Abstract
Autoreactive T cell responses have a crucial role in central nervous system (CNS) diseases such as multiple sclerosis. Recent data indicate that CNS autoimmunity can be mediated by two distinct lineages of CD4+ T cells that are defined by the production of either interferon-γ or interleukin-17. The activity of these CD4+ T cell subsets within the CNS influences the pathology and clinical course of disease. New animal models show that myelin-specific CD8+ T cells can also mediate CNS autoimmunity. This Review focuses on recent progress in delineating the pathogenic mechanisms, regulation and interplay between these different T cell subsets in CNS autoimmunity.
Autoimmune T cell responses directed against antigens that are derived from the central nervous system (CNS) are thought to trigger several diseases, including multiple sclerosis, neuromyelitis optica and acute disseminated encephalomyelitis. Multiple sclerosis is the most common of these diseases, affecting more than one million people worldwide1. Multiple sclerosis is thought to occur in genetically predisposed individuals following exposure to an environmental trigger that activates myelin- specific T cells, which allows the T cells to cross the blood–brain barrier. Reactivation of the T cells by CNS-resident antigen-presenting cells (APCs) that present myelin antigens triggers the recruitment of innate immune cells, which have important roles in mediating demyelination and axonal damage. Immune cell infiltrates and plaques of demyelination in the brain and spinal cord are hallmark features of multiple sclerosis; however, extensive heterogeneity in the clinical symptoms, disease course and detailed pathological features is seen among patients (BOX 1). This suggests that many pathways involving distinct effector mechanisms can lead to chronic autoimmune disease in the CNS2.
Box 1. The complexity of autoimmune diseases affecting the CNS.
The diverse clinical and pathological outcomes seen in patients with multiple sclerosis suggest that T cell responses in the central nervous system (CNS) are complex. Clinical signs that are associated with multiple sclerosis include ataxia, loss of coordination, hyperreflexia, spasticity, visual and sensory impairment, fatigue and cognitive difficulties1. However, the severity, frequency, specific clinical symptoms and CNS pathology vary greatly among patients with this disease, and the basis for this variation is not understood. Approximately 85% of patients with multiple sclerosis have a relapsing-remitting form of the disease, which usually converts over years into a progressive disease that is characterized by severe neurological deterioration. 10–15% of patients with multiple sclerosis follow a primary progressive disease course in which continuous neurological deterioration occurs following the initial episode. A small percentage of patients develop severe disease that leads to extreme disability or death after only months. The reasons underlying these different disease courses are not clear. Most patients develop lesions in the brain or in both the brain and spinal cord, which comprise T cells, macrophages, activated microglial cells and other inflammatory cells. By contrast, a small percentage of patients develop lesions in the spinal cord but not in the brain.
The mechanisms that determine localization of lesions in patients with multiple sclerosis are not known. Recent studies of CD4+ T cell-mediated experimental autoimmune encephalomyelitis (EAE) suggest that the local cytokine milieu generated by infiltrating T cells determines the region in which inflammatory cells localize. The pathology observed in the brains of patients with multiple sclerosis can be classified into distinct patterns depending on whether deposition of IgG and complement is observed, whether oligodendrocytes are spared or die by apoptosis or necrotic cell death and whether lesions and myelin loss occur at perivenous sites2. The mechanisms that are responsible for these patterns are not yet well defined. Importantly, all lesions in an individual seem to be of only one pattern, implying that multiple pathways involving distinct pathogenic mechanisms can lead to clinical multiple sclerosis. The remarkable heterogeneity seen in patients with this disease poses substantial challenges in developing appropriate animal models that reproduce this diversity.
Multiple sclerosis has been widely studied using the animal model experimental autoimmune encephalomyelitis (EAE). EAE is induced by immunization with myelin-derived antigens in adjuvant or by the adoptive transfer of activated myelin-specific T cells. The inflammatory infiltrates and demyelination seen in EAE have many similarities to the pathology of multiple sclerosis3. The ability to induce EAE by adoptive transfer of myelin-specific T cells reinforced the idea that multiple sclerosis is an autoimmune disease and spawned extensive investigation of autoimmune T cell responses in the CNS. Recently, our understanding of how T cells mediate EAE has greatly broadened, reflecting recent progress in elucidating distinct T cell phenotypes and their effector mechanisms, as well the development of new animal models. In this Review, I discuss the activation, infiltration, antigen specificity, pathogenicity and regulation of different T cell subsets in the CNS, all of which are crucial parameters in designing therapeutic strategies for autoimmune diseases in the CNS.
Infiltration and reactivation of T cells
To initiate CNS inflammation, myelin-specific T cells must be activated in the periphery, gain access to the CNS and then be reactivated by APCs presenting self antigen. T cell reactivation triggers the production of soluble mediators by many cell types that recruit other inflammatory cells. The anatomy of the CNS poses unique challenges to this process because it is protected from cellular infiltration by the blood–brain barrier that surrounds parenchymal venules and by the blood–cerebrospinal fluid (CSF) barrier that surrounds the choroid plexus (where CSF is synthesized) and meningeal venules. Tight junctions between the endothelial cells of the blood–brain barrier and the epithelial cells of the blood–CSF barrier limit access to the CNS by circulating cells. However, activated and memory T cells can carry out immune surveillance of the CNS because they express adhesion molecules, chemokine receptors and integrins that allow them to cross these barriers4.
The preferential ability of myelin-specific T cells to infiltrate the CNS implies that these T cells are first activated in the periphery before entering the CNS. Myelin basic protein (MBP) is unique in that it is a component of both peripheral and central myelin, and MBP presented within all lymphoid tissues can be detected by MBP-specific T cells5,6. Accordingly, inflammation in peripheral nerve roots in EAE is induced by MBP-specific but not other myelin-specific T cells7. It is not known how T cells that are specific for other CNS myelin proteins are activated in the periphery because these proteins are synthesized by oligodendrocytes that reside only in the CNS. Nevertheless, mice expressing a transgenic T cell receptor (TCR) specific for either proteolipid protein (PLP; another myelin-derived protein) or MBP develop spontaneous EAE, and the PLP- and MBP-specific T cell responses seem to be activated first in the CNS-draining cervical lymph nodes8,9. This suggests that some CNS myelin antigens are constitutively presented in cervical lymph nodes and, under some conditions, can trigger T cell activation. The conditions that cause peripheral APCs to present myelin epitopes to naive T cells in an immunogenic context have not yet been defined. Alternatively, myelin-specific T cell activation could be the result of molecular mimicry (see later), although specific pathogens have not yet been conclusively identified as triggers of multiple sclerosis.
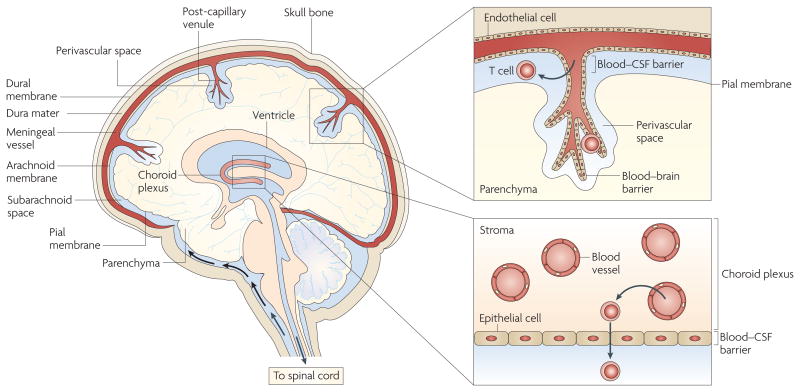
Activated T cells enter distinct CNS environments depending on their route of entry. T cells that cross the blood–CSF barrier enter the region between the arachnoid and pial membrane where the CSF circulates, known as the subarachnoid space (SAS)4. T cells that cross the blood–brain barrier enter the perivascular space that separates the basement membrane attached to the endothelial cells of the vessel from a second membrane known as the glial limitans, which comprises astrocyte feet and microglial cells (FIG. 1). In the absence of inflammation, however, endothelial cells of the brain do not express the adhesion molecules that are necessary for activated T cells to adhere to the vessel wall4,10. Therefore, immune surveillance has been proposed to occur primarily in the SAS by T cells that cross the blood–CSF barrier, where selectins and adhesion molecules are constitutively expressed4. Interestingly, the expression of CC-chemokine receptor 6 (CCR6) by a subset of pathogenic T cells has been shown to have an important role in facilitating T cell entry into the CNS in EAE11. The ligand for CCR6, CC-chemokine ligand 20 (CCL20), is constitutively expressed by epithelial cells of the choroid plexus in mice and humans, which is consistent with the idea that T cells first cross the blood–CSF barrier to initiate EAE.
Figure 1. Anatomical organization of the brain showing the possible routes of activated T cell entry.
Activated T cells can enter the subarachnoid space by migrating from blood vessels into the stroma of the choroid plexus and then crossing the blood–cerebrospinal fluid (CSF) barrier surrounding the choroid plexus stroma, which comprises epithelial cells joined by tight junctions. Activated T cells can also enter the subarachnoid space by extravasating through the cell wall of meningeal venules, which consists of endothelial cells connected by tight junctions. In addition, activated T cells can cross the blood–brain barrier surrounding post-capillary venules that penetrate the brain parenchyma, which comprises endothelial cells connected by tight junctions. T cells crossing the blood–brain barrier enter the perivascular space, which is the region between the basement membrane connected to the blood vessel endothelial cells and the glial limitans (which is composed of astrocyte feet and microglial cell; not shown).
Inflammation in EAE is detected first in the SAS, again consistent with this being the initial site of T cell infiltration12. Recent imaging studies confirm that the SAS is the first site where CD4+ T cells that had been previously activated in the periphery are reactivated by MHC class II+ APCs, and the recognition of cognate ligand results in T cell proliferation and the formation of large T cell aggregates in the SAS13. This reactivation is followed by the activation of perivascular endothelial cells, which allows the recruitment of T cells into the perivascular space. Although the details of this two-step process of inducing CNS inflammation are still poorly understood, one study suggested that T cell activation in the SAS triggers both activation of microglial cells that lie beneath the pial membrane and local axonal damage, leading to Wallerian degeneration. This causes distal activation of microglial cells and upregulation of adhesion molecules on parenchymal vasculature that is remote from the initial site of T cell infiltration in the SAS14 (FIG. 2).
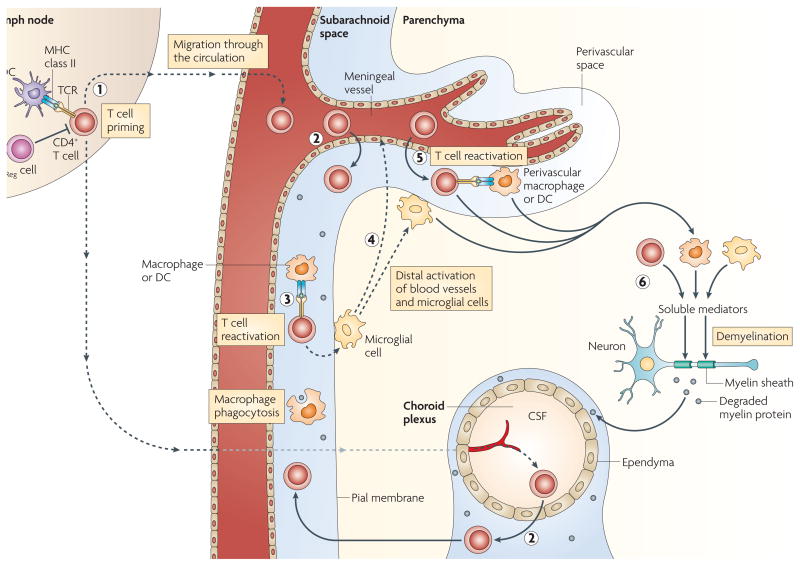
Figure 2. Peripheral and CnS activation of myelin-specific CD4+ T cells.
CD4+ T cells are primed in the periphery by dendritic cells (DCs) presenting myelin (or myelin crossreactive) epitopes. Antigen-presenting cells (APCs) residing in the central nervous system (CNS) can capture myelin antigens in situ and migrate to the cervical lymph nodes. Alternatively, soluble myelin antigens can drain from the CNS to lymph nodes to be phagocytosed by local APCs (1). CD4+ T cells enter the subarachnoid space by crossing the blood–cerebrospinal fluid (CSF) barrier either in the choroid plexus or the meningeal venules (2); the T cells are re-activated within the subarachnoid space by MHC class II-expressing macrophages and DCs expressing myelin epitopes (3). Reactivated T cells activate microglial cells in the subpial region, triggering activation of distal microglial cells and blood vessels (4). Activated T cells adhere to and cross the activated blood–brain barrier, enter the perivascular space and are reactivated by perivascular macrophages and DCs (5). T cells enter the parenchyma and, together with activated macrophages and microglial cells, secrete soluble mediators that trigger demyelination (6). TReg, regulatory T.
In addition to chemokine production, tumour necrosis factor (TNF) signalling seems to have an important role in triggering the subsequent migration of CD4+ T cells from the perivascular space into the parenchyma15. It has also been reported that it is the strength of T cell reactivation that determines the degree of ensuing parenchymal inflammation and not the ability of the T cells to infiltrate the perivascular space16. How strongly a particular T cell is reactivated depends on both the affinity of the TCR for its cognate ligand (a myelin epitope presented by an MHC molecule) and on the number of specific myelin epitope–MHC complexes available on the surface of APCs. The increased inflammation associated with the presence of strongly reactivated T cells in the SAS has been attributed to increased chemokine production triggered by the T cells. However, the higher expression of the death-inducing receptor programmed cell death 1 (PD1; also known as PCD1) by weakly activated compared with strongly activated T cells17 could also result in increased cell death of the weakly activated T cells. Thus, reactivation of T cells within the SAS seems to initiate inflammation and promote entry of T cells into the perivascular space, but the degree of reactivation may influence the amount of parenchymal inflammation that ensues.
Similarly to CD4+ T cells, CD8+ T cell-mediated CNS inflammation can also originate in the SAS. Inflammatory cells recruited by CD8+ T cells that are responding to lymphocytic choriomeningitis virus infection appear first in the SAS even though the virus is inoculated into the brain parenchyma18. However, the mechanisms of CD8+ and CD4+ T cell infiltration into the CNS differ, which may account for the preferential recruitment of CD8+ T cells into the brain that was observed in two different animal models19,20. Intravital microscopy showed that CD8+ T cells isolated from patients with multiple sclerosis adhered more strongly to inflamed mouse meningeal brain venules than either CD4+ T cells from patients with multiple sclerosis or CD8+ T cells from healthy controls21. The adherence of CD8+ T cells to the venules depended on their expression of P-selectin glycoprotein ligand 1 (PSGL1), the receptor for which is constitutively expressed in choroid plexus microvessels. By contrast, CD4+ T cells from patients with multiple sclerosis that were experiencing acute attacks upregulated the expression of vascular cell adhesion molecule 1 rather than PSGL1 (REF. 21). The development of new lesions in the CNS also correlated with the expression of CCR5 and CXC-chemokine receptor 3 (CXCR3) by peripheral CD8+ rather than CD4+ T cells22, suggesting that CD8+ T cells may contribute at this stage to the pathogenesis of CNS autoimmunity.
CD4+ T cell responses in the CNS
Antigen specificity
Myelin-specific CD4+ rather than CD8+ T cells are the primary mediators in most models of EAE because the induction protocol favours the activation of MHC class II-restricted T cells. The similarities between CD4+ T cell-mediated EAE and multiple sclerosis and the strong association of certain MHC class II molecules with genetic susceptibility to multiple sclerosis23 focused studies on the activity of the CD4+ T cells in EAE. MBP and PLP are the most abundant proteins in the myelin sheath in the CNS and were the first to be identified as CD4+ T cell targets in the initial subset of mouse strains that exhibited susceptibility to EAE. Additional mouse strains showed susceptibility to EAE when less abundant myelin proteins, such as myelin oligodendrocyte glycoprotein (MOG), were cloned and the recombinant proteins were used as immunogens. The difference in susceptibility to EAE between mouse strains correlated with their MHC haplotype, which is similar to the association of certain MHC class II alleles with susceptibility to multiple sclerosis.
Susceptibility to EAE requires not only the expression of an MHC class II molecule that can bind at least one peptide derived from a myelin antigen, but also that some myelin-specific T cells escape central tolerance so that they are available for activation in the periphery. Central tolerance is the process in which T cells that exhibit high avidity interactions with APCs presenting self antigen–MHC complexes in the thymus are deleted. T cell avidity is a function of both the affinity of the TCR for the self antigen–MHC complex and the abundance of these complexes on the surface of the APC. Therefore, central tolerance can be escaped by thymocytes that express a TCR with low affinity for a myelin peptide–MHC complex and by thymocytes that recognize a myelin peptide that does not bind well to the MHC molecule such that few specific peptide–MHC complexes are generated. One example of this is seen in B10.PL mice, in which the MBP peptide 121–140 (MBP121–140) forms a complex with the MHC molecule I-Au with a half-life of 177 hours and induces tolerance, whereas MBPAc1–11 forms an unstable complex with I-Au with a half-life of 15–30 minutes and fails to induce tolerance24. T cells that are specific for PLP139-151 also escape central tolerance because this epitope is contained in a transcript that is expressed at low levels in the thymus compared with a differentially spliced transcript that lacks this epitope and is expressed at much higher levels25,26. The observation that many myelin proteins are expressed in the thymus and mediate central tolerance suggests that myelin-specific T cells that escape to the periphery are mostly T cells with low avidity for self antigen27.
The low avidity of these T cells for self antigen in the thymus normally prevents them from engaging self antigen in the periphery and allows them to circulate in a state of ‘ignorance’. However, the avidity of T cells for self antigen can be increased under certain circumstances. Specifically, APCs responding to an immunogenic stimulus, such as an infection, increase their expression of co-stimulatory molecules and MHC complexes on the cell surface, and these changes can result in an increase in the avidity of the interaction between the APC and self-reactive T cells, such that the T cells can now respond to the self antigen that they previously ignored27.
Interestingly, elimination of high avidity MBP-specific T cells by central tolerance accounts for why immunization with MBP induces inflammation only in the CNS and proximal peripheral nervous system, where the concentration of MBP is higher than in peripheral tissues. High avidity MBP-specific T cells isolated from MBP-deficient mice can induce severe inflammation in virtually all innervated tissues28, presumably because they can respond to lower concentrations of MBP in peripheral tissues, where MBP is a smaller component of the myelin sheath and the density of myelin fibres is lower than in the CNS. However, these high avidity MBP-specific T cells would not be activated by immunization with MBP in wild-type mice because they are normally deleted in the thymus and therefore are not part of the peripheral T cell repertoire. The fact that MBP-specific T cells that are present in the periphery target only the CNS and peripheral nerve roots is consistent with a requirement for a high concentration of MBP–MHC complexes for T cell reactivation owing to lower avidity for antigen.
Numerous studies have compared the frequencies of myelin-specific T cells in patients with multiple sclerosis and healthy controls to evaluate their relevance in disease. Most studies found no differences in the frequencies of myelin-specific T cells. However, a higher frequency of T cells with a more pro-inflammatory phenotype and different epitope specificities was observed in patients with multiple sclerosis compared with healthy individuals when the T cells were stimulated in vitro with a low concentration of myelin antigen29. The authors of this study suggested that this result may reflect an increased frequency of high avidity T cells in patients with multiple sclerosis because T cells with the highest avidity for antigen are selectively expanded at low antigen concentrations. Alternatively, as memory T cells respond to lower doses of antigen than do naive T cells, this finding could reflect the previously described increased frequency of memory but not naive myelin-specific T cells in patients with multiple sclerosis compared with healthy controls30–33.
A higher frequency of T cells expressing degenerate TCRs in patients with multiple sclerosis has also been reported34. Although a direct connection between TCR degeneracy and pathogenicity has not yet been shown, this observation has interesting implications for the molecular mimicry hypothesis, in which T cells specific for self antigens are activated because of cross-reactivity with microbial epitopes35,36. Several studies have described degenerate TCRs that recognize both myelin antigens and pathogen-derived epitopes37–41. According to the molecular mimicry hypothesis, the more T cells with this type of degenerate recognition an individual has, the greater the chance that T cells activated in response to a specific pathogen will initiate an auto-immune response against a myelin antigen. Interestingly, the incidence of spontaneous EAE that develops in one model of MBP-specific TCR-transgenic mice increases as the level of microbial exposure increases42; however, it is not known whether this results from cross-reactivity of the MBP-specific TCR with environmental microorganisms. Intriguingly, when these MBP-specific TCR transgenic mice were co-housed in a specific- pathogen-free or in a conventional animal facility with transgenic mice expressing a different MBP-specific TCR that recognizes the same MBP peptide–MHC complex on the same genetic background, the incidence of spontaneous EAE increased for the TCR transgenic mice in the conventional facility compared with those housed in specific-pathogen-free conditions, but remained the same for the second TCR transgenic model43. This raises the possibility that the MBP-specific TCR in the first model exhibited greater degeneracy in its recognition of antigen. Interestingly, many T cells specific for MOG35–55–I-Ab complexes have recently been shown to crossreact with the neuronal cytoskeletal self antigen neurofilament M18–30 (REF. 44). This TCR degeneracy may enhance the ability of the MOG-specific T cells to induce CNS autoimmunity by increasing the concentration of self antigen within the CNS that is recognized by these T cells.
CD4+ T cell effector function in CNS autoimmunity
Until recently, the primary effector T cell in the pathology of both EAE and multiple sclerosis was thought to be a CD4+ T helper 1 (TH1) cell, which requires interleukin-12 (IL-12) for its differentiation and is characterized by the secretion of interferon-γ (IFNγ), IL-2 and TNF. This view stemmed in part from the finding that increased clinical activity in multiple sclerosis correlated with the expression of IFNγ and IL-12 in the CNS and CSF45. Furthermore, multiple sclerosis was exacerbated by the administration of IFNγ46. In EAE, a key role for TH1 cells was also supported by the secretion of IFNγ by CNS-infiltrating T cells, the detection of IL-12p40 in inflammatory lesions and the ability to induce disease by adoptive transfer of TH1 cells47,48. IFNγ induces MHC class II expression in the CNS, triggers the production of chemokines that attract macrophages and monocytes and activates macrophage function, which is consistent with the idea that TH1 cell-mediated responses establish pro-inflammatory responses in the CNS (FIG. 3).
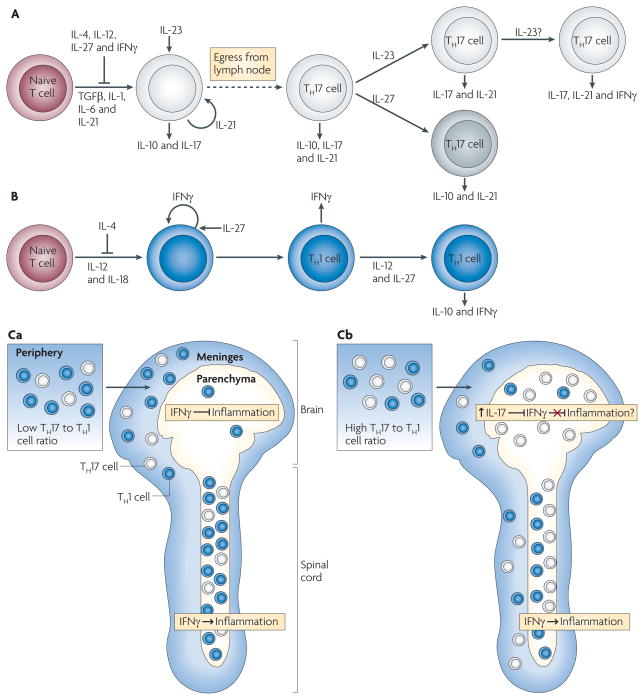
Figure 3. Model of TH1 and TH17 cell differentiation.
A. | Naive T cells differentiate in the presence of transforming growth factor-β (TGFβ), interleukin-1 (IL-1), IL-6 and IL-21 into an early T helper 17 (TH17) cell stage that also produces IL-10 and IL-17. IL-4, IL-12, IL-27 and interferon-γ (IFNγ) inhibit TH17 cell differentiation, and IL-21 acts in an autocrine manner at this stage. IL-23 promotes TH17 cell differentiation, decreases IL-10 production and stabilizes the IL-17-producing phenotype. IL-23 may facilitate co-expression of IFNγ under some circumstances. IL-27 can suppress IL-17 and induce IL-10 production by TH17 cells. B | Differentiation of TH1 cells occurs in the presence of IL-12 and IL-18, with IFNγ acting in an autocrine manner. TH1 cell differentiation is inhibited by IL-4, and IL-27 can enhance IFNγ production by differentiating TH1 cells. Exposure to IL-12 and IL-27 induces differentiated TH1 cells to produce IL-10 and IFNγ. Differentiated TH17 and TH1 cells express other pro-inflammatory cytokines (not depicted). Ca | Myelin-specific T cells with a low TH17 to TH1 cell ratio are primed in the periphery and infiltrate the meninges of both the brain and spinal cord. The T cells enter the spinal cord parenchyma and induce inflammation in an IFNγ-mediated signalling-dependent manner. The few T cells that enter the brain parenchyma, where IFNγ-mediated signalling inhibits inflammation, do not recruit inflammatory cells. Cb | Myelin-specific T cells primed in the periphery with a high TH17 to TH1 cell ratio infiltrate the meninges and parenchyma of both the brain and spinal cord. The higher TH17 to TH1 cell ratio in the brain is associated with a disproportionate increase in IL-17 production compared with the spinal cord, which may overcome the inhibitory IFNγ-mediated signalling in the brain parenchyma.
Over the past decade, however, numerous observations have led us to question this simple paradigm. Most striking were the discoveries that mice deficient in IL-12, IFNγ and TNF develop severe EAE49. By contrast, IL-23-deficient mice are completely resistant to EAE50. IL-23 is crucial for the development of pathogenic TH17 cells51, a distinct T cell lineage that is characterized by the production of IL-17A, IL-17F and IL-22 (REF. 52). Research efforts then focused on TH17 cells, the differentiation of which depends on signalling mediated by transforming growth factor-β (TGFβ), IL-6, IL-1 (REF. 146) and autocrine activity of IL-21 (REF. 52). The ability of TH17 cells to produce the pro-inflammatory cytokines IL-17A and IL-7F, and their ability to either directly synthesize or induce other cell types to produce many pro-inflammatory mediators such as IL-6, granulocyte/macrophage colony-stimulating factor (GM-CSF), matrix metalloproteinases and CXC chemokines including CXCL8 (a potent neutrophil chemoattractant), suggest that TH17 cells can contribute to inflammation in the CNS.
Support for a pathogenic role for TH17 cells comes from studies of patients with multiple sclerosis and from mouse models of EAE. Increased numbers of IL-17 transcripts are detected in chronic multiple sclerosis lesions compared with either acute lesions or control tissue from individuals without CNS pathology53. Moreover, transfer of TH17 cells seemed to induce more severe EAE compared with transfer of TH1 cells. Furthermore, neutralizing IL-17 activity ameliorated EAE, thereby strengthening the notion that TH17 cells are the ‘true’ effector cells in CNS autoimmunity54–57. Recent studies have suggested that T cells that co-express IL-17 and IFNγ may be crucial in EAE pathogenesis58,59; however, the role of these dual cytokine-producing T cells has not been well characterized. Surprisingly, the requirement for IL-17 in EAE is not the same as that for IL-23. Although Il23−/− mice are completely resistant to EAE, Il17a−/− and Il17f −/− mice are still susceptible to EAE, and the severity of disease that was observed when IL-17 was neutralized in vivo was sometimes56,60, but not always54,61, mild. IL-17F has approximately 50% sequence homology with IL-17A and has a similar expression pattern; however, no decrease in the incidence of EAE or strong differences in EAE severity were observed when IL-17A was neutralized in Il17f −/− mice compared with wild-type mice, nor was disease exacerbated in mice that overexpressed Il17a specifically in CD4+ T cells60.
On the basis of the studies described above, it is difficult to attribute a sole pathogenic role to either TH1 or TH17 cells. Indeed, several recent studies indicate that both T cell subsets can induce autoimmunity in the CNS. Both TH1 and TH17 cells can induce experimental autoimmune uveitis depending on the conditions under which the T cells were primed62. EAE can also be induced by the adoptive transfer of either TH1 or TH17 cells63,64. Consistent with the different chemotactic activities that are triggered by IFNγ and IL-17, macrophages predominated in the infiltrates of TH1 cell-induced disease, whereas neutrophils were recruited by TH17 cells64. Finally, one recent study using both cell subsets found that only TH1 and not TH17 cells could induce EAE65. These disparate outcomes reported by different laboratories on the role of TH1 and TH17 cells in EAE could in part reflect different protocols for generating TH1 and TH17 cells, as well as the use of different strains of mice and antigens. In addition, the definition of these cells is still evolving, as it has become clear that TH1 and TH17 cells differ functionally in many ways apart from just their secretion of IFNγ and IL-17, respectively. Given that neither IFNγ nor IL-17 seem to be absolutely required for the pathology of EAE, other functional differences between these T cell subsets that remain to be identified could be more relevant to the pathology of EAE. Thus, the lack of consensus on the relative pathogenicity of TH1 and TH17 cells in EAE probably reflects our limited understanding of the crucial functions that are mediated by CD4+ T cells in the pathogenesis of CNS autoimmunity. Adding to the complexity, a separate T cell lineage that expresses γδ+ TCRs has also been implicated in CNS autoimmunity (BOX 2).
Box 2. γδ cells in CNS autoimmunity.
γδ T cells have been implicated in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) by many studies, but their precise role remains unclear. Their potential importance in autoimmunity in the central nervous system (CNS) is suggested by the finding that γδ T cells accumulate in multiple sclerosis plaques and that they show evidence of oligoclonal expansion, which is consistent with their involvement in antigen-specific responses (reviewed in REF. 130). A regulatory role for γδ T cells in EAE was suggested by studies in which antibody- mediated depletion of γδ T cells resulted in exacerbated disease and because γδ T cell-deficient mice show impaired EAE remission131. By contrast, many studies suggest a pathogenic role for γδ T cells in EAE. Initial studies showed that γδ T cell-deficient mice exhibited milder EAE, and depletion of γδ T cells was reported to decrease EAE severity by dampening the production of pro-inflammatory cytokines and chemokines130. Interestingly, numerous IL-17-producing host T cells are recruited to the CNS during EAE that is induced by the adoptive transfer of T helper 1 (TH1) cells, and approximately 60% of these interleukin-17 (IL-17)+ cells were γδ T cells132. The IL-17 secretion by recipient T cells influenced the pathogenesis of disease, as EAE was ameliorated when the TH1 cells were transferred into Il17−/− mice132. The role of γδ T cells in CNS autoimmunity needs further investigation to delineate the circumstances under which γδ T cells can be regulatory or pathogenic, particularly if future therapeutic strategies target this T cell subset.
CNS region-specific regulation of inflammation
Mechanisms that determine where inflammatory lesions localize within the CNS significantly affect the clinical outcome but are not well understood. In most rodent models of EAE, inflammation predominantly targets the spinal cord, resulting in the classic symptom of ascending flaccid paralysis. By contrast, most patients with multiple sclerosis develop inflammatory lesions in the brain, which accounts for their more diverse clinical symptoms. Brain inflammation accompanied by atypical clinical signs such as leaning or circling (disequilibrium), ataxia, hyper-reflexivity and proprioception defects occurs in a few EAE models that use certain strain and antigen combinations66,67 and in models that incorporate IFNγ deficiency. The influence of IFNγ deficiency on the regional localization of inflammation was highlighted by an observation made in TCR transgenic mice expressing an MBP-specific monoclonal TCR; introducing IFNγ deficiency caused a change from predominantly spinal cord inflammation to predominantly brain inflammation in spontaneous EAE68. A crucial role for IFNγ-mediated signalling in CNS resident cells was shown by the finding that the transfer of T cells specific for MOG35–55 into wild-type mice resulted only in spinal cord inflammation, but transfer of the same cells into IFNγ receptor (IFNγR)-deficient mice led to inflammation specifically in the brain69. This suggests that IFNγR signalling in spinal cord resident cells is required to induce inflammation at this site but IFNγR signalling in brain resident cells inhibits inflammation.
The studies described above did not define the physiological mechanisms by which IFNγ-mediated signalling regulates region-specific localization of inflammation because mutant mice were used to prevent IFNγ-mediated signalling in all sites. We addressed this issue using a model of EAE in which T cells specific for three different epitopes of MOG are generated following immunization with recombinant MOG63. Adoptive transfer of T cells that were specific for MOG97–114 preferentially induced brain inflammation, whereas T cells that were specific for the other two epitopes induced only spinal cord inflammation. We found that T cells specific for all three epitopes infiltrated the brain and spinal cord meninges comparably; however, infiltration of the brain parenchyma and the induction of brain inflammation occurred only when the number of TH17 cells was higher than or equal to the number of TH1 cells in the brain meninges. This constraint did not apply to the induction of inflammation in the spinal cord. This indicates that the balance between TH17 cells and TH1 cells in the infiltrating T cell population is the crucial factor in determining whether inflammatory cells are recruited to the brain to initiate the formation of lesions (FIG. 3). Administration of recombinant MOG in complete Freund’s adjuvant resulted in the in vivo generation of a T cell population specific for MOG97–114 with a higher TH17 to TH1 cell ratio compared with that seen in T cell populations that are specific for the other two MOG epitopes. This would account for the increased ability of the MOG97–114-specific T cells to induce inflammation in the brain. The functional avidity that a T cell has for cognate antigen seems to influence the TH17 to TH1 cell ratio that is generated during priming63; however, further investigation is needed to define all of the parameters that determine the relative abundance of TH17 and TH1 cells generated in vivo.
When inflammation occurred in both the brain and spinal cord parenchyma, a disproportionate increase in IL-17 production was observed in the brain compared with the spinal cord. Furthermore, neutralization of IL-17 in vivo after transfer of TH17 cells prevented inflammation in the brain but not the spinal cord, such that the incidence of disease remained the same as when IL-17 was not neutralized; however, only classic symptoms were observed in this case. These findings suggest that IL-17 production within the brain has a crucial role in facilitating the recruitment of inflammatory cells to this site but has a non-essential role in inducing spinal cord inflammation. These differential requirements for IL-17 in the brain and the spinal cord are the opposite of the requirements for IFNγ-mediated signalling69, which is necessary for inflammation in the spinal cord but inhibits inflammation in the brain (see above). Therefore, it is tempting to speculate that the increased IL-17 production that occurs in the brain during inflammation functions to overcome the inhibitory effect of IFNγ-mediated signalling that normally prevents inflammation in this site (FIG. 3).
Mechanisms by which an increase in the local concentration of IL-17 in the brain could regulate either the strength of IFNγR signalling in brain resident cells or the downstream effects of IFNγR signalling have not yet been identified, nor is it known how IL-17- and IFNγ-mediated signalling induce different effects in distinct CNS environments. The mechanisms underlying the region-specific effects of IL-17 and IFNγ within the CNS are currently being intensely investigated, as delineating these mechanisms will provide valuable information for the design of therapeutic strategies that manipulate the activity of these cytokines in vivo.
APCs for myelin-specific CD4+ T cells
In the CNS of healthy individuals, macrophages are reported to be the predominant MHC class II-expressing cell type in the perivascular space and CSF, although some dendritic cells (DCs) are also detected13. Macrophages have an integral role in initiating EAE, as their depletion significantly inhibits disease70. Despite the low abundance of DCs in these compartments, experiments that limited MHC class II expression to DCs showed that these cells are sufficient to support the development of EAE71. DCs may also be the main APC involved in epitope spreading, which occurs when myelin-specific T cells that are recruited non-specifically to the inflamed CNS are activated by epitopes that are derived from degraded myelin. Miller and colleagues72 showed that myeloid DCs within the CNS activate naive myelin-specific T cells that were recruited to the inflamed tissue and facilitated their differentiation to TH17 cells. By contrast, Deshpande et al.73 reported that myeloid DCs isolated from the CNS support differentiation into both TH1 and TH17 cells but that the myeloid DCs isolated at the peak of disease are less efficient APCs than those isolated at disease onset, suggesting that changes in DC phenotype may contribute to subsequent remission. Interestingly, depletion of plasmacytoid DCs, which also infiltrate the CNS during inflammation but do not facilitate epitope spreading, results in exacerbation of EAE, suggesting that this DC population negatively regulates CD4+ T cell responses in the CNS74.
Although astrocytes and microglial cells comprise the glial limitans, they are unlikely to mediate initial T cell reactivation because they express negligible levels of MHC molecules in the healthy CNS. By contrast, during inflammation microglial cells express MHC class II and co-stimulatory molecules75, produce both IL-12 (REFS 75,76) and IL-23 (REF. 50) and trigger cytokine production by T cells77,78. Microglial cells seem to be crucial for maintaining autoimmune responses in the CNS, as a microglial cell-specific deficiency in CD40 expression79 and a transient inactivation of microglial cells80 reduces disease severity. However, further analysis is needed to define the precise function of microglial cells during the later stages of EAE, as they have been reported to reduce MHC class II and co-stimulatory molecule expression and become less efficient APCs during EAE remission78. Microglial cells that were activated in a graft-versus-host disease model supported T cell effector function but also caused T cell death77, suggesting that microglial cells may sustain CNS autoimmunity by triggering T cells to produce inflammatory soluble mediators before they die. Astrocytes also express MHC class II and co-stimulatory molecules following exposure to IFNγ81. Although their role as APCs in vivo is controversial, astrocytes influence the inflammatory response through the production of chemokines and cytokines. These cells also express galectin 9, which is a ligand for T cell immunoglobulin domain and mucin domain protein 3 (TIM3). The expression of this molecule is upregulated by microglial cells during disease, and TIM3 signalling in microglial cells seems to promote a pro-inflammatory phenotype in these cells82.
In summary, macrophages and DCs are probably the APCs that reactivate T cells as they infiltrate the CNS. Microglial cells become competent APCs for CD4+ T cells only after inflammation causes them to upregulate MHC and co-stimulatory molecules, but their reactivation of myelin-specific T cells can also trigger T cell death. Myeloid DCs trigger epitope spreading by activating naive T cells in the CNS, but plasmacytoid DCs dampen CD4+ T cell responses through mechanisms that are not yet well defined. B cells are also likely to have an important role as APCs in multiple sclerosis and EAE (BOX 3).
Box 3. B cells in CNS autoimmunity.
The substantial benefit of B cell depletion in patients with multiple sclerosis133 shows that B cells also have a central role in this disease. B cells could contribute to the pathogenesis of central nervous system (CNS) autoimmune disease in several ways: as a source of antibodies that recognize components of myelin, axons or neurons and contribute to demyelination or axonal damage, as antigen-presenting cells for T cells that are specific for CNS antigens and as immunoregulatory cells that produce suppressive cytokines and/or influence regulatory T cell activity (reviewed in REF. 134). The deposition of antibodies and complement on the myelin sheath of lesions in tissues from many patients with multiple sclerosis suggests that antibodies have a role in the pathogenesis of this disease. The presence of oligoclonal IgG in the cerebrospinal fluid (CSF) is also a characteristic of many patients with multiple sclerosis. Immunoglobulin gene rearrangements differ between B cells isolated from the CSF and periphery of patients with multiple sclerosis, suggesting that the antibodies are produced by B cells that mature within the CNS and are responding to specific antigen135. The formation of tertiary lymphoid follicles that contain B cells and follicular dendritic cells in the meninges of patients with secondary progressive multiple sclerosis136 further shows that B cells engage in both antigen presentation and antibody production in the CNS of these patients. The presence of these follicles is associated with earlier onset of multiple sclerosis and with more severe pathology137. However, the specificity of antibodies in the CSF has not yet been resolved.
In experimental autoimmune encephalomyelitis (EAE), antibodies that are specific for myelin oligodendrocyte glycoprotein (MOG) enhance demyelination, which is consistent with the exposed location of MOG on the surface of CNS myelin138. Recently, antibodies to neurofascin, a protein that is localized at the myelin–axon interface at the nodes of Ranvier, were detected in the periphery of patients with multiple sclerosis and were found to exacerbate EAE by mediating axonal injury139. The potential for pathogenic consequences of the collaboration between CNS antigen-specific B and T cells was revealed when MOG-specific T cell receptor (TCR) transgenic mice were crossed with mice that were engineered to express the heavy chain of a MOG-specific antibody. An aggressive autoimmune disease occurred in these mice, which targeted the optic nerves and spinal cord; this disease differed strongly from the mild clinical sign of isolated optic neuritis that was seen in mice expressing only the transgenic TCR140,141.
In contrast to a pathogenic role for B cells, several studies indicate that B cells may exert an immunoregulatory influence on CNS autoimmunity. B cells have been reported to facilitate the recruitment of regulatory T (TReg) cells to the CNS during EAE142, and interleukin-10 (IL-10) secretion by B cells are important in recovery from the disease143. A specialized subset of IL-10-secreting CD1dhiCD5+ B cells has been described in EAE, the transfer of which can ameliorate EAE at certain stages144. Interestingly, B cells from patients with multiple sclerosis produce less IL-10 than B cells from healthy controls, and B cells that emerge following treatment with rituximab (an antibody specific for CD20, which is expressed on the surface of B cells) secrete higher levels of IL-10 than B cells before treatment145. This finding, together with the observations that rituximab does not deplete plasma cells and that autoantibody titres did not change during treatment, suggests that the benefit of this treatment was not simply a result of inhibiting pathogenic antibody activity.
CD8+ T cell responses in the CNS
Although for decades the focus of research on autoimmunity in the CNS has been almost exclusively on CD4+ T cells, the potential importance of CD8+ T cells in CNS autoimmunity has recently emerged83,84. One compelling reason for this is that depletion of CD4+ T cells in patients with multiple sclerosis resulted in no improvement in disease, but treatment with alemtuzumab (Campath-1H, Genzyme Corporation and Schering AG), which depletes several leukocyte populations (including both CD4+ and CD8+ T cells), seems to be beneficial85. CD8+ T cells also outnumber CD4+ T cells in multiple sclerosis lesions, and clonal expansion is detected more frequently among CD8+ T cells isolated from multiple sclerosis lesions than among CD4+ T cells83,84,86. Preferential enrichment of CD8+ compared with CD4+ memory T cells is observed in the CSF and blood of patients with multiple sclerosis, and memory CD8+ T cells show evidence of oligoclonal expansion, which is consistent with recognition of cognate antigen. Furthermore, although no differences were detected for CD4+ T cells, the frequency of CD8+ T cells specific for CNS antigen-derived peptides was shown in vitro to be higher in patients with multiple sclerosis compared with healthy individuals87. The genetic association of MHC class I alleles with multiple sclerosis remains controversial; however, the MHC class I molecule HLA-A3 (A*0301) has been reported to increase susceptibility, whereas HLA-A2 (A*0201) seems to confer protection against disease84,88,89, suggesting that some CD8+ T cell responses can be beneficial and others are pathogenic.
Initial studies on the role of CD8+ T cells in the pathology of EAE investigated clinical disease following CD8+ T cell depletion and in Cd8−/− mice90,91. CD8+ T cell deficiency resulted in less mortality but more relapses, suggesting that CD8+ T cells may exert a regulatory influence in either promoting or sustaining periods of disease remission. However, studies of myelin-specific CD8+ T cells showed that CD8+ T cells were highly pathogenic in EAE92,93. We identified CD8+ T cells that were specific for MBP79–87 and found that transfer of these cells into wild-type mice induced a demyelinating disease that recapitulated certain pathological features seen in patients with multiple sclerosis that are not typically seen in CD4+ T cell-mediated EAE92. Although many of the MBP79–87-specific CD8+ T cells are deleted in the thymus by central tolerance, we identified one TCR that does not trigger thymocyte deletion despite having high affinity for the MBP79–87–MHC complex. A TCR transgenic mouse model was generated using this TCR in which the CD8+ T cells that populate the periphery have a seemingly naive phenotype6. In contrast to most CD4+ myelin-specific TCR transgenic models, spontaneous EAE was not observed in these mice. Interestingly, CD8+ T cell tolerance in this model could be broken by viral infection but not by many other immunogenic stimuli (Q. Ji and J.G., unpublished observations).
Recently, a humanized CD8+ TCR transgenic mouse model was generated based on a human TCR with degenerate specificity that recognizes PLP45–53 associated with the MHC class I molecule HLA-A3 (A*0301) and an unknown ligand associated with HLA-A2 (A*0201)94. In the TCR transgenic mice, positive selection occurred when only HLA-A3 (A*0301) was expressed in vivo, allowing PLP45–53-specific T cells to populate the periphery. However, negative selection of T cells expressing the transgenic TCR occurred following in vivo expression of HLA-A2 (A*0201), an allele reported to protect against multiple sclerosis in humans. The PLP45–53-specific TCR did not recognize the HLA-A2–PLP45–53 complex or respond to HLA-A2 presenting peptides from myelin peptide libraries. This result suggests that this MHC allele may decrease the risk of multiple sclerosis by eliminating PLP45–53-specific T cells from the peripheral repertoire through negative selection that does not involve the myelin antigen. Immunization of TCR transgenic mice that express HLA-A3 with the PLP45–53 peptide in complete Freund’s adjuvant induced mild disease initially, but a CD4+ T cell-dependent disease developed later that was more severe, suggesting that CD8+ T cells can initiate disease but CD4+ T cells may be required for chronic disease in this model. Although these models have begun to address the role of CD8+ T cells in the pathogenesis of autoimmunity in the CNS, much remains to be understood.
APCs for myelin-specific CD8+ T cells
Similar to CD4+ T cells, naive CD8+ T cells are inefficient at crossing the blood–brain barrier in the absence of inflammation, implying that they must also be activated in the periphery before infiltrating the CNS. APCs in cervical lymph nodes could present MHC class I-associated myelin epitopes just as they do MHC class II-associated myelin epitopes. However, MHC class I-associated peptides are typically derived from proteins that are synthesized within the cell, and the only cell types that synthesize myelin proteins in the CNS are oligodendrocytes. CD8+ T cells could still be primed in cervical lymph nodes because some APCs can generate peptide–MHC class I complexes by acquiring exogenous antigen through phagocytosis or endocytosis and cross-presenting it in the MHC class I pathway in a process known as cross-presentation (FIG. 4). Cross-presentation occurs more frequently under inflammatory conditions; for example, MHC class I-restricted tumour antigens acquired by APCs within the CNS can be presented in peripheral lymph nodes95. This suggests that, at least under inflammatory conditions, cross-presentation could activate myelin-specific CD8+ T cells in the periphery. We recently described an alternative mechanism for presenting MBP in the periphery by showing that bone marrow-derived cells can synthesize and directly present MBP in association with MHC class I molecules in the thymus96. This presentation causes most MHC class I-restricted MBP-specific T cells to be deleted. Importantly, this result indicates that peripheral APCs directly synthesize and present MBP epitopes in the MHC class I pathway, raising the possibility that other epitopes of CNS proteins might be presented by this same mechanism. As is the case for CD4+ myelin-specific T cells, the conditions under which presentation of CNS antigens by APCs in the periphery causes activation rather than tolerance induction of naive myelin-specific T cells are not yet known.
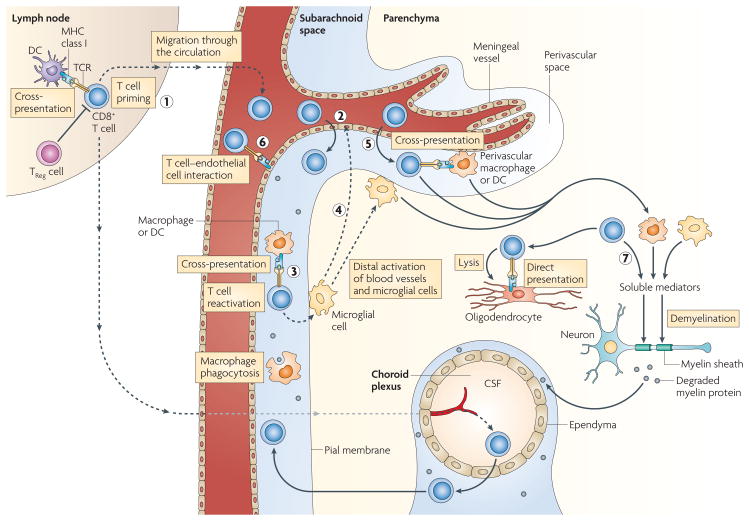
Figure 4. Peripheral and CnS activation of myelin-specific CD8+ T cells.
CD8+ T cells are primed by cross-presentation by dendritic cells (DCs) in the lymph nodes (1) and follow the same steps (2–5) as CD4+ T cells (see FIG. 2). However, CD8+ T cells are activated by macrophages, microglial cells and DCs only by cross-presentation; endothelial cells can directly present antigen if they have access to myelin epitopes (6). Activated macrophages, microglial cells and CD8+ T cells secrete soluble mediators, and CD8+ T cells can directly lyse oligodendrocytes expressing MHC class I and myelin epitopes (7). CSF, cerebrospinal fluid; TCR, T cell receptor; TReg, regulator T.
The cell types that can reactivate CD8+ T cells infiltrating the CNS before inflammation have also not yet been identified. Under non-inflammatory conditions in the CNS, endothelial cells, macrophages, DCs and some microglial cells express MHC class I molecules. As oligodendrocytes are the only cells that synthesize myelin proteins but lack expression of MHC molecules, it is probable that myelin antigens are cross-presented by DCs, macrophages or microglial cells. Endothelial cells potentially could present MHC class I-associated peptides acquired from degraded myelin proteins, as peptide injected directly into the CNS parenchyma seems to bind MHC class I molecules expressed by endothelial cells and trigger antigen-specific CD8+ T cell entry into the CNS97. Which of these different cell types reactivate CD8+ T cells allowing them to initiate CNS inflammation remains an important but unanswered question.
Once inflammation has begun, all CNS-resident cells upregulate their expression of MHC class I molecules. In TCR transgenic mice in which oligodendrocytes expressed neo-antigens, neo-antigen-specific CD8+ T cells were efficient at lysing the oligodendrocytes98,99. However, the cells presenting endogenous myelin antigens that are targeted by activated CD8+ T cells in vivo have not been identified. As cross-presentation is likely to increase under the inflammatory conditions that result in myelin degradation, multiple cell types could function as APCs for myelin-specific CD8+ T cells (FIG. 4).
Recently, Epstein–Barr virus-infected B cells have been reported to accumulate in intrameningeal follicles and white matter lesions of patients with multiple sclerosis100. Expression of viral latent proteins was consistently detected in the brain tissue from patients with multiple sclerosis, and evidence of viral reactivation was seen in the ectopic B cell follicles and acute lesions. B cells in which the virus has been reactivated may function to enhance CD8+ T cell and DC recruitment to the CNS, facilitating a chronic inflammatory response100. Under these inflammatory conditions, DCs and possibly B cells may cross-present myelin antigens that have become available by myelin degradation to myelin-specific CD8+ T cells. Further exploitation of animal models and analyses of tissues from patients with multiple sclerosis is needed to identify the cells that present MHC class I-restricted myelin epitopes to trigger autoreactive CD8+ T cell responses.
CD8+ T cell effector mechanisms
CD8+ T cell effector mechanisms include the production of soluble mediators and cell contact-mediated lysis. A role for CD8+ T cell-mediated lysis in multiple sclerosis pathology is supported by the findings that CD8+ T cells have been observed in multiple sclerosis tissues with their cytotoxic granules polarized towards oligodendrocytes and axons, and that MBP-specific CD8+ T cells isolated from patients with multiple sclerosis specifically lyse freshly isolated human oligodendrocytes83,84. Moreover, acute axonal damage is most extensive in the early stages of multiple sclerosis, and the extent of axonal damage correlates with the number of CD8+ T cells in the CNS83,84. Soluble mediators produced by CD8+ T cells may also contribute to CNS injury in multiple sclerosis. MBP-specific CD8+ T cells isolated from patients with multiple sclerosis were shown to secrete the pro-inflammatory cytokines IFNγ and TNF101. In addition, immunochemical analyses have recently shown that >70% of the T cells in acute and chronic active multiple sclerosis lesions are IL-17+, and these IL-17+ cells were equally represented among CD4+ and CD8+ T cells102. The notion that IL-17 may be contributing to lesion formation is supported by the fact that only 17% of T cells in inactive lesions were IL-17+. In our CD8+ MBP-specific T cell model, both soluble mediators and cell contact-mediated lysis were required to induce disease. Deficiency of perforin or CD95 (both of which are involved in cell contact-mediated lysis) separately did not affect the development of EAE that was induced by CD8+ MBP-specific TCR transgenic T cells. However, no disease occurred when perforin-deficient T cells were transferred into CD95-deficient recipients, suggesting a requirement for cell contact-mediated cell death (A. Perchellet and J.G., unpublished observations).
The importance of soluble mediators was revealed when CD8+ MBP-specific T cell clones were transferred into mice that were also injected in the CNS with neutralizing reagents for IFNγ and TNF; neutralizing IFNγ ameliorated disease, although TNF neutralization did not92. Thus, although CD4+ T cells are limited to recognizing only APCs expressing MHC class II molecules and exerting their effects through the secretion of soluble mediators, CD8+ T cells can recognize a wider range of targets that express MHC class I molecules and cause injury in the CNS by both cell contact-mediated lysis and production of soluble inflammatory cytokines. The pathogenic pathways that lead to disease in individual patients with multiple sclerosis could depend primarily on either T cell subset, which could result in different types of tissue damage. Alternatively, both T cell subsets could be involved, as seen in the CD8+ T cell-mediated animal model described by Friese et al.94. Differences in the relative contributions of CD4+ and CD8+ T cells in individual patients with multiple sclerosis could account for the heterogeneity seen in the tissue damage and clinical signs.
Regulating autoreactive T cell responses
CD4+FOXP3+ regulatory T cells
In myelin-specific CD4+ TCR transgenic mouse models, forkhead box P3 (FOXP3)+ regulatory T (TReg) cells prevent spontaneous EAE by suppressing the activation of the myelin-specific CD4+ T cells in the periphery9,103. High-avidity MBP-specific T cells that are suppressed following encounter with endogenous MBP presented in lymphoid tissues acquire an anti-inflammatory phenotype that is characterized by the secretion of IL-10 and TGFβ28. However, if TReg cells are absent or immunogenic stimuli are present during the initial interaction between MBP-specific T cells and peripheral APCs presenting myelin antigen, active tolerance is overcome and autoimmunity ensues.
The function of TReg cells in preventing inflammation within the CNS is controversial. Although a correlation between the presence of IL-10-producing FOXP3+ TReg cells in the CNS and disease recovery was reported104, TReg cells seem ineffective in suppressing effector T cells in the CNS until the local levels of IL-6 and TNF decrease105. Production of inflammatory cytokines may subside after the initial wave of T cells infiltrate the CNS and before epitope spreading begins because T cells typically die during acute EAE after entering the parenchyma and exerting their effector function106. Currently, the mechanisms of suppression, antigen specificity and effectiveness of TReg cells at suppressing TH1, TH17 and CD8+ T cells in the CNS are unresolved. In patients with multiple sclerosis, the function of peripheral CD4+ TReg cells seems to be impaired (reviewed in REF. 107), suggesting that they may have decreased ability to inhibit the activation of myelin-specific T cells in the periphery. FOXP3+ TReg cells have also not been detected in multiple sclerosis tissue sections102, although it is not known whether their absence reflects a defect in migration or survival in the CNS in addition to the functional impairment seen in CD4+ TReg cells isolated from the blood of patients with multiple sclerosis. Interestingly, CD25, a component of the IL-2 receptor (the expression of which is essential for TReg cell development and homeostasis108), has recently been identified as a multiple sclerosis susceptibility gene23.
Regulatory CD8+ T cells
Regulatory CD8+ T cells have also been reported to be present in EAE and patients with multiple sclerosis (reviewed in REFS 83,84,109). Naturally occurring CD8+CD122+ T cells have been described that inhibit CD8+ effector T cells by secreting IL-10 (REF. 110). CD8+HLA-G+ T cells that suppress effector T cells by secreting soluble factors have also been identified in humans111. Induced regulatory CD8+ T cells have also been described that are thought to function in the periphery. CD8+CD28− T cells seem to suppress disease by inducing tolerogenic effects on DCs112,113, and other CD8+ regulatory T cells that are induced by the expansion of CD4+ T effector cells in the periphery eliminate activated CD4+ T cells by recognizing the non-classical MHC molecule Qa-1 on the CD4+ T cell surface114,115. Studies of patients with multiple sclerosis suggest that the regulatory CD8+ T cells described in animal models may also have a functional role in patients with multiple sclerosis (reviewed in REF. 109). Indeed, CD8+ T cells were detected in patients with multiple sclerosis that lysed CD4+ myelin-specific T cells, and their numbers were decreased during exacerbation of the disease. Furthermore, vaccination of patients with multiple sclerosis with CD4+ myelin-specific clones elicited CD8+ regulatory T cells that could eliminate these effector T cells116,117. In addition, the beneficial effect of glatiramer acetate therapy may be mediated in part by regulatory CD8+ T cells118. Therefore, strategies to enhance CD8+ as well as CD4+ regulatory T cells in vivo may be beneficial for the treatment of patients with multiple sclerosis.
Cytokine-mediated regulation of CNS inflammation
The cytokine milieu in the inflamed CNS is complex and dynamic, striving on the one hand to facilitate responses to eliminate potential pathogens and on the other hand to suppress destructive immune responses. In the inflamed CNS, cytokines that promote effector T cells, such as IL-1, IL-6, IL-12, IL-23 and TGFβ, are produced by many cell types. The expression of TGFβ alone facilitates inflammation in the brain, as shown by the exacerbation of EAE in mice in which TGFβ is over-expressed by astrocytes and the amelioration of EAE following pharmacological inhibition of TGFβ activity119,120. By contrast, IL-10 has a crucial role in mediating suppression of effector T cell responses in the CNS; this is illustrated by the observations that Il10−/− mice have severe EAE and IL-10-transgenic mice are EAE resistant121. TReg cells are one source of IL-10 (REF. 105), but IL-27 can also induce IFNγ-producing TH1 and CD8+ T cells to produce IL-10 (REFS 122,123). IL-27 belongs to the same cytokine family as IL-6 and IL-12 and is produced by activated DCs and infiltrating macrophages. IL-27 also inhibits the development of IL-17-producing cells122,124,125. Deficiency of IL-27 receptor leads to severe EAE125, and recombinant IL-27 treatment suppresses the effector phase of disease123. Other cytokine-mediated pathways also exist to dampen the responses of effector T cells. For example, IL-25 expressed by microglial cells functions by inducing IL-13 to suppress the production of the TH17-polarizing cytokines IL-23, IL-1β and IL-6 by activated DCs126.
Although cytokine-mediated suppression of T cell responses in the CNS is clearly important to control inflammatory responses, T cell death within the parenchyma is another key mechanism for limiting autoimmunity in the CNS (reviewed in REF. 106). The CNS also exerts a unique immunoregulatory mechanism by upregulating αB crystallin, the most abundant gene transcript in early multiple sclerosis lesions, which both suppresses inflammatory immune cells and protects glial cells from apoptosis127.
Conclusions
Our understanding of CNS autoimmunity has advanced greatly over the past decade. Our growing knowledge of cytokines, transcription factors and signalling pathways has allowed immunologists to identify new effector and regulatory T cell subsets of both the CD4+ and CD8+ T cell lineages that may contribute to CNS autoimmunity. The heterogeneous pathology seen in tissue sections from patients with multiple sclerosis can now be viewed in the context of different types of effector T cell that initiate distinct pathogenic pathways.
Despite this enormous progress, many challenges remain. It is fundamental to determine how different T cell subsets interact with each other and with resident cells in their local environment. This requires a better understanding of the newly appreciated plasticity of T cell effector function phenotype; that is, the ability of T cell subsets that seem committed to produce one set of cytokines to secrete cytokines that are associated with different T cell subsets. For example, a subset of human TReg cells activated in the presence of the pro-inflammatory cytokines IL-1β and IL-6 can secrete IL-17, whereas a milieu dominated by TGFβ inhibits IL-17 secretion by TReg cells128. This indicates that the local milieu can exert an unexpected influence on the activity of T cell subsets that were previously assumed to be committed to a particular lineage. Interestingly, TReg cells secreting IL-17 still suppress effector T cells129, but the local environment in vivo will be altered by the chemotactic responses and metalloproteinase activity induced by IL-17. It is also important to determine the contribution of different T cell subsets at different points in disease progression and how their activity correlates with differing pathologies, clinical symptoms and/or disease course of multiple sclerosis. The mechanisms of activation and regulation of different T cell subsets must be better characterized and placed in the context of new data that define genetic susceptibility to multiple sclerosis. Most importantly, this knowledge must be applied to the development of new therapeutic strategies that take into account the fact that the brain and spinal cord may be fundamentally different environments in terms of how they respond to T cell-mediated inflammation.
Acknowledgments
J. Goverman’s research is supported by grants from the National Multiple Sclerosis Society (RG3851-A-5) and from the National Institutes of Health (AI073726, AI072737)
- Multiple sclerosis
An inflammatory neurodegenerative disorder characterized by demyelination of bundles of nerves in the central nervous system. Symptoms depend on the site of the lesion but can include sensory loss, weakness in leg muscles, speech difficulties, loss of coordination and dizziness. Multiple sclerosis is thought to be an autoimmune response against components of myelin
- Neuromyelitis optica
A relapsing, demyelinating inflammatory disorder that affects predominantly the optic nerves and spinal cord. Pathogenesis of neuromyelitis optica seems to depend on the generation of antibodies that are specific for aquaporin 4, an abundant water channel in the central nervous system
- Acute disseminated encephalomyelitis
A monophasic, inflammatory, demyelinating disease of the central nervous system, frequently affecting children
- Blood–brain barrier
A physiological barrier between blood vessels and brain parenchyma. It is formed by specialized, tight junctions between endothelial cells of the blood vessel wall, which is surrounded by a basement membrane, and an additional membrane formed from astrocyte feet and microglial cells
- Demyelination
Damage to the myelin sheath surrounding nerves in the central nervous system, which affects the function of the nerves involved
- Tight junction
An intercellular junction that joins the plasma membranes of adjacent epithelial or endothelial cells that regulate paracellular flux. Tight junction proteins include the integral membrane proteins occludin and claudin, in association with cytoplasmic zonula occludens proteins
- Oligodendrocyte
The myelin-forming cell of the central nervous system
- Mimicry
Structural similarity between epitopes contained within microbial and host proteins, leading to crossreactivity of T cells in the host
- Wallerian degeneration
The degeneration of an axon distal to a site of injury, which begins to occur about 1.5 days after a lesion is formed
- Degenerate TCR
A T cell receptor (TCR) that can bind to multiple distinct peptide–MHC complexes
- Meninges, The membraneous region surrounding the brain and spinal cord. There are three membranes contained within the meninges
the dura mater (outer), the arachnoid membrane (middle) and the pia mater (inner). The subarachnoid space lies between the arachnoid and pial membranes and is the region in which cerebrospinal fluid flows
- Epitope spreading
This term is used to describe how an immune response generated against a single peptide (or epitope) could spread to include T cell specificities for other peptides (or epitopes) not only on the same autoantigen (intramolecular spreading) but also on other self molecules that are released during the inflammatory response in the target organ
- Plasmacytoid DC
A dendritic cell (DC) that lacks myeloid markers such as CD11c and CD33 but expresses high levels of HLA-Dr and CD123. These cells produce high levels of type I interferons (IFNα and IFNβ) after activation
- Perforin
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter target cells
References
- 1.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nature Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Raine C. In: Multiple Sclerosis: Clinical and Pathogenetic Basis. Raine CS, McFarland HF, Tourtellotte WW, editors. Chapman and Hall; London: 1997. pp. 243–286. [Google Scholar]
- 4.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nature Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 5.Huseby ES, Sather B, Huseby PG, Goverman J. Age-dependent T cell tolerance and autoimmunity to myelin basic protein. Immunity. 2001;14:471–481. doi: 10.1016/s1074-7613(01)00127-3. [DOI] [PubMed] [Google Scholar]
- 6.Perchellet A, Stromnes I, Pang JM, Goverman J. CD8+ T cells maintain tolerance to myelin basic protein by ‘epitope theft’. Nature Immunol. 2004;5:606–614. doi: 10.1038/ni1073. This paper identifies a new form of tolerance that allows CD8+ T cells specific for MBP to circulate in the periphery without responding to endogenous MBP. [DOI] [PubMed] [Google Scholar]
- 7.Pender MP, Tabi Z, Nguyen KB, McCombe PA. The proximal peripheral nervous system is a major site of demyelination in experimental autoimmune encephalomyelitis induced in the Lewis rat by a myelin basic protein-specific T cell clone. Acta Neuropathol. 1995;89:527–531. doi: 10.1007/BF00571507. [DOI] [PubMed] [Google Scholar]
- 8.Furtado GC, et al. Swift entry of myelin-specific T lymphocytes into the central nervous system in spontaneous autoimmune encephalomyelitis. J Immunol. 2008;181:4648–4655. doi: 10.4049/jimmunol.181.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J Immunol. 2008;181:4638–4647. doi: 10.4049/jimmunol.181.7.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccio L, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J Immunol. 2002;168:1940–1949. doi: 10.4049/jimmunol.168.4.1940. [DOI] [PubMed] [Google Scholar]
- 11.Reboldi A, et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nature Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 12.Lassmann H, Wisniewski HM. Chronic relapsing EAE. Time course of neurological symptoms and pathology. Acta Neuropathol. 1978;43:35–42. doi: 10.1007/BF00684996. [DOI] [PubMed] [Google Scholar]
- 13.Kivisakk P, et al. Localizing central nervous system immune surveillance: Meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2008 May 21; doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DA, Sawchenko PE. Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis. J Comp Neurol. 2007;502:236–260. doi: 10.1002/cne.21307. This paper describes the temporal and spatial pattern of inflammatory and degenerative events that occur during the induction of EAE. [DOI] [PubMed] [Google Scholar]
- 15.Gimenez MA, Sim J, Archambault AS, Klein RS, Russell JH. A tumor necrosis factor receptor 1- dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. Am J Pathol. 2006;168:1200–1209. doi: 10.2353/ajpath.2006.050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami N, et al. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–197. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–197. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Disproportionate recruitment of CD8+ T cells into the central nervous system by professional antigen-presenting cells. Am J Pathol. 1999;154:481–494. doi: 10.1016/S0002-9440(10)65294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brabb T, et al. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J Exp Med. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Battistini L, et al. CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood. 2003;101:4775–4782. doi: 10.1182/blood-2002-10-3309. [DOI] [PubMed] [Google Scholar]
- 22.Eikelenboom MJ, et al. Chemokine receptor expression on T cells is related to new lesion development in multiple sclerosis. J Neuroimmunol. 2002;133:225–232. doi: 10.1016/s0165-5728(02)00374-0. [DOI] [PubMed] [Google Scholar]
- 23.Hafler DA, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 24.Harrington CJ, et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. This paper shows that the dependence of central tolerance on T cell functional avidity for antigen allows some, but not all, MBP-specific immune tolerance to escape immune tolerance. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AC, et al. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nature Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 27.Seamons A, Perchellet A, Goverman J. Immune tolerance to myelin proteins. Immunol Res. 2003;28:201–221. doi: 10.1385/IR:28:3:201. [DOI] [PubMed] [Google Scholar]
- 28.Cabbage SE, et al. Regulatory T cells maintain long-term tolerance to myelin basic protein by inducing a novel, dynamic state of T cell tolerance. J Immunol. 2007;178:887–896. doi: 10.4049/jimmunol.178.2.887. [DOI] [PubMed] [Google Scholar]
- 29.Bielekova B, et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–3904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 30.Burns J, Bartholomew B, Lobo S. Isolation of myelin basic protein-specific T cells predominantly from the memory T-cell compartment in multiple sclerosis. Ann Neurol. 1999;45:33–39. [PubMed] [Google Scholar]
- 31.Scholz C, Patton KT, Anderson DE, Freeman GJ, Hafler DA. Expansion of autoreactive T cells in multiple sclerosis is independent of exogenous B7 costimulation. J Immunol. 1998;160:1532–1538. [PubMed] [Google Scholar]
- 32.Lovett-Racke AE, et al. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponsford M, et al. Differential responses of CD45+ve T-cell subsets to MBP in multiple sclerosis. Clin Exp Immunol. 2001;124:315–322. doi: 10.1046/j.1365-2249.2001.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, et al. Degenerate TCR recognition and dual DR2 restriction of autoreactive T cells: implications for the initiation of the autoimmune response in multiple sclerosis. Eur J Immunol. 2008;38:1297–1309. doi: 10.1002/eji.200737519. [DOI] [PubMed] [Google Scholar]
- 35.Fujinami RS, Oldstone MB. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230:1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 36.Münz C, Lünemann JD, Getts MT, Miller SD. Antiviral immune responses: triggers of or triggered by autoimmunity? Nature Rev Immunol. 2009;9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. This paper shows that a search using a molecular mimicry motif based on structural rather than sequence similarity identifies distinct, multiple peptides from microorganisms that can activate human T cells specific for MBP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang HL, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature Immunol. 2002;3:940–943. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 39.Tejada-Simon MV, Zang YC, Hong J, Rivera VM, Zhang JZ. Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosis. Ann Neurol. 2003;53:189–197. doi: 10.1002/ana.10425. [DOI] [PubMed] [Google Scholar]
- 40.Holmoy T, Kvale EO, Vartdal F. Cerebrospinal fluid CD4+ T cells from a multiple sclerosis patient cross-recognize Epstein–Barr virus and myelin basic protein. J Neurovirol. 2004;10:278–283. doi: 10.1080/13550280490499524. [DOI] [PubMed] [Google Scholar]
- 41.Markovic-Plese S, et al. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: implications for the initiation of autoimmune response in multiple sclerosis. J Neuroimmunol. 2005;169:31–38. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Goverman J, et al. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. This paper is the first to show that T cells specific for some epitopes of MBP are not subject to deletion by central tolerance in vivo and that the incidence of spontaneous autoimmunity mediated by these T cells is influenced by environmental factors. [DOI] [PubMed] [Google Scholar]
- 43.Goverman J. Tolerance and autoimmunity in TCR transgenic mice specific for myelin basic protein. Immunol Rev. 1999;169:147–159. doi: 10.1111/j.1600-065X.1999.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishnamoorthy G. Cumulative autoimmunity: Myelin oligodendrocyte glycoprotein-specific T cells co-recognize neurofilament-M in a spontaneous experimental autoimmune encephalomyelitis of the C57BL/6 mouse. Nature Med. (in the press) [Google Scholar]
- 45.Gutcher I, Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 47.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal BM, Shevach EM. IL-12 unmasks latent autoimmune disease in resistant mice. J Exp Med. 1996;184:771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steinman L. A brief history of TH17, the first major revision in the T H 1/T H 2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 50.Cua DJ, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 51.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nature Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 53.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nature Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 54.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. This paper is the first to show that myelin-specific TH17 cells are more pathogenic following adoptive transfer into naive mice than TH1 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofstetter HH, et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Komiyama Y, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 58.Axtell RC, Xu L, Barnum SR, Raman C. CD5–CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ivanov II, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 60.Haak S, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. This paper questions the role of IL-17A and IL-17F by showing that the lack of these cytokines in vivo does not significantly affect the manifestation of EAE mediated by MOG35–55-specific T cells in C57BL/6 mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohn TA, et al. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol. 2006;36:2857–2867. doi: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 62.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by TH1 and TH17 cells. Nature Med. 2008;14:337–342. doi: 10.1038/nm1715. This paper shows that inflammation is regulated differently in the brain compared with the spinal cord and provides evidence that the relative abundance of TH17 and TH1 cells in the infiltrating T cell population is a crucial factor in determining where inflammation localizes within the CNS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Storch MK, et al. Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol. 1998;8:681–694. doi: 10.1111/j.1750-3639.1998.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muller DM, Pender MP, Greer JM. A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol (Berl) 2000;100:174–182. doi: 10.1007/s004019900163. [DOI] [PubMed] [Google Scholar]
- 68.Wensky AK, et al. IFN-γ determines distinct clinical outcomes in autoimmune encephalomyelitis. J Immunol. 2005;174:1416–1423. doi: 10.4049/jimmunol.174.3.1416. [DOI] [PubMed] [Google Scholar]
- 69.Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-γ determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. This paper shows that IFNγ signalling in CNS resident cells is required for inflammation in the spinal cord but inhibits inflammation in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- 71.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature Med. 2005;11:328–334. doi: 10.1038/nm1197. This paper shows that DCs, which were previously considered to be scarce or absent from the non-inflamed CNS, are present in the CNS and are sufficient to reactivate primed T cells in the CNS and induce EAE. [DOI] [PubMed] [Google Scholar]
- 72.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T H-17 cells in relapsing EAE. Nature Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 73.Deshpande P, King IL, Segal BM. Cutting edge: CNS CD11c+ cells from mice with encephalomyelitis polarize Th17 cells and support CD25+CD4+ T cell-mediated immunosuppression, suggesting dual roles in the disease process. J Immunol. 2007;178:6695–6699. doi: 10.4049/jimmunol.178.11.6695. [DOI] [PubMed] [Google Scholar]
- 74.Bailey-Bucktrout SL, et al. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 76.Becher B, Blain M, Antel JP. CD40 engagement stimulates IL-12 p70 production by human microglial cells: basis for Th1 polarization in the CNS. J Neuroimmunol. 2000;102:44–50. doi: 10.1016/s0165-5728(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 77.Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996;184:1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 79.Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heppner FL, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nature Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 81.Nikcevich KM, et al. IFN-gamma-activated primary murine astrocytes express B7 costimulatory molecules and prime naive antigen-specific T cells. J Immunol. 1997;158:614–621. [PubMed] [Google Scholar]
- 82.Anderson AC, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 83.Goverman J, Perchellet A, Huseby ES. The role of CD8+ T cells in multiple sclerosis and its animal models. Curr Drug Targets Inflamm Allergy. 2005;4:239–245. doi: 10.2174/1568010053586264. [DOI] [PubMed] [Google Scholar]
- 84.Friese MA, Fugger L. Autoreactive CD8+ T cells in multiple sclerosis: a new target for therapy? Brain. 2005;128:1747–1763. doi: 10.1093/brain/awh578. [DOI] [PubMed] [Google Scholar]
- 85.Coles AJ, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 86.Junker A, et al. Multiple sclerosis: T-cell receptor expression in distinct brain regions. Brain. 2007;130:2789–2799. doi: 10.1093/brain/awm214. [DOI] [PubMed] [Google Scholar]
- 87.Crawford MP, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–4231. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 88.Brynedal B, et al. HLA-A confers an HLA-DRB1 independent influence on the risk of multiple sclerosis. PLoS ONE. 2007;2:e664. doi: 10.1371/journal.pone.0000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burfoot RK, et al. SNP mapping and candidate gene sequencing in the class I region of the HLA complex: searching for multiple sclerosis susceptibility genes in Tasmanians. Tissue Antigens. 2008;71:42–50. doi: 10.1111/j.1399-0039.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 90.Koh DR, et al. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/− mice. Science. 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 91.Jiang H, Zhang S-l, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science. 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 92.Huseby ES, et al. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. This paper shows that CD8+ T cells specific for MBP can induce a distinct form of EAE that recapitulates some aspects of multiple sclerosis not seen in CD4+ T cell-mediated EAE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun D, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. This paper shows that CD8+ T cells specific for MOG can induce EAE pathology. [DOI] [PubMed] [Google Scholar]
- 94.Friese MA, et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nature Med. 2008;14:1227–1235. doi: 10.1038/nm.1881. This paper uses humanized mice to show that one HLA allele promotes induction of EAE mediated by CD8+ PLP-specific T cells and a different HLA allele protects from disease, thereby identifying mechanisms by which MHC class I alleles can determine susceptibility to multiple sclerosis. [DOI] [PubMed] [Google Scholar]
- 95.Calzascia T, et al. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Perchellet A, Brabb T, Goverman JM. Crosspresentation by nonhematopoietic and direct presentation by hematopoietic cells induce central tolerance to myelin basic protein. Proc Natl Acad Sci USA. 2008;105:14040–14045. doi: 10.1073/pnas.0804970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galea I, et al. An antigen-specific pathway for CD8 T cells across the blood–brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Na SY, et al. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- 99.Saxena A, et al. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181:1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- 100.Serafini B, et al. Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zang YC, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J Immunol. 2004;172:5120–5127. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- 102.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 104.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 105.Korn T, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pender MP. Treating autoimmune demyelination by augmenting lymphocyte apoptosis in the central nervous system. J Neuroimmunol. 2007;191:26–38. doi: 10.1016/j.jneuroim.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 107.O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 108.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 109.Zozulya AL, Wiendl H. The role of CD8 suppressors versus destructors in autoimmune central nervous system inflammation. Hum Immunol. 2008;69:797–804. doi: 10.1016/j.humimm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 110.Rifa’i M, et al. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I–αβTCR interaction and become IL-10-producing active regulatory cells. Int Immunol. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 111.Feger U, et al. HLA-G. expression defines a novel regulatory T-cell subset present in human peripheral blood and sites of inflammation. Blood. 2007;110:568–577. doi: 10.1182/blood-2006-11-057125. [DOI] [PubMed] [Google Scholar]
- 112.Najafian N, et al. Regulatory functions of CD8+CD28− T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vlad G, Cortesini R, Suciu-Foca N. License to heal: bidirectional interaction of antigen-specific regulatory T cells and tolerogenic APC. J Immunol. 2005;174:5907–5914. doi: 10.4049/jimmunol.174.10.5907. [DOI] [PubMed] [Google Scholar]
- 114.Jiang H, et al. Regulatory CD8+ T cells fine-tune the myelin basic protein-reactive T cell receptor Vβ repertoire during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2003;100:8378–8383. doi: 10.1073/pnas.1432871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: interruption of the NKG2A–Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J, Medaer R, Stinissen P, Hafler D, Raus J. MHC-restricted depletion of human myelin basic protein-reactive T cells by T cell vaccination. Science. 1993;261:1451–1454. doi: 10.1126/science.7690157. [DOI] [PubMed] [Google Scholar]
- 117.Correale J, et al. T cell vaccination in secondary progressive multiple sclerosis. J Neuroimmunol. 2000;107:130–139. doi: 10.1016/s0165-5728(00)00235-6. [DOI] [PubMed] [Google Scholar]
- 118.Tennakoon DK, et al. Therapeutic induction of regulatory, cytotoxic CD8+ T cells in multiple sclerosis. J Immunol. 2006;176:7119–7129. doi: 10.4049/jimmunol.176.11.7119. [DOI] [PubMed] [Google Scholar]
- 119.Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-β1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997;77:45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 120.Luo J, et al. Glia-dependent TGF-β signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bettelli E, et al. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 122.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17- producing T helper cells during chronic inflammation of the central nervous system. Nature Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 123.Fitzgerald DC, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 124.Pflanz S, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 125.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 126.Kleinschek MA, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ousman SS, et al. Protective and therapeutic role for αB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 128.Beriou G, et al. IL-17 producing human peripheral regulatory T cells retain suppressive function. Blood. 2009 Jan 26; doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blink SE, Miller SD. The contribution of γδ T cells to the pathogenesis of EAE and MS. Curr Mol Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ponomarev ED, Dittel BN. γδ T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 132.Lees JR, Iwakura Y, Russell JH. Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J Immunol. 2008;180:8066–8072. doi: 10.4049/jimmunol.180.12.8066. [DOI] [PubMed] [Google Scholar]
- 133.Hauser SL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. This paper reports the findings of a Phase II clinical trial that indicate depletion of B cells mediated by rituximab is beneficial in patients with multiple sclerosis. [DOI] [PubMed] [Google Scholar]
- 134.McLaughlin KA, Wucherpfennig KW. B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol. 2008;98:121–149. doi: 10.1016/S0065-2776(08)00404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Colombo M, et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164:2782–2789. doi: 10.4049/jimmunol.164.5.2782. [DOI] [PubMed] [Google Scholar]
- 136.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Magliozzi R, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 138.Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. T- and B-cell responses to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis and multiple sclerosis. Glia. 2001;36:220–234. doi: 10.1002/glia.1111. [DOI] [PubMed] [Google Scholar]
- 139.Mathey EK, et al. Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 143.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 144.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duddy M, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 146.Chung Y, et al. Critical regulation of early TH17 cell differentiation by interleukin-1 signalling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]