounds might induce a ventralized axial pattern.
Figure 2. Role of BMP signaling in embryonic dorso-ventral (DV) patterning.
In early zebrafish embryos, a BMP signaling gradient is formed along the DV axis, with a peak in the future ventral pole (green). Left, normal BMP signaling gradients result in embryos with normal DV axial pattern, below. Right, disruption of BMP signaling either by genetic mutations or small molecule inhibitors results in embryo with dorsalized axial pattern, below. The animal pole of 3-hpf embryo is depicted as a half-circle, above.
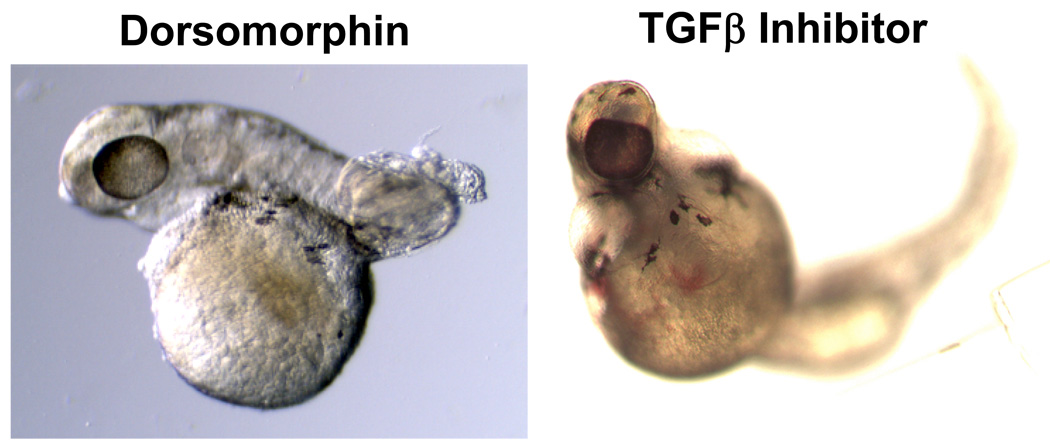
Figure 3. Dorsomorphin (DM) treatment results in the dorsalized phenotype seen in embryos with genetic defects in BMP signaling, but does not phenocopy defects caused by TGFβ inhibition.
(Left panel) Embryo treated with DM shows severe dorsalization, lacking ventrally derived posterior tissues at 36-hpf. (Right panel) treatment with a TGFβ-selective inhibitor SB431542 phenocopies cyclopia seen in cyclops mutants with mutant nodal genes72.
The validity and feasibility of the proposal were demonstrated by the discovery of dorsomorphin, the first reported selective small molecule inhibitor of the BMP pathway25. Dorsomorphin was identified based on its ability to reproducibly phenocopy the DV pattern defects seen in BMP pathway mutants. Consistent with dorsalization of body axis, dorsomorphin-treated embryos exhibited lateral expansion of the dorsal cell fate markers pax2.1 and Krox20 at the 6-somite stage25. Consequently, dorsomorphin treatment disrupted the formation of caudal and posterior structures which derive from the embryonic ventral pole. Importantly, dorsomorphin did not cause cyclopia, a phenotype associated with defective TGF-β signaling26, suggesting a degree of selectivity for the antagonism of BMP versus other TGF-β ligands (Figure 3A).
In mammalian cells, dorsomorphin blocked ligand-induced phosphorylation of SMAD1/5/8 by diverse BMP ligands in a dose-dependent manner, but had much less of an effect on the activation of Smad2 or Smad3 by TGF-β or Activin A25. In addition, dorsomorphin blocked the ability of constitutively-active forms of BMP type-I receptors ALK1, ALK2, ALK3, and ALK6 to induce BMP (BRE-Luc) transcriptional activity in transfected cells, with IC50 values of approximately ~ 1 µM, 0.2 µM, 0.5 µM, and 5 µM respectively in this cell-based assay25, 27. In addition, dorsomorphin demonstrated the ability to block the activation of SMAD1/5/8 induced by constitutively-active type I receptors. These properties strongly suggested that dorsomorphin acts to inhibit BMP type I receptor serine-threonine kinase activity25. Moreover, the heterocyclic core structure of the pyrazolopyrimidine suggested a mechanism of competition with the ATP binding site. We subsequently have found that dorsomorphin can inhibit the ability of recombinant ALK2 protein to phosphorylate substrate proteins, with an IC50 of approximately ~ 5 nM in an cell-free kinase assay (Figure 4). In fact, the structure of the ALK2 intracellular kinase domain was recently resolved in complex with dorsomorphin and regulatory protein FKBP12, demonstrating unequivocally that the pyrazolopyrimidine ring binds in an ATP-mimetic fashion within the adenine binding pocket of the ALK2 kinase domain28. An additional interesting property of dorsomorphin is its apparent ability to inhibit BMP-induced phosphorylation of BRSMADs without affecting BMP-induced activation of MAPK p3825. The ability to functionally separate BMP-mediated activation of SMAD1/5/8 and MAPK p38 with dorsormorphin suggests these effectors are activated by different aspects of BMP signaling, however, the precise mechanism of this functional separation and its biological significance remains to be elucidated.
Figure 4. Dorsomorphin antagonizes ALK2 kinase activity in a cell free system.
The phosphorylation of a substrate (casein) measured by the incorporation of 32P-ATP mediated by recombinant ALK2 protein (in the presence of 50 µM ATP) was dose-dependently inhibited by the addition of dorsomorphin at nanomolar concentrations.
In zebrafish embryonic development, treatment with dorsomorphin elicted the full spectrum of dorsalization defects seen in genetic mutants of the BMP pathway. The extent of dorsalization induced by dorsomorphin varied as a function of dose and timing. For example, when added at 6–8 hours post fertilization (hpf), dorsomorphin caused mild dorsalization manifest as the absence of the ventral tail fin similar to that of lost-a-fin (ALK8 mutant) zebrafish24. By contrast, when dorsomorphin was added at identical concentration to embryos at 1–2 hpf, tail development was more profoundly disrupted, resembling fish with more profound BMP signaling defects24. Thus, the timing of BMP signal inhibition had a substantial impact on severity of DV pattern defects, with delayed BMP inhibition leading to diminished dorsalization. Varying the concentration of dorsomorphin also impacted the severity of the dorsalization phenotype 25. More recently, a very similar relationship between the timing of BMP inhibition in zebrafish embryos and the severity of dorsalization was observed in zebrafish expressing heat-shock inducible BMP antagonist chordin29. Thus, the small molecule approach for inhibiting BMP signaling during zebrafish development faithfully recapitulates results obtained using various mutant and transgenic models, paralleling allele dosage and temporal effects while providing greater flexibility in varying the degree and timing of BMP inhibition. Such temporal control of BMP signaling permits the elucidation of developmental processes that occur later in development. For example, exposure of zebrafish to dorsomorphin starting at 24 hpf, after establishment of the dorso-ventral axis, circumvented the early requirement of BMP signaling in DV patterning, but highlighted the role of BMP signaling in osteogenesis25.
Identification of potent dorsomorphin derivatives via medicinal chemistry
While dorsomorphin has proven to be useful in various contexts as a small molecule probe of BMP function, some important limitations of this parent compound are well known. Being a known biologically active compound which was previously described to inhibit AMP activated kinase (AMPK)30, 31, compound C as it was originally known has several important off-target effects. In our hands, the effects of dorsomorphin upon AMPK activity, measured by its ability to inhibit AICAR-induced phosphorylation of the AMPK substrate Acetyl CoA-Carboyxylase, was relatively weaker than its effects on BMP signaling when both were measured in vitro in cell-based assays (IC50 ~ 2 µM vs. 0.5 µM)32. Importantly, dorsomorphin is structurally homologous to a series of compounds which are known to inhibit VEGF receptor KDR33–35. We have found that dorsomorphin exhibits significant activity against KDR and other tyrosine kinase receptors including PDGFR-β, blocking PDGF-BB-induced phosphorylation of Akt in cells with a potency approaching its ability to inhibit BMP signaling (IC50 ~ 0.8 µM)32. Because of these significant off-target effects, and because of limited metabolic stability in mouse liver microsomal degradation assays (t1/2 = 10.4 min)36 a medicinal chemistry effort was pursued to improve the selectivity of this compound for BMP signaling as well as its metabolic stability.
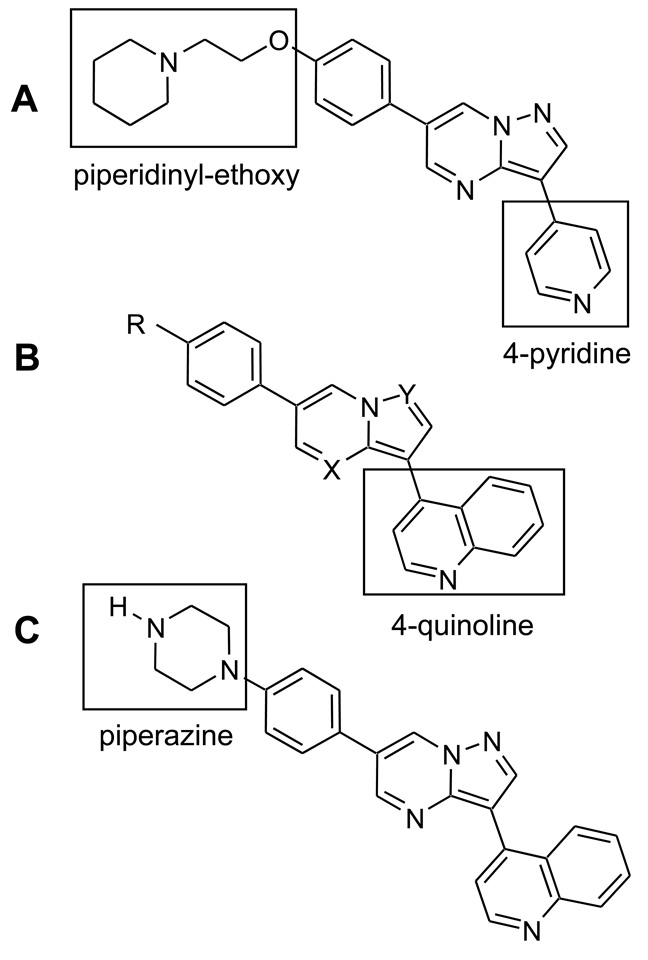
A reiterative process of modifying the pendent functional groups as well as the heterocyclic core yielded a series of compounds which were tested for metabolic stability, potency and selectivity for inhibition of BMP signaling36. In general, nitrogen substituents of the core heterocyclic pyrazolo[1,5-a]pyrimidine structure did not tolerate modification except at the N-4 position (X), while the N-1 position (Y) was necessary for potent inhibition as was the case for retaining KDR kinase inhibition in another series of compounds37 (Figure 5A–B). This finding is consistent with the observation that the N-1 atom of the parent molecule dorsomorphin is in proximity to form hydrogen bonding with the amide group of the histidine at position 286 based on the resolved structure of ALK228. By replacing the pendent 4-pyridine ring with a 4-quinolyinyl group, we were able to improve the potency for inhibiting BMP4 activity by 50- to 100-fold while simultaneously reducing the inhibition of PDGF-BB activity by 5- to 10-fold, thus improving the overall selectivity for BMP vs. PDGF activity32, 36. Molecules with modifications to the piperidinyl-ethoxy group (R) did not exhibit increased potency against BMP signaling, but rather had similar or decreased activity compared to the parent compound. However, replacement of this ether-linked moiety with piperazine in a series of compounds conferred substantial improvement in metabolic stability in microsomal degradation assays (t1/2 ~ 82 min)36. No significant impact upon AMPK activity was seen with this series of changes, but a net increase in selectivity was gained by increased potency for BMP inhibition.
Figure 5. Structures of dorsomorphin (A) and 4-quinolyinyl substituted dorsomorphin derivatives (B), and LDN-193189 (C).
Substitution of nitrogen substituents of the core heterocyclic pyrazolopyrimidine was not tolerated at the N-1 position (Y), while the N-4 position (X) was dispensable for BMP inhibition activity.
The optimized compound LDN-193189 (Figure 5C) is available in plasma after intraperitoneal injection or oral administration, with a plasma half-life of 1.6 to 4.3 hours depending on the vehicle used for administration32, 36 (and unpublished data). The improved selectivity and potency of LDN-193189 for the inhibition of BMP signaling, and its pharmacokinetic profile have suggested that this or other optimized derivative compounds may be suitable for achieving in vivo continuous blockade of BMP signaling. Preliminary data suggest that high-potency derivatives of LDN-193189 are well-tolerated in vivo, even during early postnatal life. At doses with which inhibit BMP signaling effectively, LDN-193189 does not induce skeletal, hematopoietic or developmental abnormalities, or otherwise impair normal growth and feeding when administered for greater than 60 d to newborn or adult mice 32 (and unpublished data).
Dorsomorphin and its derivatives as probes of BMP-mediated signaling in physiology
In a wide variety of systems, recombinant endogenous inhibitors such as Noggin, Follistatin, or Chordin have long been used to block BMP signaling in cell-based studies. Occasionally, these strategies have been used to inhibit BMP signaling experimentally in vivo, particularly via gene transfer of BMP antagonists or their modified IgG Fc-fusion proteins38–44. These approaches appear to be particularly effective for inhibiting ectopic bone formation in a number of animal models40–42. In some cases local blockade of BMP signaling may be achieved in anatomic compartments with recombinant ligand44, whereas systemic blockade of BMP signaling is more easily achieved with gene transfer of antagonists presumably due to the cost of repeated dosing with recombinant proteins. Due to practical limitations of gene transfer as therapy, the strategy of BMP inhibition using small molecule compounds with suitable pharmacokinetics might be preferable for applications requiring systemic blockade. By virtue of its identification in an organism-based screen for inducing a phenotype of global BMP inhibition, dorsomorphin and its derivatives are quite effective for selective antagonism of BMP signaling in vivo, a property which we and others have exploited for testing hypotheses regarding functional roles of BMPs in physiology, and as potential therapeutic strategies.
In addition to ease of administration and cost, there are several potential mechanistic advantages of employing a small molecule approach for the antagonism of BMP signaling. First, we have demonstrated that small molecule inhibition of the BMP type I receptor kinases can inhibit or truncate signaling that has been initiated prior to administration32, 45, which may be attributed to their ability to inhibit membrane bound and intracellular signaling complexes which have previously been activated by ligand. Soluble extracellular antagonists or neutralizing antibodies would not be expected to have access to internalized ligand-receptor complexes and would be more effective in preventing rather than terminating signaling. BMP type I receptor kinase inhibitors are also effective for inhibiting the activity of constitutively-active mutant BMP type I receptors25, 32, 46. To the extent that such mutations may exert their effects independently of ligand stimulation, extracellular antagonists or specific neutralizing antibodies would have limited utility in such a context. Both of these properties of BMP receptor kinase inhibitors may be advantageous in the treatment of conditions in which such activating mutations are pathogenetic, or other conditions in which excessive BMP signaling appears to contribute. The currently described BMP type I receptor antagonists have overlapping effects on all of the known BMP type I receptors which activate SMAD1/5/8 signaling, namely ALK1, ALK2, ALK3, and ALK625, 27, 32, but with varying degrees of efficiency. While type I receptor-specific reagents could be useful in situations in which a blockade of a specific receptor or ligand-receptor interaction is desirable, the lack of narrow specificity of LDN-193189 and dorsomorphin may be useful in situations in which the responsible ligand(s) and receptor(s) are either not known, or are heterogeneous due to functional redundancy of ligands and/or receptors. Due to their broad activity against BMP signaling, dorsomorphin and its derivatives have been applied as probes of BMP-mediated function in a variety of physiologic, developmental, and pathologic processes.
Dorsomorphin and derivatives as modifiers of iron homeostasis
Major advances in our understanding of systemic iron regulation have followed the identification of mutations in the gene encoding Hemojuvelin (Hjv), otherwise known as RGMc, in juvenile hemochromatosis47. Individuals with this form of hemochromatosis have a markedly impaired ability to produce hepcidin, a master control hormone of iron homeostasis which negatively regulates the transport of iron from enterocytes and other iron-storing cells into the circulation48–51, developing iron overload and multi-system organ failure as a result. The seminal observations that Hjv functions as a membrane associated BMP co-receptor15, and that hepatocytes deficient in the co-SMAD4 (required for both BMP and TGF-β-mediated signaling) are impaired in the ability to generate hepcidin52 suggested that BMP signals play a central role in iron homeostasis. We found that dorsomorphin lowered basal levels of hepcidin transcription in hepatoma (Hep3B and HepG2) cells, and inhibited BMP-, Hjv-, and IL-6-induced transcription of hepcidin25, extending the evidence that BMP signals are required for the regulation of hepcidin. In mice and in zebrafish, injection of exogenous iron acutely increased hepatic SMAD1/5/8 phosphorylation and hepcidin transcription, and both of these could be blocked with systemic administration of dorsomorphin. Consistent with these findings, Kautz et al. reported that hepatic SMAD1/5/8 phosphorylation is directly regulated by circulating iron levels in the steady state in proportion to dietary iron intake53. Dorsomorphin was thus used to demonstrate that Hjv-, IL6-, and iron-induced regulation of hepcidin require BMP type I receptor cosignaling. Consistent with a physiologically significant role of BMP co-signaling in regulating iron via hepcidin, treatment with dorsomorphin for 24 h also elevated serum iron levels. Elegant studies have subsequently extended the finding that hepatic BMP and SMAD1/5/8 signaling are necessary for Hjv-mediated regulation of hepcidin to identify BMP6 as the ligand responsible for this function53–55. In these studies it was shown that BMP6- deficient mice develop iron overload due to their inability to regulate hepcidin, and that BMP6 neutralizing antibodies can decrease hepatic hepcidin expression and thus raise serum iron levels.
Dorsomorphin and derivatives as modifiers of bone formation and metabolism
Given the well-known critical roles of BMPs in skeletogenesis and fracture repair, small molecule BMP inhibition might be useful for elucidating mechanisms of bone development and remodeling. While treatment of embryonic zebrafish after the establishment of dorsoventral axis (> 24 h post fertilization) does not induce dorsalization, dorsomorphin (10 µM in E3 media with daily changes) treatment after this time inhibits normal mineralization of the skeleton25. In neonatal mice (P7), treatment with 3 mg/kg IP twice daily with LDN-193189 does not impact skeletal mineralization or bone mineral density32, however, suggesting that BMP signals play their role in determining osteoblastogenesis and mineralization prior to P7 in mouse development.
With the expectation that BMP inhibitors could similarly inhibit de novo bone formation, we investigated small molecule BMP inhibition in a model of heterotopic ossification, or the inappropriate formation of bone in soft tissues. In these studies, a transgenic mouse model conditionally overexpressing a constitutively active mutant form of the BMP I receptor ALK2 (Q207D) was found to develop intramuscular endochondral ossification when transgene expression was induced in the context of muscle damage and inflammation, leading to joint fusion and immobility32. In these mice, the development of ectopic bone and functional impairment in the context of a constitutively active ALK2 protein contingently with soft-tissue injury appeared to model the rare human congenital heterotopic ossification disorder of fibrodysplasia ossificans progressiva (FOP)56, 57. Individuals with the classic form of FOP have constitutively-activating mutations of ALK2 affecting the neighboring residue (R206H), while individuals with a variant form have mutations affecting the same residue (Q207E) which is modified in our mice, all of which are predicted to render the kinase function constitutively active due to loss of intramolecular or intermolecular interactions required for regulation, perhaps via the binding of FKBP1258. People with FOP are born with nearly normal anatomy, but develop widespread soft tissue ossifications in the context of traumatic injury and/or inflammation. In our mouse model, administration of the dorsomorphin derivative LDN-193189 (3 mg/kg intraperitoneally each 12 h) was able to inhibit SMAD1/5/8 activation and downstream transcriptional activity induced by ALK2Q207D in affected tissues, mitigating endochondral ossification and functional impairment. Application of this potent BMP inhibitor thus confirmed the pathogenetic effect of ALK2Q207D mutant proteins as a gain-of-function defect while suggesting a potential treatment strategy. In these initial studies, endochondral ossification was partly reversed by LDN-193189 under the conditions tested, while some residual chondrogenesis appeared to contribute to functional impairment. In subsequent studies, nearly complete inhibition of heterotopic ossification was achieved with a slightly modified drug formulation at the same dosage and frequency (Figure 6), suggesting that optimal inhibition of BMP signaling could be effective for blocking ectopic bone in FOP and other heterotopic ossification disorders.
Figure 6. Micro-CT analysis of ectopic bone in Ad.Cre-injected hindlimbs of caALK2Q207D mice.
Postnatal day 7 transgenic mice carrying the conditionally expressed, constitutively-active ALK2 transgene were induced to express ALK2 in the left hindlimb with the injection of Ad.Cre (1 × 108 pfu). By postnatal day 15, ectopic calcifications appeared which infiltrated the left gastrocnemius and soleus muscles, in DMSO-treated mice (A). In mice treated with LDN-193189 (3 mg/kg each 12 hours in DMSO), consistent and essentially complete inhibition of ectopic ossification and functional impairment was observed at postnatal day 15 (B) up through postnatal day 30 (not shown).
While it has generally been expected that BMP signals promote bone mineralization, Kamiya et al. recently demonstrated that BMP signals specifically mediated by ALK3 may paradoxically downregulate bone mineral density, showing that osteoblast-targeted disruption of ALK3 in development leads to an increase in bone mineral density59–61. In the osteoblast lineage, ALK3 signals appear to inhibit Wnt signaling activity, which in turn positively regulates bone mineral density in vivo61. Since Wnt signaling in osteoblasts negatively regulates osteoclastogenesis, the phenotype can be explained by lowered bone resorption in the mutant mice. Genetic ablation of ALK3 in cultured osteoblasts, and via targeted Cre-lox recombination during murine development results in enhanced Wnt signaling, apparently via the reduced expression of Wnt inhibitors Sost and Dkk160, 61, providing a potential mechanistic explanation for the increased bone mass phenotype of the osteoblast-targeted ALK3-knockout mice59. Consistent with a negative regulatory role of BMP signaling in osteoblast Wnt signaling, the addition of BMPs induced Sost and Dkk1 expression, while their expression could be abrogated by dorsomorphin. In fact, pre-treatment of cultured osteoblasts with dorsomorphin increased basal canonical Wnt pathway transcriptional activity, similar to the effect of disrupting ALK3 expression60. Thus small molecule inhibition of BMP type I receptor kinase activity confirmed a negative regulatory effect of ALK3 signaling upon Wnt activity in osteoblasts and showed, moreover, that this effect was not due to an idiosyncratic effect of depleting ALK3 but was regulated by BMP signaling activity itself. In addition to demonstrating the utility of small molecule BMP inhibition as an experimental probe of BMP function, these studies suggest that pharmacologic BMP inhibition might be useful for the therapeutic modulation of bone mineral density, via an unexpected and somewhat paradoxical mechanism.
Dorsomorphin and derivatives as tools for stem cell manipulation
In recent years, much attention has focused on the enormous potential of stem cells as tools for regenerative medicine. Broadly speaking, regenerative medicine aims to repair damaged adult tissues through the transplantation of stem cells or the reactivation of endogenous progenitor cells. Recent developments which have raised hopes for the therapeutic application of stem cells include the reprogramming of somatic tissues to form induced pluripotent stem cells (iPSC), which, like embryonic stem cells (ESC), harbor the potential to differentiate into nearly all cell and tissue types in the body62. Despite these promising developments, realizing the full potential of regenerative medicine faces numerous hurdles, including the potential for teratoma formation, insufficient differentiation toward desired lineages, and clinically negligible regenerative capacity in vivo. In principle, pharmaceutical agents capable of directing differentiation of pluripotent stem cells to desired cell types or promoting regenerative processes in the body could be utilized to overcome these obstacles.
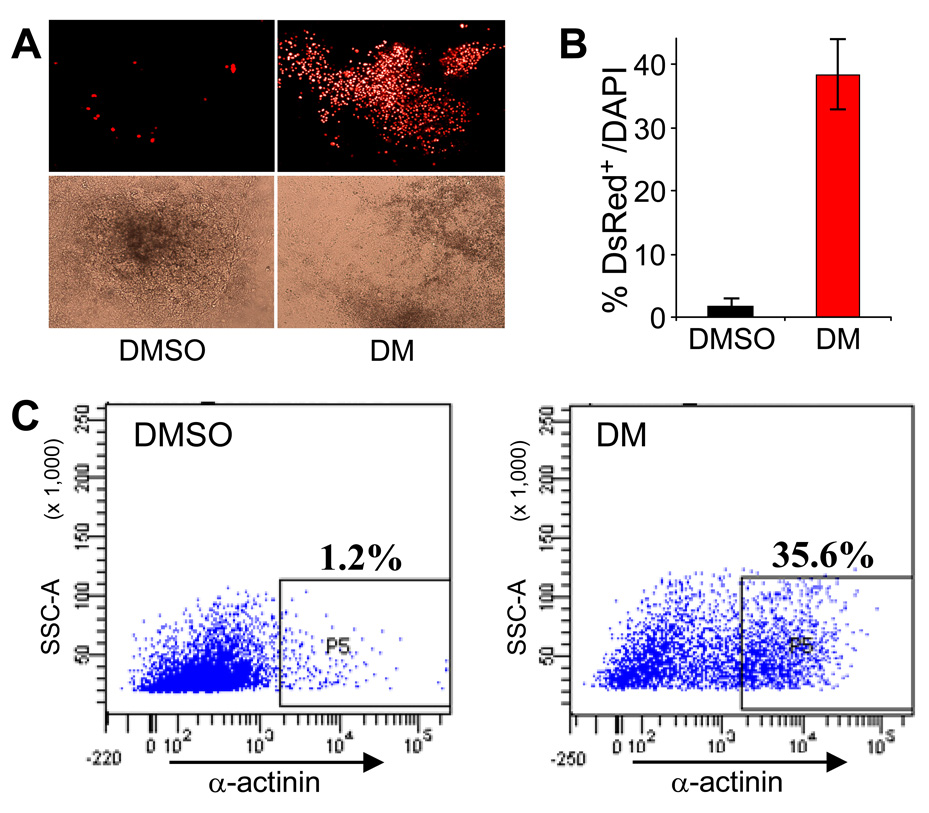
Because stem cell maintenance and differentiation utilize the same key intercellular signaling networks that regulate embryonic development, ability to precisely control these developmental pathways is a promising approach to achieve directed stem cell differentiation and to boost body’s endogenous regenerative capacity. For example, temporally-controlled inhibition of BMP signaling using endogenous BMP antagonist noggin is effective for inducing the differentiation of pluripotent stem cells to cardiomyocyte and neuronal lineages63–65. Extending these observations to use dorsomorphin as a temporally controlled inhibitor of BMP signaling, we recently used dorsomorphin to induce cardiac myocyte differentiation by greater than 30-fold in a mouse ES cell model (Figure 7)35.
Figure 7. Dorsomorphin (DM) induces cardiomyogenesis in mouse ES cells.
(A) DM-treated ES cells formed large areas of beating cardiomyocytes that expressed DsRed-Nuc under the cardiac α-MHC promoter (right), but DMSO-treated cells did not (left). Upper panels depict red fluorescence images. Lower panels show the corresponding bright-field images. (B) DM treatment dramatically increased the number of cardiomyocytes (DsRed+) as a percentage of total cells (DAPI+) (*p<0.0001). (C) FACS analysis indicates DM treatment leads to about 30-fold increase in number of α-actinin-positive cardiac cells.
Small molecule BMP inhibitors like dorsomorphin could play valuable roles for regenerative medicine. One potential advantage of small molecules over decoy BMP receptors, endogenous BMP antagonists, or neutralizing antibodies, is that in the context of differentiating embryoid bodies (EB) formed by differentiating embryonic (ES) cells, small molecules are easily accessible to cells at the center of EB clusters. Cell permeable compounds like dorsomorphin may be able to achieve uniform signal modulation within EBs or even more complex tissues, affording consistent results as compared to diffusion-limited protein inhibitors, which will also require administration at high concentrations to block ligands efficiently. Furthermore, small molecules are far less expensive than recombinant proteins, affording scale-up of various differentiation protocols for large amounts of tissue. For use in modifying progenitor cell differentiation in whole live animals or patients, these advantages of a small molecule BMP inhibitor are likely to be even more critical. Small molecule BMP inhibitors can be delivered to living organisms inexpensively with wide availability. Administration of recombinant antagonists such as noggin and follistatin in vivo would be prohibitively expensive, requiring repeated dosing of gram quantities of protein, or alternatively, require gene transfer and its associated limitations. Ultimately, small molecule BMP inhibitors may be routinely used not only to expand specific tissue types in vitro, but also be used to enhance appropriate differentiation of engrafted progenitor cells or endogenous progenitors in vivo. In conclusion, small molecule cell signaling modulators like dorsomorphin could become critical ingredients in successful strategies for translating recent stem cell advances into viable therapeutic modalities.
Dorsomorphin and derivatives as probes for discerning mechanisms of TGF-β-mediated SMAD1/5/8 signaling
It was previously observed that TGF-β mediated signaling in various cell types could induce the activation of BMP-receptor (BR-) associated SMADs 1, 5, and 8, in addition to SMADs 2 and 3 specific to TGF-β and Activin signaling66–68. It was proposed that SMAD1/5/8 activation by TGF-β was mediated principally by the multifunctional type I receptor ALK1, based on biochemical evidence of interaction between TGF-β and the type I receptor ALK1, and the ability of ALK1 to phosphorylate SMAD1/5/8. Specifically, it was found that the function of TGF-β type I receptor ALK5, normally specific for the activation of SMADs 2 and 3, was required for the recruitment of ALK1 into TGF-β signaling complexes, mediating “lateral” signaling activation of the BR-SMADs68. These data, coupled with the phenotypic similarity of ALK1 and TGF-β1 mutant phenotypes, suggested not only that TGF-β mediated activation of SMAD1/5/8 was present but functionally relevant, as exemplified by the human disease syndrome hereditary hemorrhagic telangectasia type 2 (HHT-2) which results from functional haploinsufficiency of ALK169. These data were taken to support the notion that a balance in the activity of ALK5 and ALK1 signaling in endothelial cells are required for normal vasculogenesis and remodeling, with the assumption that ALK1, being expressed primarily in the endothelium, was the principal means by which TGF-β signaling could recruit activation of SMAD1/5/8 in endothelial cells. Recently several groups have revisited the mechanism of TGF-β-induced activation of SMAD1/5/8, particularly in non-endothelial lineages which do not express abundant or significant levels of ALK1. In addition to RNAi-based, overexpression, and biochemical strategies, these groups have used small molecule inhibitors of BMP and TGF-β signaling to discern the mechanism and functional roles of TGF-β-induced activation of SMAD1/5/8 in non-endothelial cell types.
Daly et al. recently showed that TGF-β potently induced SMAD1/5/8 in a number of epithelial and fibroblast cell lines, in a manner which was independent of ALK1, but was dependent on the activity of ALK2 and ALK370, both recruited by ALK5. Specific siRNAs which selectively inhibited ALK2 and ALK3 expression, and dorsomorphin treatment were all found to inhibit TGF-β-mediated cross-activation of SMAD1/5/8, consistent with a contribution of these BMP type I receptors to this process. Importantly, noggin did not inhibit TGF-β-induced activation of SMAD1/5/8, demonstrating that this effect was independent of the activity of BMP ligands. Immunoprecipitation studies demonstrated that this signaling required the recruitment of ALK2 and ALK3 to ALK5-containing receptor complexes, and resulted in the formation of mixed SMAD1/5 and SMAD2/3 complexes which lacked the normal transcriptional activity of activated SMAD1/5 when measured via BRE-Luc. The activity of SMAD1/5 recruited by TGF-β was dispensable for the TGF-β-induced growth arrest in epithelial cell lines, but was required for anchorage-independent growth of transformed epithelial tumor lines. Thus dorsomorphin served a unique and complementary role with extracellular BMP antagonist, RNAi, and biochemical approaches for discerning the mode of functionally relevant lateral activation of BR-SMADs by TGF-β in non-endothelial cell types.
Wrighton and colleagues also investigated the mechanisms of TGF-β-induced SMAD1/5/8 activation in several other non-endothelial cell lines27. Utilizing compound SB-431542 to inhibit ALK5 signaling and dorsomorphin to inhibit BMP type I receptors, they found that TGF-β induced activation of SMAD1/5/8 appeared to be dependent upon the activity of ALK5, but not ALK1/2/3/6 (all of which were inhibited by dorsomorphin at the concentrations tested) in myofibroblast C2C12 cells and HepG2 hepatoma cells. On the other hand, in HEK293 cells and HaCaT cells, it was found that dorsomorphin inhibited TGF-β-mediated activation of SMAD1/5/8, as did SB-431542, consistent with the participation of BMP type I receptors with ALK5 and TGF-β signaling complexes as reported by Daly and colleagues. Thus by comparing the relative effects selective BMP type I receptor and TGF-β type I receptor kinase inhibitors, it was determined that the requirement for lateral recruitment of BMP type I receptors for SMAD1/5/8 activation by TGF-β is dependent on the cell type, with ALK5 having the capacity to activate SMAD1/5/8 directly in certain cells.
Liu and coworkers demonstrated that TGF-β-mediated activation of SMADs 1 and 5 in murine mammary epithelial tumor cell line 4T1 appeared to be required for TGF-β-induced migration, a property which is thought to be important for the metastatic behavior of these tumor cells71. The activation of BR-SMAD by TGF-β was apparently dependent on ALK5 kinase activity, being abolished in ALK5-depleted cells and reconstituted with ectopic ALK571. They went on to demonstrate TGF-β-induced activation of BR-SMADs could be partly inhibited with moderate to high concentrations of dorsomorphin (5 – 10 µM), while TGF-β-mediated activation of SMAD3 was not affected under these conditions. Since dorsomorphin also inhibited the SMAD1/5 activation via TGF-β signaling through mutant ALK5 expressing ALK2/3/6 L45 loop domains (which determine BRSMAD binding specificity), it was concluded by the authors that SMAD1/5 activation via TGF-β was directly mediated by ALK5, rather than by ALK2/3/6. It ought to be noted that a role of cross-activation of ALK1 could potentially contribute due to the low but detectable expression of ALK1 in these cells, and the ability of dorsomorphin to inhibit ALK127.
These experiments demonstrate that small molecule inhibitors of BMP and TGF-β receptor signaling can be useful adjuncts to RNAi, overexpression, biochemical and other kinase inhibitor strategies for the dissection of mechanisms of TGF-β ligand family signal transduction. These studies reveal complex and highly cell-and context-sensitive mechanisms of lateral activation of traditionally BMP-associated effectors by TGF-β that are likely to be functionally relevant in tumor biology.
Future directions for the development of small molecule BMP inhibitors
Most successful applications of small molecule BMP inhibitors have benefited from the ability of the dorsomorphin family to broadly inhibit BMP ligand signaling, due to its ability to inhibit ALK1, 2, 3, and 6. This property has been useful when the contributing receptor(s) or ligand(s) are not known, or when multiple functionally redundant receptors or ligands participate. It appears that certain processes can be attributed to a restricted set of ligands, or receptors, as is the case with regulation of hepcidin expression in the liver, which depends on expression of BMP653–55, or the constitutively-activating mutations of ALK2 found in FOP56, 57. In such applications it may be advantageous to improve the specificity of effect by using BMP type I receptor subselective agents. The dorsomorphin family appears to be inherently biased in its BMP receptor selectivity, generally having 2 – 10 fold increased potency against ALK2 versus ALK1 or ALK3, and ALK6. The identification of chemical modifications, or alternate compounds which may offer increased selectivity for this and other receptors in this family is a priority for investigation.
Despite significant improvements in overall selectivity achieved via a medicinal chemistry program36, some important off-target effects persist in the current dorsmorphin derivative compounds. These include the persistent effects of LDN-193189 upon AMP activated kinase32, albeit at micromolar concentrations in contrast to the nanomolar inhibition of BMP signaling. Whether any of these effects are relevant or detrimental at doses required for inhibiting BMP signaling remains to be demonstrated. At doses used to inhibit BMP signaling in our heterotopic ossification model (3 mg/kg each 12 hours), LDN-193189 did not have significant impact upon normal growth and weight gain, in contrast to the anorexigenic effects of Compound C (10 mg/kg each 12 hours) which are attributed to its effects upon AMPK signaling in the hypothalamus31. Other off-target effects thus need to be carefully considered and evaluated, and further chemical modifications could be pursued depending on the intended application of the compound.
The ability to modulate BMP signaling for therapeutic means is an exciting prospect, but will need to be balanced against the roles of BMPs in constitutive or adaptive biological processes. Such processes are likely to include bone remodeling and fracture repair. Given the paradoxical increase in bone mass observed in mice with targeted disruption of ALK3 in osteoblasts, and the conchordant effects of dorsomorphin upon Wnt pathway signaling, the effects of long-term inhibition of BMP signaling in vivo may be hard to predict, and might even promote increased bone mineral density. We have yet to find significant evidence of overt toxicity in limited preliminary studies, using short- to medium-term dosing of LDN-193189 in small animal models32. The limited toxicity encountered thus far may be related to the intermittent nature of dosing—Periodic systemic BMP signaling inhibition may be far more tolerable than continuous systemic BMP inhibition. Thus, even if some theoretical constitutive and adaptive roles of BMP signaling could limit chronic and global inhibition of BMP signaling, they might not preclude short-term, intermittent, or localized inhibition of BMP signaling. Blockade of BMP signaling with highly selective type I receptor-specific compounds might have little toxicity owing to the functional redundancy of receptors and ligands involved in constitutive biological processes, and yet still be effective in targeting pathologic processes that are relatively restricted in ligand and receptor utilization. These possibilities will need to be investigated directly by assessing the biologic effects of BMP inhibition in relevant models.
The development of potent and biologically available selective inhibitors of BMP type I receptor kinases has helped to highlight novel roles of this receptor system in development, physiologic homeostatic mechanisms, and in pathophysiologic processes. The development of further refinements in this and other compound families will yield critical tools for the elucidation and therapeutic manipulation of a highly intricate and modular signaling pathway.
Acknowledgements
The authors appreciate the critical comments and review of this manuscript by Drs. Nobuhiro Kamiya and Yuji Mishina, and additional input from Drs. Greg Cuny, Randall Peterson, Kenneth Bloch, Xiao-Fan Wang and Irwin Liu. This work was supported by funding from the NIH grants K08HL081535 (CCH) and K08HL079943 (PBY), the GSK Cardiovascular Research and Education Foundation (CCH and PBY), the Veterans Health Administration (CCH), the Center for Research in Fibrodysplasia Ossificans and Related Disorders (CCH), the Pulmonary Hypertension Association (PBY), the Massachusetts Technology Transfer Center (PBY), the Howard Hughes Medical Institute (PBY), and the Harvard Stem Cell Institute (PBY).
Biographies

Charles C. Hong received his SB from MIT, and MD and PhD from Yale University. After clinical training at Yale-New Haven Hospital and Massachusetts General Hospital, he received postdoctoral training in chemical biology with Dr. Randy Peterson at MGH/Harvard Medical School. His primary research focus is on chemical genetic analysis of zebrafish embryo to discover novel small molecules that modulate developmental pathways and developing these chemical reagents to direct differentiation of pluripotent stem cells. He is currently an Assistant Professor of Medicine and Pharmacology at Vanderbilt University School of Medicine, a member of the Vanderbilt Institute of Chemical Biology and the Vanderbilt Center for Stem Cell Biology, and the co-Director of Vanderbilt Center for Inherited Heart Disease.

Paul B. Yu is an investigator in the Cardiovascular Research Center at Massachusetts General Hospital and Harvard Medical School. He received his AB in Philosophy from Stanford University, followed by MD and PhD degrees from Duke University in 1999. He received clinical training at the University of San Francisco at California and Massachusetts General Hospital, and joined the faculty in the Division of Cardiology at Massachusetts General in 2006. His areas of focus have been to elucidate the contribution of dysregulated bone morphogenetic protein signaling in vascular and bone disease, and to develop novel tools for the manipulation of BMP signaling for experimental and therapeutic applications. He is Assistant Professor of Medicine at Harvard Medical School, and a member of the Harvard Stem Cell Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 2.Qi X, Li TG, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao GQ. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 4.Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong Gh G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 5.Monzen K, Nagai R, Komuro I. A role for bone morphogenetic protein signaling in cardiomyocyte differentiation. Trends Cardiovasc Med. 2002;12:263–269. doi: 10.1016/s1050-1738(02)00172-x. [DOI] [PubMed] [Google Scholar]
- 6.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 7.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 8.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 9.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005 doi: 10.1016/j.cytogfr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Nishimatsu S, Thomsen GH. Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech Dev. 1998;74:75–88. doi: 10.1016/s0925-4773(98)00070-7. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun. 1997;232:153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- 12.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Yu PB, Sidis Y, Beppu H, Bloch KD, Schneyer AL, Lin HY. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J Biol Chem. 2007;282:18129–18140. doi: 10.1074/jbc.M701679200. [DOI] [PubMed] [Google Scholar]
- 15.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 16.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 17.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 18.Halbrooks PJ, Ding R, Wozney JM, Bain G. Role of RGM coreceptors in bone morphogenetic protein signaling. J Mol Signal. 2007;2:4. doi: 10.1186/1750-2187-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale L, Wardle FC. A gradient of BMP activity specifies dorsal-ventral fates in early Xenopus embryos. Semin Cell Dev Biol. 1999;10:319–326. doi: 10.1006/scdb.1999.0308. [DOI] [PubMed] [Google Scholar]
- 20.MacRae CA, Peterson RT. Zebrafish-based small molecule discovery. Chem Biol. 2003;10:901–908. doi: 10.1016/j.chembiol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov. 2005;4:35–44. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 23.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 24.Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Nusslein-Volhard C. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 25.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 27.Wrighton KH, Lin X, Yu PB, Feng XH. Transforming Growth Factor {beta} Can Stimulate Smad1 Phosphorylation Independently of Bone Morphogenic Protein Receptors. J Biol Chem. 2009;284:9755–9763. doi: 10.1074/jbc.M809223200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaikuad A, Alfano I, Shrestha B, Muniz JRC, Petrie K, Fedorov O, Phillips C, Bishop S, Mahajan P, Pike ACW, von Delft F, Muller-Knapp S, Lee WH, Marsden BD, Arrowsmith CH, Edwards AM, Weigelt J, Bountra CK, S, Bullock A. RCSB PDB Protein Data Bank. Worldwide Protein Data Bank; 2009. Crystal structure of the kinase domain of type I activin receptor (ACVR1) in complex with FKBP12 and dorsomorphin. [Google Scholar]
- 29.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 32.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraley ME, Rubino RS, Hoffman WF, Hambaugh SR, Arrington KL, Hungate RW, Bilodeau MT, Tebben AJ, Rutledge RZ, Kendall RL, McFall RC, Huckle WR, Coll KE, Thomas KA. Optimization of a pyrazolo[1,5-a]pyrimidine class of KDR kinase inhibitors: improvements in physical properties enhance cellular activity and pharmacokinetics. Bioorg Med Chem Lett. 2002;12:3537–3541. doi: 10.1016/s0960-894x(02)00827-2. [DOI] [PubMed] [Google Scholar]
- 34.Fraley ME, Hoffman WF, Rubino RS, Hungate RW, Tebben AJ, Rutledge RZ, McFall RC, Huckle WR, Kendall RL, Coll KE, Thomas KA. Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a]pyrimidines: a new class of KDR kinase inhibitors. Bioorg Med Chem Lett. 2002;12:2767–2770. doi: 10.1016/s0960-894x(02)00525-5. [DOI] [PubMed] [Google Scholar]
- 35.Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008 doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Fraley ME, Bilodeau MT, Kaufman ML, Tasber ES, Balitza AE, Hartman GD, Coll KE, Rickert K, Shipman J, Shi B, Sepp-Lorenzino L, Thomas KA. Design and synthesis of 3,7-diarylimidazopyridines as inhibitors of the VEGF-receptor KDR. Bioorg Med Chem Lett. 2004;14:909–912. doi: 10.1016/j.bmcl.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Feeley BT, Krenek L, Liu N, Hsu WK, Gamradt SC, Schwarz EM, Huard J, Lieberman JR. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–166. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Feeley BT, Liu NQ, Conduah AH, Krenek L, Roth K, Dougall WC, Huard J, Dubinett S, Lieberman JR. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and rank:Fc administration. J Bone Miner Res. 2006;21:1571–1580. doi: 10.1359/jbmr.060706. [DOI] [PubMed] [Google Scholar]
- 40.Glaser DL, Economides AN, Wang L, Liu X, Kimble RD, Fandl JP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered Noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85-A:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Hannallah D, Peng H, Young B, Usas A, Gearhart B, Huard J. Retroviral delivery of Noggin inhibits the formation of heterotopic ossification induced by BMP-4, demineralized bone matrix, and trauma in an animal model. J Bone Joint Surg Am. 2004;86-A:80–91. doi: 10.2106/00004623-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–1579. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takabe K, Wang L, Leal AM, Macconell LA, Wiater E, Tomiya T, Ohno A, Verma IM, Vale W. Adenovirus-mediated overexpression of follistatin enlarges intact liver of adult rats. Hepatology. 2003;38:1107–1115. doi: 10.1053/jhep.2003.50483. [DOI] [PubMed] [Google Scholar]
- 44.Tang J, Song M, Wang Y, Fan X, Xu H, Bai Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APP(swe)/PS1(DeltaE9) transgenic mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2009;385:341–345. doi: 10.1016/j.bbrc.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 45.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone Morphogenetic Protein (BMP) Type II Receptor Is Required for BMP-mediated Growth Arrest and Differentiation in Pulmonary Artery Smooth Muscle Cells. J Biol Chem. 2008;283:3877–3888. doi: 10.1074/jbc.M706797200. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 48.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 49.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 50.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicolas G, Viatte L, Lou DQ, Bennoun M, Beaumont C, Kahn A, Andrews NC, Vaulont S. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat Genet. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 52.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Kautz L, Meynard D, Monnier A, Darnaud V, Bouvet R, Wang RH, Deng C, Vaulont S, Mosser J, Coppin H, Roth MP. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 54.Andriopoulos B, Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 56.Shore EM, Xu M, Connor JM, Kaplan FS. Mutations in the BMP type I receptor ACVR1 in patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2006;21:S75. doi: 10.1359/jbmr.060215. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- 59.Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res. 2008;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamiya N, Kobayashi T, Mochida Y, Yu PB, Yamauchi M, Kronenberg HM, Mishina Y. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the Type IA receptor (BMPRIA) in osteoblasts. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamiya N, Ye L, Kobayashi T, Mochida Y, Yamauchi M, Kronenberg HM, Feng JQ, Mishina Y. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development. 2008;135:3801–3811. doi: 10.1242/dev.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 63.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 64.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen MA, Itsykson P, Reubinoff BE. Neural differentiation of human ES cells. Chapter 23. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2307s36. Unit 23 27. [DOI] [PubMed] [Google Scholar]
- 66.Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc Natl Acad Sci U S A. 2000;97:2626–2631. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. Embo J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 69.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, Gruenig E, Kermeen F, Laitinen T, Morrell NW, Trembath RC, Halme M, Raisanen-Sokolowski A. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daly AC, Randall RA, Hill CS. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol Cell Biol. 2008;28:6889–6902. doi: 10.1128/MCB.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFbeta-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFbeta switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho DK, Ram S, Nelson KL, Bonthuis PJ, Smith AL. lgtC expression modulates resistance to C4b deposition on an invasive nontypeable Haemophilus influenzae. J Immunol. 2007;178:1002–1012. doi: 10.4049/jimmunol.178.2.1002. [DOI] [PubMed] [Google Scholar]