Abstract
Glycine is a major inhibitory neurotransmitter in the spinal cord and brainstem. Recently, in vivo analysis of glycinergic synaptic transmission has been pursued in zebrafish using molecular genetics. An ENU mutagenesis screen identified two behavioral mutants that are defective in glycinergic synaptic transmission. Zebrafish bandoneon (beo) mutants have a defect in glrbb, one of the duplicated glycine receptor (GlyR) β subunit genes. These mutants exhibit a loss of glycinergic synaptic transmission due to a lack of synaptic aggregation of GlyRs. Due to the consequent loss of reciprocal inhibition of motor circuits between the two sides of the spinal cord, motor neurons activate simultaneously on both sides resulting in bilateral contraction of axial muscles of beo mutants, eliciting the so-called ‘accordion’ phenotype. Similar defects in GlyR subunit genes have been observed in several mammals and are the basis for human hyperekplexia/startle disease. By contrast, zebrafish shocked (sho) mutants have a defect in slc6a9, encoding GlyT1, a glycine transporter that is expressed by astroglial cells surrounding the glycinergic synapse in the hindbrain and spinal cord. GlyT1 mediates rapid uptake of glycine from the synaptic cleft, terminating synaptic transmission. In zebrafish sho mutants, there appears to be elevated extracellular glycine resulting in persistent inhibition of postsynaptic neurons and subsequent reduced motility, causing the ‘twitch-once’ phenotype. We review current knowledge regarding zebrafish ‘accordion’ and ‘twitch-once’ mutants, including beo and sho, and report the identification of a new α2 subunit that revises the phylogeny of zebrafish GlyRs.
Keywords: glycine, synapse, receptor, transporter, zebrafish, behavior, motility
Introduction
Glycine receptors (GlyRs) and GABAA receptors are pentameric ligand-gated chloride channels (reviewed in Moss and Smart, 2001) that mediate inhibitory synaptic transmission. In the vertebrate CNS, GABAergic synaptic transmission is mainly used in the brain, while glycinergic synaptic transmission operates in the brainstem and spinal cord to regulate motor systems. Although GlyRs are found in all vertebrates and perhaps selected invertebrates (Kehoe et al., 2009), mammalian GlyRs have been studied most extensively. Inherited defects in genes encoding the major adult α1β GlyR causes startle disease/hyperekplexia in humans, characterized by noise or touch-induced seizures that result in muscle stiffness and life-threatening neonatal apnea episodes (Sament and Schwartz, 1957; Kirstein and Silfverskiold, 1958; Bakker et al., 2006; Harvey et al., 2008). The biological roles of GlyRs containing the α2, α3 and α4 subunits are less clear, although GlyR α3 is clearly linked to inflammatory pain pathways (Harvey et al., 2004). Glycine also binds to the NR1 subunit of the NMDA receptor, acting as an essential coagonist for excitatory synaptic transmission mediated by glutamate (Johnson and Ascher, 1987; Moriyoshi et al., 1991; Kuryatov et al., 1994). Glycine can also activate the NR3B-containing NMDA receptor in the absence of L-glutamate in heterologous expression systems (Chatterton et al., 2002). However, the in vivo physiological significance of glycine-mediated excitatory synaptic transmission is unclear.
Two glycine transporters (GlyTs) also regulate glycinergic synaptic transmission (Eulenburg et al., 2005). GlyTs are thought to take up glycine from the synaptic cleft to terminate glycine-mediated synaptic transmission (GlyT1) and resupply glycine to glycinergic presynaptic terminals (GlyT2). Mouse models of GlyT1 dysfunction exhibit severe motor deficits accompanied by lethargy, hypotonia and hyporesponsivity, and die within 6–14 h after birth as a result of respiratory failure, although wasting and dehydration caused by an inability to suckle may also play a role (Gomeza et al., 2003a; Tsai et al., 2004). Curiously, these symptoms resemble glycine encephalopathy, a disease associated with disruption of the mitochondrial glycine cleavage system, which degrades excess glycine (Applegarth and Toone, 2006). Mutations in the GlyT2 gene cause startle disease/hyperekplexia in humans and congenital muscular dystonia type 2 (CMD2) in cattle (Rees et al., 2006; Charlier et al., 2008; Harvey et al., 2008).
Intensive examination of the small freshwater fish, zebrafish, has occurred in the past several decades due to the optical clarity and accessibility of zebrafish embryos and amenability to genetic strategies (Streisinger et al., 1981; Eisen et al., 1986; Driever et al., 1996; Haffter et al., 1996). These studies have enhanced our understanding of the in vivo function of genes involved in the regulation of glycinergic synaptic transmission. Two zebrafish mutations, one defective in GlyR function (bandoneon) and the other in GlyT1 function (shocked), were isolated from behavioral mutagenesis screens (Granato et al., 1996; Cui et al., 2004, 2005; Luna et al., 2004; Hirata et al., 2005; Masino and Fetcho, 2005; Mongeon et al., 2008). Importantly, these zebrafish mutants show physiological and behavioral defects similar to non-human mammalian mutants as well as humans with startle disease and glycine encephalopathy. Thus, zebrafish mutants serve as attractive vertebrate models for childhood neurological disorders. In this review, we discuss the history of the zebrafish mutants bandoneon and shocked and report the identification of new GlyR cDNA sequences that revise the phylogeny of zebrafish GlyRs.
Advantages of Zebrafish as a Model Organism
Zebrafish (Danio rerio) have several advantages for the analysis of vertebrate development (Grunwald and Eisen, 2002). First, raising zebrafish is easy. A pair of adult zebrafish can generate 100–200 fertilized eggs in a single spawn. The generation time is 3 months, which is comparable to other vertebrate models such as mice. It is neither expensive nor difficult to maintain thousands of zebrafish in a laboratory. Second, all stages of development occur externally and rapidly, with most organs formed by 5 days of fertilization (Kimmel et al., 1995). The fast pace of development allows one to analyze development in living zebrafish. Third, the embryos are transparent, which makes them amenable for live imaging of individual cells deep within the body such as neurons in the brain. In fact, a number of transgenic zebrafish that express GFP (green fluorescent protein), RFP (red fluorescent protein) or calcium indicators under control of tissue-specific, Gal4-inducible or stress-inducible promoters has been generated to visually monitor the development of tissues and stress response as well as neuronal activity (Amsterdam et al., 1995; Peters et al., 1995; Higashijima et al., 1997, 2003; Long et al., 1997; Scheer and Campos-Ortega, 1999; Halloran et al., 2000). Fourth, the electrophysiological activity of neurons and muscles in zebrafish embryos can be recorded using standard current-clamp and voltage-clamp methods (Prugh et al., 1982; Grunwald et al., 1988; Legendre and Korn, 1995; Drapeau et al., 1999; Fetcho, 2007). Using these methods, the properties of neural circuits that underlie the earliest zebrafish behaviors are beginning to be clarified (Legendre and Korn, 1994; Ribera and Nüsslein-Volhard, 1998; Neuhauss et al., 1999; Ono et al., 2001; Saint-Amant and Drapeau, 2001; Sidi et al., 2003; Kimura et al., 2006; McLean et al., 2007; Tanimoto et al., 2009).
The aforementioned advantages make the zebrafish an excellent system for examining vertebrate development. However, it is the genetic manipulability of zebrafish that has attracted the most attention of biologists (Grunwald and Eisen, 2002; Amsterdam and Hopkins, 2006). Methods for mutagenesis of zebrafish were established in the early 1990’s (Mullins et al., 1994) with two large-scale mutant screens completed in Tübingen, Germany and Boston, USA by 1996 (Driever et al., 1996; Haffter et al., 1996). These screens used chemical mutagens and identified more than 4,000 mutants to kick-start large-scale mutagenesis analysis of a vertebrate embryo. The advent of the zebrafish genome sequencing project in 20011 has greatly improved the molecular identification of chemically-induced mutations. Retrovirus- and transposon- mediated gene disruption was also developed to generate zebrafish mutants in a systematic manner (Lin et al., 1994; Gaiano et al., 1996a,b; Amsterdam et al., 1999, 2004; Kawakami et al., 2000, 2004; Golling et al., 2002; Sivasubbu et al., 2006; Wang et al., 2007). In addition to these forward genetic approaches, targeting-induced local lesion in genome, combining ENU mutagenesis with large-scale exon sequencing has made it possible to inactivate selected zebrafish genes (Wienholds et al., 2002). More recently, a gene-targeting technique using designed sequence-specific zinc-finger proteins has been demonstrated to be effective in disrupting key genes in zebrafish (Doyon et al., 2008; Meng et al., 2008). Genes can also be knocked down during embryonic stages by the injection of antisense morpholino oligonucleotides into recently fertilized embryos (Nasevicius and Ekker, 2000). These highly stable oligonucleotides can effectively block translation or splicing of a target mRNA to interfere with gene function in vivo. Splice-site morpholinos have the major advantage that their efficacy can be monitored by RT-PCR.
Transgenesis is also a powerful tool for analysis of development and gene function in zebrafish. The first zebrafish transgenic lines were generated by injection of DNA into embryos (Stuart et al., 1988, 1990; Culp et al., 1991; Bayer and Campos-Ortega, 1992). Transgenic zebrafish exhibiting cell-specific or inducible gene expression have proven extremely useful for in vivo analysis of gene function and developmental processes (Higashijima et al., 1997; Long et al., 1997; Halloran et al., 2000). More recently, virus- and transposon-mediated transgenesis methods have greatly improved the efficiency of generating transgenic zebrafish (Davidson et al., 2003; Kawakami et al., 2004; Ellingsen et al., 2005; Sivasubbu et al., 2006; Kwan et al., 2007; Villefranc et al., 2007). The increased efficiency has significantly enhanced the application of powerful controlled expression methods such as the Gal4/UAS system (Scheer and Campos-Ortega, 1999; Inbal et al., 2006; Scott et al., 2007; Asakawa et al., 2008; Halpern et al., 2008).
Recent technical advances make zebrafish an attractive complement to invertebrate systems such as C. elegans and Drosophila on the one hand and the transgenic, knockout and knock-in mice on the other. Indeed, zebrafish mutant and transgenic embryos are useful for in vivo high-throughput chemical screening, thereby enabling discovery of novel pharmaceutical reagents that may be useful for mitigating human diseases (Peterson et al., 2000, 2004; Stern et al., 2005).
Development of Locomotion Behavior in Zebrafish
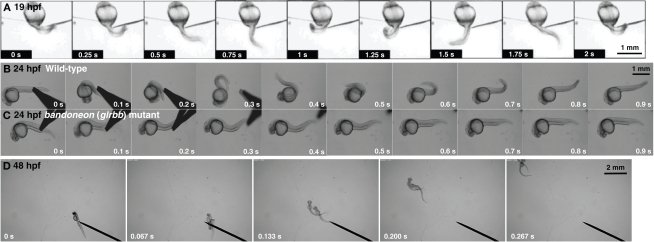
Zebrafish exhibit three distinct behaviors during embryogenesis; spontaneous coiling, touch-evoked escape contractions and swimming. Spontaneous coiling appears after 17 hours postfertilization (hpf) and consists of side-to-side alternating contractions of the axial muscles in the trunk and tail (Figure 1A; Saint-Amant and Drapeau, 1998; Downes and Granato, 2006; Pietri et al., 2009). This relatively slow coiling is independent of sensory stimulation, with the frequency of spontaneous coiling peaking at 0.3∼1 Hz at 19 hpf and gradually declining to less than 0.1 Hz by 26 hpf. Thus, locomotor circuits are functional as early as 17 hpf. Interestingly, the isolated trunk and tail following transections between somites 5 and 7 also displays spontaneous coiling with a similar time course and frequency compared to intact embryos (Downes and Granato, 2006). However, transections removing the first 10 somites eliminate all spontaneous activity (Pietri et al., 2009). These experiments suggest that the neural network triggering spontaneous coiling is located in the rostral spinal cord between somites 5 and 10.
Figure 1.
Zebrafish embryos display three early behaviors. (A) A 19 hpf wild-type embryo exhibits spontaneous coiling. (B) At 24 hpf, a wild-type embryo responds to mechanosensory stimulation with two fast, alternating contractions. (C) A bandoneon (beo) mutant embryo with a defect in glrbb responds to mechanosensory stimulation with bilateral axial muscle contractions that causes the trunk to shorten and bend dorsally. (D) At 48 hpf, wild-type embryos swim away following tactile stimulation.
After 21 hpf, zebrafish embryos respond to touch with escape contractions that typically consists of two to three rapid, alternating contractions of the tail, with muscles contralateral to the side of tactile stimulation contracting first to turn the embryo away from the stimulus (Figure 1B; Saint-Amant and Drapeau, 1998; Hirata et al., 2005). Head and yolk stimulation activates trigeminal sensory neurons (Drapeau et al., 2002), whereas trunk and tail stimulation activates Rohon-Beard neurons, which are primary sensory neurons located within the spinal cord and hindbrain of embryonic fish and amphibians. Thus, the neural circuitry responsible for locomotor responses to external stimuli is functional shortly after the appearance of spontaneous coiling. Applying tactile stimuli to spinalized embryos, which were transected rostral to somite 1, evokes the normal touch response (Pietri et al., 2009). By contrast, transections at more caudal locations (somites 1–10) result in progressively weaker responses in progressively caudal locations (Downes and Granato, 2006; Pietri et al., 2009). Thus, the rostral hindbrain is necessary for the full touch-evoked escape response. It has also been reported that the touch response is dependent on AMPA-type glutamate receptor activation (Pietri et al., 2009).
By 28 hpf, tactile stimulation initiates swimming (Figure 1D; Saint-Amant and Drapeau, 1998). The frequency of alternating contractions during swimming reaches 30 Hz at 36 hpf, which is comparable with the frequency in adult zebrafish (Buss and Drapeau, 2001). Although spinalized embryos transected between somites 5–7 respond to touch with an initial tail flip, swimming does not follow the initial response in most cases (Downes and Granato, 2006). Thus, it appears that the spinal cord can initiate a touch response, but that supraspinal input is necessary for swimming.
Forward Genetics to Identify Zebrafish Mutants Showing Motility Defects
Since zebrafish embryos display organized behaviors within the first several days of development, behavioral mutagenesis screens are an efficient way to isolate mutants that have defects in the formation and function of neural circuits, including neuronal excitability and synaptic transmission. In the Tübingen screen, Granato and his colleagues reported 166 mutants that showed defective motility at 48–60 hpf (Granato et al., 1996). Mutations that induced obvious developmental defects such as abnormal morphology and increased degeneration were eliminated, since they would also lead to defective responses. Some of the behavioral mutations were linked to muscle defects by simple visual inspection of muscle striation using polarized light. The actin-myosin structure of normal muscle fibers resulted in birefringence (double refraction) when viewed this way, while muscles with defective actin-myosin organization resulted in decreased birefringence (Felsenfeld et al., 1990). Indeed, mutations in dystrophin, laminin, titin, Hsp90 and the cognate cochaperone Unc45b were identified in this manner (Bassett et al., 2003; Etard et al., 2007; Hall et al., 2007; Steffen et al., 2007; Hawkins et al., 2008; Guyon et al., 2009). Several other muscle mutations exhibited defects in excitation-contraction (EC) coupling or muscle structure that resembled human myopathies (Schredelseker et al., 2005, 2009; Zhou et al., 2006; Hirata et al., 2007; Dowling et al., 2009). Since some of these zebrafish mutants exhibit muscle degeneration similar to human diseases, they could be useful for the biological and therapeutic analysis of these diseases (Kunkel et al., 2006; Lieschke and Currie, 2007).
Among the 166 motility mutations isolated from the Tübingen screen, 103 mutants displayed normal birefringence suggesting impairments in the nervous system, neuromuscular junction (NMJ) or functional components of muscle such as EC coupling. These mutants have been further classified into several classes by their responses to touch such as no response, normal but reduced response, vigorous but abnormal response, or simultaneous, bilateral contractions. The latter were named ‘accordion’ class mutants (Table 1), because they respond to tactile stimulation with apparent simultaneous, bilateral contractions of axial muscles, resulting in shortening of the body rather than the normal alternating contractions. Molecular genetic studies have revealed that the ‘accordion’ phenotype can arise from numerous distinct mechanisms. For example, accordion (acc) mutants have slow muscle relaxation due to defective clearance of cytosolic Ca2+ caused by mutations in atp2a1, encoding the sarcoplasmic reticulum Ca2+-ATPase SERCA1, resulting in overlap of contractions by axial muscles on the two sides of mutant fish (Gleason et al., 2004; Hirata et al., 2004). Other ‘accordion’ class mutants show defects in cholinergic transmission, such as zeihharmonika (zim) which harbors either missense or nonsense mutations in ache, encoding acetylcholinesterase (Behra et al., 2002; Downes and Granato, 2004) and bajan (baj) which harbors a splice acceptor site mutation in chat, encoding choline acetyltransferase (Wang et al., 2008). By contrast, diwanka (diw) mutants show defective primary motoneuron pathfinding as a result of nonsense mutations in plod3, encoding the procollagen lysine 2-oxoglutarate 5-dioygenase 3 (Zeller and Granato, 1999; Zeller et al., 2002; Schneider and Granato, 2006).
Table 1.
Accordion mutants.
| Mutant | Alleles | Mutation | Phenotype and gene defect | References |
|---|---|---|---|---|
| accordion | dta5 | Unknown | Embryonic lethal. Touch-induced uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, 10–20% shorter than wild type. Mutations I97N, S766F, T848I in the skeletal muscle sarcoplasmic reticulum Ca2+-ATPase SERCA1 gene (atp2a1) on chromosome 3. | Granato et al. (1996), Odenthal et al. (1996), Hirata et al. (2004), Gleason et al. (2004), Masino and Fetcho (2005) |
| acc | mi25i | I97N | ||
| mi289a | T848I | |||
| tc249a | Unknown | |||
| ti284a† | Unknown | |||
| tm286 | Unknown | |||
| tn218b | Unknown | |||
| tp72x | Unknown | |||
| tq206§ | S766F | |||
| ty20 | Unknown | |||
| bajan | tf247 | IVS2-2A > C | Embryonic lethal. Uncoordinated contraction of trunk muscles, eventually completely immotile. Intron 2 splice acceptor site mutation in the choline acetyltransferase gene (chat) on chromosome 13. | Granato et al. (1996), Odenthal et al. (1996), Wang et al. (2008) |
| baj | ||||
| bandoneon | mi106a | R275H | Embryonic lethal. Touch-induced uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, slightly bent up, 10–20% shorter than wild type. Mutations Y79X, L255R, R275H in the GlyR beta subunit gene (glrbb) on chromosome 14. | Granato et al. (1996), Odenthal et al. (1996), Hirata et al. (2005), Masino and Fetcho (2005) |
| beo | ta86d | Unknown | ||
| ta92† | Unknown | |||
| tf242 | Unknown | |||
| tm115 | Unknown | |||
| tp221 | Y79X | |||
| tu230‡ | Allele lost | |||
| tw38f§ | L255R | |||
| diwanka | ts286 | Q608X | Embryonic lethal. Touch-induced uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, slightly bent up, 10–20% shorter than wild type, small eyes and enlarged hindbrain ventricle. Intron 4 splice acceptor site and nonsense mutations W447X and Q608X in the procollagen lysine 2-oxoglutarate 5-dioxygenase 3 gene (plod3) on chromosome 23. | Granato et al. (1996), Odenthal et al. (1996), Zeller and Granato (1999), Zeller et al. (2002), Schneider and Granato (2006) |
| diw | tv205a | IVS4-1A > G | ||
| tz290 | W447X | |||
| expander exp | tu12 | Unknown | Embryonic lethal. Uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, 10–20% shorter than wild type. Unknown gene on chromosome 11. | Granato et al. (1996), Odenthal et al. (1996), Geisler et al. (2007) |
| quetschkommode | ti274 | Unknown | Embryonic lethal. Uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, 10-20% shorter than wild type. Unknown gene on chromosome 22. | Granato et al. (1996), Odenthal et al. (1996), Geisler et al. (2007) |
| que | ||||
| ziehharmonika | sb55 | S226N | Embryonic lethal. Uncoordinated contraction of trunk muscles resulting in a contracted wavy notochord, 10–20% shorter than wild type. Eventually becoming completely immotile. Mutations Y139X, G198R and S226N in the acetylcholinesterase gene (ache) on chromosome 7. | Granato et al. (1996), Odenthal et al. (1996), Behra et al. (2002), Downes and Granato (2004) |
| zim | tf222a | G198R | ||
| ache | tm205§ | Y139X | ||
| tm206‡ | Allele lost | |||
| Unresolved | ta222b | Unknown | Unknown. | Granato et al. (1996), Odenthal et al. (1996) |
‡Mutant lost, †Viable allele, §Strongest allele. Information compiled from: http://www.eb.tuebingen.mpg.de/core-facilities/zebrafish-stockcenter; http://zfin.org/
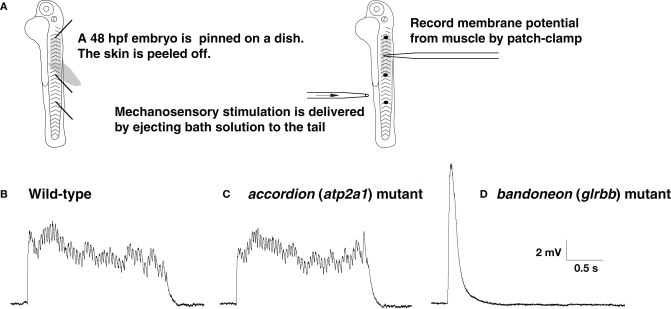
Simultaneous contraction of antagonistic muscles was also attributable to bilateral activation of motor neurons caused by impaired reciprocal inhibition in glrbb, encoding one of two zebrafish GlyR β subunits (Figure 1C; Hirata et al., 2005). Thus, defects in muscle Ca2+ storage, cholinergic transmission, motor projection and glycinergic transmission lead to very similar phenotypes in zebrafish embyros. Two additional ‘accordion’ class mutants, expander (exp) and quetschkommode (que) remain to be characterized (Table 1; Granato et al., 1996), and it will be intriguing to determine whether the underlying defects fit into one or other of the functional classes above. In order to distinguish between neuronal and muscle defects, one approach is to record the electrophysiological responses of muscles to sensory stimulation (Figure 2A). The output of the nervous system and status of the NMJ can be assayed by membrane potential and voltage-clamp recordings following tactile stimulation of zebrafish embryos. For example, recordings from the muscles of accordion (atp2a1) mutants show normal rhythmic activity corresponding to fictive swimming, indicating that the nervous system is unaffected in accordion (Figures 2B,C; Hirata et al., 2004). However, highly aberrant, arrhythmic responses can be recorded from the muscles of bandoneon (glrbb) larvae, demonstrating that the nervous system output is defective in these mutants (Figure 2D; Hirata et al., 2005).
Figure 2.
The output of the CNS is normal in accordion (atp2a1) mutants but abnormal in bandoneon (glrbb) mutants. (A) Schematic summary of the experimental procedure. (B) Wild-type muscle at 48 hpf responds to tactile stimulation with rhythmic depolarizations, representing cyclic muscle contractions during fictive swimming. (C) accordion (atp2a1) mutant muscle respond to tactile stimulation with a similar rhythmic pattern, indicating that the outputs from the CNS are normal. (D) bandoneon (glrbb) mutant muscle responds to tactile stimulation with a short and large voltage response devoid of rhythmicity, suggesting that the CNS outputs are aberrant.
Additional touch-insensitive mutants from the Tübingen screen, such as alligator (ali), macho (mao) and steifftier (ste), have defects in the excitability of sensory Rohon-Beard neurons (Granato et al., 1996; Ribera and Nüsslein-Volhard, 1998; Gnuegge et al., 2001; Pineda et al., 2005). Although the genes responsible for these mutations have not yet been identified, these mutants show reduced Na+ current amplitudes. By contrast, the zebrafish twitch twice (twt) mutant showing an aberrant unidirectional startle response was found to harbor nonsense mutations in robo3, encoding roundabout 3, a Slit ligand receptor essential for Mauthner cell axon guidance (Burgess et al., 2009). Unsurprisingly, other mutations that affect axon outgrowth also exhibit abnormal behavior (Zeller and Granato, 1999; Zhang and Granato, 2000; Zhang et al., 2004; Schneider and Granato, 2006; Palaisa and Granato, 2007; Tanaka et al., 2007). For example, the mutant unplugged (unp) is defective in muscle-specific receptor tyrosine kinase (MuSK) and exhibits defective initial outgrowth of motor axons (Lefebvre et al., 2007; Jing et al., 2009). Other behavioral mutants exhibiting decreased synaptic transmission and clustering of nAChRs at the NMJ have also been identified including nicotinic receptor (nic) and sofa potato (sop) which harbor mutations in the nAChR α and δ subunit genes, respectively. By contrast, mutants unp and twitch-once (two) have mutations in the genes encoding MuSK and the AChR clustering factor rapsyn, respectively (Westerfield et al., 1990; Sepich et al., 1998; Ono et al., 2001, 2002, 2004; Saint-Amant et al., 2008). Curiously, another mutant in the ‘twitch-once’ class of motility mutants (Granato et al., 1996), shocked (sho), was recently shown to result from mutations in slc6a9 encoding GlyT1 (Table 2; Cui et al., 2005; Mongeon et al., 2008). Once again, this highlights that central nervous system and muscle defects can result in phenocopying in zebrafish.
Table 2.
Twitch once mutants.
| Mutant | Alleles | Mutation | Phenotype and gene defect | References |
|---|---|---|---|---|
| shocked | ta51e | Unknown | Embryonic lethal. d2, twitch only once, head not straight; d5, head straight, in response to touch just jumps up and falls down, then vibrates with tip of tail. Resting position sideways or upside down. Mutations G81D and C305Y in the glycine transporter 1 gene (slc6a9) on chromosome 2. | Granato et al. (1996), Odenthal et al. (1996), Luna et al. (2004), Cui et al. (2004, 2005), Mongeon et al. (2008) |
| sho | ta229g§ | G81D | ||
| te301† | C305Y | |||
| twitch once | th26 | G130E | Embryonic lethal. d2, twitch only once, head not straight. d5, head straight, Just jumps and falls down, then vibrates with tip of tail. Resting position is sideways or upside down. Mutation G130E in the muscle rapsyn gene (rapsn) on chromosome 18. | Granato et al. (1996), Odenthal et al. (1996), Ono et al. (2002) |
| two | tq265b | Unknown | ||
| tm335 | Unknown |
‡Mutant lost, †Viable allele, §Strongest allele. Information compiled from: http://www.eb.tuebingen.mpg.de/core-facilities/zebrafish-stockcenter; http://zfin.org/
Zebrafish Glycine Receptor Genes
Inhibitory GlyRs belong to a superfamily of ligand-gated ion channels that includes nAChRs, serotonin (5HT3) receptors and GABAA receptors (Lynch, 2004). GlyRs are heteromultimers consisting of ligand-binding α and structural β subunits (Grenningloh et al., 1987, 1990a; Langosch et al., 1988), the latter contain a binding site for gephyrin, a multifunctional cytoplasmic linker protein that clusters αβ GlyRs at synapses (Kirsch et al., 1993; Meyer et al., 1995; Feng et al., 1998; Kim et al., 2006). Four α subunit genes (GLRA1, GLRA2, GLRA3 and GLRA4) and a single β subunit gene (GLRB) have been identified in mammals (Grenningloh et al., 1987, 1990a,b; Kuhse et al., 1990, 1991; Akagi et al., 1991). In humans, however, GLRA4 is a pseudogene. Several variants are also created by alternative splicing and RNA editing (Meier et al., 2005), which may modify functional properties such as agonist specificity, affinity and desensitization kinetics.
In zebrafish, four GlyR α subunits (αZ1, αZ2, αZ3 and αZ4) and two β subunits genes (βa (= βZ) and βb encoded by glrba and glrbb, respectively; Table 3) were initially reported (David-Watine et al., 1999; Imboden et al., 2001a,b,c; Hirata et al., 2005). Phylogenetic analysis suggested that αZ1, αZ3 and αZ4 showed high sequence similarity to the mammalian GlyR α1, α3 and α4 subunits, respectively (Imboden et al., 2001a), and were referred to as zebrafish GlyR α1, GlyR α3, and GlyR α4 (Figures 3A,C,D). A sequence reported as αZ2 was originally thought to encode a GlyR α2 subunit (Imboden et al., 2001b), but Imboden and colleagues subsequently reclassified this protein as a second α4 subunit based on a more detailed phylogenetic analysis (Imboden et al., 2001a). Thus, αZ2 was renamed α4a, and αZ4 was renamed α4b and the genes were renamed glra4a and glra4b, respectively. The existence of two distinct orthologs of a mammalian gene is not uncommon in the zebrafish genome, due to the suspected duplication of the whole genome during fish evolution (Amores et al., 1998). Using the most recent zebrafish genome assembly Zv82, we identified a novel zebrafish GlyR α subunit gene on chromosome 9 that is likely to encode α2 based on our own phylogenetic and sequence analysis (GenBank GQ406228; Figure 3B). We also amplified new zebrafish cDNAs encoding the correct N-terminus of α4a, containing a cleavable signal peptide sequence (GenBank GQ406229), which may explain why the originally isolated α4a (αZ2) required the signal peptide of α1 in functional expression experiments (Imboden et al., 2001b). Therefore, zebrafish have five α subunit (glra1, glra2, glra3, glra4a and glra4b) and two β subunit (glrba and glrbb) genes (Figures 3A–E). As well as considering overall sequence identity and similarity, we considered other diagnostic criteria in our assignment of orthologs. For example, exon 3a and 3b are typically alternatively spliced in the mouse, rat and human GlyR α2 transcripts. But, RT-PCR and genome analysis suggests that zebrafish glra2, glra4a and glra4b do not show alternative splicing of exon 3. However, examining diagnostic residues in the M1-M3 domains proved more useful. The second residue in M2 (also called the 2’ residue) is typically glycine in GlyR α1/α4 and alanine in GlyR α2/α3 subunits. This residue has been suggested to influence the conductance of GlyR channels (Bormann et al., 1993) and picrotoxin/picrotin blockade (Wang et al., 2007; Yang et al., 2007). From this point of view, our revised orthology appears to be more accurate, since the zebrafish GlyR α2 subunit has an alanine residue at the 2’ position, whereas both zebrafish GlyR α4 subunits harbor a glycine residue at this position.
Table 3.
Zebrafish glycinergic transmission.
| Gene | Location | Protein | Mutant/Knockdown | References |
|---|---|---|---|---|
| GLYCINE RECEPTORS | ||||
| glra1 | Chr 14 | GlyR α1 | Knockdown: no phenotype reported | David-Watine et al. (1999), Hirata et al. (2005), McDearmid et al. (2006) |
| glra2 | Chr 9 | GlyR α2 | Unknown | This review |
| glra3 | Chr 1 | GlyR α3 | Unknown | Imboden et al. (2001b) |
| glra4a | Chr 14 | GlyR α4a | Knockdown: disrupted rhythm-generating networks and reduced the number of spinal interneurons | Imboden et al. (2001a,b), McDearmid et al. (2006) |
| glra4b | Chr 5 | GlyR α4b | Unknown | Imboden et al. (2001b) |
| glrba | Chr 1 | GlyR βa | Unknown | Imboden et al. (2001c), Hirata et al. (2005) |
| glrbb | Chr 14 | GlyR βb | Bandoneon: touch-induced bilateral muscle contraction | Hirata et al. (2005) |
| GLYCINE TRANSPORTERS | ||||
| slc6a9 | Chr 2 | GlyT1 | Shocked: touch-induced single twitch | Higashijima et al. (2004), Cui et al. (2005), Mongeon et al. (2008) |
| slc6a5 | Chr 7 | GlyT2 | Unknown | Higashijima et al. (2004) |
Figure 3.
Sequence alignments of zebrafish GlyR subunits with avian and mammalian counterparts. (A) Sequence alignment of human (GenBank accession: NP_000162), rat (NP_037265), mouse (NP_065238) GlyR α1 with zebrafish (NP_571477) GlyR α1. The four membrane-spanning domains are represented as M1-M4. The 2’ residues in M2 are highlighted by grey box. Signal peptides are denoted by negative numbering. (B) Protein sequence alignment of human (NP_002054), rat (NP_036700), mouse (NP_906272), chick (XP_001234291) GlyR α2 with zebrafish (GQ406228) GlyR α2. (C) Protein sequence alignment of human (NP_006520), rat (NP_446176), mouse (NP_536686), chick (XP_420527) GlyR α3 with zebrafish (NP_694497) GlyR α3. (D) Protein sequence alignment of rat (XP_346351), mouse (NP_034427), chick (XP_001232995) with zebrafish GlyR GlyR α4a (GQ406229) and GlyR α4b (AAH85599). (E) Protein sequence alignment of human (NP_000815), rat (NP_445748), mouse (NP_034428) and chick (XP_420379) GlyR β with zebrafish GlyR βb (NP_001003587) and GlyR βa (XP_683646). Position of mutations identified in the three beo alleles are represented by arrowheads.
However, assuming orthology based on sequence identity alone is unwise, and it is also important to consider patterns of gene expression. Hindbrain neurons likely to be reticulospinal interneurons and spinal neurons express both glra1 and glra4a by 24 hpf (Imboden et al., 2001a; Hirata et al., 2005; McDearmid et al., 2006) but glra4a was lost by 48 hpf. By contrast, Imboden and colleagues showed robust expression of glra4a at 52 hpf in the olfactory pits, mesencephalon, rhombencephalon and somites (Imboden et al., 2001a). However, glra4b expression appears to be restricted to the retina in 52 hpf embryos (Imboden et al., 2001a) and the expression patterns of glra2 and glra3 remain to be determined. Interestingly, glrbb is also expressed by reticulospinal and spinal neurons by 24 hpf, while glrba is not expressed in zebrafish until 72 hpf (Hirata et al., 2005). Thus, whilst some data appear contradictory, it is likely that early embryonic GlyRs have the capacity to be heteromeric, with GlyR βa and GlyR βb forming different heteromeric GlyRs based on developmental expression profiles, and the apparent lack of compensation shown in bandoneon (beo).
Defective GlyR Clustering in Bandoneon
In the Tübingen screen, seven alleles of the ‘accordion’ class beo mutation (tp221, tw38f, ta86d, ta92, tm115, tf242 and tu230) were isolated (Granato et al., 1996; Table 1) and named after the South American accordion-like instrument. From our behavioral screen, we generated another allele (mi106a) and showed that it results from missense and nonsense mutations in glrbb, encoding the GlyR βb subunit (Hirata et al., 2005). As mentioned previously, at 24 hpf wild-type embryos responded to touch with multiple coils of the body, which was achieved by alternating trunk contractions (Figure 1B). By contrast, beo mutants displayed simultaneous contraction of the bilateral axial muscles that resulted in a dorsal flexure and shortening of the body following a tactile stimulation (Figure 1C). Later, when wild-type embryos swam in response to touch, beo mutants contracted the trunk muscle simultaneously on both sides and failed to swim in response to touch. Although beo mutants exhibited an abnormal tactile response, spontaneous coiling was not affected. In addition to behavioral perturbation, beo mutants exhibited secondary morphological defects in the notochord and axial muscles that were common to zebrafish behavioral mutants showing excessive contraction of the musculature (Hirata et al., 2004; Lefebvre et al., 2004). In fact, suppression of motor behavior either by a sodium channel inhibitor (tricaine) or a muscle myosin inhibitor (N-benzyl-p-toluene sulfonamide) prevented the morphological perturbations of mutant muscle. The beo larvae typically died at 7 days postfertilization (dpf), presumably due to their inability to swim and feed effectively, but cumulative notochord damage may have also contributed to lethality.
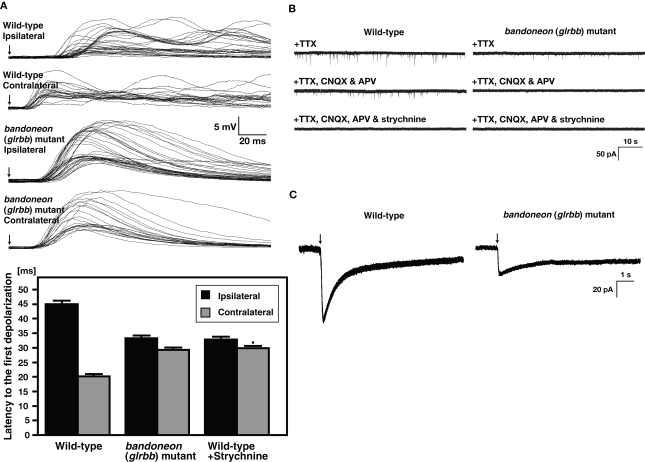
Alternation of muscle contractions on the left and the right side of animals requires reciprocal inhibition between left and right sides of the hindbrain and spinal cord (reviewed in Grillner, 2003). Disruption of this inhibition can lead to simultaneous activation of bilateral motor neurons and thus simultaneous muscle contractions on both sides. Normally, tactile stimulation delivered to one side of the body leads to contraction of the contralateral side followed by contraction of the ipsilateral side. To see whether bilateral muscles are simultaneously activated in beo, we measured the latency of muscle depolarization following mechanosensory stimulation to the contralateral side and the ipsilateral side. We found that in wild-type muscles, contralateral tactile stimulation results in a muscle response 25 ms faster than ipsilateral stimulation, corresponding to initial touch-induced activation of contralateral muscles followed by ipsilateral muscles (Figure 4A). By contrast, the latencies of response to ipsilateral and contralateral stimulation are comparable in beo, indicating that tactile stimulation activates both contralateral and ipsilateral muscles simultaneously. Thus, reciprocal inhibition appears to be deficient in beo.
Figure 4.
Simultaneously contraction of bilateral axial muscles in bandoneon (glrbb) mutants due to the loss of glycinergic synaptic transmission. (A) Superimposed voltage responses of muscles evoked by mechanosensory stimulation. Arrows indicate the time of stimulation. The latency of the muscle response to contralateral stimulation was shorter than that to ipsilateral stimulation in wild-type, whereas the latency to ipsilateral and contralateral stimulation was comparable in beo mutants. Histograms show that the latency to half-maximal amplitude of the first depolarization was shorter in contralateral stimulation compared to ipsilateral stimulation in wild-type. The latency of the response to tactile stimulation in strychnine-treated wild-type muscles was comparable to latency in beo mutants. (B) Spontaneous synaptic currents recorded from a wild-type motor neuron in the presence of TTX were decreased in frequency following block of NMDA and AMPA receptors by application of CNQX and APV, respectively. The non-glutamatergic currents in wild-type are eliminated by further application of strychnine, showing that they are glycinergic currents. In beo, non-glutamatergic currents in the presence of CNQX and APV are missing, indicating that glycinergic synaptic currents are absent. (C) A puff of exogenous glycine induced a current in a wile-type motor neuron and a smaller current in a beo mutant motor neuron.
Examination of glycinergic synaptic transmission in beo by patch-clamp recordings of motor neurons showed that glycinergic, but not glutamatergic synaptic transmission was absent in beo (Figure 4B). Concordantly, immunolabeling with an anti-GlyR α antibody confirmed that GlyRs were not clustered in beo spinal cord as they were in wild-type zebrafish. Interestingly, application of exogenous glycine directly onto motor neurons elicited currents in beo motor neurons, suggesting that non-clustered extrasynaptic GlyRs, which may represent homomeric α subunit GlyRs, existed in beo mutants (Figure 4C). In fact, fetal extrasynaptic GlyRs were thought to be homopentamers of GlyR α2 in rodents (Becker et al., 1988; Malosio et al., 1991; Watanabe and Akagi, 1995). Taken together, these results demonstrated that the GlyR βb subunit was required for synaptic aggregation of GlyRs, corroborating previous findings showing that GlyR β interacted with gephyrin (Meyer et al., 1995; Sola et al., 2004; Kim et al., 2006) a multifunctional cytoplasmic protein that is crucial for the synaptic localization of GlyRs (Kirsch et al., 1993; Feng et al., 1998). The synaptic GlyRs that were eliminated in beo could contain either GlyR α1 or α4a, since the corresponding genes appeared to be expressed by hindbrain and spinal neurons during early development. Antisense knockdown of GlyR α4a (but not GlyR α1) reduced glycinergic synaptic transmission and disrupted activity of circuits underlying swimming (McDearmid et al., 2006), suggesting that the early synaptic GlyRs could be α4a/βb heteromers. A complication in this study was that GlyR α4a was referred to as α2 based upon the initial designation of this cDNA as αZ2 (Imboden et al., 2001a,b). The behavioral phenotype associated with α1 and α4a knockdown was not reported, and the translation blocking α4a morpholino used in these studies also caused a reduction in the number of spinal interneurones (McDearmid et al., 2006), a phenotype not examined in beo (Hirata et al., 2005). However, the sequences of multiple ESTs and our own αZ4a cDNA cloning suggested that the α4a morpholino used by McDearmid et al. (2006) may have been directed against a mis-spliced intronic sequence upstream of glra4a exon 2. This does not preclude gene knockdown by interference with glra4a splicing, but further studies with other GlyR-directed morpholinos may be warranted to uncover the exact biological roles of the zebrafish GlyR genes.
In humans defects in glycinergic synaptic transmission lead to hyperekplexia. Hyperekplexia is a rare neurological syndrome that is characterized by an exaggerated startle response accompanied by transient muscle rigidity in response to unexpected acoustic or tactile stimuli (Gastaut, 1967; Bakker et al., 2006). More than 20 distinct missense mutations and several nonsense and frameshift mutations have been identified in the GlyR α1 subunit gene (GLRA1) to date (Shiang et al., 1993; Bakker, 2006; Harvey et al., 2008). Most missense mutations in the second membrane-spanning domain and its neighboring loops are dominant mutations, whereas most point mutations in the N-terminal extracellular domain and M3-M4 intracellular loop are recessive. Missense and nonsense mutations in GLRA1 that lead to exaggerated startle reflexes are also found in spontaneous and induced mouse mutants and bovine congenital myoclonus (Gundlach et al., 1988; Buckwalter et al., 1994; Ryan et al., 1994; Pierce et al., 2001; Holland et al., 2006; Traka et al., 2006). To date, no mutations in the other functional GlyR α subunit genes (GLRA2 and GLRA3) have been reported, but in one family compound heterozygous mutations in GLRB were associated with hyperekplexia (Rees et al., 2002). Similarly in mice, GlyR β hypomorphs (due to a LINE1 transposable element insertion causing mis-splicing of Glrb transcripts) also exhibit abnormal startle responses (Becker et al., 1992; Kingsmore et al., 1994; Mülhardt et al., 1994; Hartenstein et al., 1996).
The mutations underlying three alleles of beo have been identified to date (Hirata et al., 2005; Table 1). In the tp221 allele, a nonsense mutation (Y79X) is predicted to cause truncation of GlyR βb. By contrast, in tw38f and mi106a, missense mutations L255R and R275H, respectively, were found in the first membrane-spanning domain (M1) and in the intracellular loop between the first (M1) and second (M2) membrane-spanning domains. The R275H mutation in zebrafish GlyR βb affects a highly conserved arginine residue prior to M2 and in fact in human GlyR α1, the corresponding mutation R252H is known to accelerate degradation of GlyR α1 (Rea et al., 2002), suggesting that mi106a mutation is a hypomorph of GlyR βb. Characterization of the remaining beo alleles is underway, and may reveal key residues of involved in the function of GlyR βb.
Glycine Transporter 1 (GlyT1) Defects in Shocked
Glycine transporters are 12 membrane-spanning domain proteins that belong to Na+/Cl–-dependent transporter superfamily. In vertebrates, two glycine transporters, GlyT1 and GlyT2, mediate the uptake of glycine from the extracellular space to the cytosol driven by an electrogenic gradient (Eulenburg et al., 2005). GlyT1 is enriched in astrocytes and some excitatory neurons, whereas GlyT2 is enriched in inhibitory glycinergic neurons (Adams et al., 1995; Zafra et al., 1995; Jursky and Nelson, 1996; Cubelos et al., 2005). At glycinergic synapses, astroglial GlyT1 is thought to clear glycine from the synaptic cleft, so terminating neurotransmission. Since glycine serves both as ligand for GlyR activation and as a coagonist and possibly primary ligand for NMDA receptors (Johnson and Ascher, 1987; Kuryatov et al., 1994; Chatterton et al., 2002), GlyT1 regulates both inhibitory and excitatory synaptic transmission. By contrast, GlyT2 is localized to presynaptic terminals of glycinergic neurons and is essential for glycine reuptake, replenishing the pool of releasable transmitter (Zafra et al., 1995; Gomeza et al., 2003b; Mahendrasingam et al., 2003).
Zebrafish sho mutants fail to initiate swimming following tactile stimulation at 3 dpf (Granato et al., 1996; Cui et al., 2004; Luna et al., 2004). This phenotype is caused by missense mutations in slc6a9, which encodes GlyT1 (Cui et al., 2005; Mongeon et al., 2008). The ta229g allele, which displays the strongest phenotype, results from a G81D missense mutation in the second membrane-spanning domain. Expression of the recombinant zGlyT1 G81D mutant in Xenopus oocytes revealed that GlyT1 function is abolished by this mutation (Cui et al., 2005). The milder te301 allele harbors a C305Y missense mutation that is located next to a deduced glycine-binding residue in the pore-forming sixth membrane-spanning domain (Yamashita et al., 2005; Rees et al., 2006; Harvey et al., 2008; Mongeon et al., 2008). Both sho mutant alleles display severely compromised tactile-induced locomotion and the frequency of spontaneous coiling is reduced. Tactile stimuli do not evoke escape contractions in sho mutants at 24 hpf, unlike wild-type siblings. At later stages, when tactile stimuli induce swimming in wild-type fish, sho mutants respond with a few uncoordinated trunk contractions (or not at all) and do not display typical swimming behavior. While shota229g mutants normally die within 2 weeks of development, importantly they can be maintained to adulthood by careful feeding. These adult shota229g homozygous fish are less active than wild-type zebrafish but fertile, indicating some degree of functional recovery. Interestingly, the weaker shote301 mutants recover by around 4–5 dpf (Mongeon et al., 2008), suggesting that there is a degree of compensation for the loss of functional GlyT1 in zebrafish.
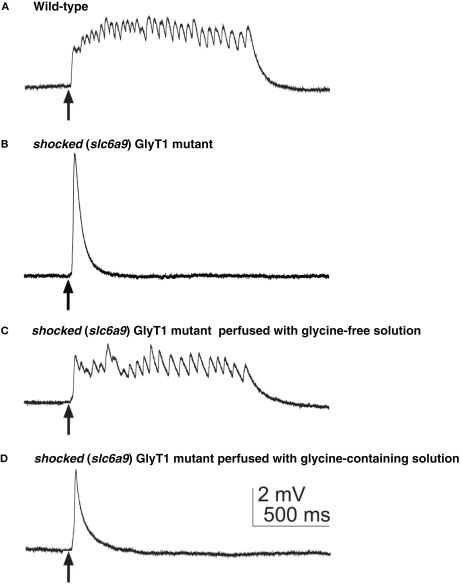
The effects of the loss of GlyT1 function on the nervous system and/or muscles were examined by electrophysiology. Voltage recordings from muscle of wild-type embryos show sustained episodes of rhythmic depolarizations corresponding to swimming following tactile stimulation, whereas sho muscle responds with one or two short, arrhythmic depolarizations corresponding to the uncoordinated ‘twitch-once’ muscle contractions exhibited by sho mutants (Figures 5A,B; Cui et al., 2004). Similarly, wild-type spinal motor neurons respond to touch with a long burst of action potentials, while sho motor neurons responded with only a short burst. Thus, the nervous system output of the CNS is aberrant in sho, signifying that the loss of GlyT1 disturbs the CNS function at glycinergic synapses (Cui et al., 2005). Interestingly, the sho mutants also exhibit aberrant electrical coupling between axial muscle fibers at 3 dpf (Luna et al., 2004), but this may be a secondary consequence of the CNS defects, since GlyT1 is not expressed in muscle. If the defect in CNS signaling in sho is attributable to increased extracellular glycine levels in the synaptic cleft due to the loss of GlyT1 function, one would expect normal responses to be restored in mutants when excess glycine is washed out. To examine this possibility, the hindbrain, which contains many of the neurons mediating responses to tactile stimulation, was exposed to various solutions by perfusion following removal of dorsal roof of the fourth ventricle and responses to tactile stimulation assayed by muscle recordings (Cui et al., 2005). When mutants were perfused with glycine-free solution, they respond with normal swimming behavior (Figure 5C) and this functional recovery is inhibited by addition of exogenous glycine (Figure 5D). Furthermore, sho mutants are more sensitive to the deleterious effects of exogenous glycine on touch-induced swimming compared to wild-type fish (Mongeon et al., 2008). Thus, the putative increase in extracellular glycine due to the loss of GlyT1 is likely to mediate aberrant signaling within the mutant CNS.
Figure 5.
The aberrant motor response of shocked (slc6a5) mutants defective in GlyT1 is due to high external glycine. (A) Muscle voltage recording from a wild-type embryo showed normal fictive swimming in response to mechanosensory stimulation. Arrows indicate the time of stimulation. (B) Muscle recording from a sho mutant embryo displaying a large, nonrhythmic depolarization. (C) Muscle recording from a sho mutant after the hindbrain was exposed and perfused with glycine-free solution demonstrated rhythmic depolarizations similar to fictive swimming. (D) Muscle recording from the same sho embryo as in (C) after switching the perfusion from glycine-free solution to saline containing 0.2 mM glycine again exhibited the aberrant response characteristic of sho mutants.
One presumption of these perfusion experiments was that extracellular glycine levels were high in the synaptic cleft of sho mutants. This excess glycine is predicted to lead to increased inhibition of neurons receiving glycinergic inputs. If this presumption is correct, then blocking glycinergic inhibition should ameliorate the effects of high glycine in sho mutants. In fact, application of low concentrations of strychnine to sho mutants led to partial recovery of spontaneous coiling in 21 hpf embryos and normal swimming responses in older (40–46 hpf) embryos (Cui et al., 2005). However, the partially recovered response might also be attributed to increased excitatory transmission via NMDA receptors in sho mutants. Taken together, it appears that the defective signaling in sho is consistent with abnormally high glycine in the synaptic cleft.
GlyT1 knockout mice showed many features that resembled those exhibited by zebrafish sho mutants, most notably motor deficits including those involving respiratory neural circuits (Gomeza et al., 2003a; Tsai et al., 2004). Recordings of spontaneous neuronal activity from hypoglossal motor neurons revealed that inspiratory cycling of the respiratory network of the brain stem was nearly eliminated in GlyT1 mutant mice. Much like the palliative effect of low concentration strychnine on the swimming circuit of zebrafish sho mutants, normal rhythmic activity was restored in hypoglossal motor neurons upon application of low strychnine to brainstem slices from GlyT1 knockout mice. Furthermore, voltage-clamp analyses of hypoglossal neurons were consistent with an increase in extracellular glycine. Thus, the neural defects seen in GlyT1-deficient mice are also likely to be due to increased levels of synaptic glycine, leading to suppression of neural networks. So far, no human GlyT1 defects have been associated with any disease. However, GlyT1 dysfunction has been suggested to play a possible role in glycine encephalopathy (Gomeza et al., 2003a; Harvey et al., 2008), as well as the psychiatric disorder schizophrenia (Freedman, 2003; Tsai et al., 2004), where NMDA receptor hypofunction is suspected. GlyT1 inhibitors elicit activation of NMDA receptors by increasing synaptic glycine levels, thus accelerating the co-agonist action of glycine may be useful pharmacological tools to mitigate some features of schizophrenia (Le Pen et al., 2003). However, whether sequence variations in the GlyT1 gene (SLC6A9) are linked to glycine encephalopathy or schizophrenia and whether GlyT1 inhibitors are useful pharmacotherapies remains to be determined. Interestingly, SNPs in the human GlyT1 gene were recently proposed to be associated with methamphetamine-use disorder (Morita et al., 2008; Bousman et al., 2009). Animal models are clearly required for investigating the biological roles of GlyT1 and for the identification of therapeutic agents for treatment of human disorders related to GlyT1. In this respect, mouse GlyT1 knockouts may be less than ideal, since they die on the day of birth (Gomeza et al., 2003a; Tsai et al., 2004), whereas zebrafish sho mutants are accessible and viable.
Concluding Remarks
Zebrafish bandoneon and shocked mutants are useful models for understanding of glycinergic synaptic transmission and for clarifying the biological consequences of gene disruption that impinge upon glycinergic signaling in vivo. Furthermore, future analysis of other zebrafish mutations may reveal new insights. For example, two ‘accordion’ class mutants (que and exp) remain to be analyzed in depth, as well as the crazy fish mutant techno trousers (tnt). Although these mutants have been suggested to harbor defects in glycinergic transmission because they exhibit exaggerated startle responses in response to touch (Granato et al., 1996), history has taught us that there are several potential phenocopies of glycinergic defects. Equally, it is unclear why defects in glra1 (encoding GlyR α1) and slc6a5 (encoding GlyT2) were not uncovered in mutagenesis screens to date, since these are highly mutable genes in other species (Harvey et al., 2008). Importantly, the genetic, developmental, and physiological accessibility of zebrafish make them useful animal models of human syndromes such as hyperekplexia. Small molecule screens using zebrafish mutants have successfully identified several drugs that ameliorate mutant phenotypes (Peterson et al., 2004; Stern et al., 2005). Such screens using zebrafish sho mutants have identified several compounds that mitigate the impairment of touch response (Hirata, unpublished). Future comparative and integrative studies using a variety of organisms including zebrafish with defects in glycinergic transmission are a promising strategy for a comprehensive understanding and development of pharmaceutical agents for human diseases defective in glycinergic synaptic transmission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to investigators whose work could not be cited in this manuscript owing to space limitations. We thank Drs. Y. Oda and S. Takagi for insightful discussion and encouragement. Research has been supported by Grant-in-Aid for Young Scientists (A) and for Scientific Research on Priority Area “System Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Nakajima Foundation and Career Development Award of the Human Frontier Science Program to Hiromi Hirata; National Science Foundation (0725976) and National Institutes of Neurological Diseases and Strokes (1RO1NS054731) to John Y. Kuwada; Medical Research Council (G0500833, G0601585) to Robert J. Harvey.
Footnotes
Abbreviations
acc, accordion; beo, bandoneon; cDNA, complementary DNA; CMD, congenital muscular dystonia; CNS, central nervous system; DNA, deoxyribonucleic acid; dpf, days post-fertilization; EC, excitation-contraction; ENU, N-ethyl-N-nitrosourea; EST, expressed sequence tag; GABA, γ-aminobutyric acid; GFP, green fluorescent protein; GlyR, glycine receptor; GlyT, glycine transporter; hpf, hours post-fertilization; mRNA, messenger ribonucleic acid; MuSK, muscle-specific kinase; nAChR, nicotinic acetylcholine receptor; NMDA, N-methyl-D-asparatate; NMJ, neuromuscular junction; NR, NMDA receptor; RFP, red fluorescent protein; RNA, ribonucleic acid; RT-PCR, reverse transcription-polymerase chain reaction sequence; TILLING, targeting-induced local lesion in genome; UAS, upstream activating sequence.
References
- Adams R. H., Sato K., Shimada S., Tohyama M., Püschel A. W., Betz H. (1995). Gene structure and glial expression of the glycine transporter GlyT1 in embryonic and adult rodents. J. Neurosci. 15, 2524–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi H., Hirai K., Hishinuma F. (1991). Cloning of a glycine receptor subtype expressed in rat brain and spinal cord during a specific period of neuronal development. FEBS Lett. 281, 161–166 10.1016/0014-5793(91)80383-E [DOI] [PubMed] [Google Scholar]
- Amores A., Force A., Yan Y.-L., Joly L., Amemiya C., Fritz A., Ho R. K., Langeland J., Prince V., Wang Y.-L., Westerfield M., Ekker M., Postlethwait J. H. (1998). Zebrafish hox clusters and vertebrate genome evolution. Science 282, 1711–1714 10.1126/science.282.5394.1711 [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Burgess S., Golling G., Chen W., Sun Z., Townsend K., Farrington S., Haldi M., Hopkins N. (1999). A large-scale insertional mutagenesis screen in zebrafish. Genes. Dev. 13, 2713–2724 10.1101/gad.13.20.2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Hopkins N. (2006). Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends. Genet. 22, 473–478 10.1016/j.tig.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Kin S., Hopkins N. (1995). The Aequorea victoria green fluorescent protein can be used as a reporter in live zebrafish embryos. Dev. Biol. 171, 123–129 10.1006/dbio.1995.1265 [DOI] [PubMed] [Google Scholar]
- Amsterdam A., Nissen R. M., Sun Z., Swindell E. C., Farrington S., Hopkins N. (2004). Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. U.S.A. 101, 12792–12797 10.1073/pnas.0403929101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegarth D. A., Toone J. R. (2006) Glycine encephalopathy (nonketotic hyperglycinemia): comments and speculations. Am. J. Med. Genet. A 140, 186–188 10.1002/ajmg.a.31030 [DOI] [PubMed] [Google Scholar]
- Asakawa K., Suster M. L., Mizusawa K., Nagayoshi S., Kotani T., Urasaki A., Kishimnoto Y., Hibi M., Kawakami K. (2008). Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 105, 1255–1260 10.1073/pnas.0704963105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M. J., van Dijk J. G., van den Maagdenberg A. M. J. M., Tijssen M. A. J. (2006). Startle syndromes. Lancet Neurol. 5, 513–524 10.1016/S1474-4422(06)70470-7 [DOI] [PubMed] [Google Scholar]
- Bassett D. I., Bryson-Richardson R. J., Daggett D. F., Gautier P., Keenan D. G., Currie P. D. (2003). Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryos. Development 130, 5851–5860 10.1242/dev.00799 [DOI] [PubMed] [Google Scholar]
- Bayer T. A., Campos-Ortega J. A. (1992). A transgene containing lacZ is expressed in primary sensory neurons in zebrafish. Development 115, 421–426 [DOI] [PubMed] [Google Scholar]
- Becker C.-M., Hoch W., Betz H. (1988). Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 7, 3717–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C.-M., Schmieden V., Tarroni P., Strasser U., Betz H. (1992). Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron 8, 283–289 10.1016/0896-6273(92)90295-O [DOI] [PubMed] [Google Scholar]
- Behra M., Cousin X., Bertrand C., Vonesch J. L., Biellmann D., Chatonnet A., Strähle U. (2002). Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 5, 111–118 10.1038/nn788 [DOI] [PubMed] [Google Scholar]
- Bormann J., Rundström N., Betz H., Langosch D. (1993). Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 12, 3729–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousman C. A., Glatt S. J., Everall I. P., Tsuang M. T. (2009) Genetic association studies of methamphetamine use disorders: a systematic review and synthesis. Am. J. Med. Genet. In press. 10.1002/ajmg.b.30936 [DOI] [PubMed] [Google Scholar]
- Buckwalter M. S., Cook S. A., Davisson M. T., White W. F., Camper S. A. (1994). A frameshift mutation in the mouse α1 glycine receptor gene (Glra1) results in progressive neurological symptoms and juvenile death. Hum. Mol. Genet. 3, 2025–2030 10.1093/hmg/3.11.2025 [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Johnson S. L., Granato M. (2009). Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors in robo3 mutant zebrafish. Genes. Brain Behav. 8, 500–511 10.1111/j.1601-183X.2009.00499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss R. R., Drapeau P. (2001). Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J. Neurophysiol. 86, 197–210 [DOI] [PubMed] [Google Scholar]
- Charlier C., Coppieters W., Rollin F., Desmecht D., Agerholm J. S., Cambisano N., Carta E., Dardano S., Dive M., Fasquelle C., Frennet J. C., Hanset R., Hubin X., Jorgensen C., Karim L., Kent M., Harvey K., Pearce B. R., Simon P., Tama N., Nie H., Vandeputte S., Lien S., Longeri M., Fredholm M., Harvey R. J., Georges M. (2008). Highly effective SNP-based association mapping and management of recessive defects in livestock. Nat. Genet. 40, 449–454 10.1038/ng.96 [DOI] [PubMed] [Google Scholar]
- Chatterton J. E., Awobuluyi M., Premkumar L. S., Takahashi H., Talantova M., Shin Y., Cui J., Tu S., Sevarino K. A., Nakanishi N., Tong G., Lipton S. A., Zhang D. (2002). Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415, 793–798 [DOI] [PubMed] [Google Scholar]
- Cubelos B., Giménez C., Zafra F. (2005). Localization of the GLYT1 glycine transporter at glutamatergic synapses in the rat brain. Cereb. Cortex 15, 448–459 10.1093/cercor/bhh147 [DOI] [PubMed] [Google Scholar]
- Cui W. W., Low S. E., Hirata H., Saint-Amant L., Geisler R., Hume R. I., Kuwada J. Y. (2005). The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J. Neurosci. 25, 6610–6620 10.1523/JNEUROSCI.5009-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W. W., Saint-Amant L., Kuwada J. Y. (2004). shocked gene is required for the function of a premotor network in the zebrafish CNS. J. Neurophysiol. 92, 2898–2908 10.1152/jn.00419.2004 [DOI] [PubMed] [Google Scholar]
- Culp P., Nüsslein-Volhard C., Hopkins N. (1991). High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc. Natl. Acad. Sci. U.S.A. 88, 7953–7957 10.1073/pnas.88.18.7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. E., Balciunas D., Mohn D., Shaffer J., Hermanson S., Sivasubbu S., Cliff M. P., Hackett P. B., Ekker S. C. (2003). Efficient gene delivery and gene expression in zebrafish using the Sleeping Beauty transposon. Dev. Biol. 263, 191–202 10.1016/j.ydbio.2003.07.013 [DOI] [PubMed] [Google Scholar]
- David-Watine B., Goblet C., de Saint Jan D., Devignot S. F. V., Bregestovski P., Korn H. (1999). Cloning, expression and electrophysiological characterization of glycine receptor alpha subunit from zebrafish. Neuroscience 90, 303–317 10.1016/S0306-4522(98)00430-8 [DOI] [PubMed] [Google Scholar]
- Dowling J. J., Vreede A. P., Low S. E., Gibbs E. M., Kuwada J. Y., Bonnemann C. G., Feldman E. L. (2009). Loss of myotubularin function results in t-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet. 5, e1000372. 10.1371/journal.pgen.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes G. B., Granato M. (2004). Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev. Biol. 270, 232–245 10.1016/j.ydbio.2004.02.027 [DOI] [PubMed] [Google Scholar]
- Downes G. B., Granato M. (2006). Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J. Neurobiol. 66, 437–451 10.1002/neu.20226 [DOI] [PubMed] [Google Scholar]
- Doyon Y., McCammon J. M., Miller J. C., Faraji F., Ngo C., Katibah G. E., Amora R., Hocking T. D., Zhang L., Rebar E. J., Gregory P. D., Urnov F. D., Amacher S. L. (2008). Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat. Biotechnol. 26, 702–708 10.1038/nbt1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Ali D. W., Buss R. R., Saint-Amant L. (1999). In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J. Neurosci. Methods 88, 1–13 10.1016/S0165-0270(99)00008-4 [DOI] [PubMed] [Google Scholar]
- Drapeau P., Saint-Amant L., Buss R. R., Chong M., McDearmid J. R., Brustein E. (2002). Development of the locomotor network in zebrafish. Prog. Neurobiol. 68, 85–111 10.1016/S0301-0082(02)00075-8 [DOI] [PubMed] [Google Scholar]
- Driever W., Solnica-Krezel L., Schier A. F., Neuhauss S. C. F., Malicki J., Stemple D. L., Stainier D. Y. R., Zwartkruis F., Abdelilah S., Rangini Z., Belak J., Boggs C. (1996). A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123, 37–46 [DOI] [PubMed] [Google Scholar]
- Eisen J. S., Myers P. Z., Westerfield M. (1986). Pathway selection by growth cones of identified motoneurons in live zebra fish embryos. Nature 320, 269–271 10.1038/320269a0 [DOI] [PubMed] [Google Scholar]
- Ellingsen S., Laplante M. A., König M., Kikuta H., Furmanek T., Hoivik E. A., Becker T. S. (2005). Large-scale enhancer detection in the zebrafish genome. Development 132, 3799–3811 10.1242/dev.01951 [DOI] [PubMed] [Google Scholar]
- Etard C., Behra M., Fisher N., Hutcheson D., Geisler R., Strähle U. (2007). The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev. Biol. 308, 133–143 10.1016/j.ydbio.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Eulenburg V., Armsen W., Betz H., Gomeza J. (2005). Glycine transporters: essential regulators of neurotransmission. Trends Biochem. Sci. 30, 325–333 10.1016/j.tibs.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Felsenfeld A. L., Walker C., Westerfield M., Kimmel C., Streisinger G. (1990). Mutations affecting skeletal muscle myofibril structure in the zebrafish. Development 108, 443–459 [DOI] [PubMed] [Google Scholar]
- Feng G., Tintrup H., Kirsch J., Nichol M. C., Kuhse J., Betz H., Sanes J. R. (1998). Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science 282, 1321–1324 10.1126/science.282.5392.1321 [DOI] [PubMed] [Google Scholar]
- Fetcho J. R. (2007). The utility of zebrafish for studies of the comparative biology of motor systems. J. Exp. Zool. B Mol. Dev. Evol. 308, 550–562 [DOI] [PubMed] [Google Scholar]
- Freedman R. (2003). Schizophrenia. N. Engl. J. Med 349, 1738–1749 10.1056/NEJMra035458 [DOI] [PubMed] [Google Scholar]
- Gaiano N., Allende M., Amsterdam A., Kawakami K., Hopkins N. (1996a). Highly efficient germ-line transmission of proviral insertions in zebrafish. Proc. Natl. Acad. Sci. U.S.A. 93, 7777–7782 10.1073/pnas.93.15.7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N., Amsterdam A., Kawakami K., Allende M., Becker T., Hopkins N. (1996b). Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 383, 829–832 10.1038/383829a0 [DOI] [PubMed] [Google Scholar]
- Gastaut H. (1967). Startle disease (pathological surprise reaction). Electroencephalogr. Clin. Neurophysiol. 23, 494–495 [PubMed] [Google Scholar]
- Geisler R., Rauch G. J., Geiger-Rudolph S., Albrecht A., van Bebber F., Berger A., Busch-Nentwich E., Dahm R., Dekens M. P., Dooley C., Elli A. F., Gehring I., Geiger H., Geisler M., Glaser S., Holley S., Huber M., Kerr A., Kirn A., Knirsch M., Konantz M., Küchler A. M., Maderspacher F., Neuhauss S. C., Nicolson T., Ober E. A., Praeg E., Ray R., Rentzsch B., Rick J. M., Rief E., Schauerte H. E., Schepp C. P., Schönberger U., Schonthaler H. B., Seiler C., Sidi S., Söllner C., Wehner A., Weiler C., Nüsslein-Volhard C. (2007). Large-scale mapping of mutations affecting zebrafish development. BMC Genomics 8, 11. 10.1186/1471-2164-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason M. R., Armisen R., Verdecia M. A., Sirotkin H., Brehm P., Mandel G. (2004). A mutation in serca underlies motility dysfunction in accordion zebrafish. Dev. Biol. 276, 441–451 10.1016/j.ydbio.2004.09.008 [DOI] [PubMed] [Google Scholar]
- Gnuegge L., Schmid S., Neuhauss S. C. F. (2001). Analysis of the activity-deprived zebrafish mutant macho reveals an essential requirement of neuronal activity for the development of a fine-grained visuotopic map. J. Neurosci. 21, 3542–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golling G., Amsterdam A., Sun Z., Antonelli M., Maldonado E., Chen W., Burgess S., Haldi M., Artzt K., Farrington S., Lin S.-Y., Nissen R. M., Hopkins N. (2002). Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 31, 135–140 10.1038/ng896 [DOI] [PubMed] [Google Scholar]
- Gomeza J., Hülsmann S., Ohno K., Eulenburg V., Szöke K., Richter D., Betz H. (2003a). Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40, 785–796 10.1016/S0896-6273(03)00672-X [DOI] [PubMed] [Google Scholar]
- Gomeza J., Ohno K., Hülsmann S., Armsen W., Eulenburg V., Richter D. W., Laube B., Betz H. (2003b). Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40, 797–806 10.1016/S0896-6273(03)00673-1 [DOI] [PubMed] [Google Scholar]
- Granato M., van Eeden F. J. M., Schach U., Trowe T., Brand M., Furutani-Seiki M., Haffter P., Hammerschmidt M., Heisenberg C.-P., Jiang Y.-J., Kane D. A., Kelsh R. N., Mullins M. C., Odenthal J., Nüsslein-Volhard C. (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399–413 [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. (1990a). Cloning and expression of the 58 kd β subunit of the inhibitory glycine receptor. Neuron 4, 963–970 10.1016/0896-6273(90)90149-A [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C.-M., Betz H. (1990b). Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 9, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. (1987). The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328, 215–220 10.1038/328215a0 [DOI] [PubMed] [Google Scholar]
- Grillner S. (2003). The motor infrastructure from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586 10.1038/nrn1137 [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Eisen J. S. (2002). Headwaters of the zebrafish-emergence of a new model vertebrate. Nat. Rev. Genet. 3, 717–724 10.1038/nrg892 [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Kimmel C. B., Westerfield M., Walker C., Streisinger G. (1988). A neural degeneration mutation that spares primary neurons in the zebrafish. Dev. Biol. 126, 115–128 10.1016/0012-1606(88)90245-X [DOI] [PubMed] [Google Scholar]
- Gundlach A. L., Dodd P. R., Grabara C. S. G., Watson W. E. J., Johnston G. A. R., Harper P. A. W., Dennis J. A., Healy P. J. (1988). Deficit of spinal cord glycine/strychnine receptors in inherited myoclonus of poll Hereford calves. Science 241, 1807–1810 10.1126/science.2845573 [DOI] [PubMed] [Google Scholar]
- Guyon J. R., Goswami J., Jun S. J., Thorne M., Howell M., Pusack T., Kawahara G., Steffen L. S., Galdzicki M., Kunkel L. M. (2009). Genetic isolation and characterization of a splicing mutant of zebrafish dystrophin. Hum. Mol. Genet. 18, 202–211 10.1093/hmg/ddn337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P., Granato M., Brand M., Mullins M. C., Hammerschmidt M., Kane D. A., Odenthal J., van Eeden F. J., Jiang Y.-J., Heisenberg C. P., Kelsh R. N., Furutani-Seiki M., Vogelsang E., Beuchle D., Schach U., Fabian C., Nüsslein-Volhard C. (1996). The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123, 1–36 [DOI] [PubMed] [Google Scholar]
- Hall T. E., Bryson-Richardson R. J., Berger S., Jacoby A. S., Cole N. J., Hollway G. E., Berger J., Currie P. D. (2007). The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin α2-deficient congenital muscular dystrophy. Proc. Natl. Acad. Sci. U.S.A. 104, 7092–7097 10.1073/pnas.0700942104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M. C., Sato-Maeda M., Warren J. T., Su F., Lele Z., Krone P. H., Kuwada J. Y, Shoji W. (2000). Laser-induced gene expression in specific cells of transgenic zebrafish. Development 127, 1953–1960 [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Rhee J., Goll M. G., Akitake C. M., Parsons M., Leach S. D. (2008). Gal4/UAS transgenic tools and their application to zebrafish. Zebrafish 5, 97–110 10.1089/zeb.2008.0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein B., Schenkel J., Kuhse J., Besenbeck B., Kling C., Becker C.-M., Betz H., Weiher H. (1996). Low level expression of glycine receptor β subunit transgene is sufficient for phenotype correction in spastic mice. EMBO J. 15, 1275–1282 [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Depner U. B., Wässle H., Ahmadi S., Heindl C., Reinold H., Smart T. G., Harvey K., Schütz B., Abo-Salem O. M., Zimmer A., Poisbeau P., Welzl H., Wolfer D. P., Betz H., Zeilhofer H. U., Müller U. (2004). GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 10.1126/science.1094925 [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Topf M., Harvey K., Rees M. I. (2008). The genetics of hyperekplexia: more than startle! Trends Genet. 24, 439–447 10.1016/j.tig.2008.06.005 [DOI] [PubMed] [Google Scholar]
- Hawkins T. A., Haramis A.-P., Etard C., Prodromou C., Vaughan C. K., Ashworth R., Ray S., Behra M., Holder N., Talbot W. S., Pearl L. H., Strähle U., Wilson S. W. (2008). The ATPase-dependent chaperoning activity of Hsp90a regulates thick filament formation and integration during skeletal muscle myofibrillogenesis. Development 135, 1147–1156 10.1242/dev.018150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S., Mandel G., Fetcho J. R. (2004). Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J. Comp. Neurol. 480, 1–18 10.1002/cne.20278 [DOI] [PubMed] [Google Scholar]
- Higashijima S., Masino M. A., Mandel G., Fetcho J. R. (2003). Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J. Neurophysiol. 90, 3986–3997 10.1152/jn.00576.2003 [DOI] [PubMed] [Google Scholar]
- Higashijima S., Okamoto H., Ueno N., Hotta Y., Eguchi G. (1997). High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev. Biol. 192, 289–299 10.1006/dbio.1997.8779 [DOI] [PubMed] [Google Scholar]
- Hirata H., Saint-Amant L., Downes G., Cui W. W., Zhou W., Li Q., Granato M., Kuwada J. Y. (2005). Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor β-subunit. Proc. Natl. Acad. Sci. U.S.A. 102, 8345–8350 10.1073/pnas.0500862102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Saint-Amant L., Waterbury J., Cui W. W., Zhou W., Li Q., Goldman D., Granato M., Kuwada J. Y. (2004). accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development 131, 5457–5468 10.1242/dev.01410 [DOI] [PubMed] [Google Scholar]
- Hirata H., Watanabe T., Hatakeyama J., Sprague S. M., Saint-Amant L., Nagashima A., Cui W. W., Zhou W., Kuwada J. Y. (2007). Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development 134, 2771–2781 10.1242/dev.004531 [DOI] [PubMed] [Google Scholar]
- Holland K. D., Fleming M. T., Cheek S., Moran J. L., Beier D. R., Meisler M. H. (2006). De novo exon duplication in a new allele of mouse Glra1 (spasmodic). Genetics 174, 2245–2247 10.1534/genetics.106.065532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden M., Devignot V., Goblet C. (2001a). Phylogenetic relationships and chromosomal location of five distinct glycine receptor subunit genes in the teleost Danio rerio. Dev. Genes. Evol. 211, 415–422 10.1007/s004270100164 [DOI] [PubMed] [Google Scholar]
- Imboden M., de Saint Jan D., Leulier F., Korn H., Goblet C., Bregestovski P. (2001b). Isolation and characterization of an alpha2-type zebrafish glycine receptor subunit. Neuroscience 103, 799–810 10.1016/S0306-4522(00)00575-3 [DOI] [PubMed] [Google Scholar]
- Imboden M., Devignot V., Korn H., Goblet C. (2001c). Regional distribution of glycine receptor messenger RNA in the central nervous system of zebrafish. Neuroscience 103, 811–830 10.1016/S0306-4522(00)00576-5 [DOI] [PubMed] [Google Scholar]
- Inbal A., Topczewski J., Solnica-Krezel L. (2006). Targeted gene expression in the zebrafish prechordal plate. Genesis 44, 584–588 10.1002/dvg.20253 [DOI] [PubMed] [Google Scholar]
- Jing L., Lefebvre J. L., Gordon L. R., Granato M. (2009). Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 61, 721–733 10.1016/j.neuron.2008.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. (1987). Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325, 529–531 10.1038/325529a0 [DOI] [PubMed] [Google Scholar]
- Jursky F., Nelson N. (1996). Developmental expression of the glycine transporters GlyT1 and GlyT2 in mouse brain. J. Neurochem. 67, 336–344 [DOI] [PubMed] [Google Scholar]
- Kawakami K., Shima A., Kawakami N. (2000). Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. U.S.A. 97, 11403–11408 10.1073/pnas.97.21.11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Takeda H., Kawakami N., Kobayashi M., Matsuda N., Mishina M. (2004). A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 10.1016/j.devcel.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Kehoe J., Buldakova S., Acher F., Dent J., Bregestovski P., Bradley J. (2009). Aplysia cys-loop glutamate-gated chloride channels reveal convergent evolution of ligand specificity. J. Mol. Evol. 69, 125–141 10.1007/s00239-009-9256-z [DOI] [PubMed] [Google Scholar]
- Kim E. Y., Schrader N., Smolinsky B., Bedet C., Vannier C., Schwarz G., Schindelin H. (2006). Deciphering the structural framework of glycine receptor anchoring by gephyrin. EMBO J. 25, 1385–1395 10.1038/sj.emboj.7601029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kimura Y., Okamura Y., Higashijima S. (2006). alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor curcuits. J. Neurosci. 26, 5684–5697 10.1523/JNEUROSCI.4993-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsmore S. F., Giros B., Suh D., Bieniarz M., Caron M. G., Seldin M. F. (1994). Glycine receptor β-subunit gene mutation in spastic mouse associated with LINE-1 element insertion. Nat. Genet. 7, 136–141 10.1038/ng0694-136 [DOI] [PubMed] [Google Scholar]
- Kirsch J., Wolters I., Triller A., Betz H. (1993). Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature 366, 745–748 10.1038/366745a0 [DOI] [PubMed] [Google Scholar]
- Kirstein L., Silfverskiold B. P. (1958). A family with emotionally precipitated drop seizures. Acta. Psychiatr. Neurol. Scand. 33, 471–476 10.1111/j.1600-0447.1958.tb03533.x [DOI] [PubMed] [Google Scholar]
- Kuhse J., Kuryatov A., Maulet Y., Malosio M. L., Schmieden V., Betz H. (1991). Alternative splicing generates two isoforms of the α2 subunit of the inhibitory glycine receptor. FEBS Lett. 283, 73–77 10.1016/0014-5793(91)80557-J [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. (1990). Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 265, 22317–22320 [PubMed] [Google Scholar]
- Kunkel L. M., Bachrach E., Bennett R. R., Guyon J., Steffen L. (2006). Diagnosis and cell-based therapy for Duchenne muscular dystrophy in human, mice, and zebrafish. J. Hum. Genet. 51, 397–406 10.1007/s10038-006-0374-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A., Laube B., Betz H., Kuhse J. (1994). Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron 12, 1291–1300 10.1016/0896-6273(94)90445-6 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 10.1002/dvdy.21343 [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. (1988). Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl. Acad. Sci. U.S.A. 85, 7394–7398 10.1073/pnas.85.19.7394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen G., Kew J., Alberati D., Borroni E., Heitz M.-P., Moreau J.-L. (2003). Prepulse inhibition deficits of the startle reflex in neonatal ventral hippocampal-lesioned rats: reversal by glycine and a glycine transporter inhibitor. Biol. Psychiatry 54, 1162–1170 10.1016/S0006-3223(03)00374-3 [DOI] [PubMed] [Google Scholar]
- Lefebvre J. L., Jing L., Becaficco S., Franzini-Armstrong C., Granato M. (2007). Differential requirement for MuSK and dystroglycan in generating patterns of neuromuscular innervation. Proc. Natl. Acad. Sci. U.S.A. 104, 2483–2488 10.1073/pnas.0610822104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J. L., Ono F., Puglielli C., Seidner G., Franzini-Armstrong C., Brehm P., Granato M. (2004). Increased neuromuscular activity causes axonal defects and muscular degeneration. Development 131, 2605–2618 10.1242/dev.01123 [DOI] [PubMed] [Google Scholar]
- Legendre P., Korn H. (1994). Glycinergic inhibitory synaptic currents and related receptor channels in the zebrafish brain. Eur. J. Neurosci. 6, 1544–1557 10.1111/j.1460-9568.1994.tb00545.x [DOI] [PubMed] [Google Scholar]
- Legendre P., Korn H. (1995). Voltage dependence of conductance changes evoked by glycine release in the zebrafish brain. J. Neurophysiol. 73, 2404–2412 [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Currie P. D. (2007). Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 8, 353–367 10.1038/nrg2091 [DOI] [PubMed] [Google Scholar]
- Lin S., Gaiano N., Culp P., Burns J. C., Friedmann T., Yee J.-K., Hopkins N. (1994). Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science 265, 666–669 10.1126/science.8036514 [DOI] [PubMed] [Google Scholar]
- Long Q., Meng A., Wang H., Jessen J. R., Farrell M. J., Lin S. (1997). GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development 124, 4105–4111 [DOI] [PubMed] [Google Scholar]
- Luna V. M., Wang M., Ono F., Gleason M. R., Dallman J. E., Mandel G., Brehm P. (2004). Persistent electrical coupling and locomotory dysfunction in the zebrafish mutant shocked. J. Neurophysiol 92, 2003–2009 10.1152/jn.00454.2004 [DOI] [PubMed] [Google Scholar]
- Lynch J. W. (2004). Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 10.1152/physrev.00042.2003 [DOI] [PubMed] [Google Scholar]
- Mahendrasingam S., Wallam C. A., Hackney C. M. (2003). Two approaches to double post-embedding immunogold labeling of freeze-substituted tissue embedded in low temperature Lowicryl HM20 resin. Brain Res. Protoc. 11, 134–141 10.1016/S1385-299X(03)00040-0 [DOI] [PubMed] [Google Scholar]
- Malosio M.-L., Marquéze-Pouey B., Kuhse J., Betz H. (1991). Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 10, 2401–2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino M. A., Fetcho J. R. (2005). Fictive swimming motor patterns in wild type and mutant larval zebrafish. J. Neurophysiol. 93, 3177–3188 10.1152/jn.01248.2004 [DOI] [PubMed] [Google Scholar]
- McDearmid J. R., Liao M., Drapeau P. (2006). Glycine receptors regulate interneuron differentiation during spinal network development. Proc. Natl. Acad. Sci. U.S.A. 103, 9679–9684 10.1073/pnas.0504871103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean D. L., Fan J., Higashijima S., Hale M. E., Fetcho J. R. (2007). A topographic map of recruitment in spinal cord. Nature 446, 71–75 10.1038/nature05588 [DOI] [PubMed] [Google Scholar]
- Meier J. C., Henneberger C., Melnick I., Racca C., Harvey R. J., Heinemann U., Schmieden V., Grantyn R. (2005). RNA editing produces glycine receptor α3P185L, resulting in high agonist potency. Nat. Neurosci. 8, 736–744 10.1038/nn1467 [DOI] [PubMed] [Google Scholar]
- Meng X., Noyes M. B., Zhu L. J., Lawson N. D., Wolfe S. A. (2008). Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat. Biotechnol. 26, 695–701 10.1038/nbt1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G., Kirsch J., Betz H., Langosch D. (1995). Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron 15, 563–572 10.1016/0896-6273(95)90145-0 [DOI] [PubMed] [Google Scholar]
- Mongeon R., Gleason M. R., Masino M. A., Fetcho J. R., Mandel G., Brehm P., Dallman J. E. (2008). Synaptic homeostasis in a zebrafish glial glycine transporter mutant. J. Neurophysiol. 100, 1716–1723 10.1152/jn.90596.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Ujike H., Tanaka Y., Kishimoto M., Okahisa Y., Kotaka T., Harano M., Inada T., Komiyama T., Hori T., Yamada M., Sekine Y., Iwata N., Iyo M., Sora I., Ozaki N., Kuroda S. (2008). The glycine transporter 1 gene (GLYT1) is associated with methamphetamine-use disorder. Am. J. Med. Genet. B 147B, 54–58 10.1002/ajmg.b.30565 [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. (1991). Molecular cloning and characterization of the rat NMDA receptor. Nature 354, 31–37 10.1038/354031a0 [DOI] [PubMed] [Google Scholar]
- Moss S. J., Smart T. G. (2001). Constructing inhibitory synapses. Nat. Rev. Neurosci. 2, 240–250 10.1038/35067500 [DOI] [PubMed] [Google Scholar]
- Mülhardt C., Fischer M., Gass P., Simon-Chazottes D., Guénet J.-L., Kuhse J., Betz H., Becker C.-M. (1994). The spastic mouse: aberrant splicing of glycine receptor β subunit mRNA caused by intronic insertion of L1 element. Neuron 13, 1003–1015 10.1016/0896-6273(94)90265-8 [DOI] [PubMed] [Google Scholar]
- Mullins M. C., Hammerschmidt M., Haffter P., Nüsslein-Volhard C. (1994). Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 4, 189–202 10.1016/S0960-9822(00)00048-8 [DOI] [PubMed] [Google Scholar]
- Nasevicius A., Ekker S. C. (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26, 216–220 10.1038/79951 [DOI] [PubMed] [Google Scholar]
- Neuhauss S. C. F., Biehlmaier O., Seeliger M. W., Das T., Kohler K., Harris W. A., Baier H. (1999). Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 19, 8603–8615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenthal J., Haffter P., Vogelsang E., Brand M., van Eeden F. J., Furutani-Seiki M., Granato M., Hammerschmidt M., Heisenberg C. P., Jiang Y. J., Kane D. A., Kelsh R. N., Mullins M. C., Warga R. M., Allende M. L., Weinberg E. S., Nüsslein-Volhard C. (1996). Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development 123, 103–115 [DOI] [PubMed] [Google Scholar]
- Ono F., Higashijima S., Shcherbatko A., Fetcho J. R., Brehm P. (2001). Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J. Neurosci. 21, 5439–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F., Mandel G., Brehm P. (2004). Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J. Neurosci. 24, 5475–5481 10.1523/JNEUROSCI.0851-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F., Shcherbatko A., Higashijima S., Mandel G., Brehm P. (2002). The zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J. Neurosci. 22, 6491–6498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaisa K. A., Granato M. (2007). Analysis of zebrafish sidetracked mutants reveals a novel role for Plexin A3 in intraspinal motor axon guidance. Development 134, 3251–3257 10.1242/dev.007112 [DOI] [PubMed] [Google Scholar]
- Peters K. G., Rao P. S., Bell B. S., Kindman L. A. (1995). Green fluorescent fusion proteins: powerful tools for monitoring protein expression in live zebrafish embryos. Dev. Biol. 171, 252–257 10.1006/dbio.1995.1276 [DOI] [PubMed] [Google Scholar]
- Peterson R. T., Link B. A., Dowling J. E., Schreiber S. L. (2000). Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. U.S.A. 97, 12965–12969 10.1073/pnas.97.24.12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. T., Shaw S. Y., Peterson T. A., Milan D. J., Zhong T. P., Schreiber S. L., MacRae C. A., Fishman M. C. (2004). Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat. Biotechnol. 22, 595–599 10.1038/nbt963 [DOI] [PubMed] [Google Scholar]
- Pierce K. D., Handford C. A., Morris R., Vafa B., Dennis J. A., Healy P. J., Schofield P. R. (2001). A nonsense mutation in the α1 subunit of the inhibitory glycine receptor associated with bovine myoclonus. Mol. Cell. Neurosci. 17, 354–363 10.1006/mcne.2000.0934 [DOI] [PubMed] [Google Scholar]
- Pietri T., Manalo E., Ryan J., Saint-Amant L., Washbourne P. (2009). Glutamate drives the touch response through a rostral loop in the spinal cord of zebrafish embryos. Dev. Neurobiol. 69, 780–795 10.1002/dneu.20741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda R. H., Heiser R. A., Ribera A. B. (2005). Developmental, molecular, and genetic dissection of Ina in vivo in embryonic zebrafish sensory neurons. J. Neurophysiol. 93, 3582–3593 10.1152/jn.01070.2004 [DOI] [PubMed] [Google Scholar]
- Prugh J. I. P., Kimmel C. B., Metcalfe W. K. (1982). Noninvasive recording of the mauthner neurone action potential in larval zebrafish. J. Exp. Biol. 101, 83–92 [DOI] [PubMed] [Google Scholar]
- Rea R., Tijssen M. A., Herd C., Frants R. R., Kullmann D. M. (2002). Functional characterization of compound heterozygosity for GlyRα1 mutations in the startle disease hyperekplexia. Eur J. Neurosci. 16, 186–196 10.1046/j.1460-9568.2002.02054.x [DOI] [PubMed] [Google Scholar]
- Rees M. I., Harvey K., Pearce B. R., Chung S. K., Duguid I. C., Thomas P., Beatty S., Graham G. E., Armstrong L., Shiang R., Abbott K. J., Zuberi S. M., Stephenson J. B., Owen M. J., Tijssen M. A., van den Maagdenberg A. M., Smart T. G., Supplisson S., Harvey R. J. (2006). Mutations in the gene encoding GlyT2 (SLC6A5) define a presynaptic component of human startle disease. Nat. Genet. 38, 801–806 10.1038/ng1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M. I., Lewis T. M., Kwok J. B. J., Mortier G. R., Govaert P., Snell R. G., Schofield P. R., Owen M. J. (2002). Hyperekplexia associated with compound heterozygote mutations in the β-subunit of the human inhibitory glycine receptor (GLRB). Hum. Mol. Genet. 11, 853–860 10.1093/hmg/11.7.853 [DOI] [PubMed] [Google Scholar]
- Ribera A. B., Nüsslein-Volhard (1998). Zebrafish touch-insensitive mutants reveal an essential role for the developmental regulation of sodium current. J. Neurosci. 18, 9181–9191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. G., Buckwalter M. S., Lynch J. W., Handford C. A., Segura L., Shiang R., Wasmuth J. J., Camper S. A., Schofield P., O'Connell P. (1994). A missense mutation in the gene encoding the α1 subunit of the inhibitory glycine receptor in the spasmodic mouse. Nat. Genet. 7, 131–135 10.1038/ng0694-131 [DOI] [PubMed] [Google Scholar]
- Saint-Amant L., Drapeau P. (1998). Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 37, 622–632 [DOI] [PubMed] [Google Scholar]
- Saint-Amant L., Drapeau P. (2001). Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron 31, 1035–1046 10.1016/S0896-6273(01)00416-0 [DOI] [PubMed] [Google Scholar]
- Saint-Amant L., Sprague S. M., Hirata H., Li Q., Cui W. W., Zhou W., Poudou O., Hume R. I., Kuwada J. Y. (2008). The zebrafish ennui behavioral mutation disrupts acetylcholine receptor localization and motor axon stability. Dev. Neurobiol. 68, 45–61 10.1002/dneu.20569 [DOI] [PubMed] [Google Scholar]
- Sament S., Schwartz M. B. (1957). Severe diabetic stupor without ketosis. S. Afr. Med. J 31, 893–894 [PubMed] [Google Scholar]
- Scheer N., Campos-Ortega J. A. (1999). Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 80, 153–158 10.1016/S0925-4773(98)00209-3 [DOI] [PubMed] [Google Scholar]
- Schneider V. A., Granato M. (2006). The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron 50, 683–695 10.1016/j.neuron.2006.04.024 [DOI] [PubMed] [Google Scholar]
- Schredelseker J., Dayal A., Schwerte T., Franzini-Armstrong C., Grabner M. (2009). Proper restoration of excitation-contraction coupling in the dihydropyridine receptor β1-null zebrafish relaxed is an exclusive function of the β1a subunit. J. Biol. Chem. 284, 1242–1251 10.1074/jbc.M807767200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J., Di Biase V., Obermair G. J., Felder E. T., Flucher B. E., Franzini-Armstrong C., Grabner M. (2005). The β1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 102, 17219–17224 10.1073/pnas.0508710102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E. K., Mason L., Arrenberg A. B., Ziv L., Gosse N. J., Xiao T., Chi N. C., Asakawa K., Kawakami K., Baier H. (2007). Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat. Methods 4, 323–326 [DOI] [PubMed] [Google Scholar]
- Sepich D. S., Wegner J., O'Shea S., Westerfield M. (1998). An altered intron inhibits synthesis of the acetylcholine receptor α-subunit in the paralayzed zebrafish mutant nic1. Genetics 148, 361–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R., Ryan S. G., Zhu Y.-Z., Hahn A. F, O'Connell P., Wasmuth J. J. (1993). Mutations in the α1 subunit of the inhibitory glycine receptor cause the dominant neurologic disorder, hyperekplexia. Nat. Genet. 5, 351–357 10.1038/ng1293-351 [DOI] [PubMed] [Google Scholar]
- Sidi S., Friedrich R. W., Nicolson T. (2003). NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301, 96–99 10.1126/science.1084370 [DOI] [PubMed] [Google Scholar]
- Sivasubbu S., Balciunas D., Davidson A. E., Pickart M. A., Hermanson S. B., Wangensteen K. J., Wolbrink D. C., Ekker S. C. (2006). Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech. Dev. 123, 513–529 10.1016/j.mod.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Sola M., Bavro V. N., Timmins J., Franz T., Ricard-Blum S., Schoehn G., Ruigrok R. W. H., Paarmann I., Saiyed T., O'Sullivan G. A., Schmitt B., Betz H., Weissenhorn W. (2004). Structural basis of dynamic glycine receptor clustering by gephyrin. EMBO J. 23, 2510–2519 10.1038/sj.emboj.7600256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen L. S., Guyon J. R., Vogel E. D., Howell M. H., Zhou Y., Weber G. J., Zon L. I., Kunkel L. M. (2007). The zebrafish runzel muscular dystrophy is linked to the titin gene. Dev. Biol. 309, 180–192 10.1016/j.ydbio.2007.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern H. M., Murphey R. D., Shepard J. L., Amatruda J. F., Straub C. T., Pfaff K. L., Weber G., Tallarico J. A., King R. W., Zon L. I. (2005). Small molecules that delay S phase suppress a zebrafish bmyb mutant. Nat. Chem. Biol. 1, 366–370 10.1038/nchembio749 [DOI] [PubMed] [Google Scholar]
- Streisinger G., Walker C., Dower N., Knauber D., Singer F. (1981). Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293–296 10.1038/291293a0 [DOI] [PubMed] [Google Scholar]
- Stuart G. W., McMurray J. V., Westerfield M. (1988). Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development 103, 403–412 [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Vielkind J. R., McMurray J. V., Westerfield M. (1990). Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development 109, 577–584 [DOI] [PubMed] [Google Scholar]
- Tanaka H., Maeda R., Shoji W., Wada H., Masai I., Shiraki T., Kobayashi M., Nakayama R., Okamoto H. (2007). Novel mutations affecting axon guidance in zebrafish and a role for plexin signaling in the guidance of trigeminal and facial nerve axons. Development 134, 3259–3269 10.1242/dev.004267 [DOI] [PubMed] [Google Scholar]
- Tanimoto M., Ota Y., Horikawa K., Oda Y. (2009). Auditory input to CNS is acquired coincidentally with development of inner ear after formation of functional afferent pathway in zebrafish. J. Neurosci. 29, 2762–2767 10.1523/JNEUROSCI.5530-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M., Seburn K. L., Popko B. (2006). Nmf1 is a novel ENU-induced mutation in the mouse glycine receptor alpha1 subunit. Mamm. Genome 17, 950–955 10.1007/s00335-006-0020-z [DOI] [PubMed] [Google Scholar]
- Tsai G., Ralph-Williams R. J., Martina M., Bergeron R., Berger-Sweeney J., Dunham K. S., Jiang Z., Caine S. B., Coyle J. T. (2004). Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. U.S.A. 101, 8485–8490 10.1073/pnas.0402662101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 10.1002/dvdy.21354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Jao L.-E., Zheng N., Dolan K., Ivey J., Zonies S., Wu X., Wu K., Yang H., Meng Q., Zhu Z., Zhang B., Lin S., Burgess S. M. (2007). Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc. Natl. Acad. Sci. U.S.A. 104, 12428–12433 10.1073/pnas.0705502104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. S., Buckinx R., Lecorronc H., Mangin J. M., Rigo J. M., Legendre P. (2007). Mechanisms for picrotoxinin and picrotin blocks of α2 homomeric glycine receptors. J. Biol. Chem. 282, 16016–16035 10.1074/jbc.M701502200 [DOI] [PubMed] [Google Scholar]
- Wang M., Wen H., Brehm P. (2008). Function of neuromuscular synapses in the zebrafish choline-acetyltransferase mutant bajan. J. Neurophysiol. 100, 1995–2004 10.1152/jn.90517.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E., Akagi H. (1995). Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci. Res. 23, 377–382 10.1016/0168-0102(95)00972-V [DOI] [PubMed] [Google Scholar]
- Westerfield M., Liu D. W., Kimmel C. B., Walker C. (1990). Pathfinding and synapse formation in a zebrafish mutant lacking functional acetylcholine receptors. Neuron 4, 867–874 10.1016/0896-6273(90)90139-7 [DOI] [PubMed] [Google Scholar]
- Wienholds E., Schulte-Merker S., Walderich B., Plasterk R. H. (2002). Target-selected inactivation of the zebrafish rag1 gene. Science 297, 99–102 10.1126/science.1071762 [DOI] [PubMed] [Google Scholar]
- Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005). Crystal structure of a bacterial homologue of Na+/Cl–-dependent neurotransmitter transporters. Nature 437, 215–223 10.1038/nature03978 [DOI] [PubMed] [Google Scholar]
- Yang Z., Cromer B. A., Harvey R. J., Parker M. W., Lynch J. W. (2007). A proposed structural basis for picrotoxinin and picrotin binding in the glycine receptor pore. J. Neurochem. 103, 580–589 10.1111/j.1471-4159.2007.04850.x [DOI] [PubMed] [Google Scholar]
- Zafra F., Aragón C., Olivares L., Danbolt N. C., Giménez C., Storm-Mathisen J. (1995). Glycine transporters are differentially expressed among CNS cells. J. Neurosci. 15, 3952–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J., Granato M. (1999). The zebrafish diwanka gene controls an early step of motor growth cone migration. Development 126, 3461–3472 [DOI] [PubMed] [Google Scholar]
- Zeller J., Schneider V., Malayaman S., Higashijima S., Okamoto H., Gui J., Lin S., Granato M. (2002). Migration of zebrafish spinal motor nerves into the periphery requires multiple myotome-derived cues. Dev. Biol. 252, 241–256 10.1006/dbio.2002.0852 [DOI] [PubMed] [Google Scholar]
- Zhang J., Granato M. (2000). The zebrafish unplugged gene controls motor axon pathway selection. Development 127, 2099–2111 [DOI] [PubMed] [Google Scholar]
- Zhang J., Lefebvre J. L., Zhao S., Granato M. (2004). Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat. Neurosci. 7, 1303–1309 10.1038/nn1350 [DOI] [PubMed] [Google Scholar]
- Zhou W., Saint-Amant L., Hirata H., Cui W. W., Sprague S. M., Kuwada J. Y. (2006). Non-sense mutations in the dihydropyridine receptor β1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium 39, 227–236 10.1016/j.ceca.2005.10.015 [DOI] [PubMed] [Google Scholar]