Abstract
OBJECTIVE: To examine clinical, etiologic, and histologic features of Stevens-Johnson syndrome and to identify possible correlates of clinical disease severity related to etiologic and histopathologic findings.
PATIENTS AND METHODS: This is a retrospective review of patients seen at Mayo Clinic between January 1, 2000, and December 31, 2007.
RESULTS: Of 27 patients (mean age, 28.1 years), 22 (81%) had involvement of 2 or more mucous membranes, and 19 (70%) had ocular involvement. Medications, most commonly antibiotics and anticonvulsants, were causative in 20 patients. Mycoplasma pneumoniae infection caused 6 of the 27 cases. Corticosteroids were the most common systemic therapy. No patients with mycoplasma-induced Stevens-Johnson syndrome had internal organ involvement or required treatment in the intensive care unit, in contrast to 4 patients each in the drug-induced group. Three patients had chronic ocular sequelae, and 1 died of complications. Biopsy specimens from 13 patients (48%) showed epidermal necrosis (8 patients), basal vacuolar change (10 patients), and subepidermal bullae (10 patients). Biopsy specimens from 11 patients displayed moderate or dense dermal infiltrate. Histologic features in drug-induced cases included individual necrotic keratinocytes, dense dermal infiltrate, red blood cell extravasation, pigment incontinence, parakeratosis, and substantial eosinophils or neutrophils.
CONCLUSION: Our clinical and etiologic findings corroborate those in previous reports. M pneumoniae—induced Stevens-Johnson syndrome manifested less severely than its drug-induced counterpart. The limited number of biopsies precludes unequivocal demonstration of histopathologic differences between drug-induced and M pneumoniae—induced Stevens-Johnson syndrome.
Mycoplasma pneumoniae—induced Stevens-Johnson syndrome manifested less severely than its drug-induced counterpart; the limited number of biopsies precludes unequivocal demonstration of histopathologic differences between drug-induced and M pneumoniae—induced Stevens Johnson syndrome.
EM = erythema multiforme; EuroSCAR = European Study of Severe Cutaneous Adverse Reactions; SJS = Stevens-Johnson syndrome; TEN = toxic epidermal necrolysis
Stevens-Johnson syndrome (SJS) is a rare, severe, immune-mediated cutaneous reaction usually secondary to an idiosyncratic reaction to medication, although infection with Mycoplasma pneumoniae is also a well-documented cause.1 In 1922, Stevens and Johnson2 described a strikingly distinct disease in 2 children as an “extraordinary, generalized eruption with continued fever, inflamed buccal mucosa and severe purulent conjunctivitis.” In ensuing years, controversy existed as to whether SJS indeed was a separate entity or merely a severe form of erythema multiforme (EM).3 More recent evidence suggests that EM with mucous membrane involvement and SJS are 2 different diseases with distinct causes.4 Although various classification systems exist, the most widely accepted consensus definition was proposed by Bastuji-Garin et al5 in 1993: SJS consists of epidermal “detachment below 10% of the body surface area plus widespread macules or flat atypical targets.” Other studies have described histopathologic correlates in patients with EM, SJS, and toxic epidermal necrolysis (TEN).6-9 The aim of the current study was to retrospectively examine clinical, etiologic, and histopathologic data from patients with SJS to identify possible correlates of clinical disease severity related to specific etiologic and histopathologic findings.
PATIENTS AND METHODS
Patients with a new diagnosis of SJS who were treated at Mayo Clinic, Rochester, MN, between January 1, 2000, and December 31, 2007, were identified from the institutional medical index and text retrieval system. Patients who had refused research authorization were excluded from analysis. This study was approved by the Mayo Clinic Institutional Review Board.
Medical records were examined to abstract the following information: demographics, clinical features including extent of mucous membrane and skin involvement, identifiable etiologic classes of SJS, specific causative drugs, laboratory tests, treatment, follow-up data including chronic sequelae secondary to SJS, and histopathologic data.
All study patients met the consensus definition for SJS proposed by Bastuji-Garin et al5 in 1993. The extent and severity of mucous membrane erosions were not used to classify a disease as SJS.5 Patients identified with a clinical diagnosis of SJS who did not meet the aforementioned consensus criteria were excluded from analysis.
Cases were designated secondary to M pneumoniae in patients who had clinical features (eg, cough, fever, or constitutional symptoms) compatible with infection and positive IgM and IgG serologic findings of M pneumoniae. Cases were considered to be medication related when patients had negative laboratory test results for infectious etiologies and were known to have been exposed to the offending drug or drugs within the preceding few weeks.
Biopsy specimen slides were reviewed by a single dermatopathologist (M.J.C.) and examined for the presence of epidermal necrosis, basal vacuolar change, subepidermal bullae, intraepidermal bullae, epidermal infiltrate (qualitatively graded as poor, mild, moderate, or dense), epidermal infiltrate cell type, dermal infiltrate (qualitatively graded as poor, mild, moderate, or dense), dermal infiltrate cell type, and dermal infiltrate pattern (described as superficial perivascular, superficial perivascular and interstitial, periadnexal, or all 3). Cell types present in the dermal infiltrate other than lymphocytes (ie, eosinophils, neutrophils, and plasma cells) were qualitatively measured as few, moderate, or many. Other less common findings (eg, red blood cell extravasation, pigment incontinence, parakeratosis, regenerating epidermis, and hair follicle necrosis) were also recorded.
RESULTS
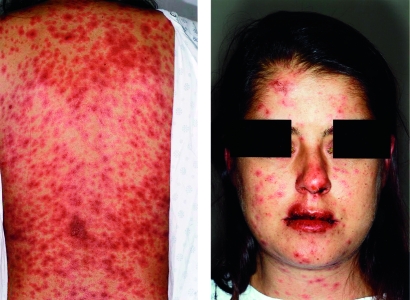
All 27 patients met published clinical criteria for SJS5 and had widespread macules or flat atypical target lesions and limited areas of epidermal detachment on less than 10% of the body surface area (Figure 1). All patients also had oral mucous membrane involvement, with 22 (81%) experiencing involvement of 2 or more mucous membranes (Figure 1). Nineteen patients (70%) manifested ocular involvement, with the most common signs and symptoms being conjunctivitis, exudate, photophobia, “grittiness,” and cicatrizing changes. Other affected mucous membranes were genitourinary (9 patients), nasopharyngeal (6 patients), rectal (2 patients), and respiratory (1 patient). Internal organ involvement was uncommon, with 4 patients experiencing cardiovascular, respiratory, or hepatic abnormalities. No patient experienced sepsis during the clinical course, although 1 patient died of complications related to SJS (ie, dyspnea, hypotension, and anemia). Clinical features of the patients are summarized in Table 1.
FIGURE 1.
29-year-old woman (patient 13 in Table 4) with Stevens-Johnson syndrome secondary to Mycoplasma pneumoniae infection. Left, Widespread macules ranging from erythematous to purpuric and flat atypical target lesions on the back. Right, Prominent hemorrhagic crust on the lips, eyes, and nasal mucosa.
TABLE 1.
Clinical Features, Laboratory Testing, and Treatment of Patients With Stevens-Johnson Syndrome
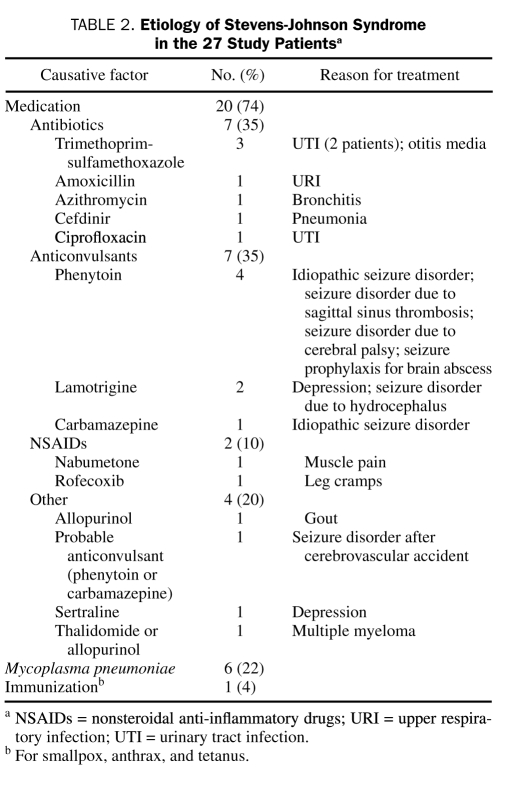
Drugs were the causative factors in 20 (74%) of the 27 patients (Table 2) (mean age, 31.4 years; range, 1-72 years). The mean interval between drug administration and SJS was 15.3 days (range, 2-42 days). Infection with M pneumoniae (confirmed with positive IgM and IgG serologic tests) led to 6 cases of SJS (mean age, 19 years; range, 10-36 years). Stevens-Johnson syndrome developed in 1 patient 20 days after immunization for smallpox, anthrax, and tetanus.
TABLE 2.
Etiology of Stevens-Johnson Syndrome in the 27 Study Patientsa
Serologic tests for M pneumoniae were performed in 11 (41%) of the 27 patients, 6 of whom had positive IgM and IgG antibodies demonstrating recent infection (Table 1). Twenty patients (74%) were tested for herpes simplex virus infection (swab cultures with or without serologic studies were obtained for 19 patients; serologic findings without a culture swab were obtained in 1 patient), and results were negative in all these patients. Various laboratory abnormalities were noted in several patients (Table 1), the most common of which were chest radiographic findings (eg, infiltrate, edema, bronchial inflammation), elevated liver function test results, and increased markers of acute inflammation (eg, erythrocyte sedimentation rate and C-reactive protein).
Of 26 patients (96%) hospitalized for treatment, 4 received treatment in the intensive care unit; it was unclear from our retrospective review of the records why 1 patient was not hospitalized (Table 1). For patients in whom data on the total length of the hospital stay were available (23/26), the mean duration of hospital stay was 8.0 days (range, 1-50 days); for the other 3 patients, our retrospective review of medical records showed that they had been hospitalized before evaluation at Mayo Clinic, resulting in a lack of complete information about the exact duration of their total hospital stay. One patient experienced complications, including deep venous thrombosis, mediastinitis, and methicillin-resistant Staphylococcus aureus tracheitis, that necessitated a prolonged hospitalization (50 days); when this outlier was excluded, the mean hospital stay for the other 22 patients was 6.1 days. Systemic therapies used in the cohort of 27 patients included corticosteroids (15 patients) and intravenous immunoglobulin (6 patients). One patient with drug-induced SJS received mycophenolate mofetil and dapsone for chronic cicatrizing ocular inflammation.
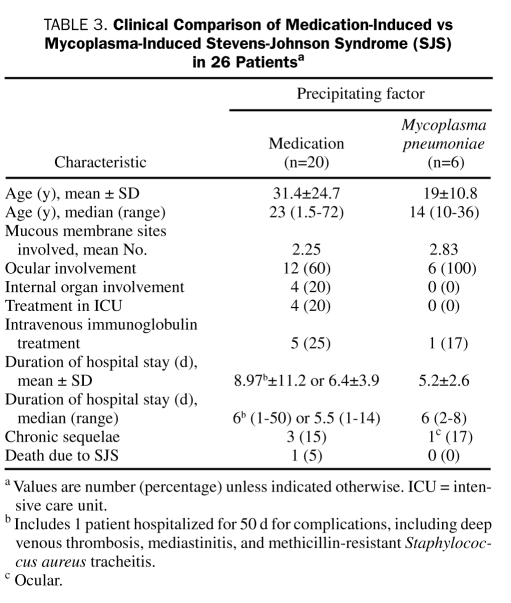
The clinical characteristics of the 20 patients with drug-induced SJS compared with those of the 6 patients with mycoplasma-induced SJS are shown in Table 3.
TABLE 3.
Clinical Comparison of Medication-Induced vs Mycoplasma-Induced Stevens-Johnson Syndrome (SJS) in 26 Patientsa
The mean follow-up after diagnosis was 16.6 months (range, 0-80 months). One patient with preexisting atrial fibrillation and chronic obstructive pulmonary disease died during hospitalization of complications related to SJS (ie, dyspnea, hypotension, and anemia), and 5 patients were lost to follow-up after hospital discharge. Three patients had chronic ocular complications; the vision was affected in 2 of these 3 patients. One of the 3 patients with clinically important ocular involvement also experienced multiple oral mucoceles and painful defecation as a result of oral and rectal involvement, respectively. One patient with urethral involvement from SJS reported gradually improving urinary retention. At follow-up, 2 patients had died of causes unrelated to SJS (1 due to multiple myeloma and 1 due to cerebrovascular disease).
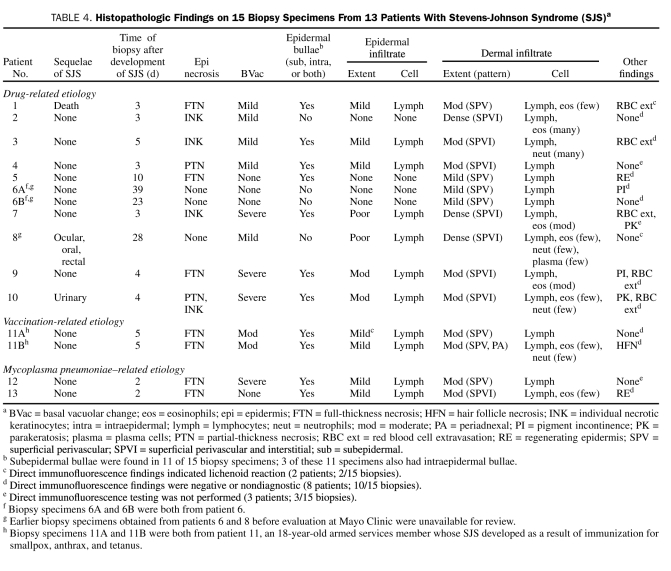
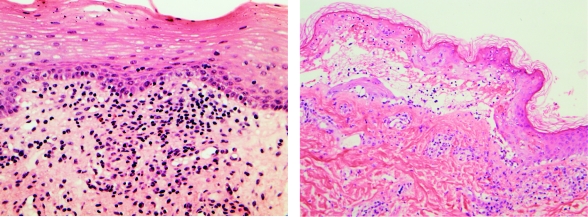
Biopsy specimens were available to review for 13 (48%) of the 27 patients; 2 patients each underwent 2 biopsies, and thus 15 biopsies were reviewed. Of the 15 biopsy specimens, 11 were from patients with drug-induced SJS; the other 4 were from 2 mycoplasma-induced cases (1 biopsy each) and 1 immunization-induced case (2 biopsies). Detailed information about the specific histopathologic findings of each biopsy specimen is provided in Table 4. Histologic features of drug-induced and mycoplasma-induced cases of SJS are illustrated in Figure 2.
TABLE 4.
Histopathologic Findings on 15 Biopsy Specimens From 13 Patients With Stevens-Johnson Syndrome (SJS)a
FIGURE 2.
Histopathology of Stevens-Johnson syndrome. Left, Focal basal cell vacuolar change with dense superficial dermal lymphocytic inflammation and occasional eosinophils in patient with Stevens-Johnson syndrome secondary to lamotrigine therapy (hematoxylin-eosin, original magnification ×40). Right, Full-thickness necrosis, basal vacuolar change, and subepidermal bullae in patient with Stevens-Johnson syndrome secondary to Mycoplasma pneumoniae infection (hematoxylin-eosin, original magnification ×20).
Epidermal necrosis was present in 1 or more biopsy specimens for 8 (62%) of 13 patients; full-thickness necrosis was present in 6 patients (46%). Basal vacuolar change was observed in 10 (77%) of 13 patients, with 5 (38%) of the 13 patients demonstrating moderate to severe change. Biopsy specimens from 10 (77%) of the 13 patients contained subepidermal bullae, and 11 patients (85%) displayed either a moderate or dense dermal infiltrate (8, moderate; 3, dense), with lymphocytes representing the predominant cell type. Eosinophils were observed in 1 or more biopsy specimens from 8 patients, and neutrophils were present in 4 patients. Other less common findings included red blood cell extravasation (5/13 patients), pigment incontinence, regenerating epidermis, parakeratosis, and necrosis of the hair follicle.
Histologic features found only in drug-induced cases included individual necrotic keratinocytes, dense dermal infiltrate, red blood cell extravasation, pigment incontinence, and parakeratosis. Biopsy specimens that demonstrated a substantial number of eosinophils or neutrophils were also found to be drug related. Three biopsy specimens were reviewed from patients with SJS sequelae (eg, ocular, urinary, or death), and all 3 showed moderate or dense dermal inflammation; the biopsy specimen from the patient with ocular sequelae (ie, cicatrizing conjunctivitis) revealed dense dermal inflammation.
DISCUSSION
Much confusion exists regarding the nosology of SJS, which poses particular challenges for researchers conducting studies of SJS and reviewing previously reported cases. The distinction between SJS and EM major is controversial for some researchers, and they suggest instead that the 2 entities are synonymous; others contend that more extensive mucous membrane involvement in SJS differentiates it from EM major. To most accurately review the 27 cases of SJS in the current study and compare them with those reported in the medical literature, we used an accepted consensus definition of SJS that relies on the pattern and distribution of skin lesions, rather than on the extent and severity of mucous membrane involvement.5,10 Although the degree of mucous membrane involvement is not an absolute criterion for SJS according to the consensus definition, mucosal erosions were reported to be present in more than 90% of patients by this classification scheme5; another study of 33 patients with SJS demonstrated mucous membrane involvement in all the patients, with 23 having 2 or more affected membranes.4 Our findings are in keeping with these data because all 27 of our study patients had mucous membrane involvement, and 22 (81%) had involvement of 2 or more mucous membranes.
The mean age of our patients was 28.1 years, with nearly half younger than 18 years. This finding is concordant with the emphasis in the medical literature on SJS as a disease that occurs predominantly in younger patients.1,11
In addition to delineating SJS as a distinct entity from EM major by the clinical pattern of cutaneous lesions, several authors have further distinguished these 2 entities by their respective etiologies.4,12 They found that SJS is usually related to drugs, whereas EM major is most commonly secondary to herpes simplex virus infection.4,12 Our data support these findings, with 20 cases secondary to the use of medications, whereas no patient had a documented herpes simplex virus infection.
In our patients with drug-induced SJS, the mean interval between drug administration and onset of cutaneous findings was 15.3 days. Previous reports in the medical literature describe similar intervals, with the greatest risk of development of SJS occurring in the first 2 months of drug treatment1 and an interval of 4 to 28 days being the most suggestive of drug causality in SJS.13
We found that antibiotics and anticonvulsants were the most common medications causing SJS, with trimethoprim-sulfamethoxazole and phenytoin the most common culprits in each drug class, respectively. Previous studies enumerating medications specifically associated with SJS have identified antibiotics, anticonvulsants, and nonsteroidal anti-inflammatory drugs.10,11,13-16 A recent multinational case-control study in Europe called EuroSCAR (European Study of Severe Cutaneous Adverse Reactions) showed a high risk of SJS with the following medications: trimethoprim-sulfamethoxazole and other anti-infective sulfonamides, lamotrigine, carbamazepine, phenytoin, phenobarbital, allopurinol, nevirapine, and oxicam non-steroidal anti-inflammatory drugs.13 Among recently marketed drugs, nevirapine and lamotrigine were strongly associated with SJS, with sertraline showing a lower but still significant risk as well.13 Our study reflects these findings, with 2 cases secondary to lamotrigine therapy and 1 case secondary to sertraline therapy. Moreover, recently reported data from the EuroSCAR study indicated that allopurinol was the most common cause of SJS and TEN in Europe and Israel17; our study found 1 case of allopurinol-induced SJS, with another case possibly due to allopurinol or thalidomide (of note, thalidomide has been reported to induce TEN18).
M pneumoniae is also a well-known cause of SJS.19,20 It usually affects children and young adults and has been reported as the most common infectious agent associated with SJS.19 Nearly all patients manifest oral involvement, and approximately two-thirds of patients have ocular involvement.19,20 Previous studies suggest that mycoplasma-induced SJS is associated with less frequent and less severe complications than those resulting from other causes.20 In our study, 6 cases were due to M pneumoniae as confirmed by positive serologic findings; previous studies have documented 10%15 to 29%21 of SJS cases as secondary to M pneumoniae infection. In our series, the mean age of patients with mycoplasma-induced SJS (19 years) was younger than that of patients with drug-induced SJS (31.4 years). No patient with mycoplasma-induced SJS manifested internal organ involvement or required treatment in the intensive care unit, and the mean hospital stay was shorter than that observed in patients with drug-induced SJS, which augments previous assertions that mycoplasma-induced SJS may manifest less severely than its drug-induced counterpart.20
Numerous reports have described patients with “atypical SJS” who were infected with M pneumoniae and presented with severe mucositis without skin lesions.22 Given that the pattern and distribution of cutaneous lesions, rather than mucous membrane involvement, are the hallmarks of the revised classification of SJS,5 some authors argue that these cases do not represent SJS and are better termed M pneumoniae—associated mucositis.23 All 6 of our patients with mycoplasma-induced SJS had both skin and mucous membrane involvement. The strong association between M pneumoniae and mucositis is further evident in our study when considering that the mean number of mucous membranes involved and the percentage of patients presenting with ocular involvement were higher in the mycoplasma-induced group than in the drug-induced SJS group.
Patients with M pneumoniae—associated SJS frequently receive antibiotics during the early stages of their respiratory illness before the onset of a mucocutaneous eruption, which at times can make it difficult to determine the precise etiology of SJS. Of the 6 cases in our study attributed to M pneumoniae, 4 were treated with antibiotics before the development of mucocutaneous lesions (median, 2 days; range, 0.5-4 days).
Interestingly, vaccinations have been associated with the development of SJS.24,25 In our study, SJS developed in an 18-year-old man in the armed forces 20 days after he underwent immunization for smallpox, anthrax, and tetanus.
The role of corticosteroids in the treatment of SJS is controversial.1 Some investigators say that corticosteroids may promote infectious complications and lead to a poorer prognosis.1 However, recently published data from the EuroSCAR study do not reflect an increased mortality in patients treated with corticosteroids but rather a possible beneficial effect that deserves further exploration.26 Of note, sepsis developed in none of the 15 of our 27 patients who received treatment with corticosteroids for SJS, although 1 patient treated with corticosteroids and intravenous immunoglobulin had a prolonged hospital stay and experienced multiple complications, including methicillin-resistant S aureus tracheitis. The patient who died of complications related to SJS also received corticosteroids (without concurrent intravenous immunoglobulin). Although the small number of patients treated with systemic agents precluded conclusions about survival benefits from therapy, patients overall generally did well with supportive care with or without systemic treatment. General diagnostic and treatment recommendations for patients with SJS are summarized in Table 5.
TABLE 5.
Recommendations for Diagnosis and Treatment of Stevens-Johnson Syndrome
Three patients in our study had chronic ocular complications due to SJS. However, SJS-related ocular disease is not only the sequelae of the acute disease process but also can occur with a variable course several years after onset of SJS and is not always the direct result of conjunctival scarring.27
In addition to developing clinical classification schemes based on the patterns and distribution of skin lesions, some authors have sought to differentiate EM from SJS and TEN by their histopathologic features. Rzany et al6 examined biopsy specimens from patients with EM major, SJS, and TEN and found no differences in histologic features (eg, eosinophils) by etiology (ie, drug vs infection). In our study, histologic features such as epidermal necrosis, basal vacuolar change, subepidermal bullae, and dermal infiltrate were common, regardless of the underlying cause. In contrast to the findings by Rzany et al,6 we identified several histologic features that were observed only in drug-induced cases; these included individual necrotic keratinocytes, dense dermal infiltrate, red blood cell extravasation, pigment incontinence, and parakeratosis. Moreover, biopsy specimens that demonstrated a substantial number of eosinophils or neutrophils were also found to be drug related. The importance of these findings is unclear, given that only 4 of the 15 biopsy specimens indicated that SJS was due to causes other than drugs (2 from M pneumoniae and 2 from immunization-induced SJS). In addition, differences in histologic findings could be related to the evolution of individual lesions because biopsies were not strictly matched between groups due to the timing of the biopsy in relation to the onset of mucocutaneous findings.
In a more recent study that focused on identifying histologic criteria with prognostic importance in patients with TEN, a dense dermal mononuclear infiltrate was found to portend a worse prognosis.7 Dense dermal inflammation was observed in the biopsy specimen from the patient who experienced ocular sequelae (ie, cicatrizing conjunctivitis) that required therapy with mycophenolate mofetil and dapsone, but only moderate dermal inflammation was found in the patient who died of SJS-related complications. The limited histologic data in our study prevent any firm conclusions about clinical and etiologic correlates based on histologic patterns.
We recognize and acknowledge the shortcomings of the current study: it is retrospective, the cohort is small, and biopsy specimens were not obtained from all patients. Given the small number of biopsy specimens obtained from patients with SJS due to causes other than drugs, it is unclear whether specific etiologic correlates (ie, drug vs M pneumoniae) can be garnered from our histologic data. Because we used a recently devised (1993) consensus definition of SJS, limitations exist when comparing our data to those of earlier studies that used alternative classification schemes.
CONCLUSION
Our findings corroborate previously reported clinical and etiologic associations with SJS. M pneumoniae—induced SJS clinically manifested less severely than its drug-induced counterpart. Confirmation of possible histopathologic differences between drug-induced and M pneumoniae—induced SJS requires further studies with larger patient cohorts.
Supplementary Material
REFERENCES
- 1.Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181-200 [DOI] [PubMed] [Google Scholar]
- 2.Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmia: report of two cases in children. Am J Dis Child. 1922;24:526-533 [Google Scholar]
- 3.Thomas BA. The so-called Stevens-Johnson syndrome. Br Med J. 1950;1(4667):1393-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assier H, Bastuji-Garin S, Revuz J, Roujeau JC. Erythema multiforme with mucous membrane involvement and Stevens-Johnson syndrome are clinically different disorders with distinct causes. Arch Dermatol. 1995;131(5):539-543 [PubMed] [Google Scholar]
- 5.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92-96 [PubMed] [Google Scholar]
- 6.Rzany B, Hering O, Mockenhaupt M, et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 1996;135(1):6-11 [PubMed] [Google Scholar]
- 7.Quinn AM, Brown K, Bonish BK, et al. Uncovering histologic criteria with prognostic significance in toxic epidermal necrolysis. Arch Dermatol. 2005;141(6):683-687 [DOI] [PubMed] [Google Scholar]
- 8.Paquet P, Pierard GE. Erythema multiforme and toxic epidermal necrolysis: a comparative study. Am J Dermatopathol. 1997;19(2):127-132 [DOI] [PubMed] [Google Scholar]
- 9.Cote B, Wechsler J, Bastuji-Garin S, Assier H, Revuz J, Roujeau JC. Clinicopathologic correlation in erythema multiforme and Stevens-Johnson syndrome. Arch Dermatol. 1995;131(11):1268-1272 [DOI] [PubMed] [Google Scholar]
- 10.Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102(6):28S-30S [DOI] [PubMed] [Google Scholar]
- 11.Schopf E, Stuhmer A, Rzany B, Victor N, Zentgraf R, Kapp JF. Toxic epidermal necrolysis and Stevens-Johnson syndrome: an epidemiologic study from West Germany. Arch Dermatol. 1991;127(6):839-842 [DOI] [PubMed] [Google Scholar]
- 12.Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schroder W, Roujeau JC, SCAR Study Group Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002;138(8):1019-1024 [DOI] [PubMed] [Google Scholar]
- 13.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs: the EuroSCAR-study. J Invest Dermatol. 2008;128(1):35-44 Epub 2007 Sep 6 [DOI] [PubMed] [Google Scholar]
- 14.Sharma VK, Sethuraman G, Minz A. Stevens Johnson syndrome, toxic epidermal necrolysis and SJS-TEN overlap: a retrospective study of causative drugs and clinical outcome. Indian J Dermatol Venereol Leprol. 2008;74(3):238-240 [DOI] [PubMed] [Google Scholar]
- 15.Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to 2006. Allergol Int. 2007;56(4):419-425 Epub 2007 Sep 1 [DOI] [PubMed] [Google Scholar]
- 16.Kamaliah MD, Zainal D, Mokhtar N, Nazmi N. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in northeastern Malaysia. Int J Dermatol. 1998;37(7):520-523 [DOI] [PubMed] [Google Scholar]
- 17.Halevy S, Ghislain PD, Mockenhaupt M, et al. EuroSCAR Study Group Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol. 2008;58(1):25-32 Epub 2007 Oct 24 [DOI] [PubMed] [Google Scholar]
- 18.Horowitz SB, Stirling AL. Thalidomide-induced toxic epidermal necrolysis. Pharmacotherapy 1999;19(10):1177-1180 [DOI] [PubMed] [Google Scholar]
- 19.Tay YK, Huff JC, Weston WL. Mycoplasma pneumoniae infection is associated with Stevens-Johnson syndrome, not erythema multiforme (von Hebra). J Am Acad Dermatol. 1996;35(5, pt 1):757-760 [DOI] [PubMed] [Google Scholar]
- 20.Levy M, Shear NH. Mycoplasma pneumoniae infections and Stevens-Johnson syndrome: report of eight cases and review of the literature. Clin Pediatr (Phila) 1991;30(1):42-49 [DOI] [PubMed] [Google Scholar]
- 21.Leaute-Labreze C, Lamireau T, Chawki D, Maleville J, Taieb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child. 2000;83(4):347-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravin KA, Rappaport LD, Zuckerbraun NS, Wadowsky RM, Wald ER, Michaels MM. Mycoplasma pneumoniae and atypical Stevens-Johnson syndrome: a case series. Pediatrics 2007;119(4):e1002-1005 Epub 2007 Mar 12 [DOI] [PubMed] [Google Scholar]
- 23.Schalock PC, Dinulos JG. Mycoplasma pneumoniae-induced Stevens-Johnson syndrome without skin lesions: fact or fiction? J Am Acad Dermatol. 2005;52(2):312-315 [DOI] [PubMed] [Google Scholar]
- 24.Ball R, Ball LK, Wise RP, Braun MM, Beeler JA, Salive ME. Stevens-Johnson syndrome and toxic epidermal necrolysis after vaccination: reports to the vaccine adverse event reporting system. Pediatr Infect Dis J. 2001;20(2):219-223 [DOI] [PubMed] [Google Scholar]
- 25.Dobrosavljevic D, Milinkovic MV, Nikolic MM. Toxic epidermal necrolysis following morbilli-parotitis-rubella vaccination. J Eur Acad Dermatol Venereol. 1999;13(1):59-61 [PubMed] [Google Scholar]
- 26.Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33-40 Epub 2007 Oct 4 [DOI] [PubMed] [Google Scholar]
- 27.De Rojas MV, Dart JK, Saw VP. The natural history of Stevens Johnson syndrome: patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br J Ophthalmol. 2007;91(8):1048-1053 Epub 2007 Feb 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.