Abstract
Tone detection and temporal gap detection thresholds were determined in CBA/CaJ mice using a Go/No-go procedure and the psychophysical Method of Constant Stimuli. In the first experiment, audiograms were constructed for five CBA/CaJ mice. Thresholds were obtained for eight pure tones ranging in frequency from 1 to 42 kHz. Audiograms showed peak sensitivity between 8 and 24 kHz, with higher thresholds at lower and higher frequencies. In the second experiment, thresholds for gap detection in broadband and narrowband noise bursts were measured at several sensation levels. For broadband noise, gap thresholds were between 1 and 2 ms, except at very low sensation levels, where thresholds increased significantly. Gap thresholds also increased significantly for lowpass-filtered noise bursts with a cutoff frequency below 18 kHz. Our experiments revised absolute auditory thresholds in the CBA/CaJ mouse strain and demonstrated excellent gap detection ability in the mouse. These results add to the baseline behavioral data from normal-hearing mice which have become increasingly important for assessing auditory abilities in genetically altered mice.
Keywords: mouse, Mus musculus, audiogram, gap detection, psychophysics
Introduction
The large number and variety of genetically engineered mouse strains make mice good models for studies that seek to identify genetic factors likely to contribute to various forms of human deafness (e.g.Zheng et al. 1999; Prosen et al. 2003). Researchers have already found nearly 100 naturally occurring mouse mutations with hearing impairments and both the human and mouse genomes have been completely mapped (Zheng et al. 1999). However, to fully understand the effects of genetic mutations on hearing, it is necessary to determine the full repertoire of hearing abilities of these mice. Common auditory assessments of hearing include absolute thresholds, temporal resolution, frequency and intensity discrimination, masking effects, and sound localization acuity.
To accurately demonstrate their full range of auditory processing abilities, it is highly desirable that the mice be awake and behaviorally responding to acoustic cues in the environment. Using operant conditioning procedures, it is possible to train an animal to become a motivated, reliable listener, allowing different aspects of auditory perception to be evaluated in different strains of normal hearing mice or mice with known genetic mutations (e.g. Heffner and Heffner 2001). However, since operant techniques are time consuming and labor intensive, indirect measures of murine auditory function, such as the auditory brainstem response (Zheng et al. 1999) and the acoustic startle response (Ison and Allen 2003), have typically been employed since they require no training, provide useful information, and can be completed quickly (hours or days vs. weeks or months for operant tasks). However, the ABR and acoustic startle responses only provide indirect estimates of auditory threshold sensitivity by tapping into electrophysiological circuits or neural-motor reflex pathways located mainly in the brainstem or midbrain. To be interpreted in any meaningful way, ABR and startle reflex data need to be compared to the “gold standard”, specifically behavioral measures of auditory sensitivity obtained with operant conditioning methods. Several studies have shown that conditioned suppression/avoidance paradigms (Heffner and Masterson 1980) and go/no-go procedures (Prosen et al. 2000, 2003; Klink et al. 2006) produce sensitive and reliable measures of auditory sensitivity in mice. However, important differences have been observed across strains, experimental conditions, and laboratories; therefore it is important to establish baseline measures of auditory sensitivity for each mouse strain and behavioral method used in a laboratory. In the first experiment reported here, the operant conditioning go/no-go procedure and the psychophysical Method of Constant Stimuli (MOCS) were used to construct audiograms for adult CBA/CaJ mice, a mouse model that retains normal sensitivity over much of its lifespan. The CBA mouse strain was originally a cross of a Bagg albino female and a DBA male, and the CaJ (Carter J) substrain is obtained solely from The Jackson Laboratory (Bar Harbor, ME, USA). The go/no-go procedure requires an animal to wait until a stimulus is presented and then respond within a given amount of time (Prosen et al. 2003). The MOCS involves the presentation of stimuli both below and well above threshold throughout each experimental session. The presentation of stimuli well above threshold ensures that the animal is capable of receiving many reinforcements and is thus more likely to complete many trials. In addition, trials are presented in random order, making it impossible for the mouse to anticipate the next stimulus (e.g., Klink et al. 2006).
Klink et al. (2006) found that the MOCS produced lower 10 kHz thresholds compared to those obtained with an adaptive tracking procedure in NMRI mice. Unfortunately, the MOCS has not been used to obtain audiograms in mice covering a wide range of frequencies. In the first experiment we measured the audiogram for CBA/CaJ mice for frequencies ranging from 1 to 42 kHz using go/no-go procedures and the MOCS. This strain is used extensively in mouse research because it maintains nearly normal hearing until a very advanced age. However, no behavioral audiogram measures have been taken on these animals, despite having become the preferred ‘normal’ mouse strain for acoustic testing.
In the second experiment, we obtained behavioral gap detection thresholds, again using operant conditioning and the MOCS. Both physiology and acoustic startle have been used to estimate gap detection in mice (e.g., Barsz et al. 1998, 2002; Ison et al. 1998, 2002, 2005; Allen et al. 2003). However, gap detection thresholds have yet to be measured in trained mice using operant conditioning procedures. Gap detection is a reflection of the temporal resolving power of the auditory system. A species' auditory temporal acuity will place limits on sound localization and the ability to process complex communication signals that contain rapid spectral and temporal changes. It is well known that impairment in one acoustic task, such as pure tone detection, does not necessarily translate to impairment in another acoustic task, such as temporal resolution. Providing baseline behavioral hearing thresholds on these animals is important for future studies on aging, for comparing genetic variants, and for studies on the prevention of hearing loss.
Materials and methods
Subjects
The animals used in this experiment were adult house mice (Mus musculus), 6 males and 4 females, of the CBA/CaJ strain. Five subjects (three males and two females) were used in the audiogram experiment and five subjects (three males and two females) were used in the gap detection experiment. Data collection began when the mice were approximately five months old. The original breeding pairs were acquired from The Jackson Laboratory. These mice were bred at the University at Buffalo, SUNY and all procedures were approved by the University at Buffalo, SUNY's Institutional Animal Care and Use Committee. All mice were housed separately and were kept on a reverse day/night cycle (lights off at 6 am and lights on at 6 pm). Accordingly, the mice were tested during the dark portion of their cycle. They were water restricted and kept at approximately 85% of their free-drinking weight during the course of the experiment. The animals had unrestricted access to food, except while they were participating in the experiments.
Apparatus
The mice were tested in a wire cage (28 × 56 × 30.5 cm for audiograms, 23 × 39 × 15.5 cm for gap detection, see Figure 1) placed in an echo reduced chamber (53.5 × 54.5 × 57 cm) lined with 4 cm thick Sonex sound attenuating foam (Illbruck, Inc., Minneapolis, MN). The chamber was illuminated at all times by a small lamp with a 25-watt red light bulb in the audiogram experiment. A white light bulb was used for the gap detection experiment to make the animals more visible to the experimenters during training. The behavior of the animals during test sessions was monitored by an overhead web-camera (Logitech QuickCam Pro, Model 4000). The test cage consisted of a speaker (Fostex Dome Tweeter, FT28D; frequency response differing by less than 5 dB up to 50 kHz), a response dipper (Med Associates Model ENV-302M-UP), one or two nose poke holes surrounded by infrared sensors (Med Associates Model ENV-254), and for the audiogram experiments, a starting platform (Radio Shack project box containing a microswitch) that was activated when the mouse sat on the front of it.
Fig 1.
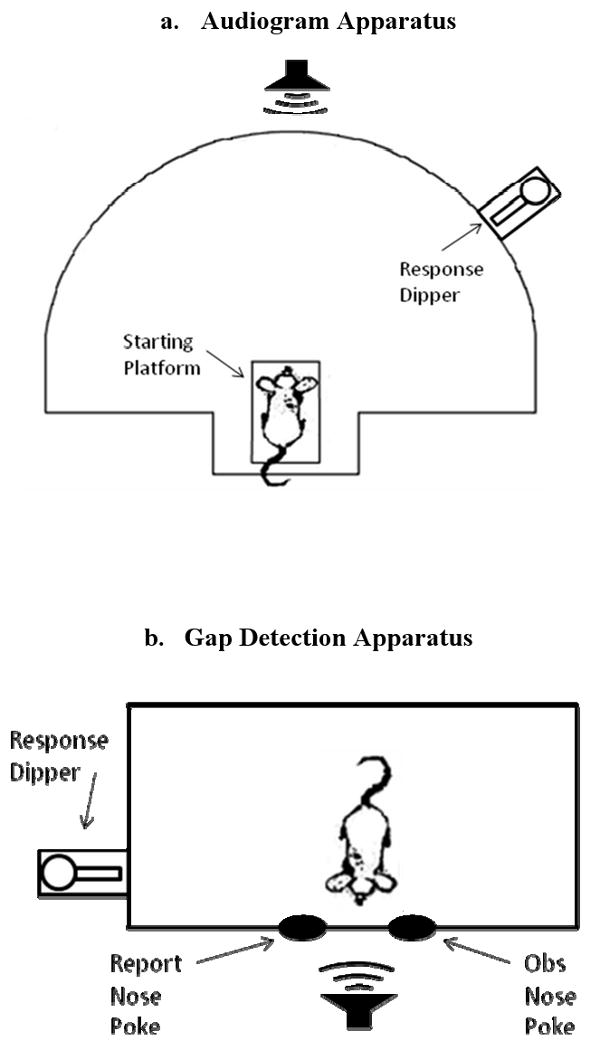
Overhead schematics of the testing apparatus for the audiogram experiment (a) and the gap detection experiment (b). Each cage contained one or more nose poke holes, a water dipper, and a loudspeaker.
The experiment was controlled by Dell Dimension 3100 computers operating Tucker-Davis Technologies (TDT, Gainesville, FL) modules and software. Stimuli were created using a 100 kHz sampling rate and were sent through an RP2 signal processor, an SA1 power amplifier, a PA5 programmable attenuator, and finally to the loudspeaker. Inputs to and outputs from the testing cages were also controlled via the RP2 processor. Power supplies were used to drive the dipper (Elenco Precision, Wheeling, IL, Model XP-603) and infrared sensors (Elenco Precision Model XP-650). The software used to control the hardware used TDT's RPvds software with a Visual Basic interface.
The stimuli were created and edited in Adobe Audition (v. 1.5). Sound pressure levels for frequencies up to 12 kHz were calibrated using a sound level meter (Larson Davis System 824) with a condenser microphone (PRM902) placed at the position where the animal's head would be during testing. Sound pressure levels for higher frequencies were calibrated using an ultrasound recording system (Avisoft, Model USG 116-200) and Raven Pro (v 1.3, Cornell University) software, with the microphone (Avisoft Bioacoustics Ultra Sound Gate CM116) placed at same location. Calibrations were conducted weekly, and did not vary by more than 2 dB at any frequency tested throughout the course of the experiments.
Data analysis
Signal detection analysis was performed to factor out the animals' motivational biases since bias is independent of sensitivity (e.g., Steckler 2001). At least 400 trials were obtained for each condition from each mouse. The first 200 trials were considered practice, and thresholds were calculated using the last 200 trials as long as thresholds improved by no more than 3-5 dB or 3-5 ms from session to session. If thresholds varied by more than that, testing continued until thresholds stabilized and the last 200 trials were used to obtain thresholds. Mean hit and false alarm rates were used to calculate thresholds using signal detection theory with a threshold criterion of d′=1.5. Although any threshold criterion is chosen arbitrarily, we set our threshold criterion at 1.5 for two main reasons. This d′ value is comparable to the values used by other animal researchers (e.g., Wagner et al., 2003; Klink et al., 2006) and is a relatively conservative threshold criterion (compared to lower d′ values such as 1.0). It also corresponds to low false alarm rates, which ensure that the animal is responding primarily to the target stimuli and not randomly (Klump and Maier, 1989).
Experiment 1: Audiogram procedures
The stimuli used in the audiogram experiment were eight pure tones (1, 2, 4, 8, 12, 16, 24, and 42 kHz) with a 2 s duration and cosine rise/fall times of 20 ms. The mice were trained using a go/no-go operant conditioning procedure to respond to pure tones; correct responses were rewarded with 0.01 ml of water reinforcement. The mouse began a trial by sitting on the starting platform, which initiated a variable waiting interval that ranged between 1 and 5 s. During this time, the mouse had to remain on the platform. After the waiting interval, a single test stimulus was presented. In the go condition, a tone was played. If the mouse perceived this stimulus it was trained to jump off the platform and run to the response dipper for a water reinforcement. In this condition, a hit was recorded if the mouse correctly responded to the tone by reaching the dipper (and breaking the infrared beam trigger at the nose poke hole entrance). The mouse had 3.5 s to reach the dipper after the onset of the tone to receive reinforcement. A miss was recorded if the mouse either failed to jump off the platform or if it did not reach the dipper within 3.5 seconds. If the mouse left the platform during the variable waiting interval, the trial was aborted.
Approximately 30% of all trials were catch trials. These constituted the no-go part of the procedure and no stimulus was presented during these trials. These trials were required to measure the false alarm rate and calculate the animal's response bias. If the subject jumped off the platform during a catch trial, a false alarm was recorded. However, if the subject remained on the platform during a catch trial, a correct rejection was recorded. In either case, no reinforcement was given and the next trial began immediately.
Training began when the animals were about three months old. The first stage in the training process was to shape the mice to sit on the platform and run to the dipper for water. The animals were then trained to remain on the platform until they heard a 10 kHz tone. Catch trials were next phased into the training, the waiting interval was systematically increased, and the tone was played at varying attenuation levels. We also trained the animals on several different frequencies to ensure that the mice would generalize the task to frequencies other than 10 kHz.
Testing began when the animals were approximately five months old, after which they ran two 30-minute sessions per day, 5 to 6 days per week. Only one frequency was tested per session. Typically, the mice ran between 50 and 100 trials within each session. All mice were tested on all eight frequencies in a random order and a different random order was used for each mouse. Testing was completed when the mice were approximately one year old. When obtaining ABR thresholds, Zheng et al. (1999) did not find age-related hearing loss in the CBA/CaJ strain when comparing 9-week old mice with 47-week old mice. Also, Jiminez et al. (1999) and Guimaraes et al. (2004) found no significant decline in distortion product otoacoustic emissions up to 15 months of age, suggesting that our mice did not suffer any age-related hearing deficits during our experiments.
We presented the pure tone stimuli to the animals according to the MOCS. Within a session, one frequency was presented in 10-trial blocks. Within each 10-trial block, the test tone was presented randomly at seven different predetermined levels using a step size of 5 or 10 dB (3 catch trials were also included in each block). The levels were chosen so that only the quietest one or two stimuli could not be heard by the mice, whereas the loudest tones were well above threshold. Starting stimulus intensities for each frequency were based on previous publications (Birch et al. 1968; Ehret 1974). As such, the intensities of the tones and the step sizes for each attenuation level had to be adjusted throughout the experiment from session to session, according to the animal's performance. Chance performance was represented by the animal's false alarm rate. Sessions were excluded from analysis if the false alarm rate was greater than 30%. Approximately 5% of all sessions were eliminated due to a high false alarm rate.
Experiment 2: Gap detection procedures
The stimuli used in the gap detection experiment were repeating noise bursts with an 800 ms duration and cosine rise/fall times of 10 ms. The stimuli were broadband or bandpassed, and only one stimulus type was presented per session. The 4 band-limited stimuli that were used consisted of (1) noise high passed at 18 kHz or noise low passed at (2) 18 kHz, (3) 12 kHz, or (4) 8 kHz. The broadband stimuli contained frequencies up to 100 kHz, although the frequency characteristics of the speakers were such that very little energy above 60 kHz was probably perceived by the mice. Broadband stimuli were presented at six sensation levels ranging from 10 to 45 dB (only one sensation level per session). The bandpassed stimuli were presented at a sensation level (SL) of 30 dB. The sensation levels for the noiseburst stimuli were determined using three mice from the audiogram experiment. The same procedures were used as in the previous audiogram experiment but broadband noise served as the target stimuli instead of pure tones. We found that the broadband noiseburst of 55 dB SPL corresponded to a SL of 30 dB.
The 800 ms noisebursts for each bandwidth condition were created and saved for use as background (and sham) trials. Target stimuli with gaps for each condition were created from the same background (no gap) stimuli. That is, for the target stimuli, silence was inserted into the center of the stimulus while the rest of the stimulus remained identical to the background stimulus. Thus, the gap contained no onset or offset ramps. Allen et al. (2002) found that human listeners do not use abrupt onset or offset information when detecting gaps in noise. To date, it is unclear whether mice could make use of such abrupt onset/offset information.
All stimuli remained 800 ms in duration, and the gaps ranged from 1-100 ms depending on the animal's performance. To determine whether thresholds from our single draw of random noise for each frozen noise condition could have been an artifact of the characteristics of that noiseburst, gap detection thresholds for five different broadband noisebursts presented at the 30 dB SL condition were measured for all five subjects. A one-way repeated measures ANOVA found no significant differences between the noiseburst thresholds (F(4,4) = 1.05, p>0.05). For all other conditions, only a single noiseburst was tested.
The mice were trained using a go/no-go operant conditioning procedure to discriminate between noisebursts containing a gap of silence in the middle (target) from ones not containing a gap (repeating background). The mouse began a trial by nose poking through the observation nose-poke hole two times, which initiated a variable waiting interval ranging from 1 to 4 s. During this time, a repeating background of noisebursts containing no gap was played at a rate of 1 burst every 2 s. After the waiting interval, a single test stimulus was presented, alternating with the background stimulus once. In the go condition, the target stimulus was the noiseburst containing a gap. If the mouse discriminated this change between background and target, it was required to nose poke through the report nose-poke hole within 2 s of the onset of the target. In this trial type, a hit was recorded if the mouse correctly responded to the gap noiseburst within the allotted time, and the animal received 0.01 ml of Ensure® as a reinforcement. Ensure® is a nutritional supplement with a thick chocolate milk consistency. We used Ensure® for this experiment instead of water to elicit more trials from the mice. A miss was recorded if the mouse either failed to nose poke through the report hole or if it did not respond within 2 seconds. If the mouse responded to the report nose-poke hole during the waiting interval, the trial was aborted and the mouse received a 3 s timeout during which no test stimulus would play.
Approximately 30% of all trials were catch trials. These constituted the no-go part of the procedure and no target stimulus was presented during these trials (the repeating background no-gap stimulus simply continued to be played). These trials were required to measure the false alarm rate and calculate the animal's response bias. If the subject nose poked to the report hole during a catch trial, a false alarm was recorded. However, if the subject continued to nose-poke to the observation nose-poke hole, a correct rejection was recorded. In either case, no reinforcement was given and the next trial began immediately. Chance performance was represented by the animal's false alarm rate. Sessions were excluded from analysis if the percentage of false alarms was greater than 30%. Approximately 1% of all sessions were thrown out due to a high false alarm rate.
Training began when the animals were about two months old. The first stage in the training process was to shape the mice to nose poke to the observation hole and run to the dipper for the Ensure®. The animals were then trained to repeatedly poke to the observation nose poke hole until they heard a noiseburst containing a 100 ms gap, after which time they would nose poke to the report nose poke hole for the reinforcement. Next, the repeating background (non-gapped) noisebursts were phased into the training in small intensity increase increments from session to session until they reached the intensity of the target stimuli. Catch trials were eventually phased into the training, the waiting interval was systematically increased, and finally, the gap durations were shortened.
Testing began when the animals were approximately seven months old and, during the data collection period, they ran two 30-minute sessions per day, 5 to 6 days per week. Typically, the mice ran between 50 and 100 trials within each session. All mice were tested on all noiseburst types and a different random order was used for each mouse. Testing was completed when the mice were approximately one year old.
The gap stimuli were presented according to the psychophysical MOCS. Within a session, one noiseburst bandwidth or intensity was tested. Within each 10-trial block in a session, seven predetermined gaps were presented randomly using a step size of 1-5 ms (3 catch trials were also included in each block). The gaps were chosen so that only the smallest one or two stimuli could not be heard by the mice, while the largest gaps were well above threshold. Gap sizes were initially 5 ms and were decreased when a preliminary threshold was obtained. Gap detection thresholds were first measured for broadband noisebursts presented at six SLs (10, 15, 25, 30, 35, 45 dB) in a random order and then for four 30 dB SL band-passed noisebursts (high passed at 18 kHz and low passed at 18, 12, and 8 kHz) in a random order.
Results
Audiograms
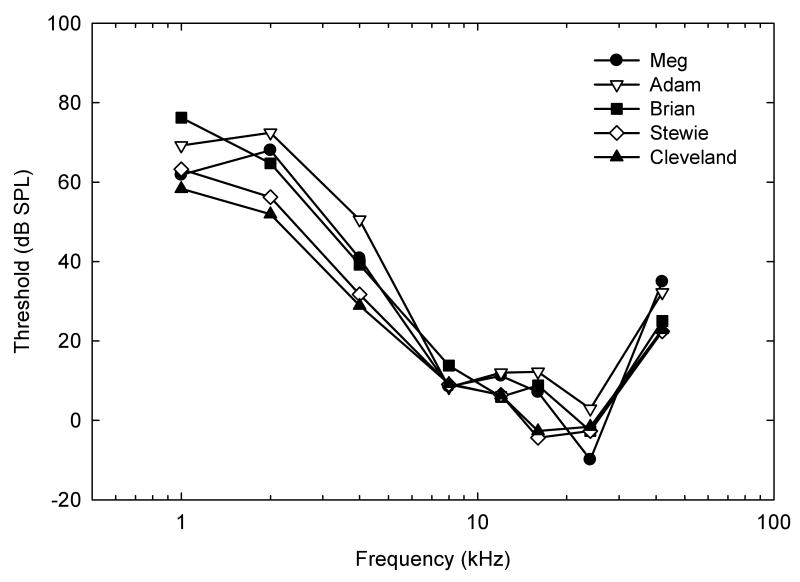
Audiograms for our five individual subjects on all eight frequencies show that the range of best hearing for these mice occurred between 8 and 24 kHz. They were most sensitive at 24 kHz, with poorer sensitivity at higher and lower frequencies (Figure 2, Table I). Importantly, these behavioral results highlight the very low between-subject variability when using this go/no-go procedure and the MOCS. Thresholds varied across the five subjects by as little as 5.5 dB at 8 kHz with greater variability only at lower and higher frequencies.
Fig 2.
Audiograms for five individual subjects tested at eight frequencies (1, 2, 4, 8, 12, 16, 24, and 42 kHz). Meg and Cleveland were females, and Adam, Brian, and Stewie were males. Absolute threshold (d′ = 1.5) is shown as a function of frequency (in kHz).
Table I.
Absolute thresholds (dB SPL) for each subject at each tone frequency (1-42 kHz) and the mean and standard deviation (SD) of all subjects (d′=1.5).
| Tone Frequency (kHz) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | 1 | 2 | 4 | 8 | 12 | 16 | 24 | 42 |
| Meg | 61.8 | 68.0 | 40.8 | 8.5 | 11.2 | 7.1 | -9.9 | 34.9 |
| Adam | 69.2 | 72.4 | 50.5 | 8.3 | 12.0 | 12.2 | 2.9 | 32.2 |
| Brian | 76.2 | 64.7 | 39.2 | 13.8 | 5.9 | 8.8 | -2.7 | 25.0 |
| Stewie | 63.2 | 56.2 | 31.7 | 9.0 | 6.4 | -4.4 | -2.7 | 22.4 |
| Cleveland | 58.3 | 51.9 | 28.9 | 9.2 | 6.3 | -2.7 | -1.6 | 22.8 |
| Mean Threshold (dB) | 65.74 | 62.64 | 38.22 | 9.76 | 8.36 | 4.20 | -2.80 | 27.46 |
| SD | 7.05 | 8.44 | 8.48 | 2.29 | 2.98 | 7.33 | 4.59 | 5.73 |
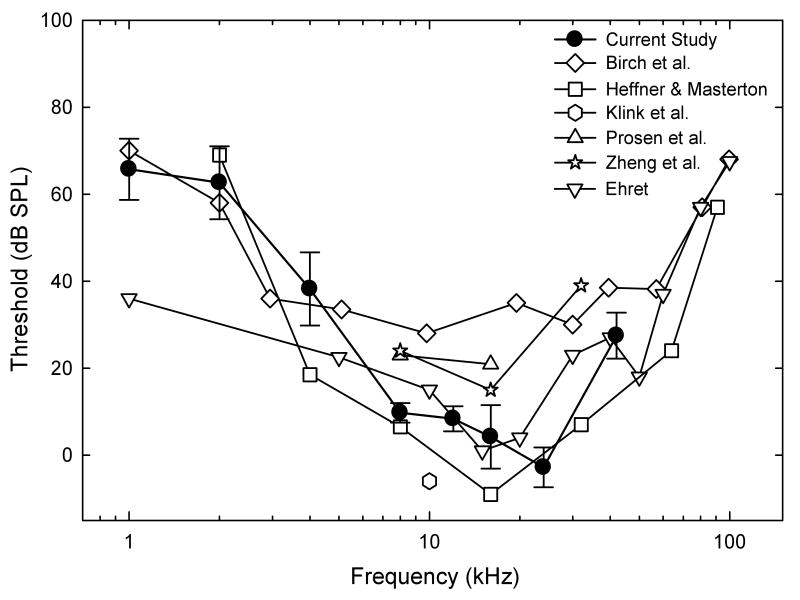
In Fig. 3, we highlight the audiograms from the current study and audiograms obtained from various strains of mice using various methods. Ehret (1974) and Klink et al. (2006) measured thresholds in NMRI mice, Birch et al. (1968) measured thresholds in CBA-J mice, and Prosen et al. 2003 measured thresholds in CBA/CaJ mice, and all used positive reinforcement conditioning methods. Heffner and Masterton (1980) used a conditioned suppression/avoidance procedure to measure thresholds in feral house mice. Zheng et al (1999) measured thresholds using ABRs. Klink et al. (2006), Heffner and Masterton (1980), and Prosen et al. (2003), like the current study, used the MOCS, presenting pre-determined stimulus intensities in a random order. Birch et al. (1968) used the psychophysical Descending Method of Limits. The range of obtained thresholds across the different experiments is as much as 40 dB at some frequencies and as little as 10 dB at other frequencies.
Fig 3.
Mean audiograms for the current study (black circles) with standard deviation error bars. Birch et al. (1968; white diamonds), Ehret (1974; white upside-down triangles), Prosen et al. (2003; white triangles), Klink et al. (2006; white hexagon), and Heffner and Masterson (1980; white squares) tested behaving mice, while Zheng et al. (1999; white stars) measured auditory thresholds using the auditory brainstem response.
Gap detection thresholds
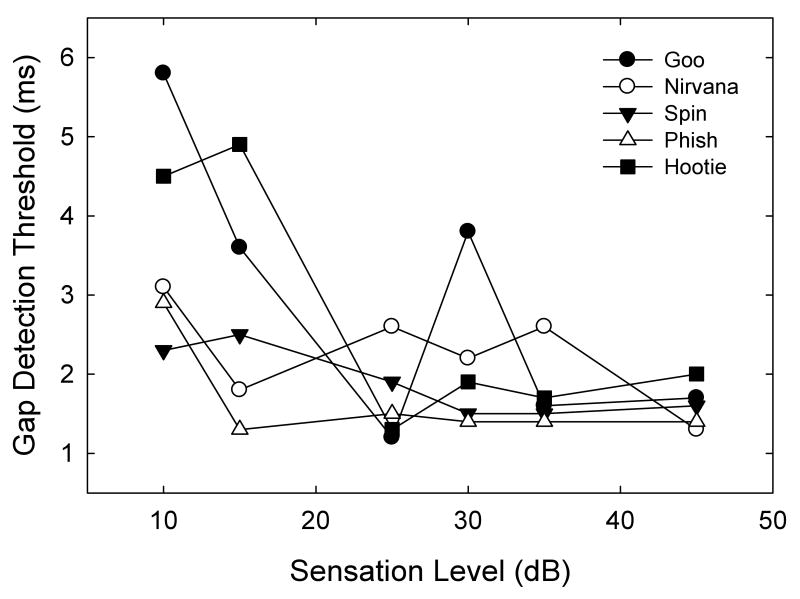
Gap detection thresholds for broadband white noisebursts increased as a function of decreasing intensity for all five subjects (Fig. 4; Table II). Although the variation was higher in these experiments than in the audiogram experiment, all subjects showed the same general pattern (except for one very high 30 dB SL threshold from Goo). Thresholds averaged 1.6 ms at the highest intensity and only 3.7 ms at the lowest intensity. There were significant differences across intensities, as calculated by a one way repeated measures ANOVA (F(4,5) = 4.44, p<.05). Holm-Sidak post-hoc analyses showed that the 10 dB SL gap thresholds were significantly higher than the 45, 35, and 25 dB SL thresholds (p<0.05). No other intensities were significantly different from each other (p>0.05).
Fig 4.
Broadband noise gap detection thresholds for five individuals tested at six sensation levels (10, 15, 25, 30, 35, and 45 dB SPL). Nirvana and Spin were females, and Phish, Goo, and Hootie were males.
Table II.
Broadband (BB) noise gap detection thresholds (ms) for each subject at each intensity (10-45 dB SL), and the mean and standard deviation (SD) of all subjects (d′=1.5).
| Sensation Level (dB) | ||||||
|---|---|---|---|---|---|---|
| Subject | 10 | 15 | 25 | 30 | 35 | 45 |
| Nirvana | 3.1 | 1.8 | 2.6 | 2.2 | 2.6 | 1.3 |
| Phish | 2.9 | 1.3 | 1.5 | 1.4 | 1.4 | 1.4 |
| Goo | 5.8 | 3.6 | 1.2 | 3.8 | 1.6 | 1.7 |
| Spin | 2.3 | 2.5 | 1.9 | 1.5 | 1.5 | 1.6 |
| Hootie | 4.5 | 4.9 | 1.3 | 1.9 | 1.7 | 2.0 |
| Mean Threshold (ms) | 3.7 | 2.8 | 1.7 | 2.2 | 1.8 | 1.6 |
| SD | 1.4 | 1.4 | 0.6 | 1.0 | 0.5 | 0.3 |
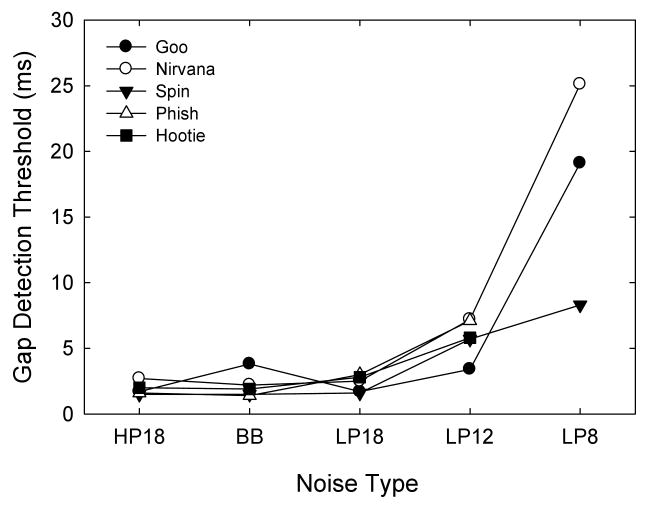
Fig. 5 and Table III show gap detection thresholds for band passed stimuli presented at 30 dB SL, along with broadband noise gap thresholds at that intensity. After several weeks of testing, we were unable to obtain thresholds at the 8 kHz low pass condition from two subjects because of very low hit and very high false alarm rates. However, results from three subjects at that condition and all five subjects at the 12 kHz low pass condition reveal that the high frequencies in noises are essential for the detection of gaps. Thresholds increased from 2.2 ms for broadband noises to 2.7 ms when frequencies above 18 kHz were removed, to 5.8 ms when frequencies above 12 kHz were removed, and finally to 17.5 ms when frequencies above 8 kHz were removed. A one way repeated measures ANOVA found significant differences between the four (not including the LP8 condition) noise types (F(4,3) = 14.58, p<0.05). Post-hoc Holm-Sidak pairwise comparisons found significant differences between the low pass 12 kHz thresholds and the broadband noise thresholds, the high pass 18 kHz thresholds, and the low pass 18 kHz thresholds (p<0.05). None of the other comparisons were significant (p>0.05).
Fig 5.
Gap detection thresholds for five subjects. Thresholds were obtained for broadband noise (BB), high pass at 18 kHz noise (HP18), low pass at 18 kHz noise (LP18), low pass at 12 kHz noise (LP12), and low pass at 8 kHz noise (LP8).
Table III.
Broadband (BB) and band-passed (HP = high pass, LP = low pass) noise gap detection thresholds (ms) for each subject at a constant 30 dB SL (BB thresholds also shown in Table II), and the mean and standard deviation (SD) of all subjects (d′=1.5). The gap detection thresholds for bandpassed noises are, in left to right order: high passed at 18 kHz, low passed at 18 kHz, low passed at 12 kHz, and low passed at 8 kHz.
| Noise Type | |||||
|---|---|---|---|---|---|
| Subject | BB | HP >18 kHz |
LP <18 kHz |
LP <12 kHz |
LP <8 kHz |
| Nirvana | 2.2 | 2.7 | 2.5 | 7.2 | 25.1 |
| Phish | 1.4 | 1.6 | 3.0 | 7.1 | -- |
| Goo | 3.8 | 1.7 | 3.5 | 3.4 | 19.1 |
| Spin | 1.5 | 1.5 | 1.6 | 5.7 | 8.3 |
| Hootie | 1.9 | 2.0 | 2.8 | 5.8 | -- |
| Mean Threshold (ms) | 2.2 | 1.9 | 2.7 | 5.8 | 17.5 |
| SD | 1.0 | 0.5 | 0.7 | 1.5 | 8.5 |
Discussion
Audiograms
The results from these two experiments reveal that operant conditioning and the psychophysical MOCS can be used to obtain reliable auditory acuity measurements in mice on both detection and discrimination tasks. Audiograms from our go/no-go procedure are comparable to findings using research methods such as conditioned suppression/avoidance in wild house mice (Heffner and Masterton 1980). However, the thresholds measured with MOCS in our CBA/CaJ mice produced thresholds that were lower than with the Descending Method of Limits in CBA/J mice, especially in the frequency range of greatest sensitivity (Birch et al. 1968). While these differences could be due to the methods employed, one cannot completely rule out CBA strain differences. The advantage of using the Descending Method of Limits over the MOCS is that more stimuli are presented closer to the animal's threshold so an audiogram can be collected with a fewer number of trials. However, since most of the stimuli are presented close to threshold using the Descending Method of Limits, the animal may have difficulty hearing the stimuli and thus predicting when it will receive reinforcement. Without the frequent opportunity to receive reinforcements, Klink et al. (2006) found that mice were less likely to pay attention to all stimuli, and that these temporary ‘lapses in participation’ led to very disparate thresholds from day to day.
When the Birch et al. (1968) study is removed from the comparison, the differences between the other obtained thresholds and our own are smaller. Still, differences of as much as 30 dB remain between audiograms measured in different laboratories. Whether these disparities are due to differences between housing conditions (Lauer et al. 2009), ages, strains, threshold criteria, equipment, or procedures (or a combination of these factors) remains unknown. Maier and Klump (2006) also found differences in the spatial acuity of Mongolian gerbils (Meriones unguiculatus) when thresholds were measured using operant conditioning for a food reinforcement instead of conditioned suppression procedures (Heffner and Heffner 1988). As a whole, these results highlight the need for multiple tests of auditory acuity from within the same laboratory and, whenever possible, within the same animals.
Gap detection thresholds
The gap detection thresholds obtained in this experiment are the first to be calculated using behavioral operant conditioning procedures in mice. This common measure of temporal resolution in animals generally yields low thresholds at high intensities and higher gap thresholds as sound intensity decreases below 30 dB SL. Gap detection thresholds range from 1.6 ms in rats (Syka et al. 2002), to 2.1 ms in Mongolian gerbils (Wagner et al. 2003), to 3.0 ms in chinchillas (Giraudi et al. 1980). At the highest intensity tested, thresholds for our mice averaged 1.6 ms, demonstrating a similar sensitivity amongst most rodents tested to date. However, it is interesting to note that gap thresholds were the lowest for mice, followed by rats, gerbils and chinchillas. Gap detection performance improves as the frequency content of the stimulus increases. Thus, the rank ordering of gap detection performance may be related to the upper frequency range of hearing; mice have the highest and chinchillas the lowest upper cutoff frequency while rats and gerbils fall in between. Gap thresholds are known to increase with age, but it is unclear whether this effect is due to peripheral hearing loss or changes in central auditory function (Giraudi-Perry et al. 1982; Salvi and Arehole 1985; Hamann et al. 2004; Walton et al. 2008).
Our mice had higher thresholds when stimuli were decreased to very low intensities (35-40 dB SPL), but generally low and stable thresholds for the four highest intensities tested (50-70 dB SPL). The only other available behavioral gap detection thresholds in mice have been obtained using acoustic startle procedures. Although acoustic startle yields absolute thresholds that are much higher than those obtained using operant conditioning procedures (Ison and Allen 2003), the differences between the two procedures in measuring gap detection appear to be much more subtle. Walton et al. (1997) measured gap detection thresholds in CBA/CaJ mice using reflex modification procedures and correlated these findings to neurophysiological recordings in the inferior colliculus. In both cases, they found gap detection thresholds of 1-2 ms (behavioral stimuli were presented at 70 dB SPL, matching our highest intensity of 45 dB SL). These thresholds are nearly the same as those reported here, even though their stimuli were much shorter than ours (100 ms compared to 800 ms). Allen et al. (2008) also recently reported similar thresholds of 1-2 ms using reflex modification procedures. Allen et al. (2003) found higher gap detection thresholds in mice when stimuli were presented at lower compared to higher intensities, increasing by more than 2.4 ms between 40 and 80 dB in young mice and increasing by more than 10.9 ms between 60 and 80 dB in 2-year old mice. The increase in gap detection thresholds with decreasing intensities in the current experiments never reached these levels, but showed similar trends.
The gap detection thresholds measured from the bandpassed stimuli highlight the importance of high frequency information for doing this task. When the frequencies above 12 kHz were filtered, thresholds increased significantly. When the frequencies above 8 kHz were filtered, thresholds more than doubled in some subjects and they were immeasurable in others. These results are comparable to those from Ison et al. (2005), who found that frequencies above 16 kHz were necessary for accuracy on the gap detection task. Although the mouse has a wider range of hearing than the human, this is another effect seen in both species (e.g. Buus and Florentine 1985).
Conclusions
We have demonstrated that our procedures yield consistent and sensitive results in both a pure tone detection and gap threshold discrimination task. Training times in this first study were somewhat longer than in more recent efforts. We find that mice can acquire these tasks within 2-4 weeks, which will enable more sophisticated experiments that are not subject to changes in auditory capability with age.
There are more than a 1000 strains of mutant and knockout mice with known genetic mutations that can affect the peripheral or central nervous system. The present study lays the foundation for assessing the effects of strain differences on auditory sensitivity and temporal acuity thereby allowing genetic variables to be linked to auditory perception. The current methods can be adapted to assessing other measures of auditory function such as frequency or intensity discrimination, sound localization or discrimination of complex sounds.
Acknowledgments
We would like to thank the many UB undergraduate research assistants, Jarrod Cone, and Kelly Beedon for their assistance. Thanks to John Cone for assistance with the artwork creation and to Dr. Amanda Lauer, Dr. Cynthia Prosen, and Dr. Georg Klump for advice. This work was funded by a University at Buffalo Interdisciplinary Research Fund grant to MAX, RJS, and MLD, and NIH Grant DC009483 to MLD. The experiments complied with the “Principles of Animal Care”, publication from the National Institutes of Health, and were approved by the University at Buffalo IACUC.
Abbreviations
- ABR
Auditory brainstem responses
- MOCS
Method of constant stimuli
- NMRI
Naval medical research institute
- SPL
Sound pressure level
- SL
Sensation level
References
- Allen PD, Virag TM, Ison JR. Humans detect gaps in broadband noise according to effective gap duration without additional cues from abrupt envelope changes. J Acoust Soc Am. 2002;112(6):2967–2974. doi: 10.1121/1.1518697. [DOI] [PubMed] [Google Scholar]
- Allen PD, Burkard RF, Ison JR, Walton JP. Impaired gap encoding in aged mouse inferior colliculus at moderate but not high stimulus levels. Hear Res. 2003;186:17–29. doi: 10.1016/s0378-5955(03)00300-9. [DOI] [PubMed] [Google Scholar]
- Allen PD, Schmuck N, Ison JR, Walton JP. Kv1.1 channel subunits are not necessary for high temporal acuity in behavioral and electrophysiological gap detection. Hear Res. 2008;246:52–58. doi: 10.1016/j.heares.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsz K, Benson PK, Walton JP. Gap encoding by inferior collicular neurons is altered by minimal changes in signal envelope. Hear Res. 1998;115:13–26. doi: 10.1016/s0378-5955(97)00173-1. [DOI] [PubMed] [Google Scholar]
- Barsz JR, Ison JR, Snell KB, Walton JP. Behavioral and neural measures of auditory temporal acuity in aging humans and mice. Neurobiol Aging. 2002;23:565–578. doi: 10.1016/s0197-4580(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Birch LM, Warfield D, Ruben RJ, Mikaelian DO. Behavioral measurements of pure tone thresholds in normal CBA-J mice. J Auditory Res. 1968;8:459–468. [Google Scholar]
- Buus S, Florentine M. Gap detection in normal and impaired listeners: The effect of level and frequency. In: Michelsen A, editor. Time resolution in auditory systems. Springer-Verlag; London: 1985. pp. 159–179. [Google Scholar]
- Ehret G. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften. 1974;11:506. doi: 10.1007/BF00622976. [DOI] [PubMed] [Google Scholar]
- Giraudi DM, Salvi RJ, Henderson D, Hamernik RP. Gap detection by the chinchilla. J Acoust Soc Am. 1980;68:802–806. doi: 10.1121/1.384818. [DOI] [PubMed] [Google Scholar]
- Giraudi-Perry DM, Salvi RJ, Henderson D. Gap detection in hearing-impaired chinchillas. J Acoust Soc Am. 1982;72:1387–1393. doi: 10.1121/1.388444. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim SH, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear Res. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hamann I, Gleich O, Klump GM, Kittel MC, Strutz J. Age-dependent changes of gap detection in the Mongolian gerbil (Meriones unguiculatus) J Assoc Res Otolaryngol. 2004;5:49–57. doi: 10.1007/s10162-003-3041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Behavioral assessment of hearing in mice. In: Willott JF, editor. Handbook of mouse auditory research. CRC Press; Boca Raton, FL: 2001. pp. 19–29. [Google Scholar]
- Heffner HE, Masterson B. Hearing in glires: Domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. [Google Scholar]
- Heffner RS, Heffner HE. Sound localization and use of binaural cues by the gerbil (Meriones unguiculatus) Behav Neurosci. 1988;102:422–428. doi: 10.1037//0735-7044.102.3.422. [DOI] [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: A study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J Acoust Soc Am. 1998;104:1696–1704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD. Low-frequency tone pips elicit exaggerated startle reflexes in C57BL/6J mice with hearing loss. J Assoc Res Otolaryngology. 2003;4:495–504. doi: 10.1007/s10162-002-3046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Rivoli PJ, Moore JA. The behavioral response of mice to gaps in noise depends on its spectral components in the noise and its bandwidth. J Acoust Soc Am. 2005;117:3944–3951. doi: 10.1121/1.1904387. [DOI] [PubMed] [Google Scholar]
- Ison JR, Castro J, Allen PD, Virag TM, Walton JP. The relative detectability for mice of gaps having different ramp durations at their onset and offset boundaries. J Acoust Soc Am. 2002;112:740–747. doi: 10.1121/1.1490352. [DOI] [PubMed] [Google Scholar]
- Jiminez AM, Stagner BB, Martin GK, Lonsbury-Martin BL. Age-related loss of distortion product otoacoustic emissions in four mouse strains. Hear Res. 1999;138:91–105. doi: 10.1016/s0378-5955(99)00154-9. [DOI] [PubMed] [Google Scholar]
- Klink KB, Bendig G, Klump GM. Operant methods for mouse psychoacoustics. Behav Res Methods. 2006;38:1–7. doi: 10.3758/bf03192744. [DOI] [PubMed] [Google Scholar]
- Klump GM, Maier EH. Gap detection in the starling (Sturnus vulgaris) J Comp Physiol A. 1989;164:531–538. [Google Scholar]
- Lauer AM, May BJ, Hao ZJ, Watson J. Analysis of environmental sound levels in modern rodent housing rooms. Lab Anim. 2009;38:1–8. doi: 10.1038/laban0509-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JK, Klump GM. Resolution in azimuth sound localization in the Mongolian gerbil (Meriones unguiculatus) J Acoust Soc Am. 2006;119:1029–1036. doi: 10.1121/1.2159429. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Bath KG, Vetter DE, May BJ. Behavioral assessments of auditory sensitivity in transgenic mice. J Neurosci Methods. 2000;97:59–67. doi: 10.1016/s0165-0270(00)00169-2. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, May BJ. The functional age of hearing loss in a mouse model of presbycusis. I. Behavioral assessments. Hear Res. 2003;183:44–56. doi: 10.1016/s0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Arehole S. Gap detection in chinchillas with temporary high-frequency hearing loss. J Acoust Soc Am. 1985;77:1173–1177. doi: 10.1121/1.392181. [DOI] [PubMed] [Google Scholar]
- Steckler T. Using signal detection methods for analysis of operant performance in mice. Behav Brain Res. 2001;125:237–248. doi: 10.1016/s0166-4328(01)00305-9. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Mazelova J, Druga R. Gap detection threshold in the rat before and after auditory cortex ablation. Hear Res. 2002;172:151–159. doi: 10.1016/s0378-5955(02)00578-6. [DOI] [PubMed] [Google Scholar]
- Wagner E, Klump GM, Hamann I. Gap detection in Mongolian gerbils (Meriones unguiculatus) Hear Res. 2003;176:11–16. doi: 10.1016/s0378-5955(02)00643-3. [DOI] [PubMed] [Google Scholar]
- Walton JP, Barsz K, Wilson WW. Sensorineural hearing loss and neural correlates of temporal acuity in the inferior colliculus of the C57BL/6 mouse. J Assoc Res Otolaryngology. 2008;9:90–101. doi: 10.1007/s10162-007-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA/CaJ mouse. J Comp Physiol [A] 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]