Abstract
OBJECTIVE: To determine incidence rate, seasonal variation, and short- and long-term outcomes of Klebsiella species bloodstream infection (BSI) in a population-based setting.
PATIENTS AND METHODS: We identified 127 unique patients in Olmsted County, Minnesota, from January 1, 1998, to December 31, 2007, who had Klebsiella spp BSI. Multivariable Poisson regression was used to examine temporal change and seasonal variation in incidence rate, and Cox proportional hazards regression was used to determine predictors of mortality.
RESULTS: The age-adjusted incidence rate of Klebsiella spp BSI per 100,000 person-years was 15.4 (95% confidence interval [CI], 11.6-19.2) in men and 9.4 (95% CI, 7.0-11.8) in women. There was no linear increase in incidence rate of Klebsiella spp BSI during the study period (P=.55). The incidence rate of Klebsiella spp BSI increased at quadratic rate with age (P=.005). No significant difference was noted in incidence rate of Klebsiella spp BSI during the warmest 4 months compared to the rest of the year (incidence rate ratio, 0.97; 95% CI, 0.66-1.38; P=.95). The overall 28-day and 1-year all-cause mortality rates of Klebsiella spp BSI were 14% (95% CI, 9%-22%) and 35% (95% CI, 27%-44%), respectively. Respiratory source of BSI was associated with a higher 28-day mortality (hazard ratio, 4.90; 95% CI, 1.73-13.84; P=.003).
CONCLUSION: The incidence rate of Klebsiella spp BSI increased with age. There was no temporal change or seasonal variation in incidence rate of Klebsiella spp BSI during the past decade. The 28-day all-cause mortality rate of Klebsiella spp BSI was relatively low; however, a respiratory source of BSI was associated with a poorer outcome.
The incidence rate of Klebsiella species bloodstream infection increased with age; the 28-day all-cause mortality rate of Klebsiella spp bloodstream infection was relatively low; however, a respiratory source of bloodstream infection was associated with a poorer outcome.
BSI = bloodstream infection; CI = confidence interval; HR = hazard ratio; REP = Rochester Epidemiology Project
Klebsiella species are the second most common cause of gram-negative bloodstream infection (BSI).1-5 Population-based studies that specifically address the epidemiology and outcome of Klebsiella spp BSI are lacking because most previous studies have been derived from referral tertiary care centers.6-8 Therefore, we performed a population-based study to determine the incidence rate of Klebsiella spp BSI and to investigate for temporal changes and seasonal variation in the incidence rate of Klebsiella spp BSI. We estimated the 28-day and 1-year mortality rates and identified predictors of mortality of Klebsiella spp BSI among inhabitants of Olmsted County, Minnesota, from 1998 through 2007.
PATIENTS AND METHODS
Olmsted County is located in southeastern Minnesota and has a population of 124,277 according to the 2000 US census.9 With the exception of a lower prevalence of injection drug use, a higher prevalence of middle-class residents, and a higher proportion of residents being employed in the health care industry, the population characteristics of Olmsted County residents are similar to those of US non-Hispanic white people.10,11 The Rochester Epidemiology Project (REP) is a unique medical records—linkage system that encompasses care delivered to residents of Olmsted County. The only 2 microbiology laboratories in Olmsted County are located at Mayo Clinic and Olmsted Medical Center. These 2 medical centers are geographically isolated from other urban centers, as previously described11-13; therefore, local residents are able to obtain health care within the community rather than seeking it at a distant geographic location.
Case Ascertainment
We used complete enumeration of Olmsted County from January 1, 1998, to December 31, 2007. Using the microbiology databases at Mayo Clinic and Olmsted Medical Center, the only 2 medical centers in Olmsted County, we identified 127 unique patients with first episodes of mono microbial Klebsiella spp BSI. The primary investigator (M.N.A.-H.) reviewed medical records to confirm the diagnosis, determine patient residency status, and obtain baseline clinical features and outcome.
Blood cultures were identified using standard microbiology techniques according to the Clinical and Laboratory Standards Institute. Both laboratories are certified by the College of American Pathologists. The study was approved by the institutional review boards of both institutions. The detailed case ascertainment and blood culture methods used have been published previously.3,12
Case Definition
Monomicrobial BSI was defined as growth of only 1 organism in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium species, and Propionibacterium species. Cases were classified according to the site of acquisition: nosocomial, health care—associated, and community-acquired.14 The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria.15
Statistical Analyses
The incidence rate, expressed as the number of new cases of Klebsiella spp BSI per 100,000 person-years, was calculated with the assumption that the entire population of Olmsted County was at risk of BSI. The 2000 Olmsted County census figures were used to compute the person-years denominator specific for age, sex, and calendar year, and the population growth rate after 2000 was projected at 1.9% per year. The 10-year study period was divided into five 2-year intervals (1998-1999, 2000-2001, 2002-2003, 2004-2005, and 2006-2007), and age was divided into 5 groups (0-18, 19-39, 40-59, 60-79, and ≥80 years). The incidence rate was directly adjusted to the 2000 US white population.9 A 95% confidence interval (CI) for each incidence rate was estimated using a Poisson distribution.
To evaluate the association between seasonal variation and incidence rate of Klebsiella spp BSI, the incidence rate for the 4 warmest months (June through September) and the incidence rate for the 8 remaining months were each calculated; the person-years denominator was multiplied by one-third and two-thirds, respectively. The incidence rate ratio is the ratio of the incidence rate for the 4 warmest months relative to the incidence rate for the remaining 8 months. A 95% CI for the incidence rate ratio was constructed using bootstrap resampling.
To create an additional measure of seasonal variation, the average monthly temperatures for Rochester, MN, were obtained from historical city records.16 Incidence rates were calculated for each of the 12 months with the assumption that the population was fixed within a given year. To test for an association between average monthly temperatures and incidence of Klebsiella spp BSI, a multivariable Poisson regression model that adjusted for sex, age, and calendar year was used. The functional form of the continuous variables age and calendar year was assessed. Incidence rate was plotted by age and by calendar year to evaluate linearity; a quadratic form of the continuous variable was formally tested in the multivariable Poisson model if the plot suggested a nonlinear relationship.
The Kaplan-Meier method was used to estimate the 28-day and 1-year all-cause mortality rates. Patients were followed up from the date of first episode of Klebsiella spp BSI until death or last health care encounter; long-term follow-up was available through the REP. Patients lost to follow-up were censored on the date of their last health care encounter. Cox proportional hazards regression was used to determine factors associated with 28-day and 1-year all-cause mortality. The following variables were each evaluated in univariate models: age (as a continuous variable), sex, infection site of acquisition, primary source of infection, species, and year of diagnosis (continuous variable). To control for the potential confounding of cumulative lifetime comorbidity up until infection, each factor was also included in a model adjusting for Charlson comorbidity index.17 Hazard ratios (HRs) along with 95% CIs are presented to demonstrate the strength of association between each factor and all-cause mortality.
All analyses were performed using the SAS statistical software package (version 8.2, SAS Institute, Cary, NC). The level of significance for statistical testing was defined as P<.05 (2-sided).
RESULTS
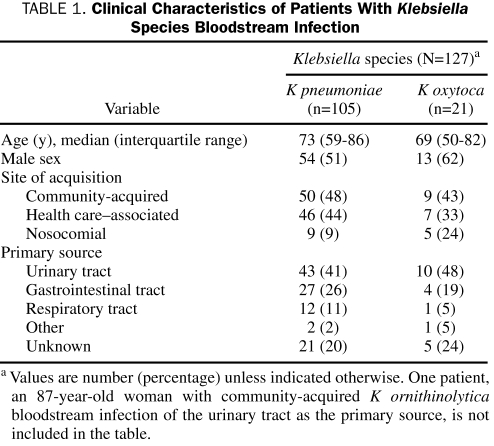
We identified 127 unique patients with Klebsiella spp BSI during the study period; 105 patients had Klebsiella pneumoniae, 21 had Klebsiella oxytoca, and 1 had Klebsiella ornithinolytica BSI (Table 1). The median age of patients with Klebsiella spp BSI was 72 years (interquartile range, 52-85 years), and 67 (52.8%) were men. Forty-seven percent of cases were community-acquired, 42% were health care—associated, and 11% were nosocomial. The urinary tract was the most common primary source of infection (43%), followed by the gastrointestinal tract (24%), the respiratory tract (10%), and other (2%). Twenty-six patients (20%) had primary BSI of unknown source. Most patients with Klebsiella spp BSI had multiple chronic comorbid conditions, as reflected by a median Charlson comorbidity index of 4 (interquartile range, 1-7).
TABLE 1.
Clinical Characteristics of Patients With Klebsiella Species Bloodstream Infection
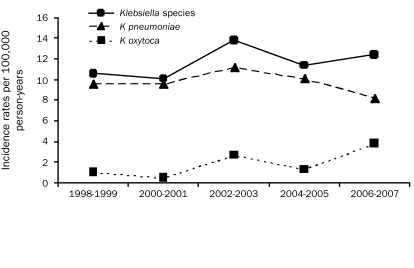
The age- and sex-adjusted incidence rate of Klebsiella spp BSI per 100,000 person-years was 11.7 (95% CI, 9.7-13.8). The age-adjusted incidence rate of Klebsiella spp BSI per 100,000 person-years was 15.4 (95% CI, 11.6-19.2) in men and 9.4 (95% CI, 7.0-11.8) in women. There was no linear temporal change in the incidence rate throughout the study period (P=.55 from multivariable Poisson model adjusting for age, sex, and average temperature; Figure 1), and a plot of the incidence rate by age (not shown) suggested an increasingly higher rate of incidence with older age. In the multivariable Poisson model, a quadratic term for age was included (P=.005).
FIGURE 1.
Age- and sex-adjusted incidence rates of Klebsiella species bloodstream infection by calendar year.
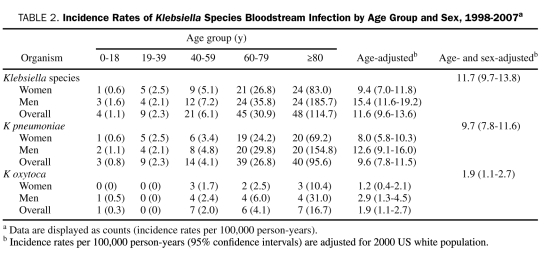
The age- and sex-adjusted incidence rates of K pneumoniae and K oxytoca BSI per 100,000 person-years were 9.7 (95% CI, 7.8-11.6) and 1.9 (95% CI, 1.1-2.7), respectively. The age-adjusted incidence rate of K pneumoniae BSI per 100,000 person-years was 12.6 (95% CI, 9.1-16.0) in men and 8.0 (95% CI, 5.8-10.3) in women. The incidence rate of K pneumoniae BSI also increased at a quadratic rate with age (P=.008). The age-adjusted incidence rate of K oxytoca BSI per 100,000 person-years was 2.9 (95% CI, 1.3-4.5) in men and 1.2 (95% CI, 0.4-2.1) in women (Table 2).
TABLE 2.
Incidence Rates of Klebsiella Species Bloodstream Infection by Age Group and Sex, 1998-2007a
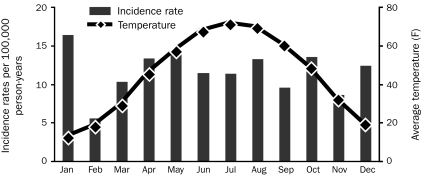
The age- and sex-adjusted incidence rate of Klebsiella spp BSI per 100,000 person-years was 11.5 (95% CI, 8.0-15.0) during the warmest 4 months of the year (June through September) compared to 11.9 (95% CI, 9.3-14.4) during the rest of the year (incidence rate ratio, 0.97; 95% CI, 0.66-1.38; P=.95). Additionally, no association existed between the incidence rate of Klebsiella spp BSI and average temperature after adjustment for age and sex (P=.74; Figure 2).
FIGURE 2.
Monthly age- and sex-adjusted incidence rates of Klebsiella species bloodstream infection and average monthly temperatures, 1998-2007.
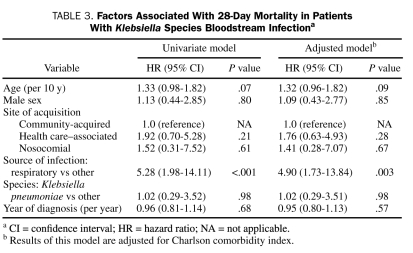
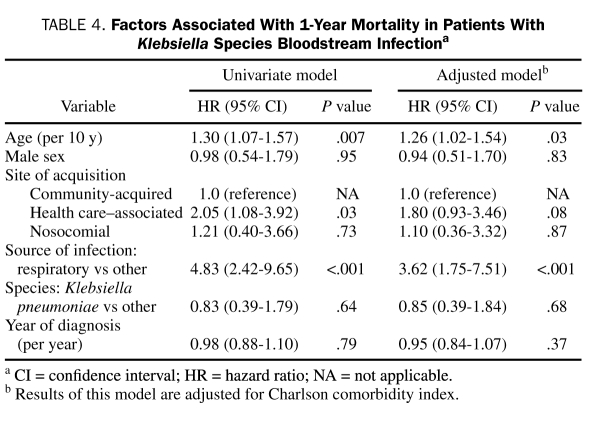
For most of the cohort, patient follow-up was complete; no patient was lost to follow-up within 28 days, and only 8 (6%) were lost to follow-up within 1 year of Klebsiella spp BSI. The overall 28-day and 1-year all-cause mortality rates of Klebsiella spp BSI were 14% (95% CI, 9%-22%) and 35% (95% CI, 27%-44%), respectively. Factors associated with 28-day and 1-year all-cause mortality are shown in Tables 3 and 4, respectively. After adjustment for Charlson comorbidity index, respiratory source of BSI was associated with a higher 28-day all-cause mortality (HR, 4.90; 95% CI, 1.73-13.84; P=.003). Factors associated with 1-year all-cause mortality after adjustment for Charlson comorbidity index were age (HR, 1.26; 95% CI, 1.02-1.54; P=.03) and respiratory source of BSI (HR, 3.62; 95% CI, 1.75-7.51; P<.001).
TABLE 3.
Factors Associated With 28-Day Mortality in Patients With Klebsiella Species Bloodstream Infectiona
TABLE 4.
Factors Associated With 1-Year Mortality in Patients With Klebsiella Species Bloodstream Infectiona
DISCUSSION
To our knowledge, this is the first population-based study to describe the epidemiology and the short- and long-term outcomes of Klebsiella spp BSI. The age- and sex-adjusted incidence rates of Klebsiella spp BSI remained stable during the past decade and ranged from 10.1 to 13.8 per 100,000 person-years.
On the basis of an age- and sex-adjusted incidence rate of 9.7 per 100,000 person-years (95% CI, 7.8-11.6), K pneumoniae was the second most common gram-negative organism to cause BSI in our population (after Escherichia coli, which had an incidence rate of 41.4 per 100,000 person-years [95% CI, 37.6-45.3]).18 The incidence rate of BSI due to each of these organisms increased with age. Unlike E coli BSI, which was more common in women, K pneumoniae BSI was more common in men. Additionally, patients with E coli were more likely than those with K pneumoniae to have a community-acquired BSI (59% vs 48%) and a urinary primary source of BSI (80% vs 41%).18
In contrast to a previous report from 4 tertiary care centers on 4 continents,19 we observed no seasonal variation in the incidence rate of Klebsiella spp BSI. One possible explanation for the difference in results is the settings in which the 2 studies were performed: the former study is hospital-based, whereas ours is population-based. It is conceivable that factors within a hospital environment contributed to the seasonality of Klebsiella spp BSI; therefore, the results were not reproduced in a population-based setting. Another possible explanation is the difference in temperature between the geographic locations where the 2 studies were performed. In other words, the average monthly temperatures in Minnesota were much lower than those in any of the 4 geographic locations where the other study was conducted (Australia, France, Taiwan, and North Carolina). Such lower average temperatures might have masked a seasonal variation in Klebsiella spp BSI in our study.
The lack of seasonal variation in Klebsiella spp BSI in the current study is also contrary to what we have previously demonstrated in E coli BSI in our local population, in which the incidence rate was higher during the warmest 4 months than the rest of the year.20 It is unclear why we observed seasonal variation in E coli BSI but not in Klebsiella spp BSI. One notable difference between the 2 pathogens is the predominance of the urinary tract as the primary source of infection in patients with E coli BSI (80%) compared with patients with Klebsiella spp BSI (only 43%). We speculate that this difference likely accounts for the presence of seasonal variation in E coli BSI and its lack in Klebsiella spp BSI. The number of patients with Klebsiella spp BSI in the current study was too small to allow for stratification by source of acquisition to examine this hypothesis. Further population-based studies are warranted to examine for a seasonal variation in the incidence rate of urinary tract infections.
The 28-day all-cause mortality rate of 14% in patients with Klebsiella spp BSI in the current study was notably lower than the short-term mortality rates reported in recent investigations from tertiary care centers, which ranged from 18% to 37%.8,21-24 This was most likely due, in part, to the lack of referral bias in this population-based study; referral patients characteristically have more complications that lead to worse outcomes.
To our knowledge, this is the first study to report the long-term outcome of Klebsiella spp BSI. More than one-third of patients did not survive beyond 1 year after Klebsiella spp BSI, most likely because of advanced age and multiple comorbidities. Patients with a respiratory source of infection had a worse outcome than did those with other sources of infection. Comparing the results of our study with those of a prior survey of Pseudomonas aeruginosa BSI in Olmsted County residents,12 patients with Klebsiella spp BSI had relatively lower 28-day and 1-year mortality rates than did patients with P aeruginosa BSI (14% vs 26% and 35% vs 48%, respectively).
The strength of our study is the population-based design and therefore lack of referral bias. Contrary to previous hospital-based studies that have estimated the incidence rate of Klebsiella spp BSI per the number of admissions to a particular hospital, we determined the incidence rate by 100,000 person-years in a well-defined population. In addition, the availability of prolonged follow-up through the REP resources is a unique advantage of our work.
Our study has limitations. First, our data are derived from 1 geographic area. Studies from multiple geographic locations may provide a more comprehensive view. Second, because the number of patients with Klebsiella spp BSI was relatively small and their mortality rate was low, the model for predictors of mortality may have been underpowered. Third, we did not evaluate the appropriateness of antimicrobial therapy in this model. Finally, the population of Olmsted County consists mainly of middle-class white people; therefore, our study results may be generalized only to communities with similar population characteristics.
CONCLUSION
To our knowledge, this is the first population-based study that defined the epidemiology and short- and long-term outcomes of Klebsiella spp BSI in the United States. The incidence rate of Klebsiella spp BSI increased with age and was higher in men than in women. No seasonal variation or temporal change was observed in the incidence rate of Klebsiella spp BSI during the 10-year study period. The relatively low mortality rate after Klebsiella spp BSI in our population as compared with rates in previously reported investigations from tertiary care centers is likely due to referral bias, which may impact data generated from those types of centers. However, a respiratory source of BSI was associated with a worse outcome.
ADDENDUM
After this manuscript was accepted for publication, a population-based study that described the incidence rate and short-term outcome of Klebsiella pneumoniae bloodstream infection in Calgary, Canada, was published.25
Acknowledgments
We thank Emily Vetter and Mary Ann Butler for providing vital data from the microbiology laboratory databases at Mayo Clinic and Olmsted Medical Center. Additionally, we thank Susan Schrage, Susan Stotz, RN, and all the staff at the Rochester Epidemiology Project for their administrative help and support.
Footnotes
This study received funding from the Small Grants Program and the Baddour Family Fund at Mayo Clinic, Rochester, MN. The funding source had no role in study design.
This work was made possible by research grant R01-AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, US Public Health Service).
REFERENCES
- 1.Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29(3):595-607 [DOI] [PubMed] [Google Scholar]
- 2.Decousser JW, Pina P, Picot F, et al. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J Antimicrob Chemother. 2003;51(5):1213-1222 [DOI] [PubMed] [Google Scholar]
- 3.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167(8):834-839 [DOI] [PubMed] [Google Scholar]
- 4.Al-Hasan MN, Wilson JW, Lahr BD, et al. β-Lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother. 2009;53(4):1386-1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hasan MN, Razonable RR, Eckel-Passow JE, Baddour LM. Incidence rate and outcome of Gram-negative bloodstream infection in solid organ transplant recipients. Am J Transplant. 2009;9(4):835-843 [DOI] [PubMed] [Google Scholar]
- 6.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum β-lactamase production in nosocomial infections. Ann Intern Med. 2004;140(1):26-32 [DOI] [PubMed] [Google Scholar]
- 7.Korvick JA, Bryan CS, Farber B, et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother. 1992;36(12):2639-2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pai H, Kang CI, Byeon JH, et al. Epidemiology and clinical features of bloodstream infections caused by AmpC-type-β-lactamase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2004;48(10):3720-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Census Bureau . State & County QuickFacts. Olmsted County, Minnesota: Accessed October 29, 2009. [Google Scholar]
- 10.Steckelberg JM, Melton LJ, III, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88(6):582-588 [DOI] [PubMed] [Google Scholar]
- 11.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266-274 [DOI] [PubMed] [Google Scholar]
- 12.Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121(8):702-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA 2005;293(24):3022-3028 [DOI] [PubMed] [Google Scholar]
- 14.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137(10):791-797 [DOI] [PubMed] [Google Scholar]
- 15.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988 [published correction appears in Am J Infect Control. 1988;16(4):177] Am J Infect Control. 1988;16(3):128-140 [DOI] [PubMed] [Google Scholar]
- 16.Weatherbase Web site Rochester, Minnesota, http://www.weatherbase.com/weather/weather.php3?s=044627&refer= Accessed October 29, 2009 [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383 [DOI] [PubMed] [Google Scholar]
- 18.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: a population-based study, 1998-2007. J Antimicrob Chemother. 2009;64(1):169-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson DJ, Richet H, Chen LF, et al. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis. 2008;197(5):752-756 [DOI] [PubMed] [Google Scholar]
- 20.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Seasonal variation in Echerichia coli bloodstream infection: a population-based study. Clin Microbiol Infect. 2009;15(10):947-950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tumbarello M, Spanu T, Sanguinetti M, et al. Bloodstream infections caused by extended-spectrum-β-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50(2):498-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thom KA, Schweizer ML, Osih RB, et al. Impact of empiric antimicrobial therapy on outcomes in patients with Escherichia coli and Klebsiella pneumoniae bacteremia: a cohort study. BMC Infect Dis. 2008;8:116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao CH, Lai CC, Hsu MS, et al. Correlation between time to positivity of blood cultures with clinical presentation and outcomes in patients with Klebsiella pneumoniae bacteraemia: prospective cohort study. Clin Microbiol Infect. 2009December; 15(12):1119-1125 Epub 2009 Apr 15 [DOI] [PubMed] [Google Scholar]
- 24.Kang CI, Kim SH, Bang JW, et al. Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci. 2006;21(5):816-822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meatherall BL, Gregson D, Ross T, et al. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866-873 [DOI] [PubMed] [Google Scholar]