Abstract
The mitogen-activated protein kinase/extracellular regulated kinase (MAPK/ERK) pathway plays a key role in mediating estrogen actions in the brain and neuronal sensitization during inflammation. Estrogen status is a risk factor in chronic temporomandibular muscle/joint disorders (TMJD); however, the basis for this relationship is not known. The present study tested the hypothesis that estrogen status acts through the MAPK/ERK signaling pathway to alter TMJ nociceptive processing. Single TMJ-responsive neurons were recorded in laminae I–II at the spinomedullary (Vc/C1–2) junction in naïve ovariectomized (OvX) female rats treated for 2 days with high (20 μg/day; HE2) or low dose estradiol (2 μg/day; LE2) and after chronic inflammation of the TMJ region by Complete Freund’s Adjuvant (CFA) for 12–14 days. Intra-TMJ injection of ATP (1 mM) was used to activate Vc/C1–2 neurons. The MAPK/ERK inhibitor (PD98059, 0.01–1 mM) was applied topically to the dorsal Vc/C1–2 surface at the site of recording 10 min prior to each ATP stimulus. In naive HE2 rats, low dose PD98059 caused a maximal inhibition of ATP-evoked activity, whereas even high doses had only minor effects on units in LE2 rats. By contrast, after chronic TMJ inflammation, PD98059 produced a marked and similar dose-related inhibition of ATP-evoked activity in HE2 and LE2 rats. These results suggested that E2 status and chronic inflammation acted, at least in part, through a common MAPK/ERK-dependent signaling pathway to enhance TMJ nociceptive processing by laminae I–II neurons at the spinomedullary junction region.
Keywords: estrogen, MAP kinase, nociception, temporomandibular joint, trigeminal brainstem
Introduction
Temporomandibular joint/muscle disorders (TMJD) consist of a heterogeneous group of conditions that cause pain in the temporomandibular joint (TMJ) region and masticatory muscles (Dworkin and LeResche, 1992). A significant feature of persistent TMJD is a higher prevalence in women than men (LeResche, 1997; Huang et al., 2002; Slade et al., 2007). The basis for this sex difference is not certain; however, clinical findings suggest that changes in estrogen status may play a significant role (Suenaga et al., 2001; Isselee et al., 2002; LeResche et al., 2003). In those TMJD cases diagnosed with disc displacement or after overt injury to the TMJ elevated levels of pro-inflammatory agents are found within the joint space and may contribute to persistent TMJD pain (Milam and Schmitz, 1995; Lobbezoo et al., 2004; Ta and Dionne, 2004). Intra-articular administration of Complete Freund’s Adjuvant (CFA) is a well-established animal model for monoarthritis that causes persistent joint inflammation (Wilson et al. 2006) and long-term changes nociceptive behavior in rodents (Butler et al. 1992; Schadrack et al. 1999; Luo et al. 2008). In male rats intra-TMJ injection of CFA produces orofacial cutaneous hyperalgesia lasting at least 2 weeks (Imbe et al., 2001; Okamoto et al., 2005a; Yamazaki et al., 2008) and increases the expression of pERK-positive neurons in Vc after jaw movement at that time (Suzuki et al., 2007). In cycling female rats, we found that 2 weeks after CFA treatment the number of Fos-positive neurons produced at the Vc/C1–2 region after TMJ stimulation was enhanced in proestrous (high E2) compared to diestrous (low E2) female rats suggesting a role for sex hormone status in TMJ-evoked responses during chronic inflammation (Bereiter et al., 2005b). Although several electrophysiological studies have shown that acute (Hu et al., 1992; Broton et al., 1988; Iwata et al., 1999; Takeshita et al., 2001) or chronic inflammation of the TMJ region (Okamoto et al., 2005b) can alter the encoding properties of Vc neurons, these studies either used only males or did not control for E2 status.
The TMJ region is supplied by small diameter sensory fibers (Kido et al., 1995; Takeuchi and Toda, 2003; Ioi et al., 2006) that project mainly to the superficial laminae at the Vc/C1–2 region (Shigenaga et al., 1986; 1988). The superficial laminae receive the vast majority of unmyelinated C-fiber input from peripheral nociceptors (Light, 1992) and express a high density of estrogen receptors (Bereiter et al., 2005a). In naïve OvX rats elevation of plasma levels of E2 for two days selectively enhanced TMJ-evoked unit activity in laminae I–II, but not lamina V, at the Vc/C1–2 junction (Tashiro et al., 2007) and increased the Fos-LI response produced by acute TMJ inflammation (Okamoto et al., 2008).
The MAPK/ERK pathway plays a key role in rapid and long-term genomic effects leading to the sensitization dorsal horn neurons in models of inflammation-induced cutaneous nociception (Ji et al., 1999; Ji and Woolf, 2001) and monoarthritis (Cruz et al., 2005). Activation of the MAPK/ERK pathway also contributes to rapid and transcriptional effects of estrogens in limbic and forebrain regions (Bi et al., 2001; Manella and Brinton, 2006; Szego et al., 2006) and may be critical for long-term changes in synaptic function underlying learning and memory (Fernandez et al., 2008). The main goals of this study were to determine if the MAPK/ERK signaling pathway contributes to E2-induced effects on TMJ nociceptive processing by neurons in superficial laminae at the Vc/C1–2 region and if these effects are altered during chronic TMJ inflammation.
Experimental Procedures
The protocols were approved by the Institutional Animal Care and Use Committee of University of Minnesota and conformed to established guidelines set by The National Institutes of Health guide for the care and use of laboratory animals (PHS Law 99–158, revised 2002).
Estradiol (E2) treatment
Adult ovariectomized (OvX) female rats (250–320 g, Sprague-Dawley, Harlan, Indianapolis, IN) were used within 3 weeks after ovariectomy. Rats were given daily injections of low (LE2, 2 μg) or high dose (HE2, 20 μg, sc) 17β-estradiol-3-benzoate (Sigma, St. Louis, MO), dissolved in 200 μl sesame oil, for two days prior to the experiment. This design was intended to mimic the cyclic nature and magnitude of plasma levels of E2 in diestrous and proestrous rats (Butcher et al., 1974). Estrogen status was confirmed by the vaginal smear cytology taken by gentle lavage on the day of the experiment. Vaginal smears from LE2 rats revealed mainly small nucleated leukocytes, while smears from HE2 rats had large nucleated epithelial cells or a combination of large nucleated and squamous epithelial cells (Montes and Luque, 1988). Data were collected without prior knowledge of the E2 treatment.
Chronic inflammation by CFA
Chronic inflammation of the TMJ region was induced by an injection of Complete Freund’s Adjuvant (CFA, Sigma, St. Louis, MO) given as a suspension (oil/saline, 1:1) in a total volume of 50 μl (i.e., 25 μg heat-killed mycobacterium) under pentothal sodium (50 mg/kg, ip) anesthesia 10–14 days prior to the experiment. The TMJ region was identified by palpation and the injection was delivered manually by advancing a 30 gauge needle through the skin immediately inferior to the posterior border of the zygomatic arch until it contacted the mandibular condyle. This dose of CFA injected into the TMJ caused persistent behavioral hyperalgesia (Imbe et al., 2001; Okamoto et al. 2005) and elevated Fos-LI in the trigeminal brainstem complex for at least 14 days (Bereiter et al. 2005b; Zhou et al., 1999), while other arthritis-related studies have reported active inflammation over this time (Donaldson et al., 1993; Wilson et al., 2006). Rats that did not receive CFA were defined in the text as “naive”, even though all rats were ovariectomized, whileCFA-treated animals were defined as “inflamed”.
Surgical preparation
Rats were anesthetized initially with pentobarbital sodium (60 mg/kg, i.p.) and after tracheotomy respired artificially with oxygen-enriched room air. Catheters were placed in the right femoral artery (blood pressure monitor) and right jugular vein (infusion of thiopental sodium). Anesthesia was maintained after surgery by continuous infusion of thiopental sodium (20–30 mg/kg/h) and switched to a mixture of thiopental and the paralytic agent, gallamine triethiodide (15–20 mg/kg/h), after completion of all surgical procedures and immediately prior to the recording session. Adequate depth of anesthesia was confirmed by the absence of corneal and hind limb withdrawal reflexes prior to gallamine, fully constricted pupils and constant arterial blood pressure and heart rate. Expiratory end-tidal CO2 (3.5–4.5%) and mean arterial pressure (90–120mmHg) were monitored throughout the experiment and body temperature was maintained 38°C with a heating blanket and thermal probe.
Animals were placed in a stereotaxic frame and portions of the C1and C2 vertebrae were removed to expose the lower brainstem and upper cervical dorsal horn and bathed in warm mineral oil. The caudal portion of trigeminal subnucleus caudalis (Vc) and the upper cervical (C1–C2) spinal cord, approximately 5 to 7 mm caudal to obex and ipsilateral to exposed condyle, was explored for TMJ-responsive units using the entrance of the C2 rootlets as a landmark. The tungsten microelectrode (9 Mohm, Frederick Haer Inc., Bowdoinham, ME) penetrated the brainstem tangential (~43° off vertical, 60° off midline) to the surface. Unit activity was amplified, discriminated, stored and analyzed offline on a computer (Apple G4) using a DAQ interface board and LabVIEW software (National Instruments, Austin, TX). Spike amplitude and shape were monitored continuously and stored on digital tape to reconfirm unit isolation later in offline analyses.
All units displayed a vigorous response to mechanical probing of the exposed dorsal surface of the posterior condyle (see Okamoto et al., 2003, Fig 1). TMJ units were further classified by the response to convergent input from the overlying facial skin. All units included in this study were classified as nociceptive specific (NS) and were activated by press or pinch of the skin but not by brush.
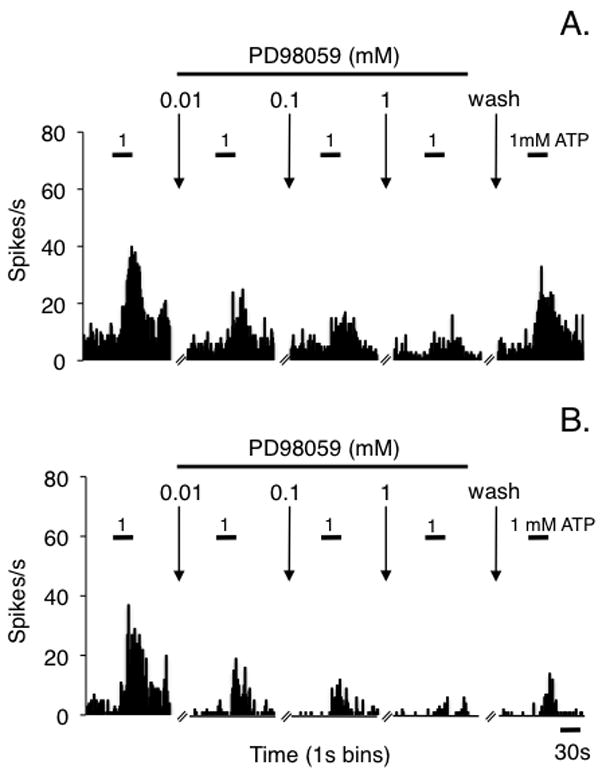
Figure 1.

Peristimulus time histograms displaying examples of the effects of cumulative doses of the MEK inhibitor, PD98059, on ATP-evoked responses of TMJ units from (A) HE2-treated and (B) LE2-treated naive rats. The small calibration bars above the histograms indicate the time of intra-TMJ injection of 1 mM ATP.
Experimental design
One TMJ-responsive neuron was recorded from each animal preparation. All units were located in the superficial dorsal horn (< 250 μm) within 1.5 mm rostral to the entrance of the C2 rootlets. After confirming a response to condyle stimulation the face and neck was explored for a possible cutaneous receptive field (RF) and the high threshold RF area was mapped using a small forceps (~3 mm2) onto a standardized series of rat face drawings, digitized and later quantified by a planimetric method using NIH Image J software. Next a guide cannula (26 gauge) was positioned at the posterior edge of the mandibular condyle in the TMJ region (~3 mm deep) by a dorsal approach. The experimental design consisted of six intra-TMJ injections: phosphate buffered saline (PBS) followed by five repeated injections of adenosine triphosphate (ATP; 1 mM, Sigma, St. Louis, MO). Intra-TMJ injections were delivered manually from a microsyringe attached by polyethylene tubing to an inner cannula (33 gauge) that protruded ~0.5 mm from the end of guide cannula. Injections were delivered slowly over 30 s (total volume of 6 × 20 μl = 120 μl) with an inter-injection interval of 20 min to reduce the likelihood of tachyphylaxis. The concentration of ATP used here (1 mM) was within the physiological range found in normal and injured rat skeletal muscle (Morris et al., 1985), caused only minor localized inflammation (Green et al., 1993). Previously we determined that this dose of ATP could be repeatedly injected into the TMJ without causing sensitization or tachyphylaxis of the evoked response (Tashiro et al., 2008). To determine the effect of MAPK/ERK inhibition, the selective MAP kinase kinase (MEK) inhibitor, PD98059 (0.01, 0.1 or 1 mM; 30μl, Tocris, Ellisville, MO), was applied topically to Vc/C1–2 surface 10 min before each ATP stimulus in an increasing cumulative dose regimen. The range of drug dose was chosen from previous reports (Ji et al., 1999). The angle of the caudal brainstem provided a natural pool for topically applied drugs. Previously we reported that small volumes of drugs applied to the Vc/C1–2 surface did not affect unit activity in rostral regions of Vc (Meng et al., 1998).
Data analysis
Neural data were acquired and displayed as peristimulus time histograms of spikes per 1 s bins, exported to a spreadsheet and analyzed off-line. Background activity (spikes/s) was calculated as the average spike count over a 1 min epoch immediately preceding each stimulus. The evoked responses were quantified as a response magnitude (Rmag), determined by subtracting the mean plus 2 times the standard deviation (SD) of background activity from the total spike count for each bin. The total Rmag for each stimulus was defined as the cumulative sum of spikes over contiguous bins in which the spike count minus the background was a positive value. The total Rmag is similar to the “area under the curve” for each stimulus and was calculated over a period of 100 s (Hirata et al., 1999). Response duration was defined as the time interval after stimulus onset until three consecutive bins with a positive spike count occurred above background (initial latency) and until the value of three consecutive bins no longer exceeded the mean + 2 SD above background activity. Response latency was defined as earliest time after stimulus onset for which three consecutive 1 s bins exceeded the mean + 2 SD of background activity (i.e., Rmag). All units included in this study displayed a total Rmag after ATP that exceeded the response to PBS by > 50% prior to PD98059 application. Total Rmag, response duration and response latency to TMJ injections as well as spontaneous activity were assessed statistically by analysis of variance (ANOVA) corrected for repeated measures and individual comparisons were made by Newman-Keuls after ANOVA.
Results
General
These experiments were performed on 20 naïve and 10 CFA-treated rats. The range of body weights was 280–320 g on the day of recording and no group differences were seen. CFA-treated rats typically lost weight (~10 %) over the first 2 days following inflammation, however, by 5 days weight gain was similar to naïve rats. CFA treatment caused visible swelling over the injected TMJ that lasted 3–5 days and resolved by 10 days consistent with previous studies in male rats (Zhou et al., 1999; Suzuki et al. 2007). Mean arterial blood pressure prior to the first ATP stimulus of naïve HE2, naïve LE2, CFA HE2 and CFA LE2 groups averaged 100 ± 4, 98 ± 4, 103 ± 5 and 112 ± 7 mmHg, respectively, and no group differences were seen.
Effect of MAP/ERK inhibition on TMJ processing in naïve rats
Intra-TMJ injections of ATP produced a transient increase in the firing rate of units in HE2 (Fig 1A) and LE2 rats (Fig 1B) as seen by the short duration response prior to drug administration and reversal after the washout period. Prior to drug application the total Rmag to the initial ATP stimulus for TMJ units from HE2 rats was significantly greater than units from LE2 rats (Fig 2A, F1,35 = 6.96, P < 0.025) in agreement with our previous study (Tashiro et al. 2007). Topical application of the MAPK/ERK inhibitor, PD98059, produced a significant decrease in the ATP-evoked response in HE2 rats (F4,72 = 33.4, P < 0.001), while evoked unit activity in LE2 rats displayed only minor changes even after the highest drug dose (Fig 2A). This was a consistent finding as all units (11 of 11) from HE2 rats had a reduction in total Rmag after high dose PD98059 of >50%, whereas only 4 of 9 units from LE2 rats were inhibited at least 50%. Response duration also was reduced in HE2 units after PD98059 (pre-drug = 62 ± 7s versus 22 ± 5s after 1 mM, P < 0.01), but not changed for units in LE2 rats (pre-drug = 36 ± 6s versus post-drug = 38 ± 5s). Response latency to ATP stimulation was increased after high dose PD98059 in HE2 units, but not affected in LE2 units (Fig 2B). All TMJ units were spontaneously active and the rate of background activity was not affected by local MAPK/ERK inhibition in either group under naïve conditions (Fig 2C).
Figure 2.

The effects of topical application of PD98059 on ATP-evoked responses in naïve rats. Sample sizes: HE2, n = 11; LE2, n = 9. *P < 0.05, **P < 0.01 versus ATP alone (0 mM PD98059); a = P < 0.05 versus LE2.
Effect of MAP/ERK inhibition on TMJ processing in inflamed rats
In chronic TMJ-inflamed rats ATP stimulation also produced a consistent increase in the firing rate of units from HE2 (Fig 3A) and LE2 rats (Fig 3B). However, in contrast to naïve rats, the ATP-evoked total Rmag prior to drug application was not different between groups (Fig 4A, P > 0.1). Topical application of the MAPK/ERK inhibitor, PD98059, produced a marked and similar dose-related decrease in the ATP-evoked response in HE2 and LE2 units (F3,32 = 19.5, P < 0.001) as summarized in Fig 4A. This was a consistent finding as all units from HE2 (n = 5) and LE2 rat (n = 5) had a reduction in total Rmag after high dose PD98059 of > 50%. Note also that the PD98059-induced inhibition of evoked activity of all units was reversed after washout (Fig 2A, Fig 4A). Response latencies to ATP stimulation were not affected by PD98059 in either group of TMJ-inflamed rats (Fig 4B). All units from CFA-treated rats were spontaneously active. In CFA-inflamed rats spontaneous activity of HE2 units was significantly elevated compared to LE2 units and compared to HE2 units from naïve rats (Fig 4C, F3,32 = 4.33, P < 0.025).
Figure 3.
Peristimulus time histograms displaying examples of the effects of cumulative doses of PD98059 on ATP-evoked responses of TMJ units classified as NS units from (A) CFA-treated HE2 and (B) CFA-treated LE2 rats. The small calibration bars above the histograms indicate the time of intra-TMJ injection of 1 mM ATP.
Figure 4.

The effect of topical application of PD98059 on ATP-evoked responses in chronically-inflamed OvX female rats. Sample size: HE2, n = 5; LE2, n = 5. **P < 0.01 versus ATP alone (0 mM PD98059); a = P < 0.05 versus LE2.
Effect of MAP/ERK inhibition on convergent cutaneous receptive fields of TMJ units
The convergent cutaneous RF area of TMJ units from naïve HE2 and LE2 rats averaged 2.1 ± 0.2 and 1.5 ± 0.2 cm2, respectively, and not different statistically. High dose PD98059 reduced significantly (F2,46 = 21.3, P < 0.001) the RF area in HE2 (1.5 ± 0.2 cm2) and LE2 rats (1.2 ± 0.2 cm2) and returned to control values after the washout period. By contrast, the RF area of CFA-treated HE2 units (1.1 ± 0.1 cm2) and LE2 units (1.4 ± 0.2 cm2) were not affected by high dose PD98059.
Discussion
The main finding of this study was that local blockade of the MAPK/ERK pathway caused a marked inhibition of TMJ-evoked activity of units in laminae I–II at the Vc/C1–2 region in HE2 females under both naïve and TMJ-inflamed conditions, while in LE2 females blockade of this pathway was effective only after inflammation. The MAP/ERK pathway likely contributed through multiple mechanisms since spontaneous activity of TMJ units was reduced by PD98059 only in the HE2 TMJ-inflamed group, while the convergent cutaneous RF area was reduced only in naïve rats independent of E2 status.
These results suggested that E2 status and chronic inflammation acted, in part, through a common MAPK/ERK-dependent mechanism to influence the encoding properties of TMJ units in superficial laminae at the Vc/C1–2 region. The MAPK/ERK pathway has been studied extensively in relation to the induction and maintenance of pain-like behavior in male rodent models (see Ji and Woolf, 2001). These studies revealed that peripheral nerve stimulation at C-fiber intensity was required to increase the number of phosphoERK (pERK)-positive neurons in spinal dorsal horn (Lever et al., 2003) and blockade of this pathway inhibited the second but not the first phase of the formalin test (Ji et al., 1999). Ongoing tissue inflammation activated the MAPK/ERK pathway that can last for several days (Ji et al., 2002; Adwanikar et al., 2004). In CFA models of monoarthritis inflammation persisted for 2–4 weeks (Wilson et al. 2006) and although inflammation scores reached a maximum 3–5 days after tibiotarsal joint injection, the number of pERK neurons in superficial dorsal horn and the extent of vocalization to joint movement remained markedly enhanced 14 days later (Cruz et al., 2005). Similarly, after CFA injection into the TMJ the signs of inflammation were greatest 2–5 days later, whereas the number of pERK-positive neurons produced in superficial laminae of Vc after jaw movement was greatly enhanced on day 14 post-injection compared to control male rats (Suzuki et al., 2007). These studies indicated that activation of the MAPK/ERK pathway was a key contributor to persistent neural responses after an initial inflammatory injury consistent with behavioral hyperalgesia in arthritic models in spinal as well as trigeminal systems.
The present study suggested that E2 status was a key factor in determining the influence of MAPK/ERK activation on the response properties of TMJ units in superficial laminae at the Vc/C1–2 region in naïve female rats. A functional relationship between E2 status and the MAPK/ERK pathway has been well established for other brain regions. For example, the variation in magnitude of long-term potentiation (LTP) of hippocampal CA1 neurons at different stages of the estrous cycle was paralleled by changes in pERK expression and mimicked by E2 replacement in female rats (Bi et al., 2001). Systemic administration of E2 caused a rapid increase in MAPK/ERK activation in several limbic and forebrain regions (Bryant et al., 2005; Mannella and Brinton, 2006). MAPK/ERK activation was necessary for E2-dependent long-term changes in structural proteins such as PSD-95 and synaptophysin (Chamniansawat and Chongthammakun, 2009). Such changes likely have functional consequences on behavior since a single injection of E2 evoked a rapid MAPK-dependent increase in memory retention in female mice that persisted for at least 48 h (Fernandez et al., 2008), while in songbirds E2-induced enhancement of neural activity related to auditory processing and song retention required MAPK/ERK activation (Tremere et al., 2009). These studies suggested that E2-induced changes in synaptic plasticity necessary for learning, memory and persistent nociceptive behavior require MAPK/ERK activation.
The upstream regulation of MAPK likely involves multiple receptor systems and intracellular cascades consistent with the hypothesis that the MAPK/ERK pathway is a critical gate for long-term sensitization of dorsal horn neurons and nociceptive behavior (Kawasaki et al., 2004). One possible upstream regulator of the MAPK/ERK pathway shared by both E2 and tissue inflammation is glutamate neurotransmission through NMDA receptors. NMDA receptor activation is critical for long-term enhancement of dorsal horn neural activity after tissue injury and inflammation (Woolf and Slater, 2000). In spinal dorsal horn E2 administration increased the expression of the NR1 subunit of the NMDA receptor and enhanced the motor responses to visceral stimulation in female rats (Tang et al., 2008), while in males blockade of NMDA-mediated neurotransmission prevented C-fiber (Lever et al., 2003) and capsaicin-evoked MAPK/ERK activation (Kawasaki et al. 2004). In hippocampus E2-induced modulation of dendritic plasticity and enhanced LTP depends mainly on NMDA receptor activation (Woolley, 1999; McEwen, 2002). Although the exact relationship between NMDA neurotransmission and MAPK/ERK activation in TMJ nociceptive processing has not yet been determined, recently we found that local application of the competitive NMDA receptor antagonist, AP5, significantly inhibited the ATP-evoked responses of TMJ units at the Vc/C1–2 region in HE2- but not LE2-treated naïve female rats (Tashiro et al., 2005), parallel to the results of the present study. Also, pretreatment with the non-competitive NMDA receptor antagonist, MK801, greatly reduced the TMJ-evoked Fos-LI response at the Vc/C1–2 region of HE2- but not LE2-treated naïve female rats (Okamoto et al., 2008). These results suggested that glutamate neurotransmission via the NMDA receptor is one possible common receptor mechanism upstream of MAPK/ERK activation that could mediate the effects of E2- and TMJ injury on spinomedullary dorsal horn neurons. It cannot be excluded that some effects of MAPK may have involved glial mechanisms (Xie et al., 2007).
The experimental design used here combined systemic E2 treatment for 2 days with local application of drugs at the site and time of recording. Although this approach was designed to selectively interrupt MAPK/ERK activation by local dorsal horn neurons, it cannot be excluded that PD98059 also acted presynaptically on central terminals of primary afferents projecting to the Vc/C1–2 region. Trigeminal ganglion neurons express estrogen receptors (Bereiter et al., 2005a) and display increased numbers of pERK-positive neurons 3 days after CFA injection into masseter muscle, an effect that was exacerbated under elevated E2 conditions (Liverman et al., 2009). However, the fact that the convergent cutaneous RF area of TMJ units was reversibly reduced soon after topical application of PD98059 would argue against a direct effect on primary afferent terminals. It is generally understood that peripheral mechanisms are not sufficient to account for changes in receptive field area of dorsal horn neurons after inflammation (Hylden et al., 1989) or high intensity sensory nerve stimulation (Cook et al., 1987). Also we found no evidence that the effects of E2 and inflammation on the response properties of TMJ units were additive, whereas additive effects were seen in trigeminal ganglion neurons on the passive properties (Flake et al., 2005) and MAPK/ERK activation (Liverman et al., 2009) 3 days after CFA during high E2 conditions. It was somewhat unexpected that neither the convergent cutaneous RF area nor the ATP-evoked responses of TMJ units from CFA-treated rats was enhanced compared to naïve rats regardless of E2 status. This may be due to several factors. First, we recorded only from units in superficial laminae, a region where neurons generally display weak windup-like responses compared to those in deep laminae (Seagrove et al., 2004). Second, all units included here were classified as NS and previously we reported that E2 status had little effect on the cutaneous RF area of this TMJ-responsive cell group (Tashiro et al., 2007). Third, we recorded from units only 12–14 days after CFA, whereas most studies assessed the response properties of dorsal horn neurons at much earlier times, typically 1–5 days post-injection, when the signs of inflammation are most prominent (Hylden et al., 1989; Ren et al., 1992; Iwata et al., 1999). Despite observing similar TMJ-evoked responses of units in naïve and CFA-treated rats prior to PD98059 application, the effectiveness of PD98059 on TMJ unit activity was markedly enhanced 2 weeks after CFA. Although testing at 2 weeks after intra-TMJ injection of CFA has confirmed ongoing behavioral hyperalgesia (Yamazaki et al., 2008), the exact role of TMJ units in superficial laminae at the Vc/C1–2 region in mediating changes in behavior is not yet known. Others have claimed that elevated E2 has an antihyperalgesic or even analgesic effect on TMJ-related behavior (Fischer et al., 2008; Kramer and Bellinger, 2009). However, these studies used severe pro-inflammatory agents to evoke behavioral changes and tested animals only immediately after inflammation (orofacial formalin test, Fischer et al., 2008) or at peak times during ongoing inflammation (3 days after bilateral intra-TMJ injections of CFA, Kramer and Bellinger, 2009). Although the relationship between estrogens and inflammation likely is complex and tissue specific (see Straub et al., 2007), it should be noted the majority of persistent TMJD pain patients are women (LeResche, 1997; Huang et al., 2002; Slade et al., 2007).
In summary these data suggested that E2 and inflammation acted, at least in part, through a common MAPK/ERK signaling pathway to enhance the activity of TMJ-responsive units laminae I–II at the Vc/C1–2. The effect of MAPK/ERK inhibition was most pronounced on TMJ-evoked activity rather than on spontaneous activity or convergent input from facial skin. These data support the hypothesis that the Vc/C1–2 is a critical site for integrating sensory signals relevant for TMJ nociceptive processing in an estrogen-dependent manner.
Acknowledgments
This study was supported by a grant from the NIDCR (DE 12758)
Abbreviations
- TMJ
temporomandibular joint
- Vc/C1–2
trigeminal subnucleus caudalis/upper cervical cord
- E2
estradiol
- OvX
ovariectomized
- PBS
phosphate buffered saline
- ATP
adenosine triphosphate
- CFA
Complete Freund’s Adjuvant
- MAPK
mitogen-activated protein kinase/extracellular regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adwanikar H, Karim F, Gereau RW. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Cioffi JL, Bereiter DF. Oestrogen receptor-immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Arch Oral Biol. 2005a;50:971–979. doi: 10.1016/j.archoralbio.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005b;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bi R, Foy MR, Voimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Nat Acad Sci. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broton JG, Hu JW, Sessle BJ. Effects of temporomandibular joint stimulation on nociceptive and nonnociceptive neurons of the cat’s trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 1988;59:1575–1589. doi: 10.1152/jn.1988.59.5.1575. [DOI] [PubMed] [Google Scholar]
- Bryant DN, Bosch MA, Ronnekleiv OK, Dorsa DM. 17-Beta estradiol rapidly enhances extracellular signal-regulated kinase 2 phosphorylation in the rat brain. Neuroscience. 2005;133:343–352. doi: 10.1016/j.neuroscience.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. A limited arthritic model for chronic pain studies in the rat. Pain. 1992;48:73–81. doi: 10.1016/0304-3959(92)90133-V. [DOI] [PubMed] [Google Scholar]
- Chamniansawat S, Chongthammakun S. Estrogen stimulates activity-regulated cytoskeleton associated protein (Arc) expression via the MAPK- and PI-3K-dependent pathways in SH-SY5Y cells. Neurosci Lett. 2009;452:130–135. doi: 10.1016/j.neulet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Neto FL, Castro-Lopes J, McMahon SB, Cruz F. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Donaldson LF, Seckl JR, McQueen DS. A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose. J Neurosci Meth. 1993;49:5–10. doi: 10.1016/0165-0270(93)90103-x. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Torres-Chavez KE, Clemente-Napimoga JT, Jorge D, Arsati F, de Arruda Veiga MC, Tambeli CH. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. J Pain. 2008;9:630–638. doi: 10.1016/j.jpain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller P, Levine JD. Modulation of bradykinin-induced plasma extravasation in the rat knee joint by sympathetic co-transmitters. Neuroscience. 1993;52:451–458. doi: 10.1016/0306-4522(93)90171-b. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Hu JW, Sessle BJ, Raboisson P, Dallel R, Woda A. Stimulation of craniofacial muscle afferents induces prolonged facilitatory effects in trigeminal nociceptive brain-stem neurones. Pain. 1992;48:53–60. doi: 10.1016/0304-3959(92)90131-T. [DOI] [PubMed] [Google Scholar]
- Huang GJ, LeResche L, Critchlow CW, Martin MD, Drangsholt MT. Risk factors for diagnostic subgroups of painful temporomandibular disorders (TMD) J Dent Res. 2002;8:284–288. doi: 10.1177/154405910208100412. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. Pain. 1989;37:229–243. doi: 10.1016/0304-3959(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- Ioi H, Kido MA, Zhang JQ, Yamaza T, Nakata S, Nakasima A, Tanaka T. Capsaicin receptor expression in the rat temporomandibular joint. Cell Tissue Res. 2006;325:47–54. doi: 10.1007/s00441-006-0183-7. [DOI] [PubMed] [Google Scholar]
- Isselee H, Laat AD, Mot BD, Lysens R. Pressure-pain threshold variation in temporomandibular disorder myalgia over the course of the menstrual cycle. J Orofac Pain. 2002;16:105–117. [PubMed] [Google Scholar]
- Iwata K, Tashiro A, Tsuboi Y, Imai T, Sumino R, Morimoto T, Dubner R, Ren K. Medullary dorsal horn neuronal activity in rats with persistent temporomandibular joint and perioral inflammation. J Neurophysiol. 1999;82:1244–1253. doi: 10.1152/jn.1999.82.3.1244. [DOI] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nature Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido MA, Kiyoshima T, Ibuki T, Shimizu S, Kondo T, Terada Y, Tanaka T. A topographical and ultrastructural study of sensory trigeminal nerve endings in the rat temporomandibular joint as demonstrated by anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) J Dent Res. 1995;74:1353–1359. doi: 10.1177/00220345950740070601. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiological factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Pezet S, McMahon SB, Malcangio M. The signaling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Mol Cell Neurosci. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Light AR. The initial processing of pain and its descending control: spinal and trigeminal systems. New York: Karger; 1992. [Google Scholar]
- Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia. 2009;29:520–531. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbezoo F, Drangsholt M, Peck C, Sato H, Kopp S, Svensson P. Topical review: new insights into the pathology and diagnosis of disorders of the temporomandibular joint. J Orofac Pain. 2004;18:181–191. [PubMed] [Google Scholar]
- Luo H, Xu IS, Chen Y, Yang F, Yu L, Li GX, Liu FY, Xing GG, Shi YS, Li T, Han JS, Wan Y. Behavioral and electrophysiological evidence for the differential functions of TRPV1 at early and late stages of chronic inflammatory nociception in rats. Neurochem Res. 2008;33:2151–2158. doi: 10.1007/s11064-008-9751-4. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Meng ID, Hu JW, Bereiter DA. Differential effects of morphine on corneal-responsive neurons in rostral versus caudal regions of spinal trigeminal nucleus in the rat. J Neurophysiol. 1998;79:2593–2602. doi: 10.1152/jn.1998.79.5.2593. [DOI] [PubMed] [Google Scholar]
- Milam SB, Schmitz JP. Molecular biology of temporomandibular joint disorders: proposed mechanisms of disease. J Oral Maxillofac Surg. 1995;53:1448–1454. doi: 10.1016/0278-2391(95)90675-4. [DOI] [PubMed] [Google Scholar]
- Montes GS, Luque EH. Effects of ovarian steroids on vaginal smears in the rat. Acta Anat (Basel) 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Morris A, Henry W, Jr, Shearer J, Caldwell M. Macrophage interaction with skeletal muscle: a potential role of macrophages in determining the energy state of healing wounds. J Trauma. 1985;25:751–757. [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ neurons in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bereiter DF, Thompson R, Tashiro A, Bereiter DA. Estradiol replacement modifies c-fos expression at the spinomedullary junction evoked by temporomandibular joint stimulation in ovariectomized female rats. Neuroscience. 2008;156:729–736. doi: 10.1016/j.neuroscience.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Kimura A, Donishi T, Imbe H, Senba E, Tamai Y. Central serotonin 3 receptors play an important role in the modulation of nociceptive neural activity of trigeminal subnucleus caudalis and nocifensive orofacial behavior in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005a;135:569–581. doi: 10.1016/j.neuroscience.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Tashiro A, Kimura A, Donishi T, Tamai Y, Senba E. The role of peripheral 5HT2A and 5HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005b;130:465–474. doi: 10.1016/j.neuroscience.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. Pain. 1992;50:331–344. doi: 10.1016/0304-3959(92)90039-E. [DOI] [PubMed] [Google Scholar]
- Schadrack J, Neto FL, Ableitner A, Castro-Lops JM, Willoch F, Bartenstein P, Zieglgansberger W, Tolle TR. Metabolic activity changes in the rat spinal cord during adjuvant monoarthritis. Neuroscience. 1999;94:595–605. doi: 10.1016/s0306-4522(99)00186-4. [DOI] [PubMed] [Google Scholar]
- Seagrove LC, Suzuki R, Dickenson AH. Electrophysiological characterisations of rat lamina I dorsal horn neurones and the involvement of excitatory amino acid receptors. Pain. 2004;108:76–87. doi: 10.1016/j.pain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S, Nishimori T, Nasution ID, Yoshida A, Sato H, Okamoto T, Sera M, Hosoi M. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol. 1986;243:388–408. doi: 10.1002/cne.902430309. [DOI] [PubMed] [Google Scholar]
- Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007;86:1120–1125. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Suenaga S, Abeyama K, Indo H, Shigeta K, Noikura T. Temporomandibular disorders: MR assessment of inflammatory changes in the posterior disk attachment during the menstrual cycle. J Comput Assist Tomogr. 2001;25:476–481. doi: 10.1097/00004728-200105000-00023. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Harada T, Asano M, Tsuboi Y, Kondo M, Gionhaku N, Kitagawa J, Kusama T, Iwata K. Phosphorylation of ERK in trigeminal spinal nucleus neurons following passive jaw movement in rats with chronic temporomandibular joint inflammation. J Orofac Pain. 2007;21:225–231. [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta LE, Dionne RA. Treatment of painful temporomandibular joints with a cyclooxygenase-2 inhibitor: a randomized placebo-controlled comparison of celecoxib to naproxen. Pain. 2004;111:13–21. doi: 10.1016/j.pain.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Hirata H, Bereiter DA. Intensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the rat. J Neurophysiol. 2001;86:2393–2404. doi: 10.1152/jn.2001.86.5.2393. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Toda K. Subtypes of nociceptive units in the rat temporomandibular joint. Brain Res Bull. 2003;61:603–608. doi: 10.1016/s0361-9230(03)00219-3. [DOI] [PubMed] [Google Scholar]
- Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549. doi: 10.1016/j.pain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Hirata H, Bereiter DA. Estrogen acts through NMDA receptors to modulate TMJ-evoked activity of trigeminal subnucleus caudalis neurons. Soc Neurosci. 2005 Abstr: No. 747.1. [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28:2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in female rats. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AW, Medhurst SJ, Dixon CI, Bontoft NC, Winyard LA, Brackenborough KT, De Alba J, Clarke CJ, Gunthorpe MJ, Hicks GA, Bountra C, McQueen DS, Chessell IP. An animal model of chronic inflammatory pain: pharmacological and temporal differentiation from acute models. Eur J Pain. 2006;10:537–549. doi: 10.1016/j.ejpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr Opinion Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Xie YF, Zhang S, Chiang CY, Hu JW, Dostrovsky JO, Sessle BJ. Involvement of glia in central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) Brain Behav Immun. 2007;21:634–641. doi: 10.1016/j.bbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Ren K, Shimada M, Iwata K. Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp Neurol. 2008;214:209–218. doi: 10.1016/j.expneurol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Imbe H, Dubner R, Ren K. Persistent Fos protein expression after orofacial deep or cutaneous tissue inflammation in rats: implications for persistent orofacial pain. J Comp Neurol. 1999;412:276–291. doi: 10.1002/(sici)1096-9861(19990920)412:2<276::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]


