Abstract
Purpose
This phase I study was conducted to evaluate the safety, tolerability, pharmacological properties and biological activity of the combination of the lonafarnib, a farnesylproteintransferase (FTPase) inhibitor, with gemcitabine and cisplatin in patients with advanced solid malignancies.
Experimental design
This was a single institution study to determine the maximal tolerated dose (MTD) of escalating lonafarnib (75–125 mg po BID) with gemcitabine (750–1,000 mg/m2 on days 1, 8, 15) and fixed cisplatin (75 mg/m2 day 1) every 28 days. Due to dose-limiting toxicities (DLTs) of neutropenia and thrombocytopenia in initial patients, these patients were considered “heavily pretreated” and the protocol was amended to limit prior therapy and re-escalate lonafarnib in “less heavily pre-treated patients” on 28-day and 21-day schedules. Cycle 1 and 2 pharmacokinetics (PK), and farnesylation of the HDJ2 chaperone protein and FPTase activity were analyzed.
Results
Twenty-two patients received 53 courses of therapy. Nausea, vomiting, and fatigue were frequent in all patients. Severe toxicities were observed in 91% of patients: neutropenia (41%), nausea (36%), thrombocytopenia (32%), anemia (23%) and vomiting (23%). Nine patients withdrew from the study due to toxicity. DLTs of neutropenia, febrile neutropenia, thrombocytopenia, and fatigue limited dose-escalation on the 28-day schedule. The MTD was established as lonafarnib 75 mg BID, gemcitabine 750 mg/m2 days 1, 8, 15, and cisplatin 75 mg/m2 in heavily pre-treated patients. The MTD in the less heavily pre-treated patients could not be established on the 28-day schedule as DLTs were observed at the lowest dose level, and dose escalation was not completed on the 21-day schedule due to early study termination by the Sponsor. No PK interactions were observed. FTPase inhibition was not observed at the MTD, however HDJ-2 gel shift was observed in one patient at the 100 mg BID lonafarnib dose. Anti-cancer activity was observed: four patients had stable disease lasting >2 cycles, one subject had a complete response, and another had a partial response, both with metastatic breast cancer.
Conclusion
Lonafarnib 75 mg BID, gemcitabine 750 mg/m2 days 1, 8, 15, and cisplatin 75 mg/m2 day 1 on a 28-day schedule was established as the MTD. Lonafarnib did not demonstrate FTPase inhibition at these doses. Despite the observed efficacy, substantial toxicity and questionable contribution of anti-tumor activity of lonafarnib to gemcitabine and cisplatin limits further exploration of this combination.
Keywords: Lonafarnib, SCH66336, Cisplatin, Gemcitabine, Farnesyltransferase, Phase I, Pharmacokinetics
Introduction
Farnesyltransferase inhibitors (FTIs) are a novel class of anti-cancer agents blocking post-translational modification of cellular polypeptides. Farnesylproteintransferase (FPTase) enzymes add a lipophilic farnesyl moiety to proteins, thereby promoting the localization of many cell proliferation proteins, including Ras and Rho, to the cellular membranes, which is necessary for activity [16, 20]. Ras gene mutations, present in over 30% of human cancers, lead to constitutive activation of Ras proteins through multiple effector pathways such as raf, MAP and phosphatidylinositol-3-activated kinases [36]. As farnesylation is essential for post-translational activation, FPTase inhibition may arrest Ras-dependent tumor proliferation. FTI effects do not depend exclusively on Ras inhibition: gain of alternate prenylated forms of Rho, such as Rho-B, also inhibit growth, [33] and affect numerous cellular and downstream signaling events.
Lonafarnib (SCH66336, Sarasar™) is a tricyclic nonpeptidyl, nonsulphydryl {(11R)4[2[4-(3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo [5, 6] cylohepta [1,2b] pyridine-11yl) -1-pyperazinyl]-2-oxoethyl]-1-piperdinecarboxamide)} specific, potent, and orally bioavailable competitive inhibitor of FPTase [26]. In vitro, lonafarnib blocks farnesylation of H-ras and K-ras 4B, and blocks anchorage-dependent growth of K-ras transformed rodent fibroblasts at low nanomolar IC50s (1.9, 5.2, and 4.0 nM, respectively), without inhibiting the related geranylgeranyl protein transferase at concentrations up to 50 µM [23, 26]. Lonafarnib has a potent in vitro and in vivo activity in non-small cell lung cancer (NSCLC), ovarian, breast, pancreatic, bladder, and prostate cancer models [23, 26]. Phase I studies of lonafarnib monotherapy in patients with advanced malignancies demonstrated dose limiting toxicities (DLTs) of diarrhea, nausea, vomiting, anorexia, reversible renal insufficiency secondary to dehydration, neutropenia, thrombocytopenia, and fatigue on the 300 and 400 mg BID schedules, establishing the maximum tolerated dose (MTD) as 200 mg BID or 300 mg once daily [5, 7, 13]. Stable disease (SD) was observed in several tumor types and one partial response (PR) in a NSCLC patient was sustained for 14 months [5, 7, 9, 13].
Gemcitabine (GEMZAR™) and cisplatin have demonstrated efficacy in multiple solid tumors, many with Ras mutations, as first-line therapy. Phase III studies have established this combination as a standard first line regimen in advanced NSCLC (similar survival with improved time-to-progression of this combination over other platinum combinations in a meta-analysis) [25, 37] and metastatic urothelial cancers (equivalent response and survival, with lower toxicity, compared to MVAC) [1, 35]. Additionally, a phase III study demonstrated a 26.4% response for the combination without significant increased toxicity, compared to 9.2% with gemcitabine alone, in advanced pancreatic cancer [10]. Activity has also been demonstrated in the phase II setting in relapsed ovarian, [15, 29] recurrent or metastatic nasopharyngeal, [30] and metastatic breast cancers [28].
Pre-clinical and clinical data support combining lonafarnib with cisplatin and gemcitabine. Lonafarnib is additive to synergistic with chemotherapy due to its cytostatic, proapoptotic, and glycoprotein inhibitory properties [46]. Lonafarnib synergistically enhanced cisplatin chemosensitivity in melanoma, NSCLC, and glioblastoma cell lines [3, 42]. FTIs combined with gemcitabine demonstrated additive cytotoxicity in xenograft models [43]. Clinically, a phase II study of advanced urothelial tract cancer patients treated with second-line lonafarnib and gemcitabine demonstrated no severe hematologic toxicities with a response rate of 32.3% (9 PR, 1 CR) [45]. Another FTI, tipifarnib, combined with gemcitabine and cisplatin was tolerable with nausea, vomiting, fatigue being the most frequent toxicities. Dose-limiting thrombocytopenia and/or neutropenia established the MTD of tipifarnib at 300 mg po BID, gemcitabine 1,000 mg/m2 day 1, 8 and cisplatin 75 mg/m2 day 1, every 21 days [2]. High efficacy (8 PRs: 4 NSCLC, 2 ovarian, 1 bile duct and 1 hepatocellular cancer), and 1 CR (NSCLC) were observed, and correlated with farnesylation inhibition in vivo in patient buccal mucosal cells [2].
The goals of this study were to determine the safety, tolerability, toxicity profile and MTD of lonafarnib combined with gemcitabine and cisplatin. Pharmacokinetic (PK), biologic interactions, and surrogate markers of biologic lonafarnib activity, along with preliminary evidence of anti-tumor activity, were investigated.
Materials and methods
Patient selection
Patients with histologically confirmed advanced solid malignancies refractory to standard therapy or for whom no effective therapy was available were eligible for this study, including clinically stable patients with treated intracranial metastases, not requiring steroids and without carcinomatous meningitis. Additional eligibility criteria included: age ≥18 years; Southwestern cooperative oncology group (SWOG) performance status of ≤2; adequate oral/enteral intake with normal gastrointestinal absorption, and adequate hematopoietic, hepatic, and renal function. Patients were excluded if they had major surgery, radiotherapy, chemotherapy, or investigational agents within 4 weeks of study entry, concurrent anti-tumor therapy, previous FTI therapy, concurrent CYP3A4 inhibitors or inducers, or baseline QTc prolongation (>440 ms). Patients who had prior high-dose chemotherapy requiring hematopoietic stem-cell rescue, prior therapy with nitrosoureas and mitomycin C, and radiation to >30% of bone-marrow containing areas were excluded. Informed consent was obtained according to federal and institutional guidelines.
Drug administration
Lonafarnib was supplied by Schering-Plough Research Institute (Keniliworth, NJ, USA) as 25 mg oral tablets. Commercially available intravenous gemcitabine and cisplatin were used. Patients were initially started on a 28-day cycle at lonafarnib doses of 75 mg po BID, gemcitabine 750 mg/m2 day 1, 8, 15 and cisplatin 75 mg/m2 day 1 with planned dose escalation as in Table 2. Due to substantial DLTs of neutropenia and thrombocytopenia after escalation to third dose level in the initial 14 patients, these initial patients were redefined as being “heavily pre-treated” (cohort 1) and the protocol was amended to allow enrolment and re-escalation of “less heavily pre-treated” patients on alternating 28-day and 21-day cycle cohorts (cohorts 2 and 3, respectively) until the MTD was defined in each cohort. Protocol amendments for the less heavily pre-treated patients in cohorts 2 and 3 included additional exclusion criteria: patients could not have had >6 cycles of any alkylating agent (excluding oxaliplatin) or >2 cycles of carboplatin (subsequently further amended to >6 cycles before enrollment), prior radiation to >25% of the bone marrow, or have wide metastatic bone disease. Intra-patient dose escalation was not permitted. Subsequent dose level enrollment occurred only after three patients completed one cycle of lonafarnib therapy with laboratory and toxicity assessments. Patients had to complete one cycle of therapy to be evaluable for efficacy (cycle 2 day 2 of the initial study cohort and cycle 2 day 8 in the amended cohorts). Patients withdrawing for reasons unrelated to drug toxicity prior to one cycle of lonafarnib were replaced.
Table 2.
Dose levels and patient treatment with lonafarnib, gemcitabine and cisplatin
| Cohort 1 (heavily pre-treated) dose levels |
Lonafarniba | Gemcitabineb | Cisplatinc | Number of Patients (# of courses) |
Patients with dose limiting toxicity (n) |
|---|---|---|---|---|---|
| −1 | 50 | 750 | 75 | None | |
| 1 | 75 | 750 | 75 | 3 (7) | None |
| 2 | 75 | 1,000 | 75 | 7 (14) | Grade 4 neutropenia and grade 4 thrombocytopenia (1) |
| Grade 4 neutropenia/neutropenic fever (1) | |||||
| 3 | 100 | 1,000 | 75 | 4 (7) | Grade 4 neutropenia, grade 4 thrombocytopenia grade 4 diarrhea (1) |
| Cohort 2 (less heavily pre-treated) dose levels | |||||
| 6 | 75 | 1,000 | 75 | 3 (16) | Grade 4 neutropenia and platelets (1) |
| Grade 4 neutropenia with fever, grade 4 platelets, grade 3 atigue (1) |
|||||
| Cohort 3 (less heavily pre-treated) dose levels | |||||
| 10 | 75 | 750 | 75 | 2 (4) | None |
| 11 | 100 | 1,000 | 75 | 3 (5) | None |
Lonafarnib dose in mg BID on days 2–28 cycle 1 and days 1–28 subsequent cycles in cohort 1, days 8–28 of cycle 1 and days 1–28 of subsequent 28-day cycles in cohort 2, and on days 8–21 of cycle 1 and days 1–21 to of subsequent 21-day cycles in cohort 3, due to nausea and vomiting noted with gemcitabine and cisplatin administration
Gemcitabine dose in mg/m2 on days 1, 8, 15 q 28 days in cohorts 1 and 2 and on days 1 and 8 q 21 days in cohort 3
Cisplatin dose in mg/m2 on day 1 of each cycle
On day 1, patients received gemcitabine over 30 min followed by cisplatin over 60 min, with hydration and standard anti-emetics (steroids and anti-5HT3 agents) on days when cisplatin and gemcitabine were administered; however, in the original protocol, systemic steroids were not allowed for post-cisplatin delayed emesis unless other measures were ineffective. Prophylactic anti-emetics were also not given prior to lonafarnib until the patient experienced >grade 1 nausea/vomiting. Due to severe emesis in cohort 1, the protocol was amended to allow steroids for delayed emesis (up to 8 days post-cisplatin) and prophylactic anti-emetics for lonafarnib for cohorts 2 and 3. Lonafarnib was initially administered with food at 12 h-intervals daily continuously beginning on cycle 1/day 2. However, due to significant nausea and emesis affecting PK assessments in the initial 14 patients (cohort 1), the protocol was amended to start lonafarnib on day 8 of cycle 1 without breaks through subsequent cycles, in cohorts 2 and 3. A maximum of 6 cycles of cisplatin could be given; responding patients continued with lonafarnib and gemcitabine until study discontinuation criteria were met.
DLT was defined as: (a) any non-hematologic toxicity ≥grade 3 including grade 3–4 nausea while on optimal anti-emetic therapy or grade 3 diarrhea while receiving an optimal anti-diarrheal regimen; (b) grade 4 neutropenia (ANC <500) >5 days or associated with fever >38.3°C; (c) any grade 4 thrombocytopenia (platelets <25,000/mm3); (d) hemoglobin ≤6.5 g/dL; (e) neuropathy ≥grade 2; or (f) treatment delay >4 weeks due to drug-related toxicity. Patients were enrolled in standard 3 + 3 cohorts. The MTD was defined as the highest dose at which ≤1/6 patients experienced DLT through cycle 2/day 1 (Cohort 1) or cycle 2/day 8 (cohorts 2 and 3).
Interval toxicities had to resolve to ≤grade 1 before proceeding with a new cycle of therapy. Prophylactic myeloid colony-stimulating factors and/or antibiotics were not permitted. No dose modifications were made for grade 1 or 2 toxicities. At the first or second occurrence of grade 3 myelosuppression, subsequent treatment was held until myelosuppression resolved to <grade 1 with dose reductions of one level for gemcitabine and lonafarnib. At the third occurrence of grade 3 myelosuppression, study participation was discontinued. If a dose reduction was required for lonafarnib due to toxicity, pharmacokinetic (PK) sampling for lonafarnib was obtained before the dose reduction (trough concentration 12 h after the last dose) and 2 weeks thereafter. For grade 3 nausea and vomiting or diarrhea, lonafarnib was held until resolution to ≤grade 1 then decreased by one dose level. With any grade 4 non-hematologic toxicity, patients were discontinued from study unless they were responding.
For responding patients with clinically significant grade 3 or 4 non-hematologic toxicity, all drugs were held until toxicity resolved to <grade 1, then restarted with the implicated agent(s) reduced one dose level. If the required dose was less than the lowest dose level in each cohort, treatment was discontinued. If subsequent cycles were completed at the reduced dose with ≤grade 1 toxicity, re-escalation to the next higher dose was permitted. Although all adverse events were noted, adverse events attributable to lonafarnib only were considered in relationship to “study drug”.
Clinical assessments
Within 14 days of starting the treatment, a complete history and physical, documentation of weight and performance status, ophthalmologic examination (ocular history, fundoscopy, and slit-lamp), electrocardiogram, laboratory tests, and pregnancy testing were performed. Weekly evaluations of clinical status and laboratory results were graded according to NCI Common Toxicity Criteria, Version 2.0. Baseline radiologic and serum tumor markers were obtained within 28 days of starting treatment, and after every even-numbered cycle, tumor status was re-assessed by WHO criteria.
Pharmacokinetic sampling
The PK effects of the combination of lonafarnib, gemcitabine, and cisplatin, were assessed independently and during co-administration. Cycle 1 lonafarnib trough blood levels were drawn prior to the morning dose of lonafarnib on days 8, 15, and 22 in the original cohort 1; however, with the amendment starting lonafarnib on day 8 in cohorts 2 and 3, trough levels were obtained only on days 15 and 22 for cohort 2, and on day 15 for cohort 3. Cycle 2 lonafarnib trough levels were drawn at baseline and then at 1, 2, 4, 6, 12, h after the first dose for all cohorts, with additional trough levels drawn for cycle 2 day 15 in cohort 3. Cisplatin levels were collected prior to the morning dose and at 5, 15, 30 min, 1, 2, 4, 6, 24, and 48 h following the end of infusion cycles 1 and 2/day 1 for cohorts 1 and 2. Gemcitabine samples were obtained from cohorts 1 and 2 patients at 5, 15, and 30 min, 1, 2, 4, and 6 h post infusion on cycle 1/days 1 and 8. Cisplatin and Gemcitabine sampling was not required for Cohort 3.
For determination of plasma lonafarnib and cisplatin concentrations, 3 mL blood samples were collected in sodium heparin tubes. For gemcitabine sampling, 5 mL blood samples were collected in specially prepared heparin Vacutainer® tubes with tetrahydrouridine (THU). A 10 mg/mL THU stock solution was made by adding 12 mL of sterile water to 10 mg THU, then 0.31 mL of the stock THU solution was added. All tubes were inverted once, immediately placed on ice, and centrifuged at 3,000 RPM × 15 min at 4°C. Resulting plasma was divided into two aliquots of 0.5 mL, labeled, transferred to cryogenic tubes (NUNC Cryotube™) and immediately stored at ≤−70°C until assayed.
Lonafarnib pharmacokinetic analysis plasma samples were shipped on dry ice to Taylor Technology Inc. (Princeton NJ, USA). Samples for gemcitabine and cisplatin pharmacokinetic analysis were shipped to MDS Pharma Services (St Laurent, PQ, Canada). After thawing plasma samples at room temperature, lonafarnib and gemcitabine concentrations were determined using liquid chromatography tandem mass spectrometry with lower limits of quantitation (linear concentration range) of 5 ng/mL (5–2,500 ng/mL) for lonafarnib and 10 ng/mL (10–2,500 ng/mL) for gemcitabine. Plasma cisplatin concentrations were determined using atomic absorption assay with a linear concentration range of 50 ng/mL (50–2,000 ng/mL).
Individual plasma concentrations of lonafarnib, gemcitabine, and cisplatin were analyzed using model-independent methods. PK parameters of lonafarnib, gemcitabine, and cisplatin were calculated by non-compartmental methods using Pharsight® Knowledgebase Server™ Version 2.0.1 with WinNonlin Software Version 40.1 (Pharsight Corporation, Cary, NC, USA). The effects of a single dose of lonafarnib on gemcitabine and cisplatin PK (Cmax and AUC48h) were assessed by analysis of variance (ANOVA). Similarly, the effect of gemcitabine and cisplatin on lonafarnib PK (Cmax and AUC12h) was evaluated. All analyses were performed on logarithmically transformed PK parameters using SAS software (Version 6.21; SAS Institute, Inc., Cary, NC, USA).
Biological Studies
Whole blood for FTPase activity was drawn on days 1 and 15 of cycles 1 and 2 in all cohorts from each patient.
HDJ2 surrogate marker detection assay for farnesylation status
Peripheral blood mononuclear cell (PBMC) collection and preparation for HDJ2 assay
Two 8-mL tubes of blood were collected in BD Vacutainer® CPT™ Cell Preparation Tubes with Sodium Heparin (Becton Dickinson, Franklin Lakes, NJ, USA). Tubes were inverted 8–10 times and centrifuged on a horizontal rotor within 2 h of collection at 1,500–1,800g RCF × 30 min. After centrifugation, the buffy coat was transferred to a 15-mL conical tube and washed twice in 10 mL phosphate buffered saline (PBS) at 1,500g × 15 min. The final PBS wash was removed and cell pellets were flash frozen and stored at −70°C until analysis.
Western blot for HDJ2 farnesylation status
Immunoblotting for HDJ2 post-translational processing was performed using previously published methods [8]. The antibody to HDJ2 was KA2A5.6 (Neomarkers, Fremont, CA, USA). The secondary antibody was an anti-mouse HRP-conjugated antibody (Amersham, Piscataway, NJ, USA). Detection was carried out using ECL Plus chemiluminescent reagents (Amersham, Piscataway, NJ, USA) and ChemiImager 5500 software (Alpha Innotech, San Leandro, CA, USA) was used to quantitate band intensity. Lonafarnib effects on the farnesylation of HDJ2 were determined by analyzing mobility gel shift comparing processed (44 kD) to the unprocessed form (46 kD). After subtracting background, the black pixels were counted and each band assigned a value proportional to the amount of HDJ2 present. The percentage of unfarnesylated HDJ2 was determined for each time point by dividing each 46 kD band value by the sum of the 46 kD and 44 kD bands. Absolute percentage change in unfarnesylated HDJ2 was calculated by subtracting pre-treatment day 1 from day 15 values.
Ex vivo farnesyltransferase enzyme assay
Peripheral blood mononuclear cell (PBMC) lysate collection and preparation
Five to seven milliliters of whole blood was drawn in sodium heparin tubes, transferred to 15-mL Accuspin Histopaque tubes (Sigma Chemical Company, St. Louis, MO, USA) and centrifuged at 200g × 30 min. The lymphocyte fraction was isolated, diluted with 10 ml of distilled water, centrifuged at 700g for 10 min, immediately frozen, and stored at −80°C.
Scintillation-proximity assay of farnesyltransferase inhibition
Changes in the enzymatic activity of FTPase in PBMC lysates were measured using the Amersham Scintillation Proximity Assay (Amersham Pharmacia Biotechnology) with lamin B peptide substrate (Biotin-YRASNRSCAIM) and 1–3H n farnesylpyrophosphate according to the manufacturer’s instructions and previously published methods [17]. Three concentrations were assayed (1, 2, and 3 µg protein lysate/µL) in triplicate, to ensure assay linearity. Counts per minute for each sample were determined on a Beckman LS1801 scintillation counter (Beckman, Fullerton, CA, USA).
Results
General
Twenty-two patients, whose baseline characteristics are depicted in Table 1, received 53 courses of lonafarnib, gemcitabine, and cisplatin. Although all 22 patients were evaluable for toxicity, only 16 were evaluable for efficacy (one patient withdrew due to early progression, two withdrew consent due to toxicity). Nine patients (41%) discontinued therapy due to treatment-related toxicity, while 13 discontinued participation due to disease progression.
Table 1.
Patient demographics and characteristics
| Characteristic | Number |
|---|---|
| Number of patients | 22 |
| Total number of assessable courses | 53 |
| Number of courses per patient | |
| Median (Range) | 2 (1–8) |
| Patients receiving >2 cycles of therapy | 5 |
| Sex | |
| Male | 11 |
| Female | 11 |
| Age | |
| Median (in years) | 54 |
| Range (in years) | 37–72 |
| Performance statusa | |
| 0 | 4 |
| 1 | 17 |
| 2 | 1 |
| Previous treatment | |
| Median (range) of number of prior chemotherapy treatments |
3.5 (0–9) |
| Less than three prior chemotherapy regimens | 6 |
| Three or more prior chemotherapy regimens | 13 |
| Prior investigational/targeted therapy/clinical trial | 4 |
| No prior therapy (including targeted therapy, chemotherapy and radiotherapy) |
2 |
| Prior radiotherapy | 10 |
| Tumor type | |
| Colorectal | 6 |
| Breast | 3 |
| Cholangiocarcinoma | 2 |
| Sarcomab | 2 |
| Othersc | 9 |
Performance status assessed by Southwestern Oncology Group criteria
Sarcoma includes synovial cell sarcoma and chondrosarcoma
Includes one patient each with unknown primary carcinoma, melanoma, mesothelioma, renal cell carcinoma, mucoepidermoid carcinoma of the parotid gland, thymoma/thymic carcinoma, ovarian carcinoma, adenocarcinoma of the pancreas, and non-small cell lung cancer
Adverse events and dose-limiting toxicities
All 22 patients experienced adverse events with the most frequent treatment-related events being nausea (86%), vomiting (86%), and fatigue (77%). Twenty of the 22 patients (91%) developed grade 3/4 treatment-related toxicities including neutropenia (41%), nausea (36%), thrombocytopenia (32%) and anemia (23%) and vomiting (23%). Nine (41%) of the 22 subjects discontinued therapy due to toxicity, with nausea and vomiting as the most common reason for discontinuation: 5 of 14 patients in cohort 1 withdrew due to severe nausea, vomiting, dehydration and malaise, and one patient due to neutropenic fever. All patients were treated at a single institution with standard anti-emetic therapy including systemic steroids on days when cisplatin and gemcitabine were administered; however, systemic steroids were not allowed for post-cisplatin delayed emesis, nor were prophylactic anti-emetics prior to lonafarnib. Due to severe emesis in cohort 1, the protocol was amended to allow steroids for delayed emesis and prophylactic anti-emetics for Cohorts 2 and 3. After the amendment, 3 of 8 patients withdrew due to hematologic (1) and gastrointestinal (2) toxicities.
As demonstrated in Table 2, there were substantial DLTs of neutropenia, neutropenia with fever, diarrhea, and thrombocytopenia in cycle 1 in the second and third dose levels. Initially only one DLT attributable to lonafarnib was observed at Dose Level 2, which prompted a dose expansion to six patients; however, as there were no other DLTs, the dose was escalated to Dose Level 3. As toxicities were high with DLTs in three patients at this dose, the fourth patient was treated at Dose Level 2 instead of the assigned Dose Level 3. Despite being treated at Dose Level 2, this patient developed grade 4 hematologic toxicity. Furthermore, there was severe nausea, vomiting, dehydration and syncopy in one patient at Dose Level 2, and nausea and vomiting in two patients in Dose Level 3 (Table 2). Because lonafarnib could not be absorbed due to emesis in cycle 1, these toxicities were not considered DLT. At Dose Level 1, cohort 1 did not have any cycle 1 DLTs; therefore, lonafarnib 75 mg BID with gemcitabine 750 mg/m2 on days 1, 8, 15 and cisplatin 75 mg/m2 on day 1 on a 28-day schedule was established as the MTD.
In Cohort 2 (less heavily pre-treated patients, 28-day cycles), dose-limiting grade 4 thrombocytopenia and neutropenia occurred in two patients at the initial dose level (lonafarnib 75 mg BID, gemcitabine 1,000 mg/m2 on days 1, 8, 15 and cisplatin 75 mg/m2 on day 1); therefore, the treatment schedule was closed. In Cohort 3 (less heavily pre-treated patients, 21-day cycles), no treatment-related DLTs were noted in the first two dose levels. Lonafarnib 100 mg BID, gemcitabine 1,000 mg/m2 days 1 and 8, and cisplatin 75 mg/m2 day 1 was the highest level examined without DLT prior to study termination.
Hematologic toxicity
Hematologic toxicities for each dose level are depicted in Table 3. Patients experienced dose-limiting hematologic toxicity regardless of pre-treatment intensity. Absolute neutrophil count (ANC) nadir typically occurred around day 8 or 15; whereas platelet nadirs occurred on day 15, necessitating gemcitabine being held or dose-reduced. Overall, neutropenia occurred in 19 (35.7%) of 53 courses: grade 3 or 4 neutropenia in 15 (28.3%) courses with 60% of severe neutropenia occurring in heavily pre-treated patients. However, on the 28-day schedule, dose-limiting neutropenia was present in two patients, each in the heavily and non-heavily pre-treated cohorts treated with the same doses. Excluding heavily pre-treated patients did not appear to decrease hematologic toxicity. Lonafarnib was only held in one course due to neutropenia. Thrombocytopenia occurred in 30 (57%) of 53 courses. Grade 3 or 4 thrombocytopenia occurred in 15 (28%) of 53 courses, and was dose limiting in one heavily pre-treated patient and two less heavily pre-treated patients at the same doses on the 28-day schedule. Dose omissions and reductions due to thrombocytopenia were required in 22 and 21% of cycles, respectively. Anemia occurred in 47% of patients; however, grade 3 and 4 anemia occurred in only 30% of patients and was not dose-limiting. Although grade 4 anemia occurred in one less heavily pre-treated patient during the first cycle, it was due to a bleeding esophageal tear secondary to severe chemotherapy-related nausea and vomiting, and was not felt to be DLT as lonafarnib was never ingested.
Table 3.
Hematological toxicities in all courses attributed to lonafarnib, gemcitabine and cisplatin
| Cohort | Lonafarnib (mg) BID continuous |
Gemcitabine (mg/m2) days 1, 8, 15 q 4 weeks, days 1 and 8 q 3 weeks |
Cisplatin (mg/m2) Day 1 |
Number of patients (courses) |
Neutropenia incidence (# of courses) |
Thrombocytopenia incidence (# of courses) |
Anemia incidence (# of courses) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1–2 | G3 | G4 | G1–2 | G3 | G4 | G1–2 | G3 | G4 | |||||
| 1 | 75 | 750 q 4 weeks | 75 | 3 (7) | 0 | 3 | 1 | 1 | 2 | 0 | 0 | 0 | 0 |
| 1 | 75 | 1,000 q 4 weeks | 75 | 7 (14) | 0 | 1 | 2 | 5 | 1 | 0 | 4 | 1 | 0 |
| 1 | 100 | 1,000 q 4 weeks | 75 | 4 (7) | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 1 |
| 2 | 75* | 1,000 q 4 weeks | 75 | 3 (16) | 4 | 1 | 3 | 4 | 8 | 1 | 4 | 11 | 1 |
| 3 | 75* | 750 q 3 weeks | 75 | 2 (4) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 3 | 100* | 1,000 q 3 weeks | 75 | 3 (5) | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 1 | 0 |
| TOTAL | 22 (53) | 4 (7.5%) | 7 (13%) | 8 (15%) | 15 (28%) | 14 (26%) | 1 (2 %) | 9 (17%) | 14 (26%) | 2 (4%) | |||
G1–G4 represent National Cancer Institute Clinical Toxicity Criteria Version 2.0 grades 1–4. The number of cycles in which each individual toxicity was observed is indicated in the body of the table
Gastrointestinal toxicity
The most frequent gastrointestinal toxicities were moderate to severe nausea (71%), vomiting (49%), and diarrhea (38%); severe grade 3 and 4 nausea (13%), vomiting (11%), and diarrhea (5%), as shown in Table 4. Mild toxicities included dyspepsia (24.5%), bloating and distension (21%), hypokalemia (17%), dysguesia (13%), and dehydration (13%). Although grade 3 nausea, vomiting, dehydration with syncopy and hypotension were observed in one patient in cohort 1/Dose Level 2, and two patients in cohort 2/Dose Level 6; these were not considered to be dose-limiting, as they were attributed to gemcitabine and cisplatin, as emesis prevented lonafarnib absorption. Due to these gastrointestinal toxicities, the protocol was amended to start lonafarnib on day 8 instead of day 1 in cohorts 2 and 3 in an attempt to separate the effects of lonafarnib from gemcitabine and cisplatin.
Table 4.
Gastrointestinal toxicities attributed to lonafarnib, gemcitabine and cisplatin
| Cohort | Lonafarnib (mg) BID continuous |
Gemcitabine (mg/m2) days 1, 8, 15 q 4 weeks, days 1 and 8 q 3 weeks |
Cisplatin (mg/m2) Day 1 |
Number of patients (courses) |
Nausea incidence (# of courses) |
Vomiting incidence (# of courses) |
Diarrhea incidence (# of courses) |
Anorexia incidence (# of courses) |
Dyspepsia incidence (# of courses) |
Bloating/ distension incidence (# of courses) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | G1–2 | G3–4 | |||||
| 1 | 75 | 750 q 4 weeks | 75 | 3 (7) | 5 | 1 | 5 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 2 | 75 | 1,000 q 4 weeks | 75 | 7 (14) | 5 | 2 | 4 | 2 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| 3 | 100 | 1,000 q 4 weeks | 75 | 4 (7) | 3 | 2 | 3 | 2 | 0 | 1 | 5 | 0 | 2 | 0 | 0 | 0 |
| 4 | 75* | 1,000 q 4 weeks | 75 | 3 (16) | 12 | 0 | 2 | 0 | 8 | 1 | 2 | 0 | 5 | 0 | 4 | 0 |
| 5 | 75* | 750 q 3 weeks | 75 | 2 (3) | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 0 | 3 | 0 | 3 | 0 |
| 6 | 100* | 1,000 q 3 weeks | 75 | 3 (6) | 4 | 1 | 4 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 3 | 0 |
| TOTAL | – | – | 22 (53) | 31 (58%) | 7 (13%) | 20 (38%) | 6 (11%) | 17 (32%) | 3 (5.6%) | 13 (24.5%) | 0 | 13 (24.5%) | 0 | 11 (20%) | 0 | |
G1–G4 represent National Cancer Institute Clinical Toxicity Criteria Version 2.0 grades 1–4. The number of cycles in which each individual toxicity was observed is indicated in the body of the table
Other toxicities
The predominant non-gastrointestinal, non-hematologic toxicity was fatigue noted in 24 courses (45%); 38% were grade 1 or 2. Grade 3 and 4 fatigue was observed in 7.5% of courses but did not necessitate dose delays or reductions. Other grade 1 and 2 toxicities included tinnitus (24.5%), sensory neuropathy (15%), arthralgia (13%), visual disturbances, and rash (11% each).
Dose intensity/dose modifications
Seventeen (77%) subjects experienced grade 3 or 4 adverse events that limited dose intensity and necessitated modification either by dose reduction or holding doses of gemcitabine on days 8, 15. Although some hematologic toxicity was evident in cycle 1, most hematologic toxicity was cumulative and more dose reductions and delays were evident in later cycles, predominantly on the 28-day schedule. Nine patients (41%) required delays in re-treatment of usually 1-week duration (seven in the 28-day schedule due to hematologic toxicity in later cycles, two in the 21-day schedule unrelated to toxicity), gemcitabine was held at least once (day 8 or day 15) due to neutropenia or thrombocytopenia in 53% of cycles (predominantly in the 28-day schedules). Approximately 22% of 143 planned doses of gemcitabine were held, and 21% of gemcitabine doses were reduced due to hematologic toxicity. Median dose intensity was estimated to be 57% of the planned gemcitabine dose. Lonafarnib doses were held due to neutropenia in one cycle of therapy, and dose reductions were required in four (7.5%) cycles due to nausea, vomiting, or diarrhea. The median dose intensity of lonafarnib was greater than 90% of the planned dose. All cisplatin doses were fixed and delivered without reduction.
Anti-tumor activity
This study was terminated early by the Sponsor, due to negative interim efficacy and toxicity results from a phase III randomized study of carboplatin and paclitaxel with lonafarnib versus placebo in advanced NSCLC. Although new enrollment was halted after Dose Level 2 in cohort 3, responding patients were allowed to continue study treatment. Response and efficacy was determined in 16 of 22 patients: two patients (Cohort 3/Dose Level 6) had confirmed responses (one R and one PR) and four patients had SD ≥2 cycles.
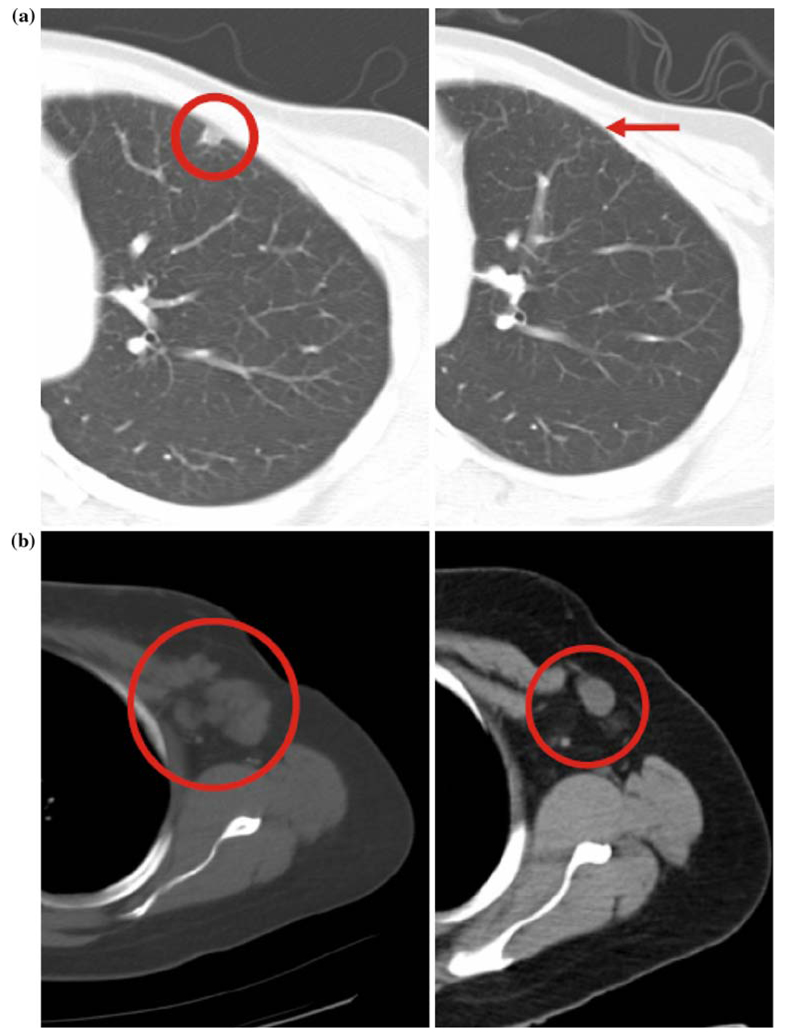
Both women with confirmed responses were 39 years old with metastatic breast cancer (ER/PR/Her-2/neu negative and ER/PR/Her2neu positive, respectively), and both patients had received local surgical/radiation therapy followed by adriamycin, cyclophosphamide, and paclitaxel/docetaxel. The first patient received low-dose gemcitabine and radiotherapy for a local recurrence, before rapidly developing lymph node, chest wall, and lung metastases. On study cycle 1/day 15 gemcitabine was held due to severe neutropenia and thrombocytopenia; however, a clinical and radiologic CR (Fig. 1a) was evident by cycle 3 and sustained to cycle 7, when she was withdrawn from study due to persistent and increasing delays due to neutropenia and thrombocytopenia. The second patient on adjuvant tamoxifen developed recurrent progressive chest wall disease, axillary lymph node and bone metastases treated with various prior regimens incorporating trastuzumab, gemcitabine, docetaxel, capecitabine, gefitinib, and celecoxib. Following cycle 2, a PR chest wall disease resolution and a 72% reduction in the left axillary adenopathy were evident (Fig. 1b). Despite holding day 15 gemcitabine due to thrombocytopenia in all cycles, PR was maintained until the end of cycle 8.
Fig. 1.
a Chest CT at baseline and after two cycles of therapy, demonstrating complete resolution of a lung lesion in a breast cancer patient. b Chest CT at baseline and after two cycles of therapy, demonstrating partial resolution (72% reduction) of axillary adenopathy in another breast cancer patient
Four patients with metastatic disease in cohort 1 had SD: one patient with pancreatic cancer was stable for four cycles (Dose Level 1); one patient with thymic carcinoma completed 3 cycles (Dose Level 2) before progressing; one patient with cholangiocarcinoma was stable after 2 cycles (Dose Level 2) but withdrew consent due to nausea, vomiting and dehydration; and one patient with a parotid mucoepidermoid cancer was stable for two cycles (Dose Level 3), before discontinuing therapy due to disease-related dysphagia. Response was not determined in six patients who had no follow-up imaging or had failed to complete >1 cycle. Overall, the response rate was 12.5% with a clinical benefit rate (PR + CR + SD) of 37.5% in 16 evaluable patients.
Pharmacokinetics (PK)
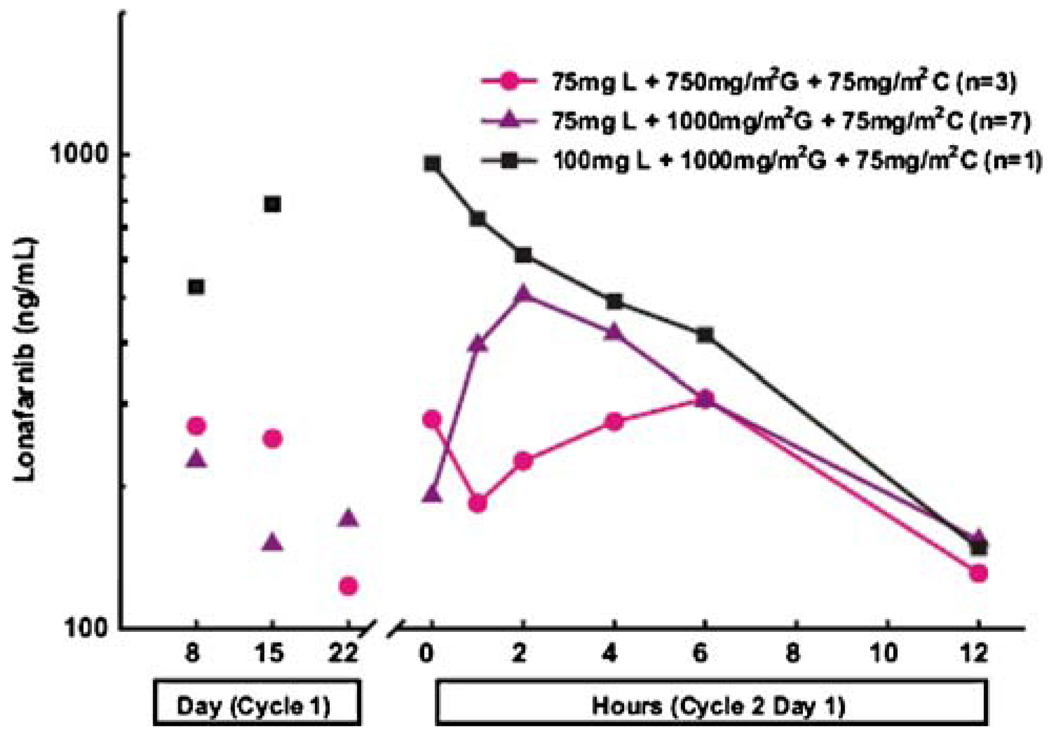
Although 17 gemcitabine and cisplatin PK analyses were performed, only 11 patients had cycle 2 day 1 lonafarnib PK profiles performed. With the amended protocol starting lonafarnib on day 8, and allowing anti-emetic prophylaxis in cohorts 2 and 3, more patients were able to ingest the pills for lonafarnib PK analyses. A representative mean plasma lonafarnib concentration versus time profile is presented in Fig. 2. Mean trough lonafarnib concentrations were similar on cycle 1/day 8, 15, and 22 and cycle 2/day 15 for each dose, indicating that steady state was attained by cycle 1/day 8 in all cohorts. Lonafarnib trough concentrations range from 123 to 347 ng/mL at 75 mg BID, and from 363 to 958 ng/mL at 100 mg BID (Table 5). Mean lonafarnib derived PK parameters were similar between Dose Levels 1 and 2, regardless of gemcitabine and cisplatin doses. The AUC for one patient treated with lonafarnib 100 mg BID with gemcitabine and cisplatin was similar to the AUC for lonafarnib 100 mg BID alone. Despite limited numbers of patients, data suggest that lonafarnib PK were not affected by co-administration with gemcitabine and cisplatin.
Fig. 2.
Mean plasma concentrations of lonafarnib versus time profiles following twice daily oral administration of lonafarnib in combination with intravenous gemcitabine and cisplatin
Table 5.
Individual pre-dose plasma concentrations and mean pharmacokinetic parameters of lonafarnib, following twice-daily multiple dose oral administration of lonafarnib in combination with IV administration of gemcitabine and cisplatin
| Cohort 1 Dose Level (dose)a |
Cohort 1 subject | C1D8 | C1D15 | C1D22 | C2D1 | Number of subjects tested |
Cmax median (range) ng/mL |
Tmax median (range) h |
AUCτ median (range) ng/h/mL |
CL/F median (range) mL/min |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 0 h | 0 h | 0 h | 12 h | |||||||
| 1 (75 + 750 + 75) | 001 | 249 | 202 | 115 | NS | NS | 1 | 429 (N/A) | 6.0 (N/A) | 3769 (N/A) | 332 (N/A) |
| 002 | 444 | 549 | 50.4 | NS | NS | ||||||
| 003 | 112 | 5.44 | 205 | 347 | 171 | ||||||
| Mean plasma levels ng/mL (CV, %) |
268 (62) | 252 (109) | 123 (63) | 347 (N/A) | 171 (N/A) | ||||||
| 2 (75 + 1,000 + 75) | 004 | 115 | 245 | 186 | 236 | 60.0 | 8 | 513 (55) | 3.0 (1–12) | 3412 (36) | 412 (37) |
| 005 | 133 | 197 | 121 | 143 | 352 | ||||||
| 006 | 64.6 | 123 | 240 | 346 | 97.1 | ||||||
| 007 | 186 | 78.8 | 279 | 67.9 | 109 | ||||||
| 008 | 775 | 237 | NS | NS | NS | ||||||
| 009 | 280 | 129 | 87.0 | 369 | 110 | ||||||
| 010 | 34.7 | 48.4 | 104 | 132 | 196 | ||||||
| 015 | NS | 151 | NS | 177 | 198 | ||||||
| 016 | NS | 347 | 308 | 139 | 149 | ||||||
| 017 | NS | 248 | NS | NS | NS | ||||||
| Mean plasma levels ng/mL (CV, %) |
227 (112) | 180 (50) | 189 (47) | 201 (53) | 159 (58) | ||||||
| 3 (100 + 1,000 + 75) | 011 | 526 | 788 | NS | 958 | 148 | 1 | 958 (N/A) | 0 (N/A) | 5219 (N/A) | 319 (N/A) |
| 012 | 132 | NS | NS | NS | NS | ||||||
| 014 | 432 | 130 | NS | NS | NS | ||||||
| Mean plasma levels ng/mL (CV, %) |
363 (57) | 459 (N/A) | NS | 958 (N/A) | 148 (N/A) | ||||||
N/A Not applicable due to sample size < 3
Level 1: 75 mg BID lonafarnib + 750 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 2: 75 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 3: 100 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
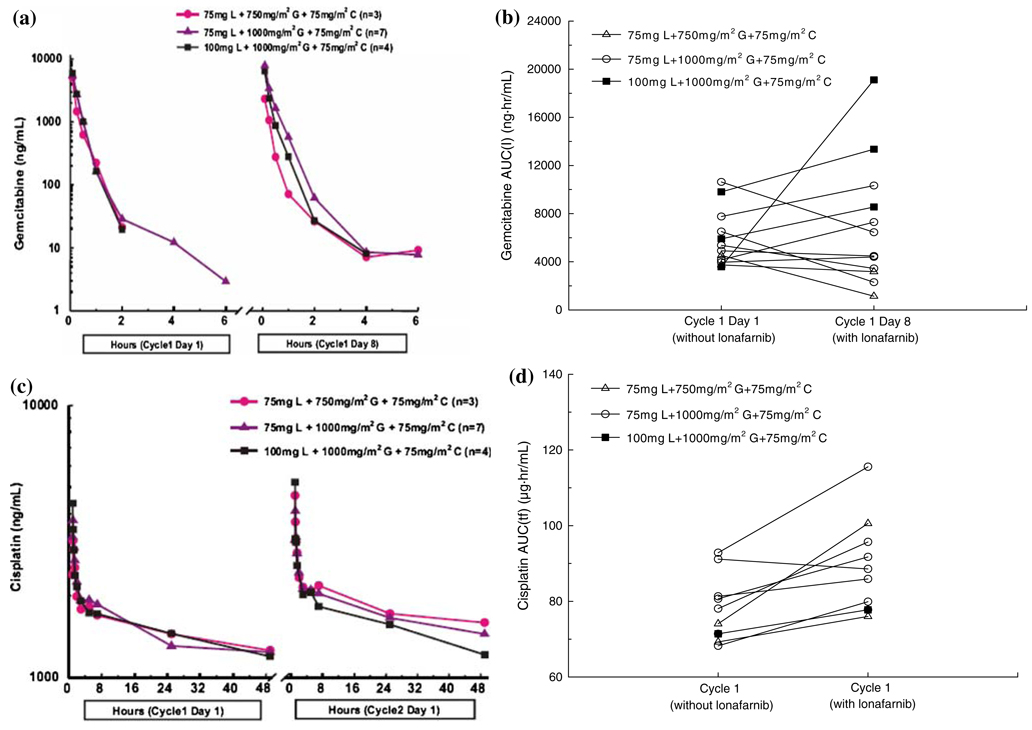
Although samples were collected from 17 subjects for both gemcitabine and cisplatin pharmacokinetics, not all samples were collected or analyzed in cycle 2. Plasma gemcitabine concentrations and PK were similar between day 1 (without lonafarnib) and day 8 (with lonafarnib) for each dose level (Fig. 3a; Table 6). Furthermore, AUC values for each subject were similar between day 1 and day 8 (Fig. 3b), indicating that lonafarnib did not affect gemcitabine PK. The mean plasma pharmacokinetic parameters of cisplatin, after gemcitabine monotherapy or with lonafarnib are provided in Table 7. AUC values were used to compare the two treatment cycles. Plasma cisplatin concentrations were similar between cycle 1 (without lonafarnib) and cycle 2 (with lonafarnib) for each dose level and were similar for all dose levels at the same cisplatin dose, regardless of lonafarnib and gemcitabine doses (Table 7; Fig. 3c). The AUC for each subject was slightly higher in cycle 2 than cycle 1; however, the difference was not clinically significant (Fig. 3d). Overall, lonafarnib did not affect cisplatin PK.
Fig. 3.
a Mean gemcitabine plasma concentrations versus time profiles following weekly 30 min intravenous infusion of gemcitabine without (day 1) and with (day 8) oral administration of lonafarnib and cisplatin. b Individual gemcitabine area under the curve (AUC) values following weekly 30 min intravenous infusion of gemcitabine without (day 1) and with (day 8) oral administration of lonafarnib and intravenous cisplatin. c Mean Plasma cisplatin concentrations of versus time profiles following day 1 60 min intravenous infusion of cisplatin in every cycle without (cycle 1) and with (cycle 2) administration of lonafarnib and gemcitabine. d Individual cisplatin area under the curve (AUC) values following day 1 60 min intravenous infusion of cisplatin in every cycle without (cycle 1) and with (cycle 2) administration of lonafarnib and gemcitabine
Table 6.
Mean pharmacokinetic parameters of gemcitabine following 30 min intravenous infusion weekly without (day 1) and with (day 8) oral administration of lonafarnib and intravenous infusion of cisplatin
| Cohort 1 Dose Level (Dose)a |
Day | n |
Cmax (ng/mL) |
AUC(I) (ng h/mL) |
AUC(tf) (ng h/mL) |
t1/2 (h) | Vd (L/m2) | tf (h) |
|---|---|---|---|---|---|---|---|---|
| 1 (75 + 750 + 75) | 1 | 3 | 6,683 (46) | 3,288 (46) | 3,276 (46) | 0.354 (27) | 157 (86) | 2.5 (0) |
| 2 (75 + 1,000 + 75) | 9 | 10,266 (51) | 5,791b (43) | 5,560 (43) | 1.67b (106) | 528b (131) | 4.81 (39) | |
| 3 (100 + 1,000 + 75) | 4 | 23,539 (80) | 8,800 (61) | 8,793 (61) | 0.271 (8) | 59.9 (63) | 2.63 (10) | |
| 1 (75 + 750 + 75) | 8 | 4 | 6,147 (35) | 3,274c (NA) | 3,320 (29) | 1.13c(NA) | 369c (N/A) | 5.58 (22) |
| 2 (75 + 1,000 + 75) | 9 | 10,986 (57) | 6,102b (49) | 6,134 (46) | 1.18b (112) | 373b(104) | 5.13 (38) | |
| 3 (100 + 1,000 + 75) | 3 | 35,606 (55) | 13,675 (39) | 13,665 (39) | 0.480 (37) | 60.7 (67) | 3.94 (32) |
N/A Not applicable, sample size<3
Level 1: 75 mg BID lonafarnib + 750 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 2: 75 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 3: 100 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
n = 8
n = 2
Table 7.
Mean pharmacokinetic parameters of cisplatin following 60-min intravenous infusion of cisplatin on day 1 of every cycle without (cycle 1) and with (cycle 2) oral administration of lonafarnib and intravenous infusion of gemcitabine
| Cohort 1 dose level (dose)a | Cycle | n | Cmax (µg/mL)b | AUC(tf) (µg h/mL)b | tf (h)b |
|---|---|---|---|---|---|
| 1 (75 + 750 + 75) | 1 | 3 | 3.73 (9) | 72.6 (4) | 48 (0) |
| 2 (75 + 1,000 + 75) | 9 | 4.01 (14) | 77.4 (20) | 48 (0) | |
| 3 (100 + 1,000 + 75) | 5 | 4.00 (24) | 73.7 (7) | 47.6(2) | |
| 1 (75 + 750 + 75) | 2 | 1 | 3.09 (N/A) | 76.0 (N/A) | 48 (N/A) |
| 2 (75 + 1,000 + 75) | 8 | 4.55 (16) | 88.4 (15) | 48 (0) | |
| 3 (100 + 1,000 + 75) | 1 | 5.22 (N/A) | 77.7 (N/A) | 48 (N/A) |
NANot applicable, sample size <3
Level 1: 75 mg BID lonafarnib + 750 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 2: 75 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Level 3: 100 mg BID lonafarnib + 1,000 mg/m2 gemcitabine + 75 mg/m2 cisplatin
Median (range)
Biological studies
Samples for pharmacodynamic (PD) marker analysis of FTPase inhibition were obtained for all 22 subjects; however, only 17 subjects were evaluable for HDJ-2 determination (four subjects had only a baseline sample submitted and one patient had no detectable HDJ-2 protein in the post-baseline sample).
Immunoblotting of HDJ2 farnesylation status
Two patients treated with lonafarnib 100 mg BID had observable HDJ-2 gel changes. In cycle 2 after administration of lonafarnib, there was a decrease in the biochemical activity and a >10% measurable shift of HDJ-2, indicating activity in one patient. This effect is not conclusive due to small patient numbers, and intra-patient variability. The biological activity of lonafarnib appears to be low or under-estimated in this study. Samples taken from patients treated at 75 mg BID did not demonstrate shift of HDJ-2 status, indicating a probable lack of PD effect.
Scintillation-proximity assay (SPA) of farnesyltransferase inhibition
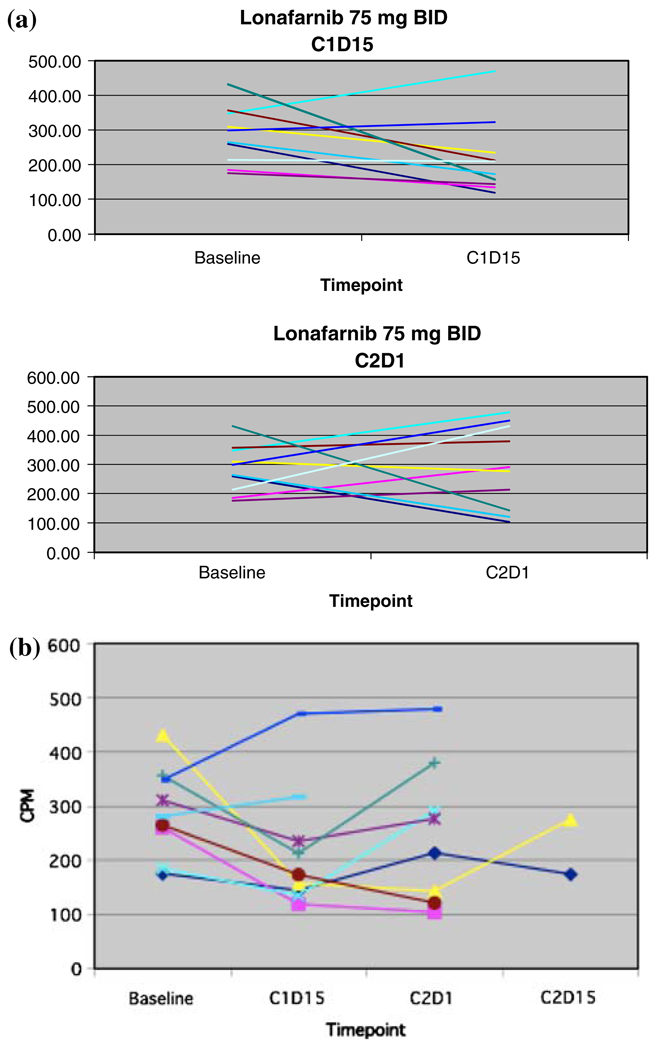
In 17 evaluable patients, a trend was noted in the percent change of FTPase inhibition: following treatment with lonafarnib, with greater inhibition of FTPase as measured by SPA in the 100 mg BID doses (Fig. 4b) rather than the 75 mg BID doses (Fig. 4a). Due to wide inter- and intra-patient variability, there were no statistically significant findings, and no significant conclusions could be drawn.
Fig. 4.
a Farnesyltransferase activity for patients treated with lonafarnib 75 mg po BID at baseline, cycles 1 and 2. b Farnesyltransferase activity for patients treated with lonafarnib 100 po BID at baseline, cycles 1 and 2
Discussion
Lonafarnib has demonstrated nanomolar potency, competitively inhibiting FTPase in preclinical models and three published phase I monotherapy studies have defined its toxicities, MTD, and anti-tumor activity [2, 7, 13]. In vitro, lonafarnib is synergistic with cisplatin and additive with gemcitabine [3, 27, 43]. Clinically, lonafarnib and gemcitabine demonstrates efficacy (two PRs and one minimal response) in pancreatic cancer [9] and urothelial cancer (9 CR, 1PR in 33 patients) [45]. This study was designed to establish the dose for further study in gemcitabine and cisplatin active malignancies with potential Ras mutations such as NSCLC.
In this study, gemcitabine and cisplatin with lonafarnib was not well-tolerated: frequent myelosuppression, gastrointestinal toxicity, malaise, anorexia, and fatigue, prompted 9 out of 22 patients to discontinue study participation. Thrombocytopenia limited the ability to give day 15 gemcitabine in >50% of patients. Limited results from the PK studies did not indicate any parameters or interactions that would predict these toxicities. Gemcitabine and cisplatin alone have substantial grade 3/4 toxicities: gemcitabine (1,000–1,250 mg/m2 weekly) and cisplatin (60–100 mg/m2 day 1 q21 or q28 days) in advanced untreated NSCLC, previously treated breast and ovarian cancers: 12–57% neutropenia, 16–52% thrombocytopenia, 20 and 25–32% nausea and emesis [15, 19, 25, 28, 29, 37]. The hematologic toxicity in our phase I study population was much higher, with 91% of patients previously treated with a median of 3.5 prior chemotherapy regimens. Most of the high hematologic toxicity limiting lonafarnib dose escalation is attributed to cisplatin and gemcitabine; the degree of lonafarnib contribution to these toxicities is not known.
The substantial gastrointestinal toxicity following gemcitabine and cisplatin was due to inadequate anti-emetic therapy in cohort 1, as corticosteroids for delayed emesis and prophylactic anti-nauseants were not allowed. Cisplatin is highly emetogenic (>90% of patients suffer emesis without anti-emetics); patients had difficulty recovering from cisplatin-induced emesis without prophylactic anti-emetics and lonafarnib induced worsening nausea. When the anti-emetic protocol was amended in cohorts 2 and 3, nausea and vomiting severity was reduced (only one grade 3 incidence) in these patients in all cycles.
By intent-to-treat analysis, the patient assigned to Dose Level 3 who actually treated at Dose Level 2 and developed hematologic DLT would have established the MTD at Dose Level 2, since the DLT would have been attributed to the assigned Dose Level 3. However, analysis of the seven patients actually treated at Dose Level 2 revealed two DLTs, and one patient with grade 3 and 4 gastrointestinal toxicities not considered DLT (unrelated to lonafarnib), which were not clinically tolerable. Therefore, based on the actual doses patients received and their first cycle toxicities, Dose Level 1 (lonafarnib 75 mg BID, gemcitabine 750 mg/m2 day 1, 8, 15 with cisplatin 75 mg/m2 day 1 q28 days) was established as the MTD. Unfortunately, the MTD was not tolerable for continuous subsequent dosing: gemcitabine was held for six doses in the seven cycles (21 doses) of therapy and was reduced twice for hematologic toxicity in three patients. Dose reductions of gemcitabine were frequent, and over 50% of patients did not receive day 8 or 15 on the 28-day schedule. Despite the study closing early without establishing the MTD for the 21-day schedule (cohort 3), it appeared better tolerated: there were no DLTs and gemcitabine was only held for three doses of nine cycles (18 doses) in five patients, with only two dose reductions. Therefore, lonafarnib 75 mg BID, gemcitabine 750 mg/m2 day 1, 8 with cisplatin 75 mg/m2 day 1 q 21 days would be the recommended dose for further studies. However it is of concern that these doses are lower than clinically established efficacious doses for gemcitabine and cisplatin [1, 10, 25, 35].
Systemic exposure (Cmax and AUClast) to gemcitabine and cisplatin did not appear influenced by concomitant administration of lonafarnib 75 mg or 100 mg BID. A phase II study of lonafarnib with gemcitabine in urothelial cancers supports that lonafarnib does not affect gemcitabine PK [45]. Mean trough plasma concentrations of lonafarnib 75 mg BID, in combination with gemcitabine, (123–347 ng/mL) were similar to previous clinical trials with lonafarnib [5, 7, 9, 13]. Minimal lonafarnib plasma concentrations (Cmin) at the MTD of 75 mg BID in this study did not reach preclinical target concentrations (>1 µM) to inhibit Ras and critical protein farnesylation and to inhibit tumor growth; [6, 26, 39, 44] however, they were achieved with 100 mg BID doses, which is consistent with other reports [2, 7, 13, 39].
Moreover, patients who were treated with lonafarnib 75 mg BID did not demonstrate a shift in HDJ-2 activity, indicating a negative PD effect with low biochemical activity of lonafarnib; whereas, the two patients treated with lonafarnib 100 mg BID showed a detectable level of unfarnesylated HDJ-2 at baseline, with one of these two patients demonstrated >10% increase in unfarnesylated HDJ-2 over pre-treatment, indicating biological activity. The HDJ-2 chaperone protein should undergo mobility shifts when FTPase is inhibited and is a validated sensitive marker of FPTase inhibition at lonafarnib levels >6.25 nM [4]. Yet no anti-tumor responses were observed at lonafarnib 100 mg BID; while they were noted at 75 mg BID, bringing into question the PD predictability of the assay in this study or possibly indicating that the anti-tumor response were due to chemotherapy alone. Data from the SPA FTPase ex vivo assays were insufficient to clarify the HDJ-2 gel findings; enzyme assays generally underestimate the degree of FTPase, [4] and small sample numbers, inter-and intra-patient variability rendered these studies inconclusive. An immunohistochemical assay of prelaminin A accumulation in buccal mucosa cells has been a sensitive PD marker of FTI in other phase I studies, and may help to clarify biological results [2, 4].
Despite phase I clinical studies determining the MTD of lonafarnib to be 300 mg daily or 200 mg BID alone, [5, 7, 13] these dose levels could not be achieved in this study due to excessive toxicity. When lonafarnib was combined with gemcitabine, hematologic and gastrointestinal DLTs limited the doses of lonafarnib and established the MTD as lonafarnib 150 mg q am, 100 mg q pm with 1,000 mg/m2 gemcitabine days 1, 8, and 15, q28 days [9]. When the feasibility of these doses was tested in the phase II setting of 33 patients with urothelial cancers, this combination was tolerated and efficacious with a response rate of 32.3% (nine PRs and one CR) [45]. However, the dose intensity of gemcitabine was 75.5% and in the 33 patients, grade 3 toxicities were observed: neutropenia (18%), thrombocytopenia (18%), anemia (27%), fatigue (30%), diarrhea (12%), and nausea and vomiting (10% of patients) indicating that there is additive hematologic and gastrointestinal toxicity when lonafarnib was combined with gemcitabine [45]. This effect is even more pronounced when combined with platinum-based combinations with higher inherent hematologic and gastrointestinal toxicity.
Another FTI, tipifarnib, combined with gemcitabine and cisplatin could be delivered with adequate tipifarnib levels for activity [2]. Tipifarnib has higher FTI activity than lonafarnib in vitro (0.45–0.57 vs. 4.9–7.8 nM IC50s in human and bovine assays, respectively), with approximately 5–10 fold higher potency in Ras processing assays over lonafarnib in human cancer cell lines [17, 18, 39]. Tipifarnib’s higher FTI potency and perhaps improved therapeutic index may allow better dose-escalation to active FTI doses in combination with chemotherapy than lonafarnib. Dose-limiting thrombocytopenia and/or neutropenia established the MTD of tipifarnib at 300 mg po BID, gemcitabine 1,000 mg/m2 day 1, 8 and cisplatin 75 mg/m2 day 1, every 21 days [2]. High efficacy [eight PRs (four NSCLC, two ovarian, one bile duct and one hepatocellular cancer), and one CR (NSCLC)] was observed, and correlated with farnesylation inhibition in vivo in patient buccal mucosal cells at these tipifarnib doses [2]. However, frequent severe nausea, vomiting, and myelosuppression occurred in the ten patients at the MTD despite excluding heavily pre-treated patients: two patients had dose limiting neutropenia and thrombocytopenia in cycle 1 and in subsequent cycles required a dose reduction of 25–30% of all drugs, and nine patients required gemcitabine and cisplatin dose reductions due to severe nausea and vomiting and fatigue. Therefore for chronic administration, tipifarnib at 300 mg BID with × 14 days, gemcitabine 750 mg/m2 day 1, 8 and cisplatin 60 mg/m2 day 1 on a 21-day schedule was recommended instead of the MTD. Of note, these doses were also significantly lower than the standard efficacious gemcitabine and cisplatin doses in clinical use [11, 12, 25, 38, 41]. As the 21-day schedule of gemcitabine and cisplatin appears to have much less hematologic toxicity and is more tolerable; from the beginning, our study should have assessed a 21-day schedule rather than the 28-day schedule, excluded heavily pre-treated patients, and explored a discontinuous dosing schedule to decrease cumulative toxicity to make this therapy more “deliver-able.”
Gemcitabine and cisplatin have demonstrated response rates of 29–62% in previously treated advanced breast cancer patients in the phase II setting; however, a high incidence of thrombocytopenia (up to 32%) was observed necessitating gemcitabine dose reduction [14, 22, 40]. The two responding breast cancer patients in this study were heavily pre-treated with prior chemotherapy but neither had any prior cisplatin, and the patient with a CR did not receive prior gemcitabine. As both gemcitabine and cisplatin are active in previously treated breast cancer, it is possible that lonafarnib did not contribute to the anti-tumor effects, as target concentrations were not reached. However, there may be other unmeasured chemotherapy or anti-tumor modulating effects. Lonafarnib is synergistic with cisplatin and additive with gemcitabine in vitro, and active against breast carcinoma xenografts [31, 32, 42]. FTIs alone are clinically very active in breast cancer [21]: a phase II study of tipifarnib dosed continuously in 76 advanced breast cancer patients demonstrated 4 PR and 6 SD lasting >24 weeks, and when dosed for 21 out of 28 days, 5 PRs and 3 prolonged SD were noted in 35 patients [21, 24]. Therefore, further exploration of lonafarnib and other FTIs with chemotherapy in breast cancer may be warranted.
This combination was originally planned to be taken forward in NSCLC, but this study was terminated early due lack of efficacy and survival benefit and high toxicity in a Phase III study of lonafarnib, paclitaxel, and carboplatin in advanced NSCLC [39]. Lonafarnib may increase nausea, vomiting, neutropenia and thrombocytopenia in combination with chemotherapy [9, 34, 45] and these toxicities prohibit achieving efficacious lonafarnib target FTPase inhibitory levels. Therefore, pursuing lonafarnib combination therapy with chemotherapy doublets, particularly platinums with high gastrointestinal and hematologic toxicities profiles, is of questionable value.
Acknowledgments
Research support: Schering-Plough Research Institute, Kenilworth, NJ, USA.
Contributor Information
Laura Q. M. Chow, University of Colorado Cancer Center, Aurora, CO, USA
S. Gail Eckhardt, University of Colorado Cancer Center, Aurora, CO, USA.
Cindy L. O’Bryant, University of Colorado Cancer Center, Aurora, CO, USA
Mary Kay Schultz, University of Colorado Cancer Center, Aurora, CO, USA.
Mark Morrow, University of Colorado Cancer Center, Aurora, CO, USA.
Stacy Grolnic, University of Colorado Cancer Center, Aurora, CO, USA.
Michele Basche, University of Colorado Cancer Center, Aurora, CO, USA.
Lia Gore, Email: lia.gore@uchsc.edu, University of Colorado Cancer Center, Aurora, CO, USA; The Children’s Hospital, Denver, CO, USA; University of Colorado Health Sciences Center at Fitzsimons, Mail Stop 8302, PO Box 6511, Aurora, CO 80045, USA.
References
- 1.Adamo V, Magno C, Spitaleri G, Garipoli C, Maisano C, Alafaci E, Adamo B, Rossello R, Scandurra G, Scimone A. Phase II study of gemcitabine and cisplatin in patients with advanced or metastatic bladder cancer: long-term follow-up of a 3-week regimen. Oncology. 2005;69:391–398. doi: 10.1159/000089993. [DOI] [PubMed] [Google Scholar]
- 2.Adjei AA, Croghan GA, Erlichman C, Marks RS, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Bruzek LM, Atherton P, Thibault A, Palmer PA, Kaufmann SH. A Phase I trial of the farnesyl protein transferase inhibitor R115777 in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2003;9:2520–2526. [PubMed] [Google Scholar]
- 3.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–1445. [PubMed] [Google Scholar]
- 4.Adjei AA, Davis JN, Erlichman C, Svingen PA, Kaufmann SH. Comparison of potential markers of farnesyltransferase inhibition. Clin Cancer Res. 2000;6:2318–2325. [PubMed] [Google Scholar]
- 5.Adjei AA, Erlichman C, Davis JN, Cutler DL, Sloan JA, Marks RS, Hanson LJ, Svingen PA, Atherton P, Bishop WR, Kirschmeier P, Kaufmann SH. A Phase I trial of the farnesyl transferase inhibitor SCH66336: evidence for biological and clinical activity. Cancer Res. 2000;60:1871–1877. [PubMed] [Google Scholar]
- 6.Ashar HR, Armstrong L, James LJ, Carr DM, Gray K, Taveras A, Doll RJ, Bishop WR, Kirschmeier PT. Biological effects and mechanism of action of farnesyl transferase inhibitors. Chem Res Toxicol. 2000;13:949–952. doi: 10.1021/tx000138v. [DOI] [PubMed] [Google Scholar]
- 7.Awada A, Eskens FA, Piccart M, Cutler DL, van der Gaast A, Bleiberg H, Wanders J, Faber MN, Statkevich P, Fumoleau P, Verweij J. Phase I and pharmacological study of the oral farnesyltransferase inhibitor SCH 66336 given once daily to patients with advanced solid tumours. Eur J Cancer. 2002;38:2272–2278. doi: 10.1016/s0959-8049(02)00379-9. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Caponigro F. Farnesyl transferase inhibitors: a major break-through in anticancer therapy? Naples, 12 April 2002. Anticancer Drugs. 2002;13:891–897. doi: 10.1097/00001813-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, Cigolari S, Testa A, Maiello E, Lopez M. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902–910. [PubMed] [Google Scholar]
- 11.Crino L. Combined platinum containing treatment in NSCLC. Lung Cancer. 2002;38 Suppl 3:S51–S52. doi: 10.1016/s0169-5002(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 12.Crino L, Calandri C. Gemzar platinum combinations: phase III trials in non-small cell lung cancer. Lung Cancer. 2002;38 Suppl 2:S9–S12. doi: 10.1016/s0169-5002(02)00351-3. [DOI] [PubMed] [Google Scholar]
- 13.Eskens FA, Awada A, Cutler DL, de Jonge MJ, Luyten GP, Faber MN, Statkevich P, Sparreboom A, Verweij J, Hanauske AR, Piccart M. Phase I and pharmacokinetic study of the oral farnesyl transferase inhibitor SCH 66336 given twice daily to patients with advanced solid tumors. J Clin Oncol. 2001;19:1167–1175. doi: 10.1200/JCO.2001.19.4.1167. [DOI] [PubMed] [Google Scholar]
- 14.Fuentes H, Calderillo G, Alexander F, Ramirez M, Avila E, Perez L, Aguirre G, Onate-Ocana LF, Gallardo D, Otero J. Phase II study of gemcitabine plus cisplatin in metastatic breast cancer. Anticancer Drugs. 2006;17:565–570. doi: 10.1097/00001813-200606000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Gallardo D, Calderillo G, Serrano A, Alexander F, Rodriguez G, Perez L, de la Garza J, Orate-Ocana L, Otero J. A phase II study of gemcitabine plus cisplatin in previously untreated advanced ovarian cancer. Anticancer Res. 2006;26:3137–3141. [PubMed] [Google Scholar]
- 16.Gibbs JB, Graham SL, Hartman GD, Koblan KS, Kohl NE, Omer CA, Oliff A. Farnesyltransferase inhibitors versus Ras inhibitors. Curr Opin Chem Biol. 1997;1:197–203. doi: 10.1016/s1367-5931(97)80010-5. [DOI] [PubMed] [Google Scholar]
- 17.Gore L, Holden SN, Cohen RB, Morrow M, Pierson AS, O’Bryant CL, Persky M, Gustafson D, Mikule C, Zhang S, Palmer PA, Eckhardt SG. A phase I safety, pharmacological and biological study of the farnesyl protein transferase inhibitor, tipifarnib and capecitabine in advanced solid tumors. Ann Oncol. 2006;17:1709–1717. doi: 10.1093/annonc/mdl282. [DOI] [PubMed] [Google Scholar]
- 18.Gu WZ, Joseph I, Wang YC, Frost D, Sullivan GM, Wang L, Lin NH, Cohen J, Stoll VS, Jakob CG, Muchmore SW, Harlan JE, Holzman T, Walten KA, Ladror US, Anderson MG, Kroeger P, Rodriguez LE, Jarvis KP, Ferguson D, Marsh K, Ng S, Rosenberg SH, Sham HL, Zhang H. A highly potent and selective farnesyltransferase inhibitor ABT-100 in preclinical studies. Anticancer Drugs. 2005;16:1059–1069. doi: 10.1097/00001813-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gupta SK, John S, Naik R, Arora R, Selvamani B, Fuloria J, Ganesh N, Awasthy BS. A multicenter phase II study of gemcitabine, paclitaxel, and cisplatin in chemonaive advanced ovarian cancer. Gynecol Oncol. 2005;98:134–140. doi: 10.1016/j.ygyno.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Haluska P, Dy GK, Adjei AA. Farnesyl transferase inhibitors as anticancer agents. Eur J Cancer. 2002;38:1685–1700. doi: 10.1016/s0959-8049(02)00166-1. [DOI] [PubMed] [Google Scholar]
- 21.Head J, Johnston SR. New targets for therapy in breast cancer: farnesyltransferase inhibitors. Breast Cancer Res. 2004;6:262–268. doi: 10.1186/bcr947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology. 2003;64:191–206. doi: 10.1159/000069315. [DOI] [PubMed] [Google Scholar]
- 23.Izbicka E, Lawrence R, Davidson K, et al. Activity of a farnesyl transferase inhibitor (SCH 66336) against a broad range of tumors taken directly from patients. (abstr 3454) Proc Am assoc Cancer Res. 1999 [Google Scholar]
- 24.Johnston SR, Hickish T, Ellis P, Houston S, Kelland L, Dowsett M, Salter J, Michiels B, Perez-Ruixo JJ, Palmer P, Howes A. Phase II study of the efficacy and tolerability of two dosing regimens of the farnesyl transferase inhibitor, R115777, in advanced breast cancer. J Clin Oncol. 2003;21:2492–2496. doi: 10.1200/JCO.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 25.Le Chevalier T, Scagliotti G, Natale R, Danson S, Rosell R, Stahel R, Thomas P, Rudd RM, Vansteenkiste J, Thatcher N, Manegold C, Pujol JL, van Zandwijk N, Gridelli C, van Meerbeeck JP, Crino L, Brown A, Fitzgerald P, Aristides M, Schiller JH. Efficacy of gemcitabine plus platinum chemotherapy compared with other platinum containing regimens in advanced non-small-cell lung cancer: a meta-analysis of survival outcomes. Lung Cancer. 2005;47:69–80. doi: 10.1016/j.lungcan.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Bryant MS, Chen J, Lee S, Yaremko B, Lipari P, Malkowski M, Ferrari E, Nielsen L, Prioli N, Dell J, Sinha D, Syed J, Korfmacher WA, Nomeir AA, Lin CC, Wang L, Taveras AG, Doll RJ, Njoroge FG, Mallams AK, Remiszewski S, Catino JJ, Girijavallabhan VM, Bishop WR, et al. Antitumor activity of SCH 66336, an orally bioavailable tricyclic inhibitor of farnesyl protein transferase, in human tumor xenograft models and wap-ras transgenic mice. Cancer Res. 1998;58:4947–4956. [PubMed] [Google Scholar]
- 27.Loprevite M, Favoni RE, De Cupis A, Scolaro T, Semino C, Mazzanti P, Ardizzoni A. In vitro study of farnesyltransferase inhibitor SCH 66336, in combination with chemotherapy and radiation, in non-small cell lung cancer cell lines. Oncol Rep. 2004;11:407–414. [PubMed] [Google Scholar]
- 28.Mohran TZ. Gemcitabine and cisplatin combination chemotherapy as a first-line treatment in patients with metastatic breast cancer. J Egypt Natl Canc Inst. 2004;16:8–14. [PubMed] [Google Scholar]
- 29.Nagourney RA, Brewer CA, Radecki S, Kidder WA, Sommers BL, Evans SS, Minor DR, DiSaia PJ. Phase II trial of gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed ovarian cancer patients. Gynecol Oncol. 2003;88:35–39. doi: 10.1006/gyno.2002.6855. [DOI] [PubMed] [Google Scholar]
- 30.Ngan RK, Yiu HH, Lau WH, Yau S, Cheung FY, Chan TM, Kwok CH, Chiu CY, Au SK, Foo W, Law CK, Tse KC. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13:1252–1258. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 31.Peters GJ, Ruiz van Haperen VW, Bergman AM, Veerman G, Smitskamp-Wilms E, van Moorsel CJ, Kuiper CM, Braakhuis BJ. Preclinical combination therapy with gemcitabine and mechanisms of resistance. Semin Oncol. 1996;23:16–24. [PubMed] [Google Scholar]
- 32.Petit T, Izbicka E, Lawrence RA, Bishop WR, Weitman S, Von Hoff D. Activity of SCH 66336, a tricyclic farnesyltransferase inhibitor, against human tumor colony-forming units. Ann Oncol. 1999;10:449–453. doi: 10.1023/a:1008313232381. [DOI] [PubMed] [Google Scholar]
- 33.Predergast J. Oncology ASoC. ASCO educational book; 1999. Targeting farnesyltransferase: is ras relevant? pp. 22–28. [DOI] [PubMed] [Google Scholar]
- 34.Ready NE, Lipton A, Zhu Y, Statkevich P, Frank E, Curtis D, Bukowski RM. Phase I study of the farnesyltransferase inhibitor lonafarnib with weekly paclitaxel in patients with solid tumors. Clin Cancer Res. 2007;13:576–583. doi: 10.1158/1078-0432.CCR-06-1262. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg JE, Carroll PR, Small EJ. Update on chemotherapy for advanced bladder cancer. J Urol. 2005;174:14–20. doi: 10.1097/01.ju.0000162039.38023.5f. [DOI] [PubMed] [Google Scholar]
- 36.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 37.Sandler A, Ettinger DS. Gemcitabine: single-agent and combination therapy in non-small cell lung cancer. Oncologist. 1999;4:241–251. [PubMed] [Google Scholar]
- 38.Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 39.Schering-Plough. Lornafarnib (SCH 66336) investigator’s brochure. Schering-Plough Research Institute; 2003 December 2003 Report.
- 40.Seo JH, Oh SC, Choi CW, Kim BS, Shin SW, Kim YH, Kim JS, Kim AR, Lee JB, Koo BH. Phase II study of a gemcitabine and cisplatin combination regimen in taxane resistant metastatic breast cancer. Cancer Chemother Pharmacol. 2007;59:269–274. doi: 10.1007/s00280-006-0266-x. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd FA, Abratt R, Crino L, Green M, Sandler A, Steward W, Iglesias J, Anglin G. The influence of gemcitabine and cisplatin schedule on response and survival in advanced non-small cell lung cancer. Lung Cancer. 2000;30:117–125. doi: 10.1016/s0169-5002(00)00135-5. [DOI] [PubMed] [Google Scholar]
- 42.Smalley KS, Eisen TG. Farnesyl transferase inhibitor SCH66336 is cytostatic, pro-apoptotic and enhances chemosensitivity to cisplatin in melanoma cells. Int J Cancer. 2003;105:165–175. doi: 10.1002/ijc.11064. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, Blaskovich M, Knowles D, et al. Anti-tumor efficacy of a novel class of non-thiol containing peptidomemimetic inhibitors of farnesyltransferase and geranylgeranyl transferase I: combination therapy with the cytotoxic agents cisplatin, taxol and gemcitabine. Cancer Res. 1999;59:4919–4926. [PubMed] [Google Scholar]
- 44.Taveras AG, Kirschmeier P, Baum CM. Sch-66336 (sarasar) and other benzocycloheptapyridyl farnesyl protein transferase inhibitors: discovery, biology and clinical observations. Curr Top Med Chem. 2003;3:1103–1114. doi: 10.2174/1568026033452104. [DOI] [PubMed] [Google Scholar]
- 45.Theodore C, Geoffrois L, Vermorken JB, Caponigro F, Fiedler W, Chollet P, Ravaud A, Peters GJ, de Balincourt C, Lacombe D, Fumoleau P. Multicentre EORTC study 16997: feasibility and phase II trial of farnesyl transferase inhibitor and gemcitabine combination in salvage treatment of advanced urothelial tract cancers. Eur J Cancer. 2005;41:1150–1157. doi: 10.1016/j.ejca.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Wang E, Casciano CN, Clement RP, Johnson WW. The farnesyl protein transferase inhibitor SCH66336 is a potent inhibitor of MDR1 product P-glycoprotein. Cancer Res. 2001;61:7525–7529. [PubMed] [Google Scholar]