Summary
Objectives
Surgical removal of pulmonary metastases from colorectal cancer is undertaken increasingly but the practice is variable. There have been no randomized trials of effectiveness. We needed evidence from a systematic review to plan a randomized controlled trial.
Design
A formal search for all studies concerning the practice of pulmonary metastasectomy was undertaken including all published articles using pre-specified keywords. Abstracts were screened, reviewed and data extracted by at least two of the authors. Information across studies was collated in a quantitative synthesis.
Results
Of 101 articles identified, 51 contained sufficient quantitative information to be included in the synthesis. The reports were published between 1971 and 2007, and reported on 3504 patients. There was little change over time in patient characteristics such as age, sex, the time elapsed since resection of the primary cancer, its site or stage. The proportion with multiple metastases or elevated carcinoma embryonic antigen (CEA) did not change over time but there was an apparent increase in the proportion of patients who also had hepatic metastasectomy. Differences in 5-year survival between groups defined by CEA or by single versus multiple metastases persisted over time. Few data were available concerning postoperative morbidity, postoperative lung function or change in symptoms.
Conclusion
The quality of evidence available concerning pulmonary metastasectomy in colorectal cancer is not sufficient to draw inferences concerning the effectiveness of this surgery. There is great variety in what was reported and its utility. Given the burdensome nature of the surgery involved, better evidence, ideally in the form of a randomized trial, is required for the continuance of this practice.
Introduction
Pulmonary metastasectomy for colorectal cancer is widespread but a survey in Europe shows great variation in belief and practice.1 There is growing pressure to operate on these patients to remove metastases from the lung, not infrequently in patients who have already undergone liver resection for metastatic disease. There are systematic reviews of surgery for both liver2 and for lung metastasectomy;3 the authors found no randomized trials in either case. In the absence of randomized trials a formal meta-analysis was not deemed possible, and for pulmonary metastasectomy Pfannschmidt et al. presented a textual summary of 20 papers chosen on the basis of size (40 cases or more) and criteria concerning follow-up.
At the time the practice was increasing the degree of selection was examined in the USA Veterans Administration database.4 Of 35,921 cases of colorectal cancer, 22,715 had the primary cancer removed. Pulmonary metastasectomy was performed in 76 patients and a five-year survival rate of 36% was projected. The possibility that survivors were a result of the extreme selection of cases rather than being attributable to the pulmonary metastasectomy was mooted by Åberg,5 a proposition that has never been refuted.
Mathematical modelling has been used to show that the survival rates presented in two of the larger and most complete of the follow-up studies6,7 are not inconsistent with what would be expected among the broader population of patients with colorectal cancer when account is taken of the stage mix and the period between treatment of the primary cancer and the pulmonary metastasectomy.8
Systematic review is a prerequisite to planning a randomized trial. Given our own uncertainties about the evidence for benefit9 and prior to embarking on a randomized trial10 we sought the best evidence available to guide practice. We performed an inclusive systematic review and a quantitative synthesis of the data they contain.
Methods
The literature was searched using a formal strategy. This is shown in Appendix 1, available online at http://jrsm.rsmjournals.com/cgi/content/full/103/2/60/DC1. Manuscript titles were searched to identify papers that might include data concerning pulmonary metastasectomy for colorectal cancer. These were reviewed by IH, KT and TT. No exclusions were made on the basis of the language of publication. Papers excluded at the first selection included teaching and review articles, technical reports and those containing no relevant data concerning surgical treatment. A second selection was made by FF, MU and TT to remove any residual reports which pertained to multiple primary cancer sites; registries that overlapped with institutional reports; single institutional studies that were superseded by later reports from the same institution; or specific subgroups of the patient population. Obvious exclusions were made on the basis of abstracts, the remainder with reference to the full articles.
In instances where uncertainty could not be resolved by careful reading of the manuscripts, the authors of the manuscripts concerned were contacted and asked to clarify matters.
The resulting selected papers were individually searched and data extracted.
Series with over 50 cases were inspected first and all items of data contained were collated in a spreadsheet. From these we identified the most commonly reported items of data and systematically searched the smaller series for these and other elements that we deemed important for informing practice. We tabulated the presence of data grouped according to whether these were preoperative features that describe the case-mix being operated upon, operative and pathological findings, or outcome data.
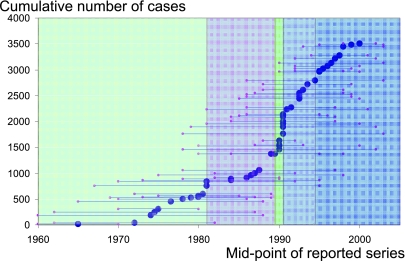
We recorded the start and end dates of the reported series. Where not reported, the series was deemed to have ended a year before the publication date and the start date was approximated with reference to the longest duration of follow-up in the publication. The mid-point of the period was used to order the papers and the cumulative sum of cases was used to define five epochs chosen to give groups of as near equal case volume as possible.
A series of graphical displays were constructed to depict the presenting features, clinicopathological findings and post-metastasectomy course, aggregating data by epoch where feasible. On inspection of these, additional graphical displays were constructed to further explore particular features of the data. Given the very large number of hypotheses that might be raised on inspection of the data, we have not performed any statistical hypothesis testing.
Results
Having excluded technical reports, teaching and review articles, and those containing no relevant data concerning surgical treatment, 101 papers remained. The flow chart ( Figure 1) shows the number of these papers rejected on the basis of each criterion; 51 papers remained for data extraction.
Figure 1.
Flow chart of papers found and progressive selection of 51 providing data in the quantitative synthesis
The 51 included series had mid-point dates from 1965 to 2000 and included a total of 3504 patients (Figure 2).4,6,7,11–58 The presence of data pertaining to each of the specified domains in each of the 51 papers is indicated by a bar in Figure 3. Where data were present for a particular domain they were often inconsistently reported, with various definitions and summary statistics.
Figure 2.
Cumulative number of pulmonary metastasectomy patients (n=3504 from 51 papers) with the mid-point and beginning and ending of case series shown. These dates were not given in two cases and were estimated as described in the text. The epochs contain 749, 626, 704, 708 and 717 cases, respectively
Figure 3.
Data available in the 51 surgical follow-up studies ranked from left to right by diminishing number of element for which data were available
For the specified factors where there were sufficient data across a number of studies, graphical displays similar in layout to Figure 4 were prepared. A further 12 figures are available online at http://jrsm.rsmjournals.com/cgi/content/full/103/2/60/DC2. Some of the data they contain are in an abbreviated form in Table 1.
Figure 4.
Percentage of patients who had multiple metastasectomy according to 38/51 papers in which data were presented
Table 1.
Prognostic factors among patients in the five epochs
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Prognostic factors and case-mix | Cases by epoch (%) | ||||
| Multiple metastases reported in 38 studies comprising 2676 patients in total | 33 | 35 | 27 | 40 | 39 |
| Bilateral metastases reported in 13 studies comprising 624 patients in total | 11 | 15 | * | 13 | 19 |
| Previous pulmonary metastasectomy reported in 21 studies comprising 1608 patients in total | 15 | 22 | 16 | 14 | 19 |
| Also with hepatic metastases reported in 34 studies comprising 2356 patients in total | 15 | 16 | 19 | 19 | 22 |
| Elevated CEA reported in 18 studies comprising 1409 patients in total | 46 | 51 | 41 | 49 | 32 |
| Prognostic factors and survival | Alive at five years (%) | ||||
| Multiple metastases reported in 15 studies comprising 1516 patients in total | 30 | 29 | 31 | 22 | 37 |
| Solitary metastasis reported in 25 studies comprising 2227 patients in total | 46 | 45 | 48 | 49 | 54 |
| CEA normal reported in 11 studies comprising 1159 patients in total | 47 | 38 | 42 | 53 | 43 |
| CEA elevated reported in 11 studies comprising 1159 patients in total | 16 | 22 | 33 | 22 | 0** |
No report in this epoch provided information on this factor
Only one series reported this factor in this epoch
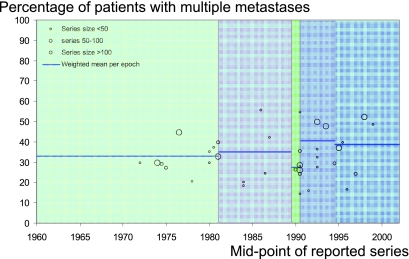
The majority of operations in case series are for a single metastasis, as opposed to two or more pulmonary metastases, but with a wide range of their relative proportions (Figure 4). In the 25 papers where five-year survival was given after single pulmonary metastasectomy, it was of the order of 50% ±5%. Fewer papers provide survival data for multiple metastasectomy and where they do it is lower.
Elevated carcinoma embryonic antigen (CEA) has been shown to be strongly associated with shorter survival in a number of multivariable analyses3 and this is in line with summary data presented here (Figure 15 online). The level of CEA was reported in fewer than half of the papers.
There may be an upward trend in the proportion of patients operated on with bilateral disease, previous pulmonary metastasectomy, and those who also have hepatic metastasectomy.
Little or no data exist within this literature concerning important considerations such as change in symptoms, change in respiratory function or other aspects of what would now be termed quality of life.
Discussion
The outcome data contained within the surgical follow-up reports found in our review are almost exclusively related to survival. There are no control groups provided of outcome among patients with similar features to those operated upon and in the absence of this information the data are not sufficient to inform judgement as to whether this surgery is effective in terms of prolonging survival.
There is very little information on symptoms. In general candidates for metastasectomy are selected as being free from cancer-related symptoms. Palliation in the sense of relieving present symptoms cannot therefore be claimed. None of the 51 papers included an account of symptoms in the time following surgery so palliation, in the sense of pre-empting imminent symptoms, cannot be claimed either. There is no published evidence on which to advocate the practice of pulmonary metastasectomy in colorectal cancer for palliation.
It should be remembered that the formal measurement of Health Related Quality of Life (QoL) started in the 1980s and requires prospective data collection, normally including baseline information prior to the intervention. The dates of the operations and the retrospective nature of the data collection in follow-up studies preclude inclusion of what we might now expect to see in the form of QoL data.
A recent systematic review provided descriptive information drawn from 20 papers deemed to be of sufficient quality.3 By adopting more liberal criteria for inclusion in our review and a pragmatic approach to aggregating data, we believe we have captured as fully as possible the present state of knowledge concerning this practice.
The weaknesses of the literature, relying on follow-up studies, are inevitably carried into this synthesis. There is variable and incomplete reporting. One conclusion that we can draw is that there is surely no purpose in compiling and reporting further small, uncontrolled studies. They are unlikely to add further knowledge59,60 and yet they continue to appear in the surgical literature.61–63
There are circumstances where the immediacy of cause and effect, the mechanistically obvious relationship between intervention and benefit, and the large and incontrovertible effect leads us to accept a treatment because we can see that it works.64 In the practice of pulmonary metastasectomy in colorectal cancer, these conditions do not prevail.
In the context of colorectal cancer, the claims of survival benefit from pulmonary metastasectomy rely on an implicit belief that survival for any of this population of patients at five years (say) is an extreme rarity, and that any survivors five years after operation are attributable to surgical metastasectomy. We explored the validity of that assumption using mathematical modelling using cancer registry data and it did not hold.8 This review of the literature does not resolve our doubts.
The reports often state that pulmonary metastasectomy is safe and indeed reported mortality rates are often zero. A recent report detailed how well these patients recover.65 Nevertheless, it should be remembered that when one operates on a patient, one does not have the opportunity to modify the dose or discontinue treatments which are not achieving the desired effect. There are certain harms which are permanent and irretrievable such as the loss of lung parenchyma for all and for some long-term pain and other complications. One of the weaknesses of the literature reviewed in this study is that these are not reported.
The authors of the previous systematic review of pulmonary metastasectomy for advanced colorectal cancer concluded by opining that randomized trials are not now possible. We draw a different conclusion – it is time for a randomized trial.10 A randomized trial is now essential because the evidence for the benefit of surgery is small, the practice is very widespread1 and probably increasing, and the certain harm being done, if there is no benefit, is too great.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding The Clinical Operational Research Unit receives funding from the UK Department of Health Policy Research Programme
Ethical approval Not applicable
Guarantor TT
Contributorship The original research was proposed by TT and IH; IH and KT conducted the literature search; the screening of papers by title and abstract was performed by IH, KT and TT; IH and KT read all papersand extracted data; the screening of papers for overlapping or duplication of content was performed by FF, TT and MU; TT, MU and FF wrote the first draft and all revisions. All authors have read successive drafts and approved the final version
Acknowledgements
None
References
- 1.Internullo E, Cassivi SD, Van Raemdonck D, Friedel G, Treasure T. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257–66 [DOI] [PubMed] [Google Scholar]
- 2.Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007;84:324–38 [DOI] [PubMed] [Google Scholar]
- 4.Wade TP, Virgo KS, Li MJ, Callander PW, Longo WE, Johnson FE. Outcomes after detection of metastatic carcinoma of the colon and rectum in a national hospital system. J Am Coll Surg 1996;182:353–61 [PubMed] [Google Scholar]
- 5.Aberg T, Malmberg KA, Nilsson B, Nou E. The effect of metastasectomy: fact or fiction? Ann Thorac Surg 1980;30:378–84 [DOI] [PubMed] [Google Scholar]
- 6.McCormack PM, Burt ME, Bains MS, Martini N, Rusch VW, Ginsberg RJ. Lung resection for colorectal metastases. 10-year results. Arch Surg 1992;127:1403–6 [DOI] [PubMed] [Google Scholar]
- 7.Okumura S, Kondo H, Tsuboi M, et al. Pulmonary resection for metastatic colorectal cancer: experiences with 159 patients. J Thorac Cardiovasc Surg 1996;112:867–74 [DOI] [PubMed] [Google Scholar]
- 8.Utley M, Treasure T, Linklater K, Moller H. Better out than in? The resection of pulmonary metastases from colorectal tumours. Operations Research for Health Care Delivery Engineering (ORAHS 2007) 2008;493–500 [Google Scholar]
- 9.Treasure T, Utley M, Hunt I. When professional opinion is not enough: surgical resection of pulmonary metastases. BMJ 2007;334:831–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treasure T, Fallowfield L, Farewell V, et al. Pulmonary metastasectomy in colorectal cancer: time for a trial. Eur J Surg Oncol 2009;35:686–9 [DOI] [PubMed] [Google Scholar]
- 11.Moore KH, McCaughan BC. Surgical resection for pulmonary metastases from colorectal cancer. ANZ J Surg 2001;71:143–6 [DOI] [PubMed] [Google Scholar]
- 12.Zanella A, Marchet A, Mainente P, Nitti D, Lise M. Resection of pulmonary metastases from colorectal carcinoma. Eur J Surg Oncol 1997;23:424–7 [DOI] [PubMed] [Google Scholar]
- 13.Negri F, Musolino A, Cunningham D, Pastorino U, Ladas G, Norman AR. Retrospective study of resection of pulmonary metastases in patients with advanced colorectal cancer: the development of a preoperative chemotherapy strategy. Clin Colorectal Cancer 2004;4:101–6 [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa K, Hashiguchi Y, Mochizuki H, Ozeki Y, Ueno H. Extranodal cancer deposit at the primary tumor site and the number of pulmonary lesions are useful prognostic factors after surgery for colorectal lung metastases. Dis Colon Rectum 2003;46:629–36 [DOI] [PubMed] [Google Scholar]
- 15.Watanabe I, Arai T, Ono M, et al. Prognostic factors in resection of pulmonary metastasis from colorectal cancer. Br J Surg 2003;90:1436–40 [DOI] [PubMed] [Google Scholar]
- 16.Shirouzu K, Isomoto H, Hayashi A, Nagamatsu Y, Kakegawa T. Surgical treatment for patients with pulmonary metastases after resection of primary colorectal carcinoma. Cancer 1995;76:393–8 [DOI] [PubMed] [Google Scholar]
- 17.Mineo TC, Ambrogi V, Tonini G, et al. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg 2003;197:386–91 [DOI] [PubMed] [Google Scholar]
- 18.van Halteren HK, van Geel AN, Hart AA, Zoetmulder FA. Pulmonary resection for metastases of colorectal origin. Chest 1995;107:1526–31 [DOI] [PubMed] [Google Scholar]
- 19.Baron O, Hamy A, Roussel JC, et al. [Surgical treatment of pulmonary metastases of colorectal cancers. 8-year survival and main prognostic factors]. Rev Mal Respir 1999;16:809–15 [PubMed] [Google Scholar]
- 20.Irshad K, Ahmad F, Morin JE, Mulder DS. Pulmonary metastases from colorectal cancer: 25 years of experience. Can J Surg 2001;44:217–21 [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata Y, Matono K, Hayashi A, et al. Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonry resection in colorectal cancer. World J Surg 2005;29:363–8 [DOI] [PubMed] [Google Scholar]
- 22.Yedibela S, Klein P, Feuchter K, et al. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol 2006;13:1538–44 [DOI] [PubMed] [Google Scholar]
- 23.Mori M, Tomoda H, Ishida T, et al. Surgical resection of pulmonary metastases from colorectal adenocarcinoma. Special reference to repeated pulmonary resections. Arch Surg 1991;126:1297–301 [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest 2001;119:1069–72 [DOI] [PubMed] [Google Scholar]
- 25.Goya T, Miyazawa N, Kondo H, Tsuchiya R, Naruke T, Suemasu K. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer 1989;64:1418–21 [DOI] [PubMed] [Google Scholar]
- 26.Vogelzang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg 2004;91:1066–71 [DOI] [PubMed] [Google Scholar]
- 27.Girard P, Ducreux M, Baldeyrou P, et al. Surgery for lung metastases from colorectal cancer: analysis of prognostic factors. J Clin Oncol 1996;14:2047–53 [DOI] [PubMed] [Google Scholar]
- 28.Higashiyama M, Kodama K, Higaki N, et al. Surgery for pulmonary metastases from colorectal cancer: the importance of prethoracotomy serum carcinoembryonic antigen as an indicator of prognosis. Jpn J Thorac Cardiovasc Surg 2003;51:289–96 [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238–44 [DOI] [PubMed] [Google Scholar]
- 30.McAfee MK, Allen MS, Trastek VF, Ilstrup DM, Deschamps C, Pairolero PC. Colorectal lung metastases: results of surgical excision. Ann Thorac Surg 1992;53:780–5 [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007–13 [DOI] [PubMed] [Google Scholar]
- 32.Reddy RH, Kumar B, Shah R, et al. Staged pulmonary and hepatic metastasectomy in colorectal cancer – is it worth it? Eur J Cardiothorac Surg 2004;25:151–4 [DOI] [PubMed] [Google Scholar]
- 33.Yano T, Hara N, Ichinose Y, Yokoyama H, Miura T, Ohta M. Results of pulmonary resection of metastatic colorectal cancer and its application. J Thorac Cardiovasc Surg 1993;106:875–9 [PubMed] [Google Scholar]
- 34.Scheele J, Altendorf-Hofmann A, Stangl R, Gall FP. Pulmonary resection for metastatic colon and upper rectum cancer. Is it useful? Dis Colon Rectum 1990;33:745–52 [DOI] [PubMed] [Google Scholar]
- 35.Ike H, Shimada H, Togo S, Yamaguchi S, Ichikawa Y, Tanaka K. Sequential resection of lung metastasis following partial hepatectomy for colorectal cancer. Br J Surg 2002;89:1164–8 [DOI] [PubMed] [Google Scholar]
- 36.Koga R, Yamamoto J, Saiura A, Yamaguchi T, Hata E, Sakamoto M. Surgical resection of pulmonary metastases from colorectal cancer: Four favourable prognostic factors. Jpn J Clin Oncol 2006;36:643–8 [DOI] [PubMed] [Google Scholar]
- 37.Rena O, Casadio C, Viano F, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg 2002;21:906–12 [DOI] [PubMed] [Google Scholar]
- 38.Pfannschmidt J, Muley T, Hoffmann H, Dienemann H. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg 2003;126:732–9 [DOI] [PubMed] [Google Scholar]
- 39.Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. Prognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2007;31:167–72 [DOI] [PubMed] [Google Scholar]
- 40.Zapatero J, Flandes J, Lago J, Devesa M, Glope A, Candelas J. Prognostic factors in pulmonary metastases from colorectal cancer. Respiration 1994;61:280–2 [DOI] [PubMed] [Google Scholar]
- 41.Brister SJ, de Varennes B, Gordon PH, Sheiner NM, Pym J. Contemporary operative management of pulmonary metastases of colorectal origin. Dis Colon Rectum 1988;31:786–92 [DOI] [PubMed] [Google Scholar]
- 42.Fujisawa T, Yamaguchi Y, Saitoh Y, et al. Factors influencing survival following pulmonary resection for metastatic colorectal carcinoma. Tohoku J Exp Med 1996;180:153–60 [DOI] [PubMed] [Google Scholar]
- 43.Casali C, Stefani A, Storelli E, Morandi U. Prognostic factors and survival after resection of lung metastases from epithelial tumours. Interact Cardiovasc Thorac Surg 2006;5:317–21 [DOI] [PubMed] [Google Scholar]
- 44.Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278–82 [DOI] [PubMed] [Google Scholar]
- 45.Zink S, Kayser G, Gabius HJ, Kayser K. Survival, disease-free interval, and associated tumor features in patients with colon/rectal carcinomas and their resected intra-pulmonary metastases. Eur J Cardiothorac Surg 2001;19:908–13 [DOI] [PubMed] [Google Scholar]
- 46.Saclarides TJ, Krueger BL, Szeluga DJ, Warren WH, Faber LP, Economou SG. Thoracotomy for colon and rectal cancer metastases. Dis Colon Rectum 1993;36:425–9 [DOI] [PubMed] [Google Scholar]
- 47.Wilking N, Petrelli NJ, Herrera L, Regal AM, Mittelman A. Surgical resection of pulmonary metastases from colorectal adenocarcinoma. Dis Colon Rectum 1985;28:562–4 [DOI] [PubMed] [Google Scholar]
- 48.Wang CY, Hsie CC, Hsu HS, et al. Pulmonary resection for metastases from colorectal adenocarcinomas. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65:15–22 [PubMed] [Google Scholar]
- 49.Iizasa T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg 2006;82:254–60 [DOI] [PubMed] [Google Scholar]
- 50.Mansel JK, Zinsmeister AR, Pairolero PC, Jett JR. Pulmonary resection of metastatic colorectal adenocarcinoma. A ten year experience. Chest 1986;89:109–12 [DOI] [PubMed] [Google Scholar]
- 51.Patel NA, Keenan RJ, Medich DS, et al. The presence of colorectal hepatic metastases does not preclude pulmonary metastasectomy. Am Surg 2003;69:1047–53 [PubMed] [Google Scholar]
- 52.Watanabe M, Deguchi H, Sato M, et al. Midterm results of thoracoscopic surgery for pulmonary metastases especially from colorectal cancers. J Laparoendosc Adv Surg Tech A 1998;8:195–200 [DOI] [PubMed] [Google Scholar]
- 53.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg 2004;91:112–20 [DOI] [PubMed] [Google Scholar]
- 54.Marincola FM, Mark JB. Selection factors resulting in improved survival after surgical resection of tumors metastatic to the lungs. Arch Surg 1990;125:1387–92 [DOI] [PubMed] [Google Scholar]
- 55.Joseph WL, Morton DL, Adkins PC. Prognostic significance of tumor doubling time in evaluating operability in pulmonary metastatic disease. J Thorac Cardiovasc Surg 1971;61:23–32 [PubMed] [Google Scholar]
- 56.Kodama K, Doi O, Higashiyama M, Tatsuta M, Iwanaga T. Surgical management of lung metastases. Usefulness of resection with the neodymium:yttrium-aluminum-garnet laser with median sternotomy. J Thorac Cardiovasc Surg 1991;101:901–8 [PubMed] [Google Scholar]
- 57.Jaklitsch MT, Mery CM, Lukanich JM, et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg 2001;121:657–67 [DOI] [PubMed] [Google Scholar]
- 58.Cheng LC, Chiu CS, Lee JW. Surgical resection of pulmonary metastases. J Cardiovasc Surg (Torino) 1998;39:503–7 [PubMed] [Google Scholar]
- 59.Chalmers I. Comment on Fergusson et al: The scandalous failure of science to cumulate evidence scientifically. Clin Trials 2005;2:229 [Google Scholar]
- 60.Fergusson D, Glass KC, Hutton B, Shapiro S. Randomized controlled trials of aprotinin in cardiac surgery: could clinical equipoise have stopped the bleeding? Clin Trials 2005;2:218–29 [DOI] [PubMed] [Google Scholar]
- 61.Chen F, Hanaoka N, Sato K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World J Surg 2009;33:505–11 [DOI] [PubMed] [Google Scholar]
- 62.Rama N, Monteiro A, Bernardo JE, Eugenio L, Antunes MJ. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardiothorac Surg 2009; January 9 [DOI] [PubMed] [Google Scholar]
- 63.Saisho S, Nakata M, Sawada S, et al. Evaluation of video-assisted thoracoscopic surgery for pulmonary metastases: 11-years of experience. Surg Endosc 2009;23:55–61 [DOI] [PubMed] [Google Scholar]
- 64.Glasziou P, Chalmers I, Rawlins M, McCulloch P. When are randomised trials unnecessary? Picking signal from noise. BMJ 2007;334:349–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrella F, Chieco P, Solli P, et al. Which factors affect pulmonary function after lung metastasectomy? Eur J Cardiothorac Surg 2009;35:792–6 [DOI] [PubMed] [Google Scholar]