Abstract
Background:
Rectal bleeding is a recognised early symptom of colorectal cancer. This study aimed to assess the diagnostic accuracy of symptoms, signs and diagnostic tests in patients with rectal bleeding in relation to risk of colorectal cancer in primary care.
Methods:
Diagnostic accuracy systematic review. Medline (1966 to May 2009), Embase (1988 to May 2009), British Nursing Index (1991 to May 2009) and PsychINFO (1970 to May 2009) were searched. We included cohort studies that assessed the diagnostic utility of rectal bleeding in combination with other symptoms, signs and diagnostic tests in primary care. An eight-point quality assessment tool was produced to assess the quality of included studies. Pooled positive likelihood ratios (PLRs), sensitivities and specificities were calculated.
Results:
Eight studies incorporating 2323 patients were included. Average weighted prior probability of colorectal cancer was 7.0% (range: 3.3–15.4%, median: 8.1%). Age ⩾60 years (pooled PLR: 2.79, 95% confidence interval (CI) 2.00–3.90), weight loss (pooled PLR: 1.89, 95% CI: 1.03–3.07) and change in bowel habit (pooled PLR: 1.92, 95% CI: 0.54–3.57) raise the probability of colorectal cancer into the range of referral to secondary care but do not conclusively ‘rule in’ the diagnosis. Presence of severe anaemia has the highest diagnostic value (pooled PLR: 3.67, 95% CI: 1.30–10.35), specificity 0.95 (95% CI: 0.93–0.96), but still only generates a post-test probability of 21.6%.
Conclusions:
In patients with rectal bleeding who present to their general practitioner, additional ‘red flag’ symptoms have modest diagnostic value. These findings have implications in relation to recommendations contained in clinical practice guidelines.
Keywords: rectal bleeding, diagnosis, colorectal cancer, primary care
Patients presenting with symptoms of rectal bleeding commonly seek medical advice in primary care (Chaplin et al, 2000). A Dutch national survey on primary care revealed an incidence of rectal bleeding of 1.6 per 1000 (95% confidence interval (CI): 1.4–1.8) people in the general population seeking medical help from their general practitioner (Linden et al, 2004). However, the majority of patients with rectal bleeding in primary care do not have serious disease, with estimates of the risk of colorectal cancer varying between 2.4 and 11.0% (Douek et al, 1999; du Toit et al, 2006).
As rectal bleeding is a recognised early symptom of colorectal cancer, primary care has an important role in its early detection (Jones and Kennedy, 1999). Timely and efficient referral leading to early diagnosis of colorectal cancer may contribute to improved survival (Gondos et al, 2008). Current UK guidelines recommend urgent referral of patients aged 40 years and older who report rectal bleeding with a change of bowel habit towards looser stools and/or increased stool frequency persisting for 6 weeks or more. Patients aged above 60 years should be urgently referred if they have rectal bleeding alone or changed bowel habit without anal symptoms for 6 weeks or more (National Institute for Health and Clinical Excellence, 2005). Referring patients at low risk of colorectal cancer may lead to unnecessary harm (patient anxiety and iatrogenic harm from further diagnostic investigations) and longer waiting time for high-risk patients. An observational study in the United Kingdom reported an average time interval of 47 days between symptom presentation and diagnosis (Barrett et al, 2006).
The incidence of colorectal cancer in people experiencing rectal bleeding in the general population is <1 per 1000 people, and increases to 20–110 per 1000 patients in a primary care setting, and to 360 per 1000 patients in a secondary care setting (Fijten et al, 1994). These differing incidences reflect the increasing probability of colorectal cancer in each community and hospital setting. In general, two selection processes occur as a patient seeks medical help and is assessed in primary care. First, a patient with rectal bleeding decides whether or not to visit a general practitioner regarding his/her symptom of rectal bleeding. About 28–41% of people experiencing rectal bleeding consult their general practitioner (Crosland and Jones, 1995; Thompson et al, 2000). Second, their general practitioner performs a gate-keeping function, prioritising patients in terms of probability of colorectal cancer.
The aim of this diagnostic accuracy systematic review is to assess the additional diagnostic value of concurrent symptoms, signs and diagnostic tests in patients presenting to their general practitioner with rectal bleeding to stratify patients into low, medium or high probability groups of colorectal cancer and assist clinical decision making in primary care.
Materials and methods
Search strategy
An electronic search was performed using Medline (1966 to May 2009), Embase (1988 to May 2009), British Nursing Index (1991 to May 2009) and PsychINFO (1970 to May 2009). Combinations of MeSH terms and text words were used including: ‘Anal/ Rectal/ Colorectal/ Gastrointestinal’, ‘Bleeding/ Haemorrhage’, ‘Colorectal cancer/ Neoplasm’, ‘General Practice’, ‘Family Practice’ and ‘Primary Care’. Bibliographies and references of included studies, review articles and clinical guidelines were also searched. An unrestricted electronic search filter was used (Leeflang et al, 2008). No restrictions were placed on language.
Study selection
Studies were independently selected by MOB and GF. If no consensus was achieved, studies were assessed by a third independent reviewer (TF). The inclusion and exclusion criteria were as follows:
Population: unselected symptomatic patients recruited from a primary care setting presenting with the symptom of rectal bleeding.
Study design: prospective cohort studies in a general practice setting. Other forms of observational studies, such as case–control studies were excluded. Screening studies and all types of retrospective studies were also excluded.
Index test and reference standard: studies that investigate the diagnostic accuracy of symptoms, signs and diagnostic tests in relation colorectal cancer. Reference standard includes colonoscopy, flexible sigmoidoscopy, rigid sigmoidoscopy, barium enaema as well as follow-up over time.
Outcome measures: Presence of colorectal cancer with data enabling the construction of 2 × 2 tables for the assessment of diagnostic accuracy of individual symptoms, signs or diagnostic tests.
Quality assessment
An eight-point quality assessment tool was created to assess the quality of included studies. The assessment tool was applied by two independent reviewers (MOB, GF) and includes criteria from the studies by Whiting et al (2006) (QUADAS) and Laupacis et al (1997).
Data extraction
Data from individual studies were independently extracted in duplicate by two reviewers (MOB, GF). If studies were eligible for inclusion, but data were insufficient to construct 2 × 2 tables, authors were contacted and asked to provide additional information.
Statistical analysis
A weighted average prior probability was calculated by adding up the priors of the sub-studies, but multiplying the individual priors by the proportion of patients in the sub-study in relation to the total number of patients in all studies together, therefore, allowing larger studies to have more influence on the prior. It is calculated in a following way: ((prior study Xi × (nstudyXi/ntotal)+…+prior study Xi+x × (nstudyXi+x/ntotal))/number of included studies.
For the meta-analyses, a bivariate, random effects approach was used (Reitsma et al, 2005). The bivariate, random effects model focuses on estimating an average sensitivity and specificity, also estimating the unexplained variation in these parameters and the correlation between them. A summary estimate with a corresponding confidence bound of the average sensitivity and specificity across studies was computed for each symptom and sign. The bivariate, random-effects model along with the hierarchical summary receiver operating characteristic method are recommended over the more traditional methods of meta-analysis (Harbord et al, 2008). The DiagMeta package in R was used for the meta-analyses in which data from at least four studies were available (R Development Core Team, 2008), otherwise summary receiver operating characteristic curves were constructed using the random effects DerSimonian–Laird model (DerSimonian and Laird, 1986).
In terms of estimating the clinical value of symptoms, signs and test results, pooled likelihood ratios are estimated. Likelihood ratios are the most accessible way to refine clinical diagnosis on the basis of symptoms, signs and test results (Grimes and Schulz, 2005). A likelihood ratio >1 indicates an increase in probability of colorectal cancer, whereas a likelihood ratio <1 is associated with a decrease in the probability colorectal cancer.
Results
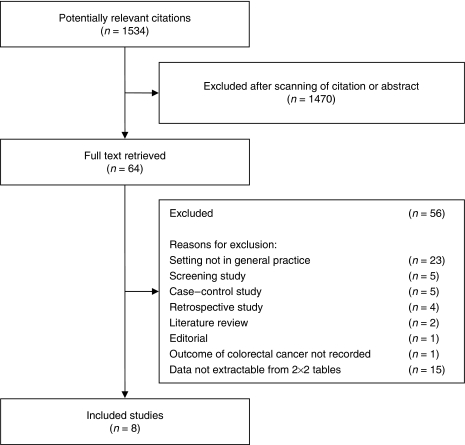
The search strategy identified 1534 potential relevant citations. Eight studies met our inclusion criteria and were included in the final analysis (Figure 1; Mant et al, 1989; Fijten et al, 1995; Metcalf et al, 1996; Norrelund and Norrelund, 1996; Wauters et al, 2000; Ellis and Thompson, 2005; Heintze et al, 2005; du Toit et al, 2006).
Figure 1.
Flow of studies through review process.
Characteristics of the included studies
The eight studies included 2323 patients and were carried out in primary care settings in England (Metcalf et al, 1996; Ellis and Thompson, 2005; du Toit et al, 2006), the Netherlands (Fijten et al, 1995), Germany (Heintze et al, 2005), Denmark (Norrelund and Norrelund, 1996), Belgium (Wauters et al, 2000) and Australia (Mant et al, 1989). The mean weighted prior probability of colorectal cancer was 7.0% (range: 3.3–15.4%, median: 8.1%). All studies included patients presenting with rectal bleeding in primary care and assessed the diagnostic accuracy of additional symptoms, signs and diagnostic tests. Summary characteristics of each included study are presented in Table 1.
Table 1. Summary of the included studies.
| Author, Year | Number of patients Mean age (range) Sex (♂:♀) | Patient population Setting | Prior colorectal cancer | Reference standard and number or percentage of patients receiving it Follow-up | Prevalence of symptoms/signs/patient characteristics | PLR | |
|---|---|---|---|---|---|---|---|
| Du Toit et al, 2006 | 265 pt ND years (45–ND years)♂ND: ♀ND | Pt ⩾ 45 years with new onset rectal bleeding, irrespective of other symptoms. Rural practice in England; four doctors; one registrar. | 5.7% (15 of 265) | Rigid sigmoidoscopy with barium enaema (most patients), flexible sigmoidoscopy, or colonoscopy. (p 69) Follow-up: unclear | Patient characteristics Age 45–54 years Age 55–64 years Age 65–74 years Age ⩾75 years | % Population 19% 28% 24% 29% | PLR 0.7 0.2 1.8 1.4 |
| Ellis and Thompson 2005 | 319 pt 59 years (35–94 years) ♂143: ♀176 | Pt 35 years consulting their GP with rectal bleeding 19 GPs in 3 practices in the United Kingdom: 1 market town/rural community; 1 suburban; 1 inner-city | 3.4% (11 of 319) | -Flexible sigmoidoscopy (219 pt) -Patient questionnaire (47 pt) -Flexible sigmoidoscopy & questionnaire (53 pt) -Barium enaema (37 pt) -Colonoscopy (24 pt) Follow-up:18 months | Symptoms/signs/patient characteristics Bleeding and CIBH (n=119) Bleeding and CIBH (loose +/− frequent) (n=83) | % Population 37% 26% | PLR 2.4 1.3 |
| Bleeding and no perianal symptoms (n=63) | 20% | 2.9 | |||||
| Bleeding CIBH and abdominal pain (n=67) | 21% | 1.0 | |||||
| Dark blood (n=31) | 10% | 2.1 | |||||
| Age ⩾ 60 years (n=155) | 49% | 1.5 | |||||
| Blood on paper only (n=82) | 26% | 0.6 | |||||
| Large volume of blood (n=79) | 25% | 0.3 | |||||
| First time rectal bleeding (n=106) | 33% | 1.2 | |||||
| Blood mixed with stool (n=33) | 10% | 0.7 | |||||
| Fijten et al, 1995 | 269 pt 42 years (18–75 years) ♂118: ♀151 | Patients ⩾ 18 years and ⩽75 years with overt rectal bleeding as a reason for consult or history of recent (<3 month) blood loss visible. 83 GPs in the South of the Netherlands | 3.3% (9 of 269) | A total of 31% had further investigations initiated by the GP by means of sigmoidoscopy (9%) colon roentenography (9%), proctoscopy (8%), sonography (6%) and colonoscopy (2%). Some patients underwent more than one investigation. Follow-up: at least 1 year (mean 20 months) Medical records and information of the GP. | Symptoms/signs Blood seen: Mixed with stool only On stool or mixed with stool only Others or combinations | % Population 5% 20% 45% | PLR 8.0 3.8 0.4 |
| Abdominal pain | 50% | 0.7 | |||||
| Change in bowel habit (more frequently or diarrhoea or variously, but not constipation) | 29% | 2.9 | |||||
| Pain at night | 19% | 0.0 | |||||
| Decreased appetite | 16% | 0.7 | |||||
| Nausea | 25% | 0.4 | |||||
| Weight loss | 16% | 3.0 | |||||
| Family history of abdominal disease | 31% | 0.0 | |||||
| Previous history of rectal bleeding | 36% | 0.0 | |||||
| Pale conjunctivae | 2% | 5.8 | |||||
| Perianal eczema | 6% | 6.2 | |||||
| Rectal palpation (n=208): | |||||||
| Haemorrhoid | 7% | 2.5 | |||||
| Tumour | 0% | 1.0 | |||||
| Abnormal prostate | 1% | 22.3 | |||||
| Patient characteristics | % Population | PLR | |||||
| Age 18–29 years | 23% | 0.0 | |||||
| Age 30–39 years | 26% | 0.0 | |||||
| Age 40–49 years | 20% | 0.0 | |||||
| Age 50–59 years | 15% | 0.7 | |||||
| Age 60–75 years | 15% | 7.2 | |||||
| Male | 44% | 1.8 | |||||
| Laboratory test results | % Population | PLR | |||||
| Anaemia (Hb♀ < 7.5 mmol l−1 ♂<8.5 mmol l−1) | 5% | 6.6 | |||||
| ESR high (♀>28 mm h−1♂>8.5 mm h−1) | 9% | 4.2 | |||||
| ESR high (>30 mm h−1) | 4% | 8.8 | |||||
| High white blood cell count (>109 per litre) (n=219) | 9% | 5.8 | |||||
| Haemoccult ⩾1 positive out of 3 | 15% | 2.3 | |||||
| Heintze et al, 2005 | 422 pt ND years (ND–ND years) ♂199: ♀222 | Patients >15 years 94 GPs in Germany | 4.0% (17 of 422) | Diagnostic work-up: Sonography (52 pt) Rectoscopy (29 pt) Sigmoidscopy (26 pt) Colonoscopy (195 pt) Treatment by GP (93pt) Follow-up: Unclear | Symptoms/signs/patient characteristics Male Age <50 years Age ⩾50 years Age 15–24 years | % Population 53% 38% 62% 2% | PLR 1.3 0.2 1.5 0.0 |
| Age 25–34 years | 11% | 0.4 | |||||
| Age 35–44 years | 14% | 0.3 | |||||
| Age 45–54 years | 16% | 0.5 | |||||
| Age 55–64 years | 28% | 1.3 | |||||
| Age 65–74 years | 18% | 1.7 | |||||
| Age 75–84 years | 8% | 0.5 | |||||
| Age 85–94 years | 2% | 8.4 | |||||
| Weight loss | 3% | 1.3 | |||||
| Changed bowel habit | 18% | 1.2 | |||||
| Abdominal pain | 24% | 0.7 | |||||
| Anaemia | 6% | 2.4 | |||||
| Dark red blood | 12% | 1.1 | |||||
| Blood mixed with stool | 19% | 1.9 | |||||
| Family history of colon carcinoma | 7% | 3.6 | |||||
| Mant et al, 1989 | 145 pt 58 years (40–95 years) ♂77: ♀68 | Pt ⩾ 40 years who consulted the GP for rectal bleeding 48 GPs in Australia | 11% (16 of 145) | -Total colonoscopy (104 pt) -Endoscopy to at least 30 cm and an air-contrast barium enaema (32 pt)-Investigations not complete, but an obvious source was found, e.g. rectal cancer at proctoscopy. (9 pt) Follow-up: unclear | Symptoms/signs/patient characteristics Male First-degree relative with CRC (n=143) Abdominal pain (n=144) | % Population 53% 14% 30% | PLR 0.8 0.9 0.8 |
| Change in bowel habit (n=143) | 39% | 1.0 | |||||
| Feeling of incomplete evacuation of rectum | 29% | 1.1 | |||||
| Weight Loss (n=143) | 10% | 1.3 | |||||
| Anal itch | 25% | 0.2 | |||||
| Pain on defecation | 21% | 0.6 | |||||
| Anal protrusion noticed by patient | 21% | 0.3 | |||||
| Dark red blood (n=144) | 16% | 1.7 | |||||
| Blood mixed with faeces (n=140) | 36% | 2.2 | |||||
| Haemorrhoids identified by GP | 51% | 0.5 | |||||
| Metcalf et al, 1996 | 99 pt 58 years (40–86 years) ♂42: ♀57 | Patients ⩾ 40 years 17 GPs in Newcastle upon Tyne, England | 8.1% (8 of 99) | Questionnaire (99pt) Colonoscopy (98pt) Barium enaema in any patients whom a satisfactory colonoscopy was not completed. (1pt) Follow-up: Unclear (Practices participated between 1–9 months) | Symptoms/signs/patient characteristics Dark red blood loss Blood mixed with stool Diarrhoea Associated slime | % Population 31% 46% 32% 28% | PLR 1.2 1.4 0.9 1.4 |
| Constipation | 39% | 0.3 | |||||
| Change in bowel habit | 39% | 1.3 | |||||
| Abdominal pain | 42% | 0.9 | |||||
| Weight loss | 15% | 1.8 | |||||
| Norrelund and Norrelund 1996(1) | 208 pt 42 years (18–75 years) ♂97: ♀111 | Patients ⩾ 40 years presenting with a first episode of rectal bleeding 96 GPs from Denmark | 15.4% (32 of 208) | GPs were asked to arrange either a barium enaema or a colonoscopy at the first consultation. Follow-up: 32 months Colorectal cancer microscopically verified or yearly letter to GP | Symptoms/signs/patient characteristics Male Age 40–69 years Age 70–79 years Age 80+ years | % Population 47% 68% 25% 7% | PLR 1.3 0.3 3.3 2.2 |
| Weight loss | 11% | 1.6 | |||||
| Abdominal pain | 23% | 1.5 | |||||
| Change in bowel habits | 29% | 2.6 | |||||
| Discomfort | 27% | 1.3 | |||||
| Norrelund and Norrelund 1996(2) | 156 pt 42 years (18–75 years) ♂71: ♀85 | Patients ⩾ 40 years first bleeding episode or change in usual bleeding pattern 112 GPs from Denmark | 14.1% (22 of 156) | GPs were asked to arrange either a barium enaema or a colonoscopy at the first consultation. Follow-up: 22 months CRC microscopically verified or yearly letter to GP | Symptoms/signs/patient characteristics Male Age 40–69 years Age 70–79 years Age 80+ years | % Population 46% 72% 21% 7% | PLR 1.0 0.7 2.4 0.6 |
| Weight loss | 14% | 1.8 | |||||
| Abdominal pain | 27% | 2.2 | |||||
| Change in bowel habits | 31% | 1.6 | |||||
| Discomfort | 26% | 0.9 | |||||
| New rectal bleeding | 69% | 0.8 | |||||
| Wauters et al, 2000 | 386 pt ND years (ND-ND years) ♂ND: ♀ND | Network of sentinel practices in Belgium | 7.0% (27 of 386) | Investigations such as endoscopy were not systematically performed. ‘To obtain the number of all new cases of cancer, we sent recall letters to the practices every six months and at the end of the follow-up period.’ (p 998) Follow-up (clinical): 18–30 months | Symptoms/signs/patient characteristics Age <50 years Age 50–59 years Age 60–69 years Age 70–79 years Age ⩾ 80 years Pain | % Population 37% 15% 18% 17% 13% 9% | PLR 0.1 0.2 1.7 3.6 0.8 0.0 |
| Spasms | 29% | 0.8 | |||||
| Weight loss | 6% | 2.5 | |||||
| Palpable tumour | 5% | 6.1 | |||||
Abbreviations: CIBH=change in bowel habit; ND=no data available. The page numbers refer to the original text of the included studies.
Quality of the included studies
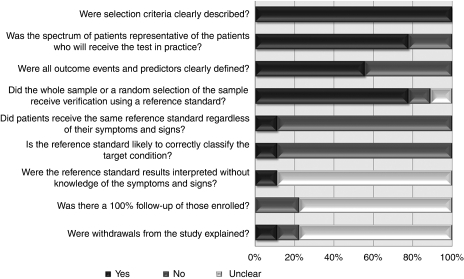
The quality assessment of individual studies is presented in Table 2. In six studies, either the entire population or a random selection of the eligible population were subjected to a reference standard. The remaining two studies did not apply a reference standard to any of the included participants (Heintze et al, 2005; du Toit et al, 2006). Only one study applied the same reference standard (colonoscopy) to all included participants (Metcalf et al, 1996). Blinding of outcome assessment was poorly reported (Table 2). A summary diagram of the quality assessment is shown in Figure 2.
Table 2. Methodological standards for quality assessment of included studies.
| Study ID | Were selection criteria clearly described? (Whiting et al, 2006) | Was the spectrum of patients representative of the patients who will receive the test in practice? (Whiting et al, 2006) | Were all outcome events and predictors clearly defined? (Laupacis et al, 1997) | Did the whole sample or a random selection of the sample receive verification using a reference standard? (Whiting et al, 2006) | Did patients receive the same reference standard regardless of their symptoms and signs? (Whiting et al, 2006) | Is the reference standard likely to correctly classify the target condition? (Whiting et al, 2006) | Were the reference standard results interpreted without knowledge of the symptoms and signs? (Whiting et al, 2006) | Was there a 100% follow-up of those enrolled? (Laupacis et al, 1997) Were withdrawals from the study explained? (Whiting et al, 2006) |
|---|---|---|---|---|---|---|---|---|
| Du Toit et al (2006) | Yes | Yes | No | Unclear “A small number of patients may not have entered the diagnostic protocol, despite of frequent reminders.” (p 69) | No -Rigid sigmoidoscopy -Flexible sigmoidoscopy -Colonoscopy | Suboptimal Suboptimal Best method available | Unclear | -No -Withdrawals not explained |
| Ellis and Thompson (2005) | Yes | Yes | No | Yes | No -Flexible sigmoidoscopy (219 pt) -Patient questionnaire (47 pt) -Flexible sigmoidscopy & questionnaire (53 pt) -Barium enaema (37 pt) -Colonoscopy (24 pt) | Suboptimal Not suitable Suboptimal Suboptimal Best method available | Unclear | Unclear |
| Fijten et al (1994) | Yes | Yes | Yes | Yes | No -Sigmoidoscopy (8 pt) -Colon-roentenography (8 pt) -Proctoscopy (7 pt) Sonography (5 pt) -Colonoscopy (2 pt) | Suboptimal Suboptimal Suboptimal Suboptimal Best method available | Yes | -No -Yes “21 patients excluded because lost of follow-up. (moved to an unknown destination)” |
| Heintze et al (2005) | Yes | Yes | Yes | No The selection of patients having further investigation was not at random | No -Sonography (52 pt) -Rectoscopy (29 pt) -Sigmoidoscopy (26 pt) -Colonoscopy (195 pt) | Suboptimal Suboptimal Suboptimal Best method available | Unclear | Unclear |
| Mant et al (1989) | Yes | Yes | No | Yes | No -Total colonoscopy (104 pt) -Endoscopy to at least 30 cm and an air-contrast barium enaema (32 pt) - Investigations not complete, but an obvious source was found, for example, rectal cancer at proctoscopy. (9 pt) | Best method available Suboptimal Suboptimal | Unclear | Unclear |
| Metcalf et al (1996) | Yes | Yes | Yes | Yes | Yes Colonoscopy 98 pt Barium enaema 1 pt (because colonoscopy was impossible) | Best method available | Unclear “The questionnaire was re-administered by the colonoscopist before the procedure” (p162) | Unclear |
| Norrelund and Norrelund (1996) (1) | Yes | No Selection made during recruitment. GPs were allowed to include a maximum of 3 patients. | Yes | Yes | No GPs were asked to arrange either a barium enaema or a colonoscopy at the first consultation. (p161) | Suboptimal “Although the authors asked the GP to refer all patients for a full colon examination, but this was no inclusion criterion.” (p165) | Unclear | Unclear |
| Norrelund and Norrelund (1996) (2) | Yes | No Selection made during recruitment. GPs were allowed to include a maximum of 4 patients. | Yes | Yes | No GPs were asked to arrange either a barium enaema or a colonoscopy at the first consultation. (p161) | Suboptimal | Unclear | Unclear |
| Wauters et al (2000) | Yes | Yes | No Predictors clearly defined, but outcomes only colorectal cancer reported. | Yes | No “Our reference standard was colorectal cancer diagnosed during a clinical follow-up of 18–30 months. Investigations, such as endoscopy, were not systematically performed.” (p 998) | Suboptimal | Unclear | Unclear |
Abbreviations: GP=general practitioners; pt=patient. The page numbers refer to the original text of the included studies.
Figure 2.
Summary diagram of the quality assessment of included studies.
Definition of the reference standard test and follow up
A variety of reference standards were used: colonoscopy, rigid sigmoidoscopy with (double contrast) barium enaema, air-contrast barium enaema, flexible sigmoidoscopy, flexible sigmoidoscopy and questionnaire, a questionnaire only, barium enaema only, and proctoscopy with sonography. Only two studies describe how many patients underwent a particular reference standard investigation (Mant et al, 1989; Metcalf et al, 1996). Follow-up was adequately described in three of the eight studies and ranged from, at least, 12 to 32 months (Fijten et al, 1995; Norrelund and Norrelund, 1996; Wauters et al, 2000). Follow-up was carried out by either sending recall letters to the general practitioner to obtain the number of all the new cases of cancer (Wauters et al, 2000), microscopic verification of colorectal cancer or an yearly letter to the general practitioner (Norrelund and Norrelund, 1996), or by checking medical records and information provided by the general practitioner (Fijten et al, 1995).
Diagnostic value of rectal bleeding and related symptoms, signs and diagnostic tests
In the primary studies, all patients had rectal bleeding and presented with additional symptoms. The pooled positive likelihood ratios (PLRs), sensitivities and specificities for individual symptoms, signs and diagnostic tests are presented in Table 3. Overall, the magnitudes of the pooled PLRs are modest, with no individual symptom, sign or diagnostic test able to alter the probability of colorectal cancer into a definite range of ‘ruling in’ or ‘ruling out’ the diagnosis of colorectal cancer. Even classical symptoms, such as a history of weight loss and anaemia yield a modest pooled positive likelihood ratio of 1.89 (95% CI: 1.03–3.07) and 3.67 (95% CI: 1.30–10.35), respectively. Pooled sensitivities are low, varying from 0.17 to 0.62. Weight loss and anaemia yield a pooled specificity of 0.91 (95% CI: 0.83–0.96) and 0.95 (95% CI: 0.93–0.96), respectively (Table 3).
Table 3. Clinical value of symptoms and signs in patients presenting with rectal bleeding in terms of colorectal cancer.
| No of studiesa | No of patients | Sens | (95% CI) | Spec | (95% CI) | Pooled PLR | (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | ||||||||
| Male | 5 | 1253 | 0.58 | (0.48–0.67) | 0.52 | (0.48–0.56) | 1.21 | (1.00–1.46) |
| Age <40 yearsb | 2 | 745 | 0.03 | (0.00–0.16) | 0.73 | (0.69–0.76) | 0.32 | (0.05–2.21) |
| Age 40–59 yearsb | 4 | 1387 | 0.09 | (0.04–0.19) | 0.79 | (0.70–0.86) | 0.41 | (0.18–0.90) |
| Age ⩾ 60 yearsb | 6 | 1760 | 0.66 | (0.45–0.83) | 0.76 | (0.68–0.83) | 2.79 | (2.00–3.90) |
| Family history colorectal cancer | 3 | 886 | 0.15 | (0.06–0.28) | 0.85 | (0.82–0.87) | 1.05 | (0.16–6.88) |
| Symptoms | ||||||||
| Dark red bloodc | 4 | 949 | 0.22 | (0.13–0.34) | 0.84 | (0.69–0.93) | 1.37 | (0.59–3.30) |
| Weight loss | 7 | 1737 | 0.17 | (0.06–0.37) | 0.91 | (0.83–0.96) | 1.89 | (1.03–3.07) |
| Abdominal pain | 7 | 1739 | 0.25 | (0.04–0.62) | 0.73 | (0.52–0.89) | 0.94 | (0.19–1.59) |
| Changed bowel habit | 5 | 1254 | 0.62 | (0.18–0.94) | 0.68 | (0.53–0.80) | 1.92 | (0.54–3.57) |
| Blood mixed with the stool | 5 | 1225 | 0.40 | (0.04–0.93) | 0.81 | (0.23–0.98) | 1.91 | (0.75–5.51) |
| Previous history of rectal bleedingd | 2 | 425 | 0.30 | (0.05–0.41) | 0.66 | (0.63–0.71) | 0.58 | (0.14–1.41) |
| Perianal symptoms – pain on defecation | 2 | 411 | 0.22 | (0.13–0.36) | 0.41 | (0.22–0.78) | 0.49 | (0.25–0.97) |
| Perianal symptoms – itch/eczema | 2 | 414 | 0.17 | (0.07–0.33) | 0.87 | (0.73–0.95) | 1.31 | (0.25–6.21) |
| Signs and diagnostic tests | ||||||||
| Rectal palpation – haemorrhoid | 2 | 354 | 0.24 | (0.09–0.45) | 0.73 | (0.46–0.91) | 0.51 | (0.09–2.97) |
| Anaemia (Hb ♀<12.0 g per 100 ml ♂<13.3 g per 100 ml) | 2 | 700 | 0.17 | (0.05–0.35) | 0.95 | (0.93–0.96) | 3.67 | (1.30–10.35) |
Abbreviations: CI=confidence interval; Hb, haemoglobin; PLR=positive likelihood ratio.
Norrelund and Norrelund (1996) consists of two independent sub-studies, and therefore are independently assessed. In the column ‘no of studies’ these two substudies are counted as two separate studies.
There is a slight age overlap between the individual studies.
The reference category of dark red blood consists of patients having bright red blood or a colour in between.
The reference category of previous history of rectal bleeding consists of patients having a first episode of rectal bleeding.
Discussion
Principal results
No individual symptom, sign or diagnostic test in patients with rectal bleeding is likely to shift the probability of colorectal cancer to the extent of ‘ruling in’ or ‘ruling out’ the diagnosis with any degree of certainty. Even the presence of anaemia (<12.0 g per 100 ml for women and <13.3 g per 100 ml for men) produces a shift in post-test probability to 21.6% (assuming a prior probability of 7.0%), a level that requires further diagnostic testing before colorectal cancer diagnosis is confirmed. ‘Red flag’ symptoms, such as weight loss and blood mixed with stool, seem to have only modest diagnostic value. Although the presence of these symptoms nearly doubles the post-test probability of colorectal cancer to about 13%, and their presence should ensure referral for further investigation, caution is needed when counselling patients about the possible reasons for their referral in terms of likely diagnoses. The fact that a presenting patient may be aged over 60 years also only provides modest diagnostic value in terms of probability of colorectal cancer (Table 3).
The findings from this systematic review have implications for clinical practice guidelines, showing that considerable diagnostic uncertainty is likely to exist in patients presenting to their general practitioner, even when they have additional symptoms, signs or test results that are conventionally associated with an increased risk of colorectal cancer (Hamilton and Sharp, 2004). The ideal threshold for referral is subject to several factors: individual patient's utilities or values regarding timely identification of colorectal cancer balanced against the iatrogenic harm and psychological damage of unnecessary investigation, and potential harm in patients free from disease. In addition, cost effectiveness of different referral thresholds in relation to probability of colorectal cancer also needs to be considered. To resolve these difficulties, formal cost utility estimates are required, which incorporate patient's utilities and cost at different referral thresholds.
Context of previous studies
Our results differ from a recent UK case–control study that assessed the diagnostic value of clinical features of colorectal cancer before diagnosis. This study identified cases from a cancer registry and controls selected and matched in terms of age and registration with a general practice (Hamilton et al, 2005). In this case–control study, PLRs were considerably higher than found in this systematic review of cohort studies. For example, PLRs for weight loss (5.1), abdominal pain (4.5) and anaemia <10 g per 100 ml (9.5) would all be associated with definitive shifts in the probability of colorectal cancer (Grimes and Schulz, 2005). The most likely explanation for this discordant finding is that recall bias amongst controls may have produced a comparison group that did not remember having colorectal symptoms in the past, thus inflating estimates of diagnostic utility for symptoms, signs and diagnostic tests when compared with individuals with colorectal cancer (Grimes and Schulz, 2002).
Our results are more consistent with a recent diagnostic accuracy review of cohort studies that included patients in both primary and secondary care presenting with ‘alarm’ features (Ford et al, 2008). In their systematic review the overall conclusion was that most alarm features of colorectal cancer had poor sensitivity and specificity for the diagnosis of colorectal cancer. The presence of rectal bleeding (PLR: 1.32, 95% CI: 1.19–1.47), weight loss (PLR: 1.96, 95% CI: 1.25–3.08) or iron deficiency anaemia (PLR: 1.43, 95% CI: 0.75–2.74) do raise the probability of colorectal cancer but only to a modest extent. The results from this systematic review of cohort studies in primary care, in which rectal bleeding was an inclusion criterion, are broadly similar in relation to diagnostic utility of symptoms, signs and diagnostic tests.
Our findings suggest that older age and iron deficiency anaemia are predictive of colorectal cancer. These findings are consistent with several previous studies. Panzuto et al (2003) showed that age >50 years and iron deficiency anaemia are independently associated with colorectal cancer in primary care (odds ratios: 9.0 and 8.8 on multivariable analysis, respectively). Patients with right-sided bowel cancers have a significantly lower haemoglobin level at presentation than those with left-sided cancers (Yates et al, 2004; Masson et al, 2007). There seems to be a trade-off in relation to the diagnostic and prognostic value of the presence of anaemia and colorectal cancer, whereas presence of anaemia is most useful in ruling in a diagnosis of colorectal cancer, it is also associated with a more advanced disease and a poorer prognosis (Stapley et al, 2006).
Lastly, having a positive family history for colorectal cancer has been cited as being associated with an increased risk of current colorectal cancer (Bonelli et al, 1988; Slattery et al, 2003). The three included studies assessing family history of colorectal cancer yield varying and inconsistent likelihood ratios (Mant et al, 1989; Fijten et al, 1995; Heintze et al, 2005). Heintze et al (2005) calculated a PLR of 3.65, whereas Fijten et al (1995) and Mant et al, 1989) reported a PLR <1. More research is needed regarding the definition of positive family history, how it might relate to risk of colorectal cancer and the impact of using family history as a preliminary screening question prior to Faecal Occult Blood (FOB) screening programs (Polmear and Glasziou, 2008).
Limitations of the present study
The validity of the results of this systematic review is determined by an independent, unbiased selection process. However, any systematic review may be susceptible to publication bias (Irwig et al, 1994, 1995; Deeks, 2001). The quality of the review is dependent on the quality of the included cohort studies. Several dimensions that relate to the quality of the included studies are unclear or inadequately reported (Table 2, online). This finding is not intended as a criticism of the original studies, but is more a reflection on the considerable challenges of undertaking cohort studies in primary care settings that rely on complete identification and follow-up of all eligible incident cases of rectal bleeding. For instance, in one included study, general practitioners were asked to include a maximum of three to four patients (Norrelund and Norrelund, 1996). This prior selection may lead to a preferential selection of more severe cases and subsequent spectrum bias, producing spurious clinical associations and overestimation of likelihood ratios (Jelinek, 2008).
The other feature of included studies was the application of a variety of different reference standards. In the detection of colorectal cancer, the most sensitive and specific diagnostic test is colonoscopy, followed by a flexible sigmoidoscopy in combination with a barium enaema (Irvine et al, 1988; Rex et al, 1990; Helfand et al, 1997). In the included studies, a variety of reference standard tests were used with a possibility of work-up bias in some studies as lower-risk patents were subject to less rigorous reference standard tests (Table 2). Other methodological problems include incomplete or inadequate blinding of outcome assessment and incomplete reporting on losses to follow-up (Table 2).
We found significant between-study heterogeneity for a range of symptoms and signs (Table 3). This might be due to whether or not rectal bleeding was the principal reason for consultation and also the duration of rectal bleeding. Three included studies report rectal bleeding as the primary complaint in 15%, 51% and 100% of patients, respectively, (Fijten et al, 1995; Metcalf et al, 1996; Wauters et al, 2000), and in terms of duration two studies excluded patients with rectal bleeding longer than six and twelve months, respectively (Mant et al, 1989; Metcalf et al, 1996).
For age categories, we calculated sensitivities, specificities and likelihood ratios. However, primary studies use different age cut-off points, which complicate the generation of reference categories. In our review, there is a slight overlap in some of the age categories, which may have affected the precision of pooled estimates. In terms of dark red blood, the reference category includes both patients with bright red blood and a colour in between. For a history of rectal bleeding the reference category consists of patients having a first episode of rectal bleeding.
Future studies
There are considerable challenges with undertaking cohort studies of rectal bleeding in primary care, including recruitment of consecutive patients; eliciting full history of symptoms, signs and diagnostic tests; consenting ‘low risk’ patients to potentially unpleasant and invasive reference standard tests, and ensuring adequate follow-up of patients. Furthermore, the relative rarity of colorectal cancer in primary care makes it difficult to design a study large enough to yield adequate power to detected significant clinical predictors of colorectal cancer (Hamilton and Sharp, 2004).
Despite these challenges, this systematic review shows that the evidence base is not substantial at present and that further studies are required, which assess the diagnostic accuracy of lower gastrointestinal symptoms in community settings. A study assessing the combined value of rectal bleeding and additional symptoms has been undertaken in secondary care, showing that patients presenting with rectal bleeding and a change in bowel habit without perianal symptoms are at highest risk of colorectal cancer (PLR: 4.2). Patients with rectal bleeding and perianal symptoms, but without a change in bowel habit had lowest risk of colorectal cancer (negative likelihood ratio: 1.3) (Thompson et al, 2007). Similar studies, focussing on patients presenting in primary care are needed.
Future studies should recruit consecutive patients, apply an agreed reference standard to all patients and evaluate the combined value of symptoms, signs and diagnostic tests in the form of a clinical prediction rule (McGinn et al, 2008). Future primary studies should also report their data completely, preferably using the Standards for Reporting of Diagnostic Accuracy (STARD) criteria as guidelines (Bossuyt et al, 2003).
In conclusion, this systematic review shows that no individual symptom, sign or diagnostic test in patients with rectal bleeding is likely to conclusively raise the probability of colorectal cancer in primary care settings. Even conventionally stated ‘red flag’ symptoms, such as weight loss and blood mixed with stool, have modest diagnostic value. Future studies are needed to establish the diagnostic value of individual and combined symptoms, signs and diagnostic tests so that the evidence base for appropriate and timely referral is more secure.
Acknowledgments
We thank Dr C Heintze for providing extra information from his study. We also thank Dr W Hamilton for clarifications regarding the Du Toit study. This study was supported by funds from the Health Research Board, Ireland.
References
- Barrett J, Jiwa M, Rose P, Hamilton W (2006) Pathways to the diagnosis of colorectal cancer: an observational study in three UK cities. Fam Pract 23: 15–19 [DOI] [PubMed] [Google Scholar]
- Bonelli L, Martines H, Conio M, Bruzzi P, Aste H (1988) Family history of colorectal cancer as a risk factor for benign and malignant tumours of the large bowel. A case-control study. Int J Cancer 41: 513–517 [DOI] [PubMed] [Google Scholar]
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J 44: 635–638 [PubMed] [Google Scholar]
- Chaplin A, Curless R, Thomson R, Barton R (2000) Prevalence of lower gastrointestinal symptoms and associated consultation behaviour in a British elderly population determined by face-to-face interview. Br J Gen Pract 50: 798–802 [PMC free article] [PubMed] [Google Scholar]
- Crosland A, Jones R (1995) Rectal bleeding: prevalence and consultation behaviour. BMJ 311: 486–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ (2001) Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 323: 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188 [DOI] [PubMed] [Google Scholar]
- Douek M, Wickramasinghe M, Clifton MA (1999) Does isolated rectal bleeding suggest colorectal cancer? Lancet 354: 393. [DOI] [PubMed] [Google Scholar]
- du Toit J, Hamilton W, Barraclough K (2006) Risk in primary care of colorectal cancer from new onset rectal bleeding: 10 year prospective study. BMJ 333: 69–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BG, Thompson MR (2005) Factors identifying higher risk rectal bleeding in general practice. Br J Gen Pract 55: 949–955 [PMC free article] [PubMed] [Google Scholar]
- Fijten GH, Blijham GH, Knottnerus JA (1994) Occurrence and clinical significance of overt blood loss per rectum in the general population and in medical practice. Br J Gen Pract 44: 320–325 [PMC free article] [PubMed] [Google Scholar]
- Fijten GH, Starmans R, Muris JW, Schouten HJ, Blijham GH, Knottnerus JA (1995) Predictive value of signs and symptoms for colorectal cancer in patients with rectal bleeding in general practice. Fam Pract 12: 279–286 [DOI] [PubMed] [Google Scholar]
- Ford AC, Veldhuyzen van Zanten SJ, Rodgers CC, Talley NJ, Vakil NB, Moayyedi P (2008) Diagnostic utility of alarm features for colorectal cancer: systematic review and meta-analysis. Gut 57: 1545–1553 [DOI] [PubMed] [Google Scholar]
- Gondos A, Bray F, Brewster DH, Coebergh JW, Hakulinen T, Janssen-Heijnen ML, Kurtinaitis J, Brenner H (2008) Recent trends in cancer survival across Europe between 2000 and 2004: a model-based period analysis from 12 cancer registries. Eur J Cancer 44: 1463–1475 [DOI] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF (2002) Bias and causal associations in observational research. Lancet 359: 248–252 [DOI] [PubMed] [Google Scholar]
- Grimes DA, Schulz KF (2005) Refining clinical diagnosis with likelihood ratios. Lancet 365: 1500–1505 [DOI] [PubMed] [Google Scholar]
- Hamilton W, Round A, Sharp D, Peters TJ (2005) Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 93: 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W, Sharp D (2004) Diagnosis of colorectal cancer in primary care: the evidence base for guidelines. Fam Pract 21: 99–106 [DOI] [PubMed] [Google Scholar]
- Harbord RM, Whiting P, Sterne JA, Egger M, Deeks JJ, Shang A, Bachmann LM (2008) An empirical comparison of methods for meta-analysis of diagnostic accuracy showed hierarchical models are necessary. J Clin Epidemiol 61: 1095–1103 [DOI] [PubMed] [Google Scholar]
- Heintze C, Matysiak-Klose D, Krohn T, Wolf U, Brand A, Meisner C, Fischer I, Wehrmeyer H, Braun V (2005) Diagnostic work-up of rectal bleeding in general practice. Br J Gen Pract 55: 14–19; discussion 18 [PMC free article] [PubMed] [Google Scholar]
- Helfand M, Marton KI, Zimmer-Gembeck MJ, Sox Jr HC (1997) History of visible rectal bleeding in a primary care population. Initial assessment and 10-year follow-up. JAMA 277: 44–48 [PubMed] [Google Scholar]
- Irvine EJ, O’Connor J, Frost RA, Shorvon P, Somers S, Stevenson GW, Hunt RH (1988) Prospective comparison of double contrast barium enema plus flexible sigmoidoscopy v colonoscopy in rectal bleeding: barium enema v colonoscopy in rectal bleeding. Gut 29: 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig L, Macaskill P, Glasziou P, Fahey M (1995) Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 48: 119–130; discussion 131–2 [DOI] [PubMed] [Google Scholar]
- Irwig L, Tosteson AN, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mosteller F (1994) Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med 120: 667–676 [DOI] [PubMed] [Google Scholar]
- Jelinek M (2008) Spectrum bias: why generalists and specialists do not connect. Evid Based Med 13: 132–133 [DOI] [PubMed] [Google Scholar]
- Jones R, Kennedy T (1999) The early detection of colorectal cancer in primary care. Br J Gen Pract 49: 956–958 [PMC free article] [PubMed] [Google Scholar]
- Laupacis A, Sekar N, Stiell IG (1997) Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 277: 488–494 [PubMed] [Google Scholar]
- Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM (2008) Systematic reviews of diagnostic test accuracy. Ann Intern Med 149: 889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MW, van der Westert GP, de Bakker DH, Schellevis FG (eds) (2004) Tweede Nationale Studie naar ziekten en verrichtingen in de huisartspraktijk: klachten en aandoeningen in de bevolking en in de huisartspraktijk]. NIVEL/ RIVM: Utrecht/ Bilthoven [Google Scholar]
- Mant A, Bokey EL, Chapuis PH, Killingback M, Hughes W, Koorey SG, Cook I, Goulston KJ, Dent OF (1989) Rectal bleeding. Do other symptoms aid in diagnosis? Dis Colon Rectum 32: 191–196 [DOI] [PubMed] [Google Scholar]
- Masson S, Chinn DJ, Tabaqchali MA, Waddup G, Dwarakanath AD (2007) Is anaemia relevant in the referral and diagnosis of colorectal cancer? Colorectal Dis 9: 736–739 [DOI] [PubMed] [Google Scholar]
- McGinn T, Jervis R, Wisnivesky J, Keitz S, Wyer PC (2008) Tips for teachers of evidence-based medicine: clinical prediction rules (CPRs) and estimating pretest probability. J Gen Intern Med 23: 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf JV, Smith J, Jones R, Record CO (1996) Incidence and causes of rectal bleeding in general practice as detected by colonoscopy. Br J Gen Pract 46: 161–164 [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence (2005) Referral Guidelines for Suspected Lower Gastrointestinal Cancer. NICE: London [Google Scholar]
- Norrelund N, Norrelund H (1996) Colorectal cancer and polyps in patients aged 40 years and over who consult a GP with rectal bleeding. Fam Pract 13: 160–165 [DOI] [PubMed] [Google Scholar]
- Panzuto F, Chiriatti A, Bevilacqua S, Giovannetti P, Russo G, Impinna S, Pistilli F, Capurso G, Annibale B, Delle Fave G (2003) Symptom-based approach to colorectal cancer: survey of primary care physicians in Italy. Dig Liver Dis 35: 869–875 [DOI] [PubMed] [Google Scholar]
- Polmear A, Glasziou P (2008) Evidence Based Diagnosis in Primary Care-Practical Solutions to Common Problems. Butterworth Heinemann Elsevier: Philadelphia, USA [Google Scholar]
- R Development Core Team (2008) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria [Google Scholar]
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58: 982–990 [DOI] [PubMed] [Google Scholar]
- Rex DK, Weddle RA, Lehman GA, Pound DC, O’Connor KW, Hawes RH, Dittus RS, Lappas JC, Lumeng L (1990) Flexible sigmoidoscopy plus air contrast barium enema versus colonoscopy for suspected lower gastrointestinal bleeding. Gastroenterology 98: 855–861 [DOI] [PubMed] [Google Scholar]
- Slattery ML, Levin TR, Ma K, Goldgar D, Holubkov R, Edwards S (2003) Family history and colorectal cancer: predictors of risk. Cancer Causes Control 14: 879–887 [DOI] [PubMed] [Google Scholar]
- Stapley S, Peters TJ, Sharp D, Hamilton W (2006) The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: a cohort study using medical records. Br J Cancer 95: 1321–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Pond CL, Ellis BG, Beach A, Thompson MR (2000) Rectal bleeding in general and hospital practice; ′the tip of the iceberg′. Colorectal Dis 2: 288–293 [DOI] [PubMed] [Google Scholar]
- Thompson MR, Perera R, Senapati A, Dodds S (2007) Predictive value of common symptom combinations in diagnosing colorectal cancer. Br J Surg 94: 1260–1265 [DOI] [PubMed] [Google Scholar]
- Wauters H, Van Casteren V, Buntinx F (2000) Rectal bleeding and colorectal cancer in general practice: diagnostic study. BMJ 321: 998–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J (2006) Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JM, Logan EC, Stewart RM (2004) Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J 80: 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]