Abstract
Autosomal dominant polycystic kidney disease (ADPKD) caused by mutations in PKD1 is significantly more severe than PKD2. Typically, ADPKD presents in adulthood but is rarely diagnosed in utero with enlarged, echogenic kidneys. Somatic mutations are thought crucial for cyst development, but gene dosage is also important since animal models with hypomorphic alleles develop cysts, but are viable as homozygotes. We screened for mutations in PKD1 and PKD2 in two consanguineous families and found PKD1 missense variants predicted to be pathogenic. In one family, two siblings homozygous for R3277C developed end stage renal disease at ages 75 and 62 years, while six heterozygotes had few cysts. In the other family, the father and two children with moderate to severe disease were homozygous for N3188S. In both families homozygous disease was associated with small cysts of relatively uniform size while marked cyst heterogeneity is typical of ADPKD. In another family, one patient diagnosed in childhood was found to be a compound heterozygote for the PKD1 variants R3105W and R2765C. All three families had evidence of developmental defects of the collecting system. Three additional ADPKD families with in utero onset had a truncating mutation in trans with either R3277C or R2765C. These cases suggest the presence of incompletely penetrant PKD1 alleles. The alleles alone may result in mild cystic disease; two such alleles cause typical to severe disease; and, in combination with an inactivating allele, are associated with early onset disease. Our study indicates that the dosage of functional PKD1 protein may be critical for cyst initiation.
Keywords: ADPKD, mutation, hypomorphic allele, childhood, PKD1
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by progressive bilateral cyst development and expansion, often resulting in end-stage renal disease (ESRD). The disease is genetically heterogeneous with two loci identified: PKD1, ∼85% of cases (16p13.3) and PKD2, ∼15% (4q21).1-3 The ADPKD phenotype displays marked variability4 that is greatly influenced by the gene type: PKD1 has an average age at ESRD of 54.3 years compared to 74 years for PKD2.5 Significant intrafamilial variability also highlights a role for the genetic background in the disease presentation.6 Extreme intrafamilial variability manifests in a small proportion of cases (∼1−2%) with early onset ADPKD, clinical symptoms before 15 years, and rarely results in in utero cystic enlargement more typical of autosomal recessive polycystic kidney disease.7,8 Most early onset cases have been linked to PKD1, but recently a PKD2 family with perinatal death in two severely affected infants was described.9-13 As illustrated in this case, siblings of early onset cases have a significantly enhanced risk of severe disease.8 Early onset ADPKD can be caused by contiguous deletion of the adjacent PKD1 and tuberous sclerosis gene (TSC2), characterized by childhood polycystic kidney disease (PKD) with additional clinical signs of tuberous sclerosis.14 Another genetic factor that can modulate the disease presentation and result in marked intrafamilial variability is mosaicism.15,16 Bilineal inheritance of a PKD1 and a PKD2 mutant allele can also result in a modest enhancement to the single gene phenotypes.17
Comprehensive base-pair mutation screening of PKD1 and PKD2 has identified definite, truncating mutations in ∼61% of cases; a further ∼4% have larger deletion/duplication mutations.3,16 Rigorous testing of amino-acid substitutions using a Grantham matrix score,18 plus analysis of segregation and other detected variants, has resulted in likely missense changes being defined in an additional ∼26% of cases. The molecular basis of disease in approximately 9% of ADPKD remains unclear. Genotype/phenotype studies have suggested a modest influence of mutation position in PKD1, but have not shown a difference in severity between truncating and missense changes associated with either gene, suggesting likely inactivation of these missense variants.19,20 This contrasts with autosomal recessive polycystic kidney disease where less severe disease is associated with at least one PKHD1 missense mutation, indicating the importance of incompletely penetrant alleles.21
Evidence from animal models of ADPKD and analysis of cystic epithelia indicate that renal cysts develop from loss of functional PKD protein (polycystin) with somatic inactivation of the normal allele suggesting a two-hit mechanism.22-24 However, the dosage level of functional polycystin may also be important because mouse models expressing low levels (<20%) of correctly spliced product develop cysts but are viable as homozygotes.25,26 No corresponding human hypomorphic or incompletely penetrant PKD1 or PKD2 alleles have been described. Here we describe a number of unusual families with atypical presentations of PKD that suggest a role for incompletely penetrant PKD1 alleles in causing and modulating cystic disease.
RESULTS
Analysis of over 100 apparent ADPKD families, including ones with unusually severe and mild disease and marked intrafamilial variability, identified three that did not fit the normal ADPKD paradigm of a single dominantly inherited mutation.
Family M34
The proband (II2) had bilaterally enlarged kidneys and ESRD at age 75 years whereas a brother (II3) required a renal transplant at 62 years (Figure 1a). The appearance of the kidneys in the two siblings was similar and atypical for ADPKD in that multiple, relatively uniformly sized cysts were found (Figure 1b), compared to the marked heterogeneity in cyst size typical of this disorder. Mild dilatation of the calyces was also seen in II2 (Figure 1b). Unusually for older ADPKD patients, neither had liver cysts. Multiple small cortical renal cysts were identified in the father at autopsy at 79 years. The mother died at 83 from a stroke with PKD unknown. The proband's children (III1, III2, and III4) each had a small number of renal cysts (see Figure 1a and b) without renal enlargement, in their 40s. The granddaughter (IV1) had a single cyst at 28 years, whereas another brother (II5) had five cysts at 76 years (Figure 1a and b). Although this cyst number was suggestive of a positive diagnosis in these individuals with the family history of ADPKD, the severity of disease was very different between generations.
Figure 1. Homozygous inheritance of PKD1: R3277C in a consanguineous family.

(a) Pedigree of family M34 showed inheritance of PKD through four generations, but only II2 and II3 (black shading) have renal impairment; age at ESRD is shown. Individuals with mild disease are shaded and total cyst number detected by CT or magnetic resonance imaging at the indicated age are shown, including multiple cysts (MC) in I1 at autopsy. Segregation of the R3277C alleles (R or C) is indicated below each patient where information is available, parenthesis indicate inferred genotypes. (b) (II2) Unenhanced coronal MR image (top) and axial MR image (bottom) following administration of gadolinium and (II3) unenhanced CT analysis shows bilaterally enlarged kidneys with numerous small and uniform cysts. Following the administration of gadolinium, there is layering of the contrast (white arrows) in dilated calyces (II2). Single cysts are indicated with arrowheads on CT (III1 and III2) or magnetic resonance imaging (III4) of mildly affected individuals. (c) Wild-type (wt), heterozygous (het), and homozygous (hom) sequence of R3277C showing the normal and variant DNA and amino-acid sequence. (d) Multisequence alignment of PC1 orthologues as indicated shows R3277 is invariant. PC1-like human sequences, as indicated, illustrate a high level of conservation of R3277.
Mutation analysis by direct sequencing of PKD1 and PKD2 exons revealed homozygosity for 13 PKD1 intragenic polymorphisms. Subsequently, the parents (I1 and I2) were found to be first cousins. In addition, the novel PKD1 substitution 9829C→T, resulting in R3277C (Figure 1c), was found homozygous in II2 and II3 and heterozygous in other family members with renal cysts (Figure 1a). No large deletion mutation was detected in II2 or II3 using a multiple ligation-dependent probe amplification assay for the PKD1 and PKD2 genes, and no PKHD1 mutations were detected.
R3277C is at a highly conserved site in polycystin-1 (PC1), completely conserved to fish (Grantham variation, GV = 0) and is a highly nonconservative substitution (Grantham distance, GD = 180) (Figure 1d).3,18 The missense change is located in the first intracellular loop of PC1, close to transmembrane region 2, and is highly conserved in other PC1-like proteins (Figure 1d). To more rigorously test the significance of this substitution, three tools to predict pathological mutations were tested. These tools (SIFT, PolyPhen, and AlignGVGD) were used utilizing default conditions and/or by using an alignment of PC1 orthologues (see Materials and Methods, Table S1 , and Supplementary Results for details). Evaluation of R3277C showed that it was predicted to be a strong pathogenic mutation in each case (Table 1). Overall, this family suggested that R3277C was an incompletely penetrant mutant allele that resulted in occasional cyst development in heterozygotes and more severe PKD in homozygosity.
Table 1.
Scoring of PC1 variants for likely pathogenicity
| SIFTa |
SIFTb |
PolyPhena |
AlignGVGDb |
Consensus |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Variant | MG | VS | MG | VS | MG | VS | MG | VS | MG |
| R2765C | C | 0.01 | C | 0.01 | B | 2.17 | C | C35 | B/C |
| R3105W | B | 0 | B | 0 | B | 2.60 | C | C35 | B |
| N3188S | C | 0.03 | B | 0 | C | 1.84 | C | C45 | C |
| R3277C | B | 0 | B | 0 | B | 2.65 | B | C65 | B |
B, highly likely; C, likely; MG, mutation group; PC1; polycystin-1; VS, variant score.
Default alignment.
PC1 orthologue alignment.
Family P192
A second consanguineous family of Pakistani origin presented a pattern typical of dominant inheritance (Figure 2a). The father (I1) had bilateral PKD, but as above, the finding of multiple small cysts (4−7 mm) was not completely characteristic of ADPKD, with clubbing of the calyces also detected. II2 had PKD diagnosed at 22 weeks gestation with large hyperechogenic kidneys and at 14 years had slightly enlarged kidneys with multiple (10−12 mm) cysts scattered throughout the parenchyma. No liver cysts were detected. Glomerular filtration rate at 15.5 years was 67 ml/min per 1.73 m2. Her sister (II3) had bilateral renal cysts detected at 9 years and at 15 years had a normal glomerular filtration rate (86 ml/min per 1.73 m2) with several cortical cysts, including ones of 12 and 17 mm, but no liver cysts. An earlier pregnancy that resulted in a stillbirth (II1) was found to have bilateral PKD but no biliary dysgenesis (typical of autosomal recessive polycystic kidney disease) at autopsy.
Figure 2. Homozygous inheritance of PKD1: N3188S in a consanguineous Pakistani family.
(a) Pedigree showing renal phenotype; multiple cysts (MC), in utero (IU) PKD, or negative ultrasound (–ve U/S) and the genotype of N3188S (N or S). (b) Sequence of N3188S showing the wild-type (wt), heterozygous (het), and homozygous (hom) DNA and amino-acid sequence. The position of IVS27 is also indicated. (c) Sequence alignment of PC1 orthologues and human PC1-like proteins as indicated. N3188 is completely conserved in the orthologues and well conserved in homologues, but is serine in PC1L1.
Haplotype analysis with five markers flanking PKD1 (see Materials and Methods for details) indicated homozygosity of ∼450 kb around PKD1 in I1, II2, and II3 with a similar single copy of the same haplotype in the mother (I2) and unaffected children (II4 and II5). Sequence analysis of PKD1 and PKD2 in I1 showed homozygosity of four intragenic PKD1 single nucleotide polymorphisms. A novel substitution in PKD1, 9563A→G; N3188S, was homozygous in the affected cases and heterozygous in the others (Figure 2a and b). This residue is completely conserved to fish (GV = 0), is a moderately conservative change (GD = 46), and highly conserved in other PC1-like proteins (Figure 2c). It lies in the PLAT domain27 but is not at a highly conserved position in the domain. Analysis with the prediction tools indicated that it was most likely a pathogenic change (Table 1). Deletion of N318828 as a mutation has been described. This substitution is close to the junction with IVS27, but reverse transcription–PCR analysis of this change did not reveal abnormal splicing. A possible large deletion mutation causing hemizygosity across the PKD1 region was excluded by multiple ligation-dependent probe amplification analysis of PKD1, and no PKHD1 mutations were detected.
Family M390
The proband (II5) in M390 (Figure 3a) was diagnosed by excretory urography at 11 years with medullary sponge kidney and bilateral renal cysts consistent with PKD, following multiple urinary tract infections. Images at 32 years showed multiple renal cysts and three liver cysts (Figure 3b). The kidneys were not particularly enlarged (RK, 12.2 cm; LK, 12.1 cm at 22 years). Cortical scars consistent with reflux nephropathy were also seen, with a history of multiple urinary tract infections during childhood. The sister (II2) had one 2.2 cm cyst (42 years; Figure 3c), while II3 had one kidney cyst and liver cyst (2.5 cm) but the parents were apparently unaffected. Mutation screening of II5 revealed two PKD1 missense variants, 9313C→T; R3105W, and 8293C→T; R2765C (Figure 3d). R3105W is a novel, nonconservative change (GD = 101) at a well-conserved site in orthologues (GV = 26) and homologues (Figure 3e). R2765C is a nonconservative change (GD = 180) at a conserved site of basic residues (GV = 29), but is not part of a conserved domain (Figure 3f). Formal analysis of these variants predicted that both are likely pathogenic changes (Table 1). Various other members of the family had one or other of the variants but not both (Figure 3a), including the sisters with single renal cysts. The pattern of inheritance suggested that both incompletely penetrant variants are required for polycystic kidney disease development and that a single variant can be associated with rare cyst development.
Figure 3. Family M390-inherited PKD1 variants: R2765C and R3105W.
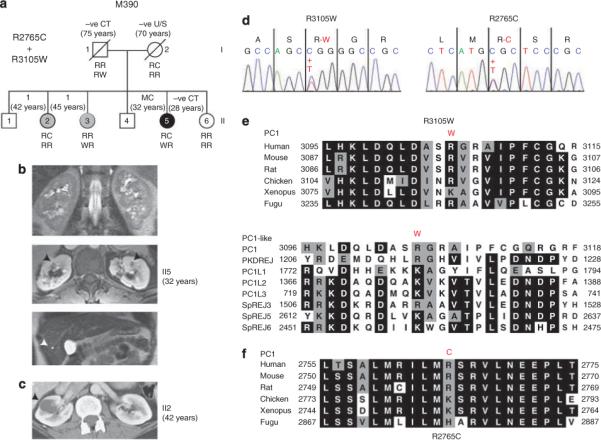
(a) Pedigree of M390 showing the proband II5, who has multiple cysts (MC) in the kidney, and mildly affected individuals (II2 and II3) with single cysts; negative imaging data and age are illustrated. The R2765C (R or C) and R3105W (R or W) genotypes are shown below. Only II5 is a compound heterozygote. (b) CT images of II5 kidney (top and middle) showing multiple renal cysts and (bottom) a single liver cyst (arrowheads). (c) CT of II2 showing a single large cyst. (d) Sequence data illustrating the two variants showing the nucleotide and amino-acid changes. (e) Sequence alignment of PC1 orthologues and human PC1-like homologous proteins. R3105 is highly conserved as a basic residue in all homologues. (f) Multisequence alignment of PC1 orthologues shows that R2765 is a basic residue in all species.
In utero onset ADPKD
We reasoned that the identified incompletely penetrant PKD1 alleles in trans with an inactivating allele may cause early onset ADPKD; hence, we screened families with an in utero presentation of PKD and a family history of ADPKD. In P438; III1 (Figure 4a), PKD was diagnosed in utero with bilateral, substantially enlarged hyperechogenic kidneys (RK = 9 cm; LK = 10 cm) at 7 days, and hypertension diagnosed at 5 months. The kidneys had no corticomedullary differentiation and multiple small cysts, with an SC = 1.7 mg/100 ml at 17 years. The father was diagnosed with ADPKD at 15 years and had an SC = 1.5 mg/100 ml at 44 years. The grandmother had ESRD at 43 years. Screening the ADPKD genes showed PKD1: Q2158X as the likely disease-causing mutation, but that the in utero case (III1) also had the R3277C variant, presumably inherited from the apparently unaffected mother.
Figure 4. Pedigrees of three families with in utero ADPKD presentations that have inherited a truncating and a hypomorphic ADPKD allele.
Each family has an in utero (IU) case, and renal phenotypes of other family members are shown: multiple cysts (MC); negative ultrasound (–ve U/S), or ESRD. Genotypes of the PKD1 variants: a, Q2158X and R3227C; b, Y3819X and R2765C; and c, 7915dup20 and R2765C, are shown below each pedigree. The etiology of cysts in P118 I1 is unclear but could potentially represent a low level of mosaicism not detected by sequence analysis.
In P117, one (III2) of a pair of dizygotic twins was diagnosed in utero at 31 weeks with enlarged bilaterally cystic kidneys that were at the 95th percentile (8 cm) at 10 months.12 III2 was hypertensive since age 2 years and had multiple bilateral cysts and a glomerular filtration rate of 89 ml/min per 1.73 m2 at 15 years. Her twin brother III1 had more typical ADPKD with two cysts in the left kidney and one in the right at 10 years. Their father had multiple renal cysts, but normal renal function at 28 years (Figure 4b). In P118, III1 died perinatally of pulmonary hypoplasia with massively enlarged cystic kidneys.19 The mother II2 had multiple cysts and enlarged kidneys at 35 years, typical of ADPKD, and five cysts were found (up to 5 cm in diameter) in I1. In both families the nonconservative variant R2765C was found inherited in trans with a truncating mutation (P117; Y3819X: P118; 7915dup20)12,29 in the severely affected cases (Figure 4b and c). Cases with just the R2765C variant did not have renal cysts by ultrasound examination. Unlike the other variants described here that have only been seen in these families, R2765C is a more common variant found on ∼1% of normal alleles and described in three studies.30 In two other families analyzed with typical ADPKD, R2765C segregated in cis with the likely pathogenic mutation, whereas segregation data were not available in three other families with that variant.
DISCUSSION
We have analyzed three families with ADPKD-like disease that are not explained by dominant inheritance of a single mutation to PKD1 or PKD2. Several pieces of data indicate a novel mechanism, including the pattern of inheritance and haplotypes, unusual distribution of cysts, sequence analysis of PKD1 and PKD2 and scoring of variants, and exclusion of other causes of disease. Consistent with the ADPKD-like phenotype, we provide strong data that atypical PKD1 alleles underlie the disease etiology in these families.
The inheritance pattern in M34 is consistent with autosomal dominance but it exhibits extreme differences in severity between generations. Although intrafamilial variability is seen in ADPKD,31 it does not usually range from ESRD in 60s to the minimal cyst development in the 40s, as seen here. The homozygosity of a highly conserved PKD1 mutation in the cases with ESRD, plus heterozygosity in those with a few cysts, suggested the involvement of an incompletely penetrant allele. In P192, the inheritance pattern is apparently dominant, but haplotype and sequence data again showed a PKD1 homozygous mutation associated with moderate to severe cystic disease. In the final family, M390, inheritance appears recessive and only the compound heterozygote with two highly conserved PKD1 variants has significant cystic disease.
PKD1 is highly polymorphic with ∼10 neutral variants found per patient from exonic sequencing.3 However, the majority are known variants, whereas most novel neutral changes are at poorly conserved sites or are conservative substitutions (Table S1). The four variants highlighted in this study bear all the characteristics of pathogenic missense changes. This is reflected in both the Grantham matrix score and the more formal analysis of likely pathogenicity that rates them as ‘highly likely’ (Mutation Group B) or ‘likely’ (Mutation Group C)3 mutations. However, it is clear from the heterozygous phenotypes that none are fully penetrant mutations as at the most extreme they are only associated with a handful of renal cysts by middle age. We propose that these partially penetrant alleles, associated with a protein with some residual function (similar to some PKHD1 missense changes) are functionally analogous to described murine Pkd1 hypomorphic alleles.25,26
To prove the significance of these variants, a functional test for PC1 is required, but unfortunately no such assay yet exists. In parts of the protein (the REJ and GPS regions), the significance of missense changes has been assessed by their ability to prevent cleavage at the GPS site.32 However, the variants described here in the transmembrane region are unlikely to influence cleavage. Furthermore, as we propose that these are incompletely penetrant alleles, obtaining clear results from any functional assay may be difficult. Mimicking these changes in a mouse knock-in model (a time-consuming process) may be the only clear way to prove their significance.
Recently, a homozygous PKD2 variant, F482C, that alters polycystin-2 channel activity, was suggested to modulate disease due to a PKD1 splicing mutation.33 Syndromic forms of PKD also exhibit genetic complexity, including oligogenic inheritance and phenotypic modulation by hypomorphic mutations.34-36 We propose here that specific PKD1 variants can be important in modulating cyst development. In the heterozygous state, they may be a significant cause of simple renal cysts, a small number of which often develop in normal individuals as they age.37 In unusual cases (sometimes associated with consanguinity), they can cause typical to severe PKD as a homozygote or a compound heterozygote. The pattern of inheritance may appear recessive or with large intergenerational differences in severity. The disease gene may be impossible to map in such cases, which could underlie some unlinked ADPKD families, akin to the described family with bilineal disease.17,38 These incompletely penetrant alleles could also act as a disease modifier that in trans with an inactivating mutation can result in early onset disease, as a result of only a low level of available functional protein. These alleles would explain the recurrence risk within families, and the R2765C allele found in ∼1% of alleles could be a major modulator of disease. This mechanism is unlikely to explain all early onset cases (including ones with more complex family relationships)7,39 and stochastic factors and genetic background also likely impact the severity of disease. Nevertheless, we propose that these incompletely penetrant alleles are important.
It is intriguing that the three homozygous/compound heterozygous cases had likely developmental defects of the collecting system, an abnormality rarely seen in ADPKD. The uniform pattern of multiple small cysts in the homozygous cases, as also often seen in childhood cases40 (including here), suggests that the mechanism of cystogenesis may be different than in typical, dominantly inherited PKD1. In that case, somatic events are thought to be important for cyst development;22 the wide variety in cyst size may reflect somatic changes occurring at different times, although differences in growth rates between cysts may also be important.41 The uniformity of cyst size seems to indicate that cyst initiation may have occurred at a similar time without a secondary genetic event, perhaps at a time during development when a critical level of functional PC1 is most important. Recently, conditional knockout models of Pkd1 have identified a critical period up to shortly after birth (P13d) when inactivation of both alleles results in severe cystic disease, whereas inactivation after P14d causes much milder disease.42,43 The unusual cases described here raise the question of how much a threshold level of PC1 during development may also be critical to cystogenesis in typical ADPKD patients.
MATERIALS AND METHODS
Sample collection
The study was approved by the relevant institutional review board or ethics committee and participants gave informed consent. Families were collected in the United States (M34 and M390), UK (P192, P117, and P118), and France (P438). Blood samples and clinical/imaging data were collected on as many cases as possible, and new computed tomography (CT) or magnetic resonance imaging analysis was performed when necessary. Pedigrees P117 and P118 were published previously and further clinical details were described.12,44
Haplotype analysis
Family members were screened with microsatellite markers within and flanking PKD1: KG8, SM6, 16AC2.5, CW2, and SM745 using methods previously described.46
Mutation screening and classification of variants
DNA was isolated from whole blood using the Puregene DNA Purification System. Mutations were screened in the proband in each family by direct sequencing of exonic and flanking introgenic regions.3 Segregation was tested by sequence analysis of the relevant genomic fragment in family members. All variants are numbered as previously indicated.3 The significance of missense variants (GD) was assessed using the Grantham matrix score.18 The GV was assessed as the largest Grantham matrix score between orthologues in a multisequence alignment of human, mouse, rat, chicken, Xenopus, and Takifugu sequence as previously defined.3 Homologous proteins to PC1 were: PC1L1, PC1L2, PC1L3, PKDREJ, and the sea urchin (Strongylocentrotus purpuratus) proteins REJ3, 4, 5, 6, and 7.47 Evidence for previous descriptions of the variants was obtained from the ADPKD Mutation Database (http://pkdb.mayo.edu).
Formal analysis of the likelihood that substitutions were pathogenic was performed using three programs: SIFT (http://blocks. fhcrc.org/sift/SIFT.html), PolyPhen (http://genetics.bwh.harvard.edu/pph/), and Align GVGD (http://agvgd.iarc.fr/agvgd_input.php) that assess the changes in light of conservation in a multisequence alignment. Orthologous and homologous sequences detected by the program were used for SIFT and PolyPhen, whereas a multisequence alignment of PC1 orthologues as above, plus dog, opossum, and Tetraodon nigroviridis was used for AlignGVGD and SIFT. Scores from these programs were interpreted similar to the four categories of changes used to define ADPKD mutations:3 Mutation Group (MG) = B (highly likely); MG = C (likely); Indeterminate (I); and Neutral (N). Specifically for SIFT: variant score 0.0 (B), 0.01−0.04 (C), 0.05−0.09 (I), >0.01 (N); PolyPhen: >2.0 (B), 1.99−1.5 (C), 1.49−1.4 (I), <1.39 (N); AlignGVGD: C55–C65 (B), C35−45 (C), C15−25 (I), C00 (N). To assess the programs, 21 previously scored variants were assayed (See Supplementary Results and Table S1).
Multiple ligation-dependent probe amplification
Large deletions or duplications of PKD1 or PKD2 were screened using a multiple ligation-dependent probe amplification assay as previously described.16
PKHD1 mutation analysis
Exonic and flanking intronic regions of PKHD1 were screened by direct sequencing for mutations using a clinical test (Molecular Genetics, Mayo Clinic, Rochester, MN, USA).
Reverse transcription–PCR
RNA isolation and cDNA generation was performed as previously described.3 To analyze 9829C→T (R3277C) in exon 28 and 9563A→G (N3188S) in exon 27, PKD1-specific LR-RT-PCR primers in exons 25 and 34 were used.48 The exon 25−29 region was subsequently amplified and analyzed by agarose gel electrophoresis and sequencing. No evidence of alternative splicing specific to the mutation was detected.
Supplementary Material
ACKNOWLEDGMENTS
The many families and physicians are thanked for their involvement in the study, and for providing comprehensive clinical records. This study was supported by NIDDK grant DK58816, the PKD Foundation (SR), and KH was supported by the Mayo Summer Undergraduate Research Fellowship program (Mayo Graduate School).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Table S1. Scoring of previously evaluated variants for likely pathogenicity.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
REFERENCES
- 1.European Polycystic Kidney Disease Consortium The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 2.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 3.Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. doi: 10.1681/ASN.2006121387. [DOI] [PubMed] [Google Scholar]
- 4.Rossetti S, Harris PC. Genotype–phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–1380. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 5.Hateboer N, van Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353:103–107. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 6.Paterson AD, Magistroni R, He N, et al. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:755–762. doi: 10.1681/ASN.2004090758. [DOI] [PubMed] [Google Scholar]
- 7.Kaariainen H. Polycystic kidney disease in children: a genetic and epidemiological study of 82 Finnish patients. J Med Genet. 1987;24:474–481. [PMC free article] [PubMed] [Google Scholar]
- 8.Zerres K, Rudnik-Schöneborn S, Deget F, et al. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. J Med Genet. 1993;30:583–588. doi: 10.1136/jmg.30.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann C, Bruchle NO, Frank V, et al. Perinatal deaths in a family with autosomal dominant polycystic kidney disease and a PKD2 mutation. N Engl J Med. 2008;359:318–319. doi: 10.1056/NEJMc0801868. [DOI] [PubMed] [Google Scholar]
- 10.MacDermot KD, Saggar-Malik AK, Economides DL, et al. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J Med Genet. 1998;35:13–16. doi: 10.1136/jmg.35.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaud J, Russo P, Grignon A, et al. Autosomal dominant polycystic kidney disease in the fetus. Am J Med Genet. 1994;51:240–246. doi: 10.1002/ajmg.1320510314. [DOI] [PubMed] [Google Scholar]
- 12.Peral B, Ong ACM, San Millán JL, et al. A stable, nonsense mutation associated with a case of infantile onset polycystic kidney disease 1 (PKD1). Hum Mol Genet. 1996;5:539–542. doi: 10.1093/hmg/5.4.539. [DOI] [PubMed] [Google Scholar]
- 13.Watnick T, Phakdeekitcharoen B, Johnson A, et al. Mutation detection of PKD1 identifies a novel mutation common to three families with aneurysms and/or very-early-onset disease. Am J Hum Genet. 1999;65:1561–1571. doi: 10.1086/302657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson JR, Maheshwar MM, Aspinwall R, et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am J Hum Genet. 1997;61:843–851. doi: 10.1086/514888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor A, Lunt PW, Dolling C, et al. Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am J Transplant. 2008;8:232–237. doi: 10.1111/j.1600-6143.2007.02030.x. [DOI] [PubMed] [Google Scholar]
- 16.Consugar MB, Wong WC, Lundquist PA, et al. Characterization of large rearrangements associated in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int. 2008;74:1468–1479. doi: 10.1038/ki.2008.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei Y, Paterson AD, Wang KR, et al. Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet. 2001;68:355–363. doi: 10.1086/318188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham R. Amino acid difference formula to help explain protein evolution. Science. 1974;185:862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- 19.Rossetti S, Burton S, Strmecki L, et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J Am Soc Neph. 2002;13:1230–1237. doi: 10.1097/01.asn.0000013300.11876.37. [DOI] [PubMed] [Google Scholar]
- 20.Magistroni R, He N, Wang K, et al. Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:1164–1174. doi: 10.1097/01.asn.0000061774.90975.25. [DOI] [PubMed] [Google Scholar]
- 21.Bergmann C, Senderek J, Sedlacek B, et al. Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1). J Am Soc Nephrol. 2003;14:76–89. doi: 10.1097/01.asn.0000039578.55705.6e. [DOI] [PubMed] [Google Scholar]
- 22.Qian F, Watnick TJ, Onuchic LF, et al. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type 1. Cell. 1996;87:979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targeted Pkd1 mutation. Nat Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 24.Wu G, D'Agati V, Cai Y, et al. Somatic inactivation of Pkd2 results in polycystic kidney disease. Cell. 1998;93:177–188. doi: 10.1016/s0092-8674(00)81570-6. [DOI] [PubMed] [Google Scholar]
- 25.Jiang ST, Chiou YY, Wang E, et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am J Pathol. 2006;168:205–220. doi: 10.2353/ajpath.2006.050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lantinga-van Leeuwen IS, Dauwerse JG, Baelde HJ, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 27.Bateman A, Sandford R. The PLAT domain: a new piece in the PKD1 puzzle. Curr Biol. 1999;9:R588–R590. doi: 10.1016/s0960-9822(99)80380-7. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti S, Chauveau D, Kubly V, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–2201. doi: 10.1016/S0140-6736(03)13773-7. [DOI] [PubMed] [Google Scholar]
- 29.Rossetti S, Strmecki L, Gamble V, et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am J Hum Genet. 2001;68:46–63. doi: 10.1086/316939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ADPKD Mutation Database (PKDB) 2008 http://pkdb.mayo.edu.
- 31.Geberth S, Ritz E, Zeier M, et al. Anticipation of age at renal death in autosomal dominant polycystic kidney disease (ADPKD)? Nephrol Dial Transplant. 1995;10:1603–1606. [PubMed] [Google Scholar]
- 32.Qian F, Boletta A, Bhunia AK, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci USA. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dedoussis GV, Luo Y, Starremans P, et al. Co-inheritance of a PKD1 mutation and homozygous PKD2 variant: a potential modifier in autosomal dominant polycystic kidney disease. Eur J Clin Invest. 2008;38:180–190. doi: 10.1111/j.1365-2362.2007.01913.x. [DOI] [PubMed] [Google Scholar]
- 34.Badano JL, Kim JC, Hoskins BE, et al. Heterozygous mutations in BBS1, BBS2 and BBS6 have a potential epistatic effect on Bardet–Biedl patients with two mutations at a second BBS locus. Hum Mol Genet. 2003;12:1651–1659. doi: 10.1093/hmg/ddg188. [DOI] [PubMed] [Google Scholar]
- 35.Hoefele J, Wolf MT, O'Toole JF, et al. Evidence of oligogenic inheritance in nephronophthisis. J Am Soc Nephrol. 2007;18:2789–2795. doi: 10.1681/ASN.2007020243. [DOI] [PubMed] [Google Scholar]
- 36.Leitch CC, Zaghloul NA, Davis EE, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet–Biedl syndrome. Nat Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 37.Ravine D, Gibson RN, Donlan J, et al. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis. 1993;22:803–807. doi: 10.1016/s0272-6386(12)70338-4. [DOI] [PubMed] [Google Scholar]
- 38.Daoust MC, Reynolds DM, Bichet DG, et al. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics. 1995;25:733–736. doi: 10.1016/0888-7543(95)80020-m. [DOI] [PubMed] [Google Scholar]
- 39.Zerres K, Hansmann M, Knopfle G, et al. Prenatal diagnosis of genetically determined early manifestation of autosomal dominant polycystic kidney disease? Hum Genet. 1985;71:368–369. doi: 10.1007/BF00388467. [DOI] [PubMed] [Google Scholar]
- 40.Shamshirsaz A, Reza Bekheirnia M, Kamgar M, et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 2005;68:2218–2224. doi: 10.1111/j.1523-1755.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 41.Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–116. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piontek K, Menezes LF, Garcia-Gonzalez MA, et al. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, et al. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- 44.Peral B, San Millán JL, Ong ACM, et al. Screening the 3′ region of the polycystic kidney disease 1 (PKD1) gene reveals six novel mutations. Am J Hum Genet. 1996;58:86–96. [PMC free article] [PubMed] [Google Scholar]
- 45.Peral B, Ward CJ, San Millán JL, et al. Evidence of linkage disequilibrium in the Spanish polycystic kidney disease 1 (PKD1) population. Am J Hum Genet. 1994;54:899–908. [PMC free article] [PubMed] [Google Scholar]
- 46.Consugar MB, Anderson SA, Rossetti S, et al. Haplotype analysis improves molecular diagnostics of autosomal recessive polycystic kidney disease. Am J Kidney Dis. 2005;45:77–87. doi: 10.1053/j.ajkd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Gunaratne HJ, Moy GW, Kinukawa M, et al. The 10 sea urchin receptor for egg jelly proteins (SpREJ) are members of the polycystic kidney disease-1 (PKD1) family. BMC Genomics. 2007;8:235. doi: 10.1186/1471-2164-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peral B, Gamble V, Strong C, et al. Identification of mutations in the duplicated region of the polycystic kidney disease 1 (PKD1) gene by a novel approach. Am J Hum Genet. 1997;60:1399–1410. doi: 10.1086/515467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




