Abstract
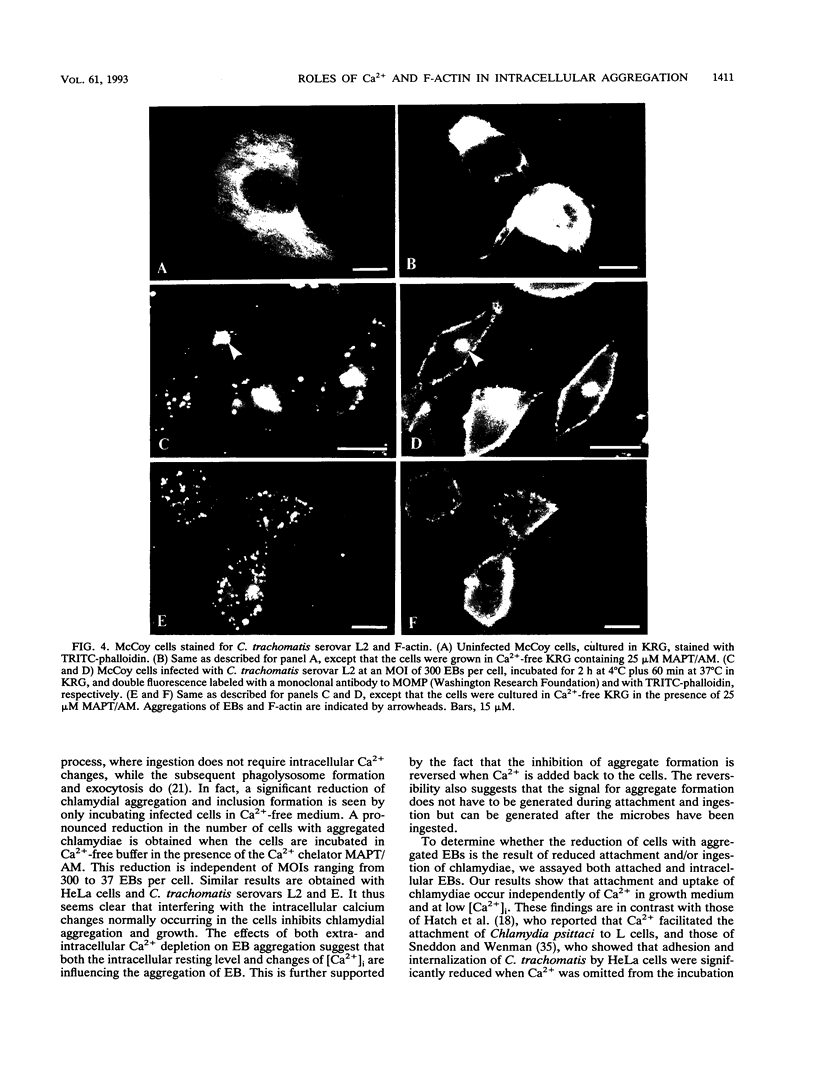
The effect of intracellular free Ca2+ ([Ca2+]i) on the intracellular aggregation of Chlamydia trachomatis serovars L2 and E in McCoy and HeLa cells is investigated. Loading the cells with the Ca2+ chelator MAPT/AM (1,2-bis-5-methyl-amino-phenoxylethane-N,N-n'-tetra-acetoxymethyl acetate), thereby decreasing the [Ca2+]i from 67 to 19 nM, decreased the number of cells with a local aggregation of chlamydiae in a dose-dependent manner. Neither the attachment nor the uptake of elementary bodies (EBs) was, however, affected after depletion of Ca2+ from the cells. There was no significant difference in the level of measured [Ca2+]i between infected and uninfected cells. Reducing the [Ca2+]i also significantly inhibited chlamydial inclusion formation. Differences in the organization of the actin filament network were observed in response to [Ca2+]i depletion. In Ca(2+)-depleted cells, where few EB aggregates were formed, few local accumulations of F-actin were observed in the cytosol. These results suggest that the aggregation of EBs in eucaryotic cells requires a normal homeostasis of intracellular Ca2+. By affecting F-actin reorganization and putatively certain Ca(2+)-binding proteins, [Ca2+]i plays a vital role in the infectious process of chlamydiae.
Full text
PDF

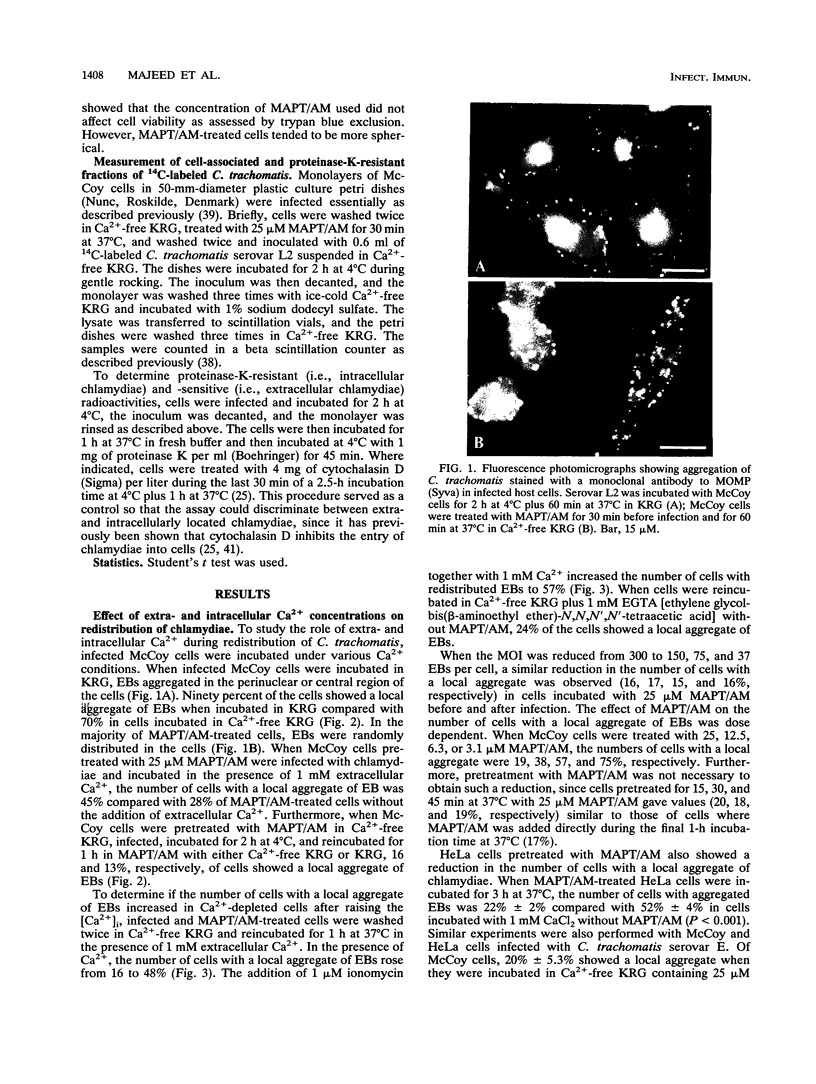




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- Baldwin T. J., Ward W., Aitken A., Knutton S., Williams P. H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991 May;59(5):1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden J. A., Miki M., Hambly B. D., Dos Remedios C. G. Localization of the phalloidin and nucleotide-binding sites on actin. Eur J Biochem. 1987 Feb 2;162(3):583–588. doi: 10.1111/j.1432-1033.1987.tb10679.x. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Ernst J. D. Characterization of Ca2(+)-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem J. 1990 Feb 15;266(1):195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth W. A., Taverne J. Some consequences of the multiple infection of cell cultures by TRIC organisms. J Hyg (Lond) 1972 Mar;70(1):33–37. doi: 10.1017/s0022172400022063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., McNeill H., Zigmond S. H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J Cell Biol. 1990 Apr;110(4):1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P. L., Berthon B., Claret M., Sansonetti P. J. Internalization of Shigella flexneri into HeLa cells occurs without an increase in cytosolic Ca2+ concentration. Infect Immun. 1989 Sep;57(9):2919–2922. doi: 10.1128/iai.57.9.2919-2922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Lea P., Takai A., Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J Cell Biol. 1992 Feb;116(3):695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg L. G., Wyrick P. B., Davis C. H., Rumpp J. W. Chlamydia psittaci elementary body envelopes: ingestion and inhibition of phagolysosome fusion. Infect Immun. 1983 May;40(2):741–751. doi: 10.1128/iai.40.2.741-751.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J. D., Hoye E., Blackwood R. A., Jaye D. Purification and characterization of an abundant cytosolic protein from human neutrophils that promotes Ca2(+)-dependent aggregation of isolated specific granules. J Clin Invest. 1990 Apr;85(4):1065–1071. doi: 10.1172/JCI114537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulstich H., Zobeley S., Rinnerthaler G., Small J. V. Fluorescent phallotoxins as probes for filamentous actin. J Muscle Res Cell Motil. 1988 Oct;9(5):370–383. doi: 10.1007/BF01774064. [DOI] [PubMed] [Google Scholar]
- Grant N. J., Aunis D. Effects of phorbol esters on cytoskeletal proteins in cultured bovine chromaffin cells: induction of neurofilament phosphorylation and reorganization of actin. Eur J Cell Biol. 1990 Jun;52(1):36–46. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gustafson M., Magnusson K. E. A novel principle for quantitation of fast intracellular calcium changes using Fura-2 and a modified image processing system--applications in studies of neutrophil motility and phagocytosis. Cell Calcium. 1992 Aug;13(8):473–486. doi: 10.1016/0143-4160(92)90016-l. [DOI] [PubMed] [Google Scholar]
- Gustafsson M., Magnusson K. E. A distributed image-processing system for measurements of intracellular calcium in living cells. Comput Methods Programs Biomed. 1991 Dec;36(4):199–221. doi: 10.1016/0169-2607(91)90090-g. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Attachment of Chlamydia psittaci to formaldehyde-fixed and unfixed L cells. J Gen Microbiol. 1981 Aug;125(2):273–283. doi: 10.1099/00221287-125-2-273. [DOI] [PubMed] [Google Scholar]
- Hodinka R. L., Davis C. H., Choong J., Wyrick P. B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988 Jun;56(6):1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. H., Wang D. Calcium ionophore, phorbol ester, and chemotactic peptide-induced cytoskeleton reorganization in human neutrophils. J Clin Invest. 1987 May;79(5):1359–1364. doi: 10.1172/JCI112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M. E., Lew D. P., Carpentier J. L., Magnusson K. E., Sjögren M., Stendahl O. Cytosolic free calcium elevation mediates the phagosome-lysosome fusion during phagocytosis in human neutrophils. J Cell Biol. 1990 May;110(5):1555–1564. doi: 10.1083/jcb.110.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Wenman W. M. Cyclic AMP inhibits developmental regulation of Chlamydia trachomatis. J Bacteriol. 1986 Nov;168(2):722–727. doi: 10.1128/jb.168.2.722-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Lew D. P. Receptor signalling and intracellular calcium in neutrophil activation. Eur J Clin Invest. 1989 Aug;19(4):338–346. doi: 10.1111/j.1365-2362.1989.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Majeed M., Kihlström E. Mobilization of F-actin and clathrin during redistribution of Chlamydia trachomatis to an intracellular site in eucaryotic cells. Infect Immun. 1991 Dec;59(12):4465–4472. doi: 10.1128/iai.59.12.4465-4472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S. Cyclic AMP second messenger systems. Curr Opin Cell Biol. 1991 Apr;3(2):213–217. doi: 10.1016/0955-0674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Meigs J. B., Wang Y. L. Reorganization of alpha-actinin and vinculin induced by a phorbol ester in living cells. J Cell Biol. 1986 Apr;102(4):1430–1438. doi: 10.1083/jcb.102.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randriamampita C., Bismuth G., Trautmann A. Ca(2+)-induced Ca2+ release amplifies the Ca2+ response elicited by inositol trisphosphate in macrophages. Cell Regul. 1991 Jul;2(7):513–522. doi: 10.1091/mbc.2.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderhof J. C., Barnes R. C. Fusion of inclusions following superinfection of HeLa cells by two serovars of Chlamydia trachomatis. Infect Immun. 1989 Oct;57(10):3189–3193. doi: 10.1128/iai.57.10.3189-3193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Brown J. E. Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infect Immun. 1987 Feb;55(2):298–303. doi: 10.1128/iai.55.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Winikoff Y., Kol R., Chaimovitz C., Sarov I. Inhibition of growth of Chlamydia trachomatis by the calcium antagonist verapamil. J Gen Microbiol. 1989 Jun;135(6):1619–1623. doi: 10.1099/00221287-135-6-1619. [DOI] [PubMed] [Google Scholar]
- Sneddon J. M., Wenman W. M. The effect of ions on the adhesion and internalization of Chlamydia trachomatis by HeLa cells. Can J Microbiol. 1985 Apr;31(4):371–374. doi: 10.1139/m85-071. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Attachment and internalization of a Chlamydia trachomatis lymphogranuloma venereum strain by McCoy cells: kinetics of infectivity and effect of lectins and carbohydrates. Infect Immun. 1983 Dec;42(3):930–935. doi: 10.1128/iai.42.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Physicochemical surface properties of elementary bodies from different serotypes of chlamydia trachomatis and their interaction with mouse fibroblasts. Infect Immun. 1982 Jun;36(3):893–899. doi: 10.1128/iai.36.3.893-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Pavlotsky N., Lin J. S., Rice P. A. Inhibition of human neutrophil NADPH oxidase by Chlamydia serovars E, K, and L2. Infect Immun. 1989 Apr;57(4):1108–1112. doi: 10.1128/iai.57.4.1108-1112.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Watts R. G., Howard T. H. Evidence for a gelsolin-rich, labile F-actin pool in human polymorphonuclear leukocytes. Cell Motil Cytoskeleton. 1992;21(1):25–37. doi: 10.1002/cm.970210104. [DOI] [PubMed] [Google Scholar]
- Wieland T., de Vries J. X., Schäfer A., Faulstich H. Spectroscopic evidence for the interaction of phalloidin with actin. FEBS Lett. 1975 Jun 1;54(1):73–75. doi: 10.1016/0014-5793(75)81071-4. [DOI] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Nibbering P. H., van Furth R. Cytosolic free calcium is essential for immunoglobulin G-stimulated intracellular killing of Staphylococcus aureus by human monocytes. Infect Immun. 1992 Aug;60(8):3092–3097. doi: 10.1128/iai.60.8.3092-3097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]