Abstract
Lung transplant is an effective treatment for patients with end-stage lung disease but is limited because of the shortage of acceptable donor organs. Organ donation after cardiac death is one possible solution to the organ shortage because it could expand the pool of potential donors beyond brain-dead and living donors. We report the preliminary experience of Mayo Clinic with donation after cardiac death, lung procurement, and transplant.
Lung transplant is accepted therapy for extending the life of patients with end-stage lung disease.1 Unfortunately, its application is limited for the growing number of indications and lung transplant candidates because of the shortage of acceptable donor organs.2 Efforts, including public awareness and legislative initiatives, are ongoing to increase the size of the current donor pool for the nearly 2000 lung transplant candidates.2 Introduction of the lung allocation score in 2005 has resulted in a decrease in wait-list times and wait-list mortality, but the mean waiting time for lung transplant is still more than 200 days.3,4 Despite progress, waiting-list mortality remains unacceptably high.5 Organ donation after cardiac death (DCD), also known as non—heart-beating donors, is one proposed solution to increase the supply of transplant organs. We report our institution's initial experience with DCD, lung procurement, and transplant.
REPORT OF A CASE
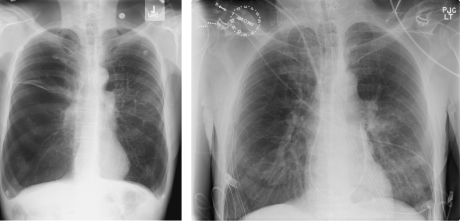
A 59-year-old man (blood type A) with chronic obstructive pulmonary disease (Figure 1, left) secondary to α1-antitrypsin deficiency and previous tobacco abuse underwent bilateral orthotopic lung transplant at Mayo Clinic, with organs procured from a donor after controlled cardiac death.
FIGURE 1.
Preoperative (left) and postoperative (right) radiographs of patient who underwent bilateral lung transplant with organs from donor after cardiac death.
Donor and Organ Procurement
After initially evaluating the available online data, our procurement team traveled to an outside hospital (approximately 160 km) to retrieve lungs from a 21-year-old (blood type A) trauma victim. Although anatomic brain injury was severe, persistently high levels of barbiturates prevented meeting all criteria for brain death. Consent for organ DCD was obtained. Organ quality (Pao2 >300 mm Hg and acceptable chest radiographic and bronchoscopic findings) and necessary donor-recipient compatibilities (blood type, serologies, virtual prospective cross-match) were confirmed. The donor was taken to the operating room for withdrawal of life support consisting of cessation of vasoactive medications and mechanical ventilation. The donor was positioned, prepped, and draped for thoracic and abdominal organ procurement before being covered with a sterile sheet. The family was allowed into the operating room during cessation of life support. The surgical teams coordinated their cooperative plans, and a detailed preoperative briefing was given to operating room personnel who were waiting in an adjacent room until initial loss of cardiac activity; then the family was ushered from the operating room. After an additional 6 minutes of cardiac inactivity, an independent physician pronounced death, at which time organ procurement was begun. Cardiac death was pronounced 16 minutes after cessation of support (reference time of t=0). Incisions by both the thoracic and abdominal procurement teams were made at 6 minutes; at 8 minutes, the aorta was cross-clamped, for a total warm ischemic time of 24 minutes. Lung procurement was then completed in the usual fashion: topical cooling, pulmonary artery cannulation, and administration of prostaglandin and pneumoplegia solution (Perfadex, Vitrolife, Göteborg, Sweden) with venting through the left atrial appendage. Once the heart was removed from the thoracic cavity, the lungs were removed together en bloc by stapling and dividing the trachea with the lungs at approximately midinspiratory volume. The lungs were flushed retrograde with pneumoplegia solution and packaged on ice for transportation to our institution.
Procedure
Our patient had undergone evaluation and was listed for lung transplant 12 months previously. He was given a detailed explanation and understood the process of DCD organ procurement. Afterward, he was taken to the operating room and underwent general anesthesia with a double-lumen endotracheal tube. The patient was positioned supine with an intrascapular pillow for shoulder extension and standard monitoring, including a pulmonary artery catheter. After confirmation of successful organ procurement and blood type compatibilities, bilateral anterolateral thoracotomy incisions were made. A standard off-pump, bilateral sequential lung transplant was performed; a postoperative radiograph of the patient's lung is shown in Figure 1, right. Total ischemic time for the right and left lung was 227 and 321 minutes, respectively.
Postoperative Course
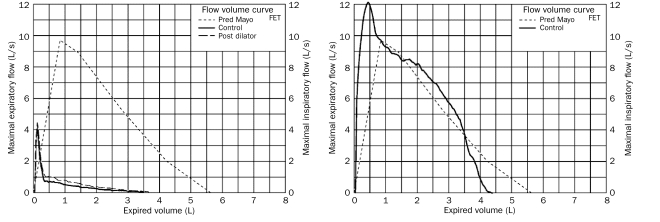
The patient had a satisfactory postoperative convalescence and was dismissed from the hospital on postoperative day 13. Spirometric lung function at 3 months demonstrated a forced expiratory volume in 1 second of 4.11 L, which was 95% of predicted, with a maximum forced expiratory flow of 125% of predicted (Figure 2). Surveillance transbronchial biopsy at 3 months showed no rejection (grade A0, B0, C0).4
FIGURE 2.
Preoperative (left) and postoperative (right) flow-volume curves for patient who underwent bilateral lung transplant with organs from donor after cardiac death. FET = forced expiratory time.
DISCUSSION
To our knowledge, we report Mayo Clinic's and Minnesota's first lung transplant from organ DCD. Even though DCD is one potential solution to expanding the number of potential organ donors, current experience is limited to a few transplant centers with fewer than 60 lung transplants having been performed in the United States to date.4 Generalized acceptance and adoption of DCD have been slow and gradual,6,7 primarily because of concerns of both the logistics of the procurement procedure and an overriding ethical dilemma regarding appropriate donor selection. Placed in proper perspective, DCD organ procurement has brought the ethics of organ transplant full circle because the earliest organ donations were from deceased donors after cardiac death,5,6 with consensus criteria for brain death organ donation having been established later. The critical shortage of organs has brought about renewed interest in DCD organ procurement and rekindled the ethical dialogue.
Another reason for limited utilization of DCD organ procurement and transplant may involve lack of familiarity with the multiple types of DCD organ procurement circumstances. Criteria for DCD organ procurement have been established to guide clinicians involved in critical care and organ transplant.7 Procurement of DCD organs is categorized into 2 broad groups: controlled and uncontrolled. Uncontrolled DCD procurement consists of organ procurement after unsuccessful cardiopulmonary resuscitation or from patients pronounced dead on arrival at a hospital. In contrast, controlled DCD organ procurement consists of withdrawal of life support from non—brain-dead donors and unexpected cardiac arrest of brain-dead donors. Similar to other transplant centers, our initial experience with controlled DCD organ procurement and transplant was successful, but offers from donors are infrequent. More active education among physicians at referring hospitals within our organ allocation region about this option and different types of DCD may remedy this problem and should be pursued on a national level. Additionally, in light of the understandably devastating circumstances, discussing the process with appropriate empathy for the donor family will certainly improve public acceptance of such endeavors. Moreover, there are concerns that lungs obtained from DCD organ procurement may be subject to primary graft dysfunction and poor outcome. Although data are inconclusive to date, early data from other centers suggest that this may be most problematic in organs from uncontrolled organ donation.7,8
The key to successful DCD organ procurement is discussion and planning by the procurement teams followed by a thorough preoperative briefing of operating room personnel. This serves to eliminate redundancy and improves necessary efficiency of the initiation of the procurement process, when time is a premium. Economy of motion and collaboration move the process along smoothly with a minimum of wasted time and effort.
CONCLUSION
Our initial experience with controlled DCD organ procurement and lung transplant is promising. Because our approach with this type of transplant deviates minimally from our standard procurement and lung transplant protocols, wider implementation should be possible. We are confident that donation of lungs after cardiac death presents a viable additional solution to the national organ shortage. This option should be encouraged when appropriate in an attempt to increase the number of potential donors for the growing number of recipients in need of lung transplant.
Acknowledgments
We thank Meg Rogers, RN, BSN, CPTC, and Tina Abdelnour, RN, BSN, CPTC, of the LifeSource organ procurement organization for their important contributions in assisting the clinical and logistical progress of lung DCD in the Upper Midwest region of the United States.
REFERENCES
- 1.Cassivi SD, Meyers BF, Battafarano RJ, et al. Thirteen-year experience in lung transplantation for emphysema. Ann Thorac Surg. 2002;74(5):1663-1669 [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Health Resources and Services Administration OPTN Organ Procurement and Transplantation Network Web site. http://optn.transplant.hrsa.gov/latestData/rptData.asp. http://optn.transplant.hrsa.gov/latestData/rptData.asp Accessed September 8, 2009.
- 3.Iribarne A, Russo MJ, Davies RR, et al. Despite decreased wait-list times for lung transplantation, lung allocation scores continue to increase. Chest 2009April;135(4):923-928 Epub 2008 Nov 18 [DOI] [PubMed] [Google Scholar]
- 4.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. J Thorac Cardiovasc Surg. 2008January;135(1):166-171 Epub 2007 Nov 12 [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Shiboski SC, Golden JA, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009September1;180(5):468-474 Epub 2009 Jun 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Alessandro AM, Fernandez LA, Chin LT, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant. 2004;9(1):68-71 [PubMed] [Google Scholar]
- 7.Mason DP, Murthy SC, Gonzalez-Stawinski GV, et al. Early experience with lung transplantation using donors after cardiac death. J Heart Lung Transplant. 2008;27(5):561-563 [DOI] [PubMed] [Google Scholar]
- 8.de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant. 2007;26(5):529-534 [DOI] [PubMed] [Google Scholar]