Abstract
Chronic heart failure (CHF) remains the only cardiovascular disease with an increasing hospitalization burden and an ongoing drain on health care expenditures. The prevalence of CHF increases with advancing life span, with diastolic heart failure predominating in the elderly population. Primary prevention of coronary artery disease and risk factor management via aggressive blood pressure control are central in preventing new occurrences of left ventricular dysfunction. Optimal therapy for CHF involves identification and correction of potentially reversible precipitants, target-dose titration of medical therapy, and management of hospitalizations for decompensation. The etiological phenotype, absolute decrease in left ventricular ejection fraction and a widening of QRS duration on electrocardiography, is commonly used to identify patients at increased risk of progression of heart failure and sudden death who may benefit from prophylactic implantable cardioverter-defibrillator placement with or without cardiac resynchronization therapy. Patients who transition to advanced stages of disease despite optimal traditional medical and device therapy may be candidates for hemodynamically directed approaches such as a left ventricular assist device; in selected cases, listing for cardiac transplant may be warranted.
ACC = American College of Cardiology; ACEI = angiotensin-converting enzyme inhibitor; ADHF = acute decompensated heart failure; AF = atrial fibrillation; AHA = American Heart Association; ARB = angiotensin II receptor blocker; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CHF = chronic heart failure; CRT = cardiac resynchronization therapy; ICD = implantable cardioverter-defibrillator; LV = left ventricular; LVAD = LV assist device; LVEF = LV ejection fraction; MI = myocardial infarction; MR = mitral regurgitation; SCD = sudden cardiac death; UF = ultrafiltration
Chronic heart failure (CHF) is a progressive syndrome that results in a poor quality of life for the patient and places an economic burden on the health care system. Despite advances in the control of cardiovascular diseases such as myocardial infarction (MI), the incidence and prevalence of CHF continue to increase.1 An accurate estimate of disease burden is difficult to gather because of the vast number of patients with asymptomatic left ventricular (LV) dysfunction. As the population ages, there is an epidemiological shift toward a greater prevalence of clinical heart failure with preserved LV function, the so-called stiff-heart syndrome. In fact, heart failure with preserved systolic function may account for up to two-thirds of cases in patients older than 70 years.2 Regardless of age, the lifetime risk of developing heart failure is approximately 20% for all patients older than 40 years.3
Despite the growing prevalence, novel screening techniques and therapeutic directions have improved the outlook for patients with heart failure by focusing not only on symptom control but also on ameliorating the pathophysiology toward a corrective phenotype. This review discusses accepted and emerging therapeutic directions, with an emphasis on practical implications. In light of the available literature and clinical trials, the primary emphasis will be on systolic dysfunction, with a separate brief discussion of heart failure with preserved systolic function.
DIAGNOSIS
No single test can be used to establish the clinical diagnosis of heart failure. Instead, history and physical examination findings showing signs and symptoms of congestion and/or end-organ hypoperfusion are used to make the diagnosis. Imaging studies documenting systolic or diastolic dysfunction and biomarkers are helpful adjuncts. Physical examination is not helpful in discriminating between systolic and diastolic heart failure because similar findings, including cardiomegaly and an S3 gallop, can be seen in both conditions.4 Pulmonary rales, often considered a sign of pulmonary venous congestion, are often absent in CHF despite elevated left-sided filling pressures. This absence is due to chronic lymphatic hypertrophy, which prevents alveolar edema despite elevated interstitial pressures.5 Framingham criteria, widely used in clinical research, comprise a series of major and minor criteria that aid in the diagnosis of heart failure and emphasize the importance of jugular venous pressure elevation, an S3 gallop, and a positive hepatojugular reflex in establishing a diagnosis, while minimizing the importance of lower extremity edema.6 The use of brain-type natriuretic peptides, in their active or inactive circulating forms, has evolved during the past decade, but the most well-established use remains in discriminating between causes of dyspnea when the diagnosis is in doubt.7 Comorbid conditions must be taken into account because renal insufficiency increases these levels and obesity lowers them.8,9
The etiology of systolic heart failure dramatically affects prognosis and treatment. Coronary artery disease (CAD) accounts for the vast majority of cases of systolic heart failure in the United States, followed by hypertensive and dilated cardiomyopathies.10 In the acute setting of newly diagnosed cardiomyopathy, the exclusion of underlying CAD and potential “at-risk” myocardium that might benefit from revascularization is critical. Patients with CAD and concomitant heart failure have a worse prognosis than those with nonischemic cardiomyopathy, but myocardial function may substantially improve after revascularization in selected cases, highlighting the importance of making the appropriate diagnosis early and accurately.
RISK MARKERS, PREVENTION, AND SCREENING
Risk Markers
Multiple cardiovascular conditions, ranging from arrhythmias to valvular heart disease, may ultimately lead to heart failure. Strict adherence to guideline-based management of these conditions is paramount in preventing heart failure. Advanced age is the most potent, albeit nonmodifiable, risk factor. Hypertension, which is easily diagnosed and treated, increases the risk of heart failure 2- to 3-fold.10,11 Although the relative risk of developing heart failure is modest, the sheer prevalence renders it a cause in approximately one-third of cases, giving it a high population-attributable risk.12 In this regard, this risk marker serves as a most viable target for preventive therapy. Analysis of the Framingham heart study revealed the median blood pressure for patients who ultimately developed heart failure was 150/90 mm Hg,12 emphasizing that risk is increased in suboptimally treated hypertension even at modest levels of severity. Multiple studies across a broad range of agents have unequivocally shown that treatment of blood pressure leads to a marked reduction in heart failure.13
Incident risk factors for CAD, including diabetes and dyslipidemia, increase the probability of an MI, another important risk factor for heart failure.14 Although moderate alcohol consumption has been correlated with a reduced incidence of heart failure in several large patient cohorts,15,16 mild to moderate alcohol consumption is associated with an increase in blood pressure,17 and cardiomyopathy is a well-described complication of long-standing alcohol abuse.
Obesity, defined as a body mass index greater than 30 kg/m2, is increasingly being recognized as an independent risk factor for heart failure.18 Obesity leads to alterations in LV chamber size and mass, which may progress over time to systolic and diastolic dysfunction.19 Intentional weight loss may lead to regression of some of these structural changes and is generally advisable.20 The association between obesity and heart failure is complex, as excess weight is correlated with a reduction in hospitalizations and improved survival in patients with established heart failure.21,22
Prevention
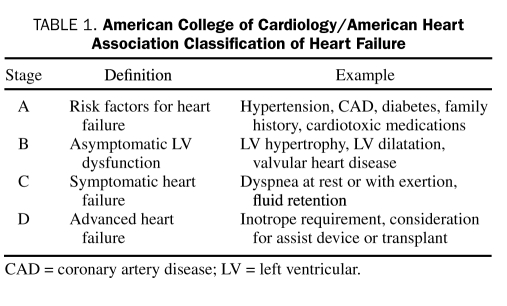
In light of the high mortality, functional limitation, and health care costs that accompany a diagnosis of heart failure, recognition of the importance of prevention is ever-increasing. To highlight the role of prevention in the overall management strategy of heart failure, the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines have identified 4 stages of heart failure23 (Table 1). Important in this schema is delineation of a preclinical class (stage A) consisting of patients with conditions that are associated with an increased likelihood for developing heart failure and who should be targeted for aggressive risk factor reduction. Patients with asymptomatic structural LV disease constitute stage B. SOLVD (Study of Left Ventricular Dysfunction), a landmark study, examined angiotensin-converting enzyme inhibitor (ACEI) treatment in this population, demonstrating a 33% reduction in clinical heart failure and hospitalizations.24 Although no randomized controlled trials of β-blockers in patients with asymptomatic LV dysfunction have been completed, the most recent version of the ACC/AHA guidelines recommend using β-blockers in patients with stage B disease.23
TABLE 1.
American College of Cardiology/American Heart Association Classification of Heart Failure
Screening
Screening asymptomatic patients for heart failure remains controversial and is an area of active investigation. Evidence in support of this practice comes from the Cardiovascular Health Study. Only 9% of patients who ultimately developed systolic heart failure had a reduced LV ejection fraction (LVEF) on study enrollment.25 Biomarkers, such as N-terminal prohormone brain natriuretic peptide and troponin, may potentially function in this role; however, the cost-effectiveness and target populations for these strategies remain unsettled.26,27 Clearly, meaningful strides in heart failure reduction can be attained simply through adherence to existing guidelines and elimination of the financial and psychosocial barriers that deter patients from taking prescribed medical therapy. In a primary care practice, it is incumbent upon the practitioner to develop a focused approach to screening for latent structural heart disease and to develop a clinical screen for manifest CHF. Such screening can be accomplished by asking a simple series of questions related to the occurrence of such symptoms as easy fatigability, functional limitations, and development of lower extremity swelling.
PATHOPHYSIOLOGY AND THERAPEUTIC IMPLICATIONS: SYSTOLIC HEART FAILURE
Pathophysiology
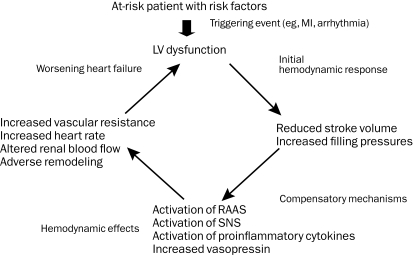
Multiple models have been conceptualized to explain the complex clinical syndrome of heart failure, which stems from a combination of structural pathology, neurohormonal activation, and altered cardiorenal dynamics with end-organ hypofunction (Figure 1). The development of heart failure is characterized by an inciting cardiac injury that triggers a cascade of neurohormonal responses. The previously normal heart may be subject to either an acute (MI) or a chronic (hypertension, valvular heart disease) insult, resulting in altered loading conditions. Subsequent stretching of myocardial fibers or their loss evokes a neurohormonal response characterized by activation of the renin-angiotensin-aldosterone system and the sympathetic nervous system. In the short term, these mechanisms are beneficial and adaptive, sustaining heart rate, blood pressure, and cardiac output, thereby maintaining organ perfusion. Over time, these responses become detrimental, resulting in disruptions of β-adrenergic signaling and impaired mobilization of intracellular calcium.28,29 Left untreated, this abnormal neurohormonal milieu leads to myocyte hypertrophy, apoptosis, fibroblast proliferation, and interstitial collagen accumulation, culminating in adverse remodeling and pump dysfunction.30 The consequences of these pathologic structural changes are a reduction in stroke volume, an increase in systemic vascular resistance, and development of signs and symptoms of congestion and hypoperfusion. These principles have guided the development of therapeutic agents and clinical trial design.
FIGURE 1.
Pathophysiology of chronic heart failure. LV = left ventricular; MI = myocardial infarction; RAAS = renin-angiotensin-aldosterone system; SNS = sympathetic nervous system.
Clinical Insights
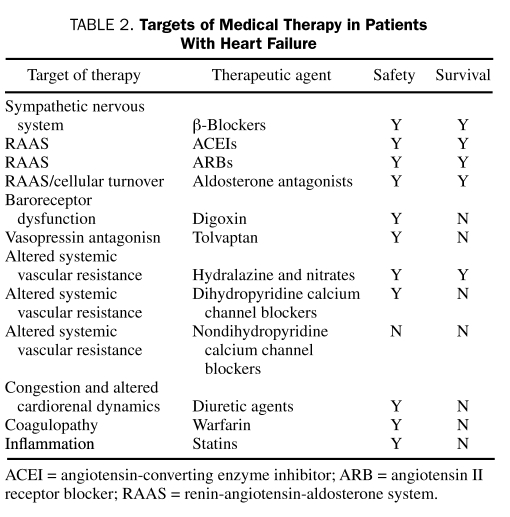
As a general rule, caution must be exercised with the use of all drugs in the setting of impaired myocardial function. The absorption, distribution, and elimination of drugs are all dependent on circulation, and in the patient with heart failure, blood flow is altered both to sites of drug metabolism and storage. Furthermore, intestinal edema from passive congestion may alter oral absorption, and concomitant liver or kidney dysfunction is frequently encountered. Table 2 summarizes the drugs routinely studied in clinical practice.
TABLE 2.
Targets of Medical Therapy in Patients With Heart Failure
CLINICAL PEARLS IN SUCCESSFUL HEART FAILURE THERAPY
Behavioral and Lifestyle Modifications Are Essential to Ensuring Success of Heart Failure Pharmacotherapy
Before initiation of pharmacotherapy, patients must be counseled regarding the importance of dietary discretion, and nutritional consultation should be provided. Strict adherence should be emphasized, and the importance of daily weight measurements addressed. Patients should be provided with instructions regarding diuretic dosing adjustments for sudden changes in weight.
Angiotensin-Converting Enzyme Inhibitors and β-Blockers Form the Cornerstone of CHF Pharmacotherapy
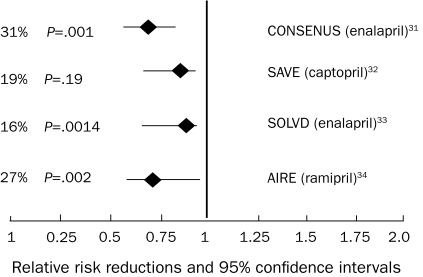
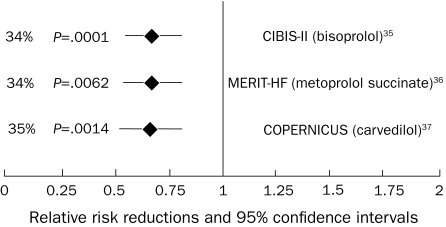
Figures 231-34 and 335-37 summarize the results of the effects of treatment with angiotensin-converting enzyme inhibitors (ACEIs) and β-blockers, respectively, on all-cause mortality. On a cellular level, ACEIs slow the progression of cardiovascular disease by multiple pleiotropic effects, including improved endothelial function; antiproliferative effects on smooth muscle cells, neutrophils, and monocytes; and antithrombotic effects.38 Meta-analyses suggest a 23% reduction in mortality and a 35% reduction in the combination end point of mortality and hospitalizations for heart failure in patients treated with ACEIs.39 β-Blockers up-regulate β-1 receptor density, blunt norepinephrine and renin production, and mitigate production of deleterious cytokines, including tumor necrosis factor α.40 Large-scale clinical trials demonstrated a 35% reduction in mortality in patients treated with β-blockers on top of the benefit provided by ACEIs alone.35,36 Increased experience with both agents in a broad range of patients with heart failure has shown the safety of ACEIs in treating patients with mild renal insufficiency and the tolerability of β-blockers in patients with moderately controlled diabetes, asthma, and obstructive lung disease. The benefits of β-blockers and ACEIs extend to patients with class IV heart failure.37 Patients with advanced disease may not be able to tolerate escalating doses of β-blockers, and the need to withdraw or reduce doses of established medications due to dizziness or hypotension may be an ominous sign of worsening heart failure.
FIGURE 2.
Angiotensin-converting enzyme inhibitor mortality trials: all-cause mortality results. AIRE = Acute Infarction Ramipril Efficacy; CONSENSUS = Cooperative North Scandinavian Enalapril Survival Study; SAVE = Survival and Ventricular Enlargement; SOLVD = Studies of Left Ventricular Dysfunction.
FIGURE 3.
β-Blocker mortality trials: all-cause mortality results. CIBIS II = Cardiac Insufficiency Bisoprolol Study II; COPERNICUS = Carvedilol Prospective Randomized Cumulative Survival; MERIT-HF = Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure.
Whereas the Benefits of ACEIs Appear to Be Class Specific, β-Blocker Use Should Be Relegated to Clinical Trials
Although there was initial optimism surrounding the use of tissue ACEIs vs non-tissue ACEIs, there appears to be no significant difference in outcomes between agents, and the benefits appear to be a class effect.41 Conversely, the beneficial effects of β-blockers are thought to be limited to specific drugs. β-Blockers with intrinsic sympathomimetic activity (xamoterol) and other agents, including bucindolol, have not demonstrated a survival benefit.42,43 On the basis of these studies, we recommend that β-blocker use be restricted to carvedilol, bisoprolol, and metoprolol succinate, agents proven to improve survival in clinical trials.
In Patients With Newly Diagnosed CHF, It Is Safe to Use Either a β-Blocker or an ACEI as First-Line Therapy
The long-established paradigm had typically been to initiate ACEI therapy before β-blocker therapy, primarily because β-blocker studies were performed with background ACEI therapy. Which of these agents was used as initial therapy and which was added subsequently did not affect outcomes in CIBIS (Cardiac Insufficiency Bisoprolol Study) III.44 It is key to use both agents at target doses and to follow up in a timely manner, titrating the dose as necessary.
Attempts Should Be Made to Attain Doses of Drugs Studied in Clinical Trials, and Rapid Outpatient Titration of Drugs Is Feasible
Clinical trial data support a dose-dependent improvement in LV function and reductions in mortality and hospitalizations with β-blocker use.45 Although a variable dosing trial demonstrated no additional survival benefits with higher doses of ACEI, higher doses were associated with reduced hospitalizations.46 Clinical experience suggests that, in the absence of symptoms to suggest hypotension (eg, fatigue and dizziness), pharmacotherapy may be up-titrated every 2 to 3 weeks in otherwise hemodynamically stable and euvolemic outpatients.
Aldosterone Antagonism Is Beneficial in Patients With Advanced (New York Heart Association III and IV) Heart Failure
The elevated aldosterone levels seen in patients with heart failure47 promote sodium retention, electrolyte imbalances, and endothelial dysfunction and may directly contribute to myocardial fibrosis.48 Both the selective agent eplerenone and the nonselective antagonist spironolactone reduce mortality and hospitalizations, with significant reductions in sudden cardiac death (SCD).49,50 Hyperkalemia is a concern, especially in patients with underlying chronic kidney disease, and renal function and serum potassium levels must be closely monitored.51
Angiotensin II Receptor Blockers Should Be Used in Patients Intolerant of ACEIs, But Triple Neurohormonal Blockade (ACEIs, β-Blockers, and Angiotensin II Receptor Blockers) Should Be Avoided
Circulating levels of angiotensin II increase to pretreatment levels with long-term angiotensin-converting enzyme inhibition.52 Angiotensin II receptor blockers (ARBs) bind competitively to the AT1 receptor, providing a downstream effect and thereby blunting this escape phenomenon.53 A large meta-analysis of 24 randomized trials showed the superiority of ARBs to placebo in patients with intolerable adverse effects with ACEIs and their noninferiority in all-cause mortality or hospitalizations when directly compared with ACEIs.54 Val-HeFT (Valsartan Heart Failure Trial) suggested that addition of valsartan in patients already receiving treatment with ACEIs and β-blockers was associated with a trend toward worse outcomes.55 Similarly, adding valsartan to captopril in patients with heart failure after MI who were receiving background β-blocker therapy was associated with an increase in adverse events without any added benefit compared with monotherapy for either group.56 A ceiling effect appears to exist beyond which additional neurohormonal blockade may no longer be beneficial and may even trend toward harm. Thus, the clinical dictum should be to use a 2-drug combination first (ACEI and β-blocker; if β-blocker intolerant: ACEI and ARB; if ACEI intolerant: ARB and β-blocker).
The Combination of Hydralazine and Nitrates Should Be Limited to Special Populations: Those Patients Who Remain Hypertensive With Neurohormonal Blockade and Those With Renal Insufficiency Prohibiting Use of ACEIs or ARBs
Hydralazine produces arterial vasodilatation and systemic vascular resistance reduction via modulation of intracellular calcium kinetics,57 and nitrates are transformed in smooth muscle cells into nitric oxide, which stimulates cyclic guanosine monophosphate production and subsequent arterial vasodilation.58 This combination improves survival, but not to the magnitude evidenced by ACEIs or ARBs.59,60 A-Heft (African-American Heart Failure Trial) studied an African American population and added fixed-dose isosorbide dinitrate with hydralazine in patients with advanced heart failure who were receiving standard background therapy.61 The study was terminated ahead of time because of an early difference in survival and hospitalizations. Adherence to this regimen is often limited by the thrice-daily dosing schedule.
In Patients With Residual Symptoms Despite Optimization of Volume Status and Pharmacotherapy, Addition of Digoxin Should Be Considered
Digitalis glycosides exert a mild inotropic effect, but more importantly, attenuate carotid sinus baroreceptors and have sympathoinhibitory effects that result in a decrease in serum norepinephrine levels, plasma renin levels, and possibly aldosterone levels.62,63 The landmark DIG (Digitalis Investigation Group) trial demonstrated a reduction in hospitalizations for heart failure in the treatment group but showed no reduction in mortality or significant changes in quality of life.64 Importantly, treatment with digoxin resulted in a higher mortality rate in women than men. Furthermore, the effects of digoxin in reducing hospitalizations were lower in women than in men.65 It should be noted that low doses of digoxin are sufficient to achieve the potentially beneficial outcomes, and higher doses tend to breach the therapeutic safety index. Trough digoxin levels are checked to minimize the risk of toxicity, and although dose reductions are indicated for higher levels, no adjustment is made for low levels.
Adequate Dosing of Diuretic Agents Is Critical in Managing Symptoms and Functional Status
Neurohormonal activation results in avid salt and water retention. Loop diuretic agents are often required because of their increased potency, and frequent dose adjustments may be necessary because of variable oral absorption and fluctuations in renal function. Importantly, clinical trial data confirming efficacy are limited, and no data suggest that these agents improve survival. Thus, diuretic agents should ideally be used in tailored dosing schedules to avoid excessive exposure.
Routine Anticoagulation Has No Role in the Patient With Heart Failure
Although heart failure is accompanied by a hypercoagulable state,66 data are insufficient to support the use of warfarin in patients in normal sinus rhythm without a history of thromboembolic events or echocardiographic evidence of LV thrombus. Aspirin blunts ACEI-mediated prostaglandin synthesis, but the clinical importance of this finding remains unclear.67,68 Current guidelines support the use of aspirin in patients with ischemic cardiomyopathy.
The Second-Generation Calcium Channel—Blocking Agents Amlodipine and Felodipine Are Safe and Effective in Reducing Blood Pressure But Have No Effects on Morbidity, Mortality, or Quality of Life
Amlodipine and felodipine, second-generation calcium channel—blocking agents, safely and effectively reduce blood pressure but do not affect morbidity, mortality, or quality of life.69-71 The first-generation agents, including verapamil and diltiazem, may exert negative inotropic effects and destabilize previously asymptomatic patients.72 Their use should be discouraged.
UNSUCCESSFUL THERAPIES
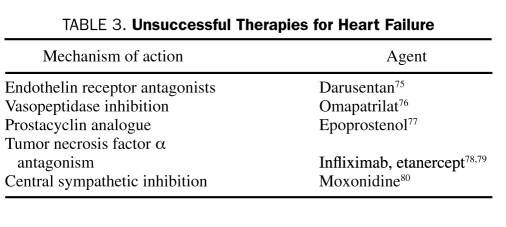
Success has been limited in extending the pharmacotherapy for heart failure beyond targeting the renin-angiotensin-aldosterone system and the sympathetic nervous system. Despite an abundance of animal and clinical data demonstrating the deleterious effects of various cytokines, therapies directed at reducing their levels have largely been unsuccessful. As an example, vasopressin plasma concentrations are elevated in patients with heart failure, but treatment with vasopressin antagonists has not translated into improved long-term outcomes.73,74 A list of these agents is summarized in Table 3. Given the apparent limitations on pharmacotherapy, additional areas of treatment should be considered.
TABLE 3.
Unsuccessful Therapies for Heart Failure
NOVEL TREATMENT TARGETS
Sleep-Disordered Breathing
Sleep-disordered breathing encompasses obstructive sleep apnea and Cheyne-Stokes breathing in its extreme form. Sleep-disordered breathing is common and may coexist in patients with CHF despite optimal pharmacological treatment.81 The frequent periods of hypoxia and repeated nighttime arousals trigger adrenergic surges, which can worsen hypertension and impair systolic and diastolic function. Obstructive sleep apnea is an independent predictor of worsening outcomes in heart failure.82 Diagnosis of obstructive sleep apnea requires a high index of suspicion and should be considered in all patients, but especially in high-risk patients (ie, those with predominant symptoms of fatigue and those with favorable LV remodeling while receiving therapy with worsening of right ventricular function). The diagnosis of sleep-disordered breathing is made via overnight polysomnography. Treatment with nocturnal positive airway pressure improves oxygenation, ejection fraction, and 6-minute walk distance. However, no firm data support improved survival with treatment.83
Atrial Fibrillation
Atrial fibrillation (AF) is common in patients with heart failure, and the rapid ventricular rates are often poorly tolerated. Despite the burden of AF in heart failure, convincing data are lacking that AF incrementally increases mortality. Primary reasons to treat AF are to stabilize LV function and to manage symptoms. Multiple studies have shown no superiority of rhythm vs rate control in this patient population.84 Rate control is typically achieved with β-blockers and digoxin. Diltiazem and verapamil should be avoided. Given the high risk of thromboembolism, warfarin should be administered to all patients when possible, with strict monitoring of the international normalized ratio.
When rate control is inadequate or symptoms persist, pursuing a rhythm control strategy is reasonable. Rhythm control may be achieved via pharmacotherapy or by percutaneous or surgical techniques, and referral to practitioners or centers experienced in these modalities is recommended. Current antiarrhthymic drug therapy should be restricted to amiodarone and dofetilide, both of which have been shown to be safe and effective. ANDROMEDA (Antiarrhythmic Trial with Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease) studied the effects of the novel antiarrhythmic agent dronedarone, finding increased mortality related to worsening treatment-associated heart failure.85 Catheter ablation and pulmonary vein isolation appear to be safe and effective in this high-risk cohort and compare favorably with the more established practice of atrioventricular node ablation and biventricular pacing.86
Exercise Training
Exercise training is recommended as an adjunctive treatment in patients with heart failure. Until recently, this recommendation stemmed from small clinical trials with varying end points. HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) investigated the short- (3-month) and long-term (12-month) effects of a supervised exercise program in patients with moderate heart failure.87 Exercise was safe, improved patients' sense of well-being, and correlated with a trend toward mortality reduction.87,88 Maximal changes in 6-minute walk distance were evident at 3 months, but the effects were durable, with significant improvements in cardiopulmonary exercise time and peak oxygen consumption persisting at 12 months. Thus, it is critical that primary care physicians emphasize the importance of exercise to most patients with heart failure and ensure adherence to this recommendation during follow-up.
DEVICE THERAPY
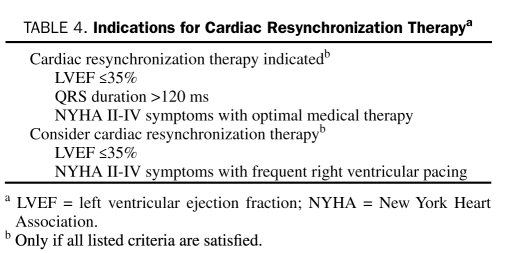
Device therapy adds incremental benefit in patients with systolic heart failure or in those who remain ill after receiving medical therapy and can be used to prevent a crisis such as a sudden arrhythmic event.89 Mechanical dyssynchrony, defined as nonsynchronous contraction between the walls of the left ventricle (intraventricular) or between the ventricular chambers (interventricular), impairs systolic function, adversely affects ventricular filling, increases wall stress, and worsens mitral regurgitation (MR). Dyssynchrony is most readily defined by the presence of QRS widening on the electrocardiogram and can be visualized on 2-dimensional echocardiography. Placement of a pacing lead via the coronary sinus to the lateral wall of the ventricle enables a more synchronous ventricular contraction. Current indications for cardiac resynchronization therapy (CRT) placement are summarized in Table 4. Early studies showed improved exercise capacity, reduction in symptoms, and evidence of reverse remodeling.90 The CARE-HF (Cardiac Resynchronization in Heart Failure Study) trial was the first study to demonstrate a statistically significant reduction in all-cause mortality with CRT placement.91 A meta-analysis of 14 randomized trials of CRT confirmed significant reductions in morbidity and mortality.92 Attempts to further optimize risk stratification and expand indications for CRT using modalities other than electrocardiography have proven disappointing. In particular, echocardiographically derived measures of dyssynchrony vary tremendously, and narrow QRS dyssynchrony has not proven to be a good target for treatment.93,94 At this time, CRT should not be used as salvage therapy in patients admitted with acute decompensated heart failure (ADHF). Current indications for CRT implantation are summarized in Table 4.
TABLE 4.
Indications for Cardiac Resynchronization Therapya
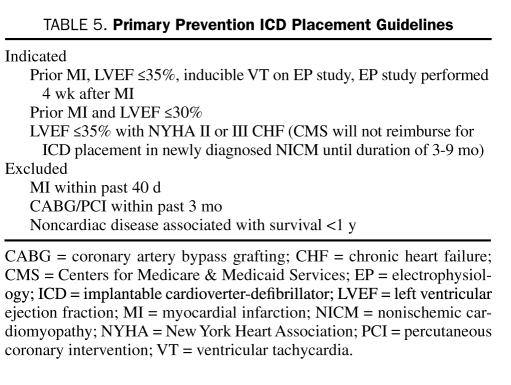
Sudden cardiac death is the mode of death in approximately half of patients with heart failure and is particularly devastating to those with mild symptoms.95 Patients who survive an episode of SCD are considered to be at very high risk and qualify for placement of an implantable cardioverter-defibrillator (ICD). Identifying patients for primary prevention of SCD is challenging. The patients at highest risk are those who have experienced an MI and have impaired LVEF. MADIT II (Multicenter Automatic Defibrillator Trial II) demonstrated that patients with a nonrecent MI and an LVEF of less than 30% derived a significant survival benefit after ICD implantation.96 Criteria for ICD placement were further expanded by the publication of the findings of SCD-HeFT (SCD in Heart Failure Trial), which evaluated all patients with cardiomyopathy (nonischemic and ischemic) and an LVEF of less than 35%, finding significant mortality reductions with ICD treatment.97 Additional efforts to further clarify arrhythmic risk using techniques such as microvolt T-wave alternans have proven disappointing.98 Importantly, current guidelines for ICD implantation capture only a small fraction of the overall number of patients who experience SCD annually, so continued investigation into risk stratification criteria is warranted. In general, a patient with heart failure who is doing well but who has mild symptoms and whose LVEF remains compromised should be considered for ICD implantation. Similarly, the presence of LV dysfunction in survivors of an MI should prompt such consideration even if the patient has no symptoms. In patients with a terminal illness and a predicted life span of fewer than 6 months or in those with New York Heart Association class IV symptoms that are refractory to medications and who are not candidates for transplant, the risks of multiple ICD shocks must be carefully weighed against the survival benefits. Table 5 summarizes current indications for ICD implantation.
TABLE 5.
Primary Prevention ICD Placement Guidelines
SURGICAL TREATMENT
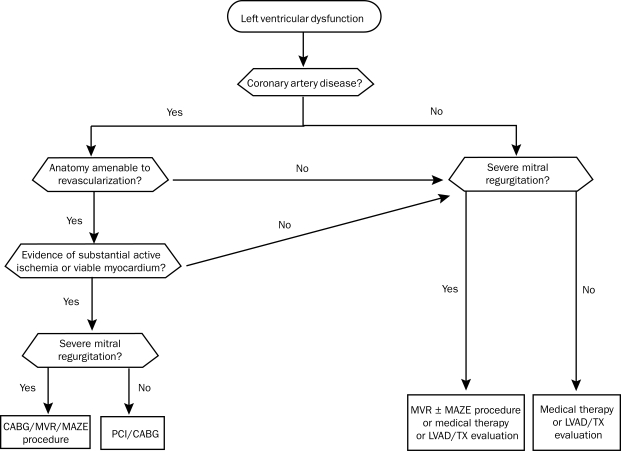
Patients with ischemic cardiomyopathy often have multivessel CAD. The recognition that hibernating myocardium, defined as myocardial tissue with abnormal function but maintained cellular function, could recover after revascularization greatly affected treatment of patients with ischemic cardiomyopathy. Allman et al99 performed a meta-analysis of 24 studies investigating late survival in 3088 patients with ischemic cardiomyopathy treated with revascularization or medical therapy. In patients with myocardial viability (42% of patients), a marked 79% reduction in annual mortality (16% vs 3%) was observed, with the greatest benefit derived among patients with the poorest LV function and the most viability. Furthermore, patients without substantial viability showed no incremental benefit with revascularization. Revascularization is most robustly supported in individuals with ongoing angina and LV failure. Revascularizing those with LV failure in the absence of angina remains controversial, but many clinicians opt for revascularization if a substantial amount of hibernating silently ischemic myocardium is discovered.
Varying degrees of MR are common in dilated cardiomyopathy. Functional MR is characterized by annular dilatation and leaflet noncoaptation in the setting of anatomically normal papillary muscles, chordal structures, and valve leaflets. In patients who are not candidates for surgical coronary revascularization, mitral valve repair remains controversial. Ischemic MR (or infarct-related MR) is typically associated with leaflet tethering and displacement related to abnormal LV wall motion and geometry. In this cohort, mitral valve repair appears safe and feasible; however, the long-term benefits are unclear.100 Concomitant AF is found in a large number of these patients, and a surgical MAZE procedure can be performed at the time of mitral valve surgery with durable maintenance of sinus rhythm.
Multiple surgical techniques to reduce LV volume and thereby alleviate LV wall stress have been used. The recently published STICH (Surgical Treatment for Ischemic Heart Failure) trial randomized patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting (CABG) to CABG alone vs CABG plus surgical ventricular reconstruction.101 Although surgical reconstruction reduced LV volumes and LV wall stress, no difference in mortality or hospitalizations was found. On the basis of these study results, routine surgical LV reconstruction with CABG is discouraged. However, LV volume reduction may still play a role when nonviability of the akinetic segment can be established and when the procedure is likely to provide a volume reduction of a magnitude approaching 30%. Figure 4 summarizes the surgical approach to the patient with heart failure.
FIGURE 4.
Surgical evaluation of heart failure. CABG = coronary artery bypass grafting; LVAD = left ventricular assist device; MVR = mitral valve repair; PCI = percutaneous coronary intervention; TX = heart transplant.
ACUTE DECOMPENSATED HEART FAILURE
Acute decompensated heart failure represents a unique clinical syndrome resulting from interrelated abnormalities of decreased cardiac performance, renal dysfunction, and alterations in vascular compliance. Admission with a diagnosis of ADHF carries a grim prognosis. Half of patients admitted with acute heart failure are readmitted within 6 months,102 and the mortality after an admission is 12% at 30 days.103 ADHERE (Analysis of the Acute Decompensated Heart Failure Registry) revealed that in-hospital mortality after hospital admission for ADHF ranges from 5% to 8%, with 1-year mortality averaging 40% to 60%.104 Hospitalization with ADHF is a sentinel event that signals a progression in disease status.
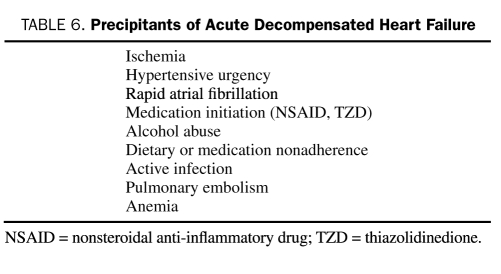
The known precipitants of ADHF are summarized in Table 6. When possible, attempts should be made to identify and treat potential precipitating factors by controlling heart rate or restoring sinus rhythm in patients with poorly tolerated rapid AF, by correcting ischemia with coronary revascularization, and by promptly removing offending medications. Specific attention should be focused on the use of nonsteroidal anti-inflammatory drugs, cold and flu preparations, and herbal preparations, including licorice, ginseng, and ma huang. Routine use of a pulmonary artery catheter is not recommended and should be restricted to those who respond poorly to diuresis or experience hypotension or signs and symptoms suggestive of a low cardiac output.105 Analysis of the ADHERE registry has identified 4 parameters associated with worse outcomes: a blood urea nitrogen level greater than 43 mg/dL (to convert to mmol/L, multiply by 0.357), systolic blood pressure less than 115 mm Hg, a serum creatinine level greater than 2.75 mg/dL (to convert to μmol/L, multiply by 88.4), and an elevated troponin I level.106,107
TABLE 6.
Precipitants of Acute Decompensated Heart Failure
Intravenous diuretic agents rapidly and effectively relieve symptoms of congestion and are essential when oral drug absorption is impaired.108 When high doses of diuretic agents are required, or when the effect is suboptimal, a continuous infusion may be needed to reduce toxicity and maintain stable serum drug levels.109 Addition of a thiazide diuretic agent such as metolazone in combination provides a synergistic effect and is often required in patients receiving long-term therapy with loop diuretic agents.110 Change in weight is often used as a surrogate for adequate diuresis, but this objective measure of volume status may be surprisingly difficult to interpret, and weight loss during hospitalization does not necessarily correlate with outcomes.111 It is generally advisable to continue diuresis until euvolemia has been achieved. Physical examination findings, specifically the jugular venous pressure coupled with biomarker trends, are useful in timing discharge planning.
The cardiorenal syndrome is being recognized increasingly as a complication of ADHF. Multiple definitions have been proposed for the cardiorenal syndrome, but at its simplest it can be thought to reflect the interplay between abnormalities of heart and kidney function, with deteriorating function of one organ while therapy is administered to preserve the other.112 Approximately 30% of patients hospitalized with ADHF have abnormal renal function at baseline, correlating with longer hospitalizations, higher costs, and increased mortality.113 Interestingly, recent studies have found no correlation between deterioration in renal function, cardiac output, filling pressures, and reduced renal perfusion; in fact, most patients who develop cardiorenal syndrome do not have a low cardiac output.114,115 It is hypothesized that in patients with established heart failure, this syndrome represents a complex interplay of neurohormonal factors, potentially exacerbated by “backward failure” resulting from increased intra-abdominal pressure and impairment in return of renal venous blood flow.116 Continued use of diuretic therapy is associated with a reduction in glomerular filtration rate and a worsening of the cardiorenal syndrome.
Ultrafiltration (UF) is an invasive fluid removal technique that may supplement or obviate the need for diuretic therapy. Benefits of UF include adjustable fluid removal rates, neutral effects on serum electrolytes, and decreased neurohormonal activity. Current UF systems can function with 2 large-bore peripherally inserted venous lines. In a pivotal study evaluating UF with conventional therapy, fluid removal was improved and subsequent heart failure hospitalizations and urgent clinic visits were reduced; however, no improvement in renal function and no subjective differences in dyspnea scores or adverse outcomes were noted.117 Ultrafiltration appears safe and effective, but available data do not suggest expanding its role beyond patients who do not respond adequately to conventional diuretic therapy.
Nesiritide is a recombinant brain-type natriuretic peptide. When administered as a continuous infusion with concomitant diuretic therapy, it reduces pulmonary capillary wedge pressure and augments cardiac output.118 Despite these benefits, enthusiasm for nesiritide has waned in recent years. Post hoc analysis of the findings from the pivotal trials has questioned its kidney-sparing effects, and data regarding reductions in hospital stays and improvements in long-term outcomes are lacking.119 Current clinical practice largely restricts the use of nesiritide to normotensive patients who remain volume overloaded despite adequate doses of diuretic therapy. Current research focuses on dose titrations to minimize hypotension and on further identification of patients who are most likely to benefit from treatment.
Inotropic therapy augments cardiac output, improves perfusion, and relieves congestion. Milrinone and dobutamine, the most commonly used inotropes in clinical practice, have similar hemodynamic profiles; however, milrinone is renally excreted and thus requires dose adjustments in the setting of kidney dysfunction. Milrinone acts downstream from the β1-adrenergic receptor, providing a theoretical advantage in patients who are taking high doses of β-blockers when admitted to the hospital. In patients with clinical or laboratory evidence of reduced cardiac output and pulmonary congestion who cannot tolerate further afterload reduction, inotropic support may be indicated but must be used with caution.
Studies are in universal agreement that long-term inotropic therapy increases mortality. More recent studies suggest that even short-term inotrope use may be associated with increased risks.120,121 The OPTIME-CHF (Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure) investigators compared inotropic therapy to standard care in patients admitted with ADHF and found no reductions in subsequent hospitalizations between groups but more frequent hypotension and arrhythmias in the inotrope-treated group.121 The use of these agents remains controversial and highly dependent on the institution or practitioner. They are currently indicated as bridge therapy (to either LV assist device [LVAD] support or to transplant) or for palliation in end-stage heart failure.
In patients who fail to respond adequately to medical therapy, mechanical assist devices may be required. The intra-aortic balloon pump, a percutaneously implanted device inserted via the femoral artery into the descending thoracic aorta, deflates with systole and expands in diastole. Balloon expansion rapidly unloads the LV, enhances coronary arterial blood flow, reduces LV diastolic pressure, and augments cardiac output up to 50%.122,123 Moderate to severe aortic insufficiency and aortic dissection are contraindications to implantation, and care must be taken in the setting of underlying peripheral arterial disease. Intraaortic balloon pump duration is typically 1 to 27 days, with the likelihood of vascular, infectious, and hematologic complications increasing with longer durations.
When organ hypoperfusion persists despite maximal medical support and balloon pump support, a percutaneously or surgically placed LVAD should be considered. The percutaneous devices augment cardiac output but typically require placement of a larger sheath and carry an increased risk of vascular complications. Additional temporary mechanical circulatory support, including venoarterial extracorporeal membrane oxygenation, should be considered in the critically ill patient. Use of long-term mechanical circulatory support via surgically placed LVADs is becoming more common in the management of advanced heart failure. Left ventricular assist devices can be used as a bridge to transplant or in patients who are too ill or hemodynamically unstable to wait on the list.124 In those patients who have conditions that preclude transplant (recent malignancy, high antibody titers), LVAD should be considered as a destination therapy.125 The newer generation of LVADs are smaller axial flow pumps that are easier to implant and carry a lower risk of thromboembolic and infectious complications.
HEART FAILURE WITH PRESERVED SYSTOLIC FUNCTION
“Diastolic” heart failure describes patients with a preserved LVEF, classic signs and symptoms of CHF, and invasive or imaging-based evidence of abnormal diastolic function. Diastole is composed of 2 distinct phases: an initial, energy-dependent, rapid untwisting-and-relaxing phase that creates a suction effect, followed by a phase of ventricular filling, in which elasticity and distensibility of the ventricle facilitate filling at low pressures. The mere presence of diastolic dysfunction does not satisfy the requirements for diastolic heart failure. The incidence of diastolic dysfunction is difficult to estimate because asymptomatic disease is more common than symptomatic diastolic dysfunction.126 Diastolic heart failure is more common in women and in elderly persons.127 Conflicting data have been reported regarding the overall prognosis for patients with diastolic heart failure vs those with systolic heart failure.128
Heart failure with preserved LVEF is seen in a spectrum of disorders not limited solely to diastolic dysfunction, including valvular heart disease, pericardial disease, and disease resulting in right ventricular dilatation and dysfunction. Left ventricular ejection fraction is a commonly used, albeit imperfect, measure of systolic function. Recent studies have identified abnormalities in torsional mechanics, demonstrating that diastolic heart failure is accompanied by abnormalities in systolic function.129 Stroke volume and cardiac output are often reduced despite a normal ejection fraction. In diastolic heart failure, the LV exhibits characteristic remodeling changes: near-normal end-diastolic volumes, increased wall thickness, and increased ratio of wall thickness to chamber diameter.
Signs and symptoms of diastolic heart failure are identical to those of systolic heart failure. Exercise is poorly tolerated.130 The heart with diastolic dysfunction cannot relax to accommodate the increased blood flow required to maintain a higher cardiac output, and perfusion is maintained via elevations in left atrial pressures, resulting in symptoms of dyspnea. Atrial fibrillation is particularly problematic, and the combined effects of the loss of atrial kick and the rapid heart rates further impair diastolic filling. Plasma brain-type natriuretic peptide levels are elevated in diastolic heart failure but are not helpful in discerning between diastolic and systolic heart failure.131
Identification of specific therapeutic agents has been disappointing. Regression of hypertrophy is often used as an end point in the hypertension literature. Although drug classes variably affect wall thickness, whether this surrogate end point translates to improved outcomes in diastolic heart failure remains unclear.132 The unimpressive experience with lusitropic agents, including β-blockers and calcium channel blockers, has challenged the notion that symptoms may be improved by simply enhancing LV compliance. Emerging data suggest that lowering blood pressure alleviates symptoms more effectively than therapy with specific agents.133 The CHARM (Candesartan in Heart Failure—Assessment of Mortality And Morbidity) Preserved study showed a statistically significant reduction in hospitalizations but no difference in all-cause mortality in patients with diastolic heart failure who were treated with candesartan.134 The I-PRESERVE (Irbesartan in Heart Failure with Preserved Systolic Function) trial demonstrated no differences in meaningful end points in patients with diastolic heart failure treated with irbesartan.135 A subset of the DIG trial found no role for digoxin in the treatment of heart failure with preserved LVEF.136 OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) results showed no change in outcomes in patients hospitalized with diastolic heart failure in whom β-blocker therapy was initiated.137
The recently updated 2005 ACC/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults identified only 4 areas for treatment: blood pressure control, rate/rhythm control in underlying AF, control of pulmonary congestion with diuretic agents, and revascularization and correction of underlying ischemia when indicated.23 Identification and appropriate treatment of sleep-disordered breathing should be considered as well. Venodilators should be used with caution because decreases in preload may lead to underfilling, resulting in hypotension and syncope.
PREDICTING PROGNOSIS
Patients and physicians routinely overestimate survival in patients with heart failure. Furthermore, differences exist in the survival estimated by the physician, that which is communicated to the patient, and that which is understood by the patient. Moderately symptomatic patients remain at high risk of SCD, highlighting the disassociation between symptoms and prognosis.96 Several models have been devised and have proven beneficial to the physician in delivering a realistic expectation of prognosis, setting treatment goals, and guiding the escalation of therapy.138
The Heart Failure Survival Score, which was designed in ambulatory patients with heart failure and advanced symptoms (New York Heart Association III and IV), incorporates 7 variables into a model: heart rate, serum sodium concentration, etiology, QRS duration, ejection fraction, peak exercise oxygen consumption, and blood pressure.139 Although this score performs well in the β-blocker era, it was developed before widespread use of aldosterone antagonists and does not take into account newer prognostic markers, including renal function and anemia.140 A newer model, the Seattle Heart Failure Model,141 which incorporates a broader range of patients along with multiple clinical predictors, laboratory data, and medical therapy, correlates well with 1-, 2-, and 3-year survival, similar to the Framingham Coronary Heart Disease Risk Model. An advantage of the Seattle Heart Failure Model is the ability to help with predicting the mode of death in heart failure: pump failure vs SCD.142 A simpler approach evaluates the number of hospitalizations because an increasing number of hospitalizations correlates with increased mortality.143
In patients who continue to decline despite optimal pharmacological and device-based therapy, referral to a heart failure center is appropriate. Heart failure care can be enhanced through participation in a multidisciplinary clinic, with increased attention to patient education and frequent nursing visits.144 Progression of disease is heralded by weight loss and medication intolerance, resulting in dose reductions, recurrent hospitalizations, and diminished functional capacity.
Cardiopulmonary exercise testing provides an assessment of a patient's global exercise capacity and is useful in risk stratification. Patients with a maximum oxygen consumption of less than 14 mL/kg per minute are considered at high risk of clinical worsening and should be referred for advanced therapy.145 Cardiac transplant has emerged as the definitive treatment for patients with refractory, symptomatic heart failure. In properly selected candidates, cardiac transplant can be expected to provide a 1-year survival in excess of 90% and a 10-year survival of 50%.146 The age of suitable candidates continues to expand, and carefully selected candidates older than 65 years have a prognosis comparable to that of the transplant population at large.147 Candidates are selected via a careful multidisciplinary screening process, which involves surgeons, social workers, financial planners, psychiatrists, and transplant physicians. Patients with fixed pulmonary hypertension fare poorly after transplant, and those with chronic disease (diabetes, connective tissue diseases, kidney disease, peripheral arterial disease) must be carefully assessed. The main obstacle to transplant remains the limited number of available donors. The donor pool has remained relatively fixed in recent years despite an increase in the number of candidates listed for transplant.
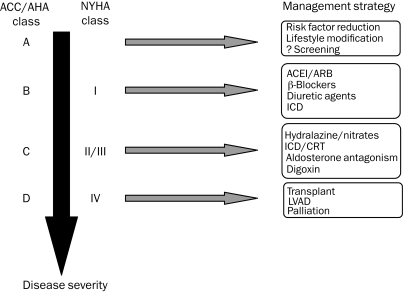
In patients who are not candidates for advanced heart failure therapy and who have worsening symptoms despite maximal therapy, consideration should be given for palliation. Involvement of the family as well as a palliative care specialist is advisable and helpful in transitioning medical care toward palliation. Figure 5 summarizes a stepwise approach to heart failure care.
FIGURE 5.
Stepwise approach to heart failure care. ACC = American College of Cardiology; ACEI = angiotensin-converting enzyme inhibitor; AHA = American Heart Association; ARB = angiotensin II receptor blocker; CRT = cardiac resynchronization therapy; ICD = implantable cardioverter-defibrillator; LVAD = left ventricular assist device; NYHA = New York Heart Association.
NOVEL THERAPIES
Adenosine is well known to have renal effects, causing constriction of the afferent arteriole, with subsequent reduction in renal blood flow and glomerular filtration rate as well as direct effects on enhancing sodium reabsorption in the proximal tubule of the kidney.148 In patients admitted to the hospital with ADHF, adenosine A1 receptor antagonists enhance the effects of loop diuretic agents and have renal protective effects.149 Clinical trials are under way to evaluate the role of A1 receptor antagonists in patients with ADHF.
Anemia is associated with a poorer prognosis in patients with heart failure,150 and registry data have identified anemia in approximately one-third of such patients.151 Small studies suggest a potential benefit for treatment of anemia in patients with heart failure, but larger randomized trials are necessary to determine when to initiate therapy and to identify the goals of treatment.152
Abundant animal data support the membrane-stabilizing effects of fish oils in patients with heart failure. The GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto)-HF trial demonstrated a small but statistically significant reduction in mortality in patients with heart failure who received adequate background therapy and were randomized to treatment with fish oils.153 Given the extensive pharmacotherapy already prescribed to patients with heart failure, concerns regarding potential polypharmacy and cost must be carefully weighed when prescribing additional medications.
CONCLUSION
As the population ages and cardiovascular risk factors become increasingly prevalent, health care professionals in multiple disciplines will encounter patients at risk of heart failure. Successful management of this population depends on risk factor reduction via lifestyle modification and application of currently established guidelines. During the past generation, a combination of behavioral, pharmacological, device-based, and surgical treatment modalities has tremendously enhanced the survival and quality of life of patients with heart failure. In light of the increasing prevalence of heart failure, continued application of these principles and research into novel treatment strategies remain vital.
Supplementary Material
On completion of this article, you should be able to: (1) apply evidence-based pharmacotherapy for systolic heart failure, (2) use multiple treatment strategies for the management of acute decompensated heart failure, and (3) recognize the importance of device therapy in heart failure treatment.
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
The contributions to the Symposium on Cardiovascular Diseases are now a CME activity. For CME credit, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39(1):60-69 [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I; diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105(11):1387-1393 [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002;106(24):3068-3072 [DOI] [PubMed] [Google Scholar]
- 4.Ghali JK, Kadakia S, Cooper RS, Yiao YL. Bedside diagnosis of preserved versus impaired left ventricular systolic function in heart failure. Am J Cardiol. 1991;67(11):1002-1006 [DOI] [PubMed] [Google Scholar]
- 5.Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 1989;261(6):884-888 [PubMed] [Google Scholar]
- 6.Mckee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441-1446 [DOI] [PubMed] [Google Scholar]
- 7.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167 [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA, Duc P, Omland T, et al. Breathing Not Properly Multinational Study Investigators B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579 [DOI] [PubMed] [Google Scholar]
- 9.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43(9):1590-1595 [DOI] [PubMed] [Google Scholar]
- 10.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996-1002 [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure: a clinical mechanistic overview. Arch Intern Med. 1996;156(16):1789-1796 [PubMed] [Google Scholar]
- 12.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275(20):1557-1562 [PubMed] [Google Scholar]
- 13.Moser M, Herbert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27(5):1214-1218 [DOI] [PubMed] [Google Scholar]
- 14.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4, suppl A):6A-13A [DOI] [PubMed] [Google Scholar]
- 15.Walsh CR, Larson MG, Evans JC, et al. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2002;136(3):181-191 [DOI] [PubMed] [Google Scholar]
- 16.Bryson CL, Mukamal KJ, Mittleman MA, et al. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006;48(2):305-311 [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW, Cook NR, Evans DA, et al. Relationship of alcohol intake with blood pressure in young adults. Hypertension 1995;25(5):1106-1110 [DOI] [PubMed] [Google Scholar]
- 18.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305-313 [DOI] [PubMed] [Google Scholar]
- 19.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321(4):225-236 [DOI] [PubMed] [Google Scholar]
- 20.Alpert MA, Terry BE, Mulekar M, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and the effects of weight loss. Am J Cardiol. 1997;80(6):736-740 [DOI] [PubMed] [Google Scholar]
- 21.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891-894 [DOI] [PubMed] [Google Scholar]
- 22.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13-22 [DOI] [PubMed] [Google Scholar]
- 23.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology Foundation/American Heart Association 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53(15):e1-e90 [DOI] [PubMed] [Google Scholar]
- 24.SOLVD Investigators Effects of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left-ventricular ejection fractions [published correction appears in N Engl J Med. 1992;327(24):1768] N Engl J Med. 1992;327(10):685-691 [DOI] [PubMed] [Google Scholar]
- 25.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35(6):1628-1637 [DOI] [PubMed] [Google Scholar]
- 26.Betti I, Castelli G, Barchielli A, et al. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure: The PROBE-HF study. J Card Fail. 2009;15(5):377-384 [DOI] [PubMed] [Google Scholar]
- 27.Sundström J, Ingelsson E, Berglund L, et al. Cardiac troponin-I and risk of heart failure: a community-based cohort study. Eur Heart J. 2009;30(7):773-781 [DOI] [PubMed] [Google Scholar]
- 28.O'Brien PJ, Gwathmey JK. Myocardial Ca2+ and ATP-cycling imbalances in end-stage dilated and ischemic cardiomyopathies. Cardiovasc Res. 1995;30(3):394-404 [DOI] [PubMed] [Google Scholar]
- 29.Bristow MR, Ginsburg R, Minobe W, et al. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in the failing human heart. N Engl J Med. 1982;307(4):205-211 [DOI] [PubMed] [Google Scholar]
- 30.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 2000;101(25):2981-2988 [DOI] [PubMed] [Google Scholar]
- 31.CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;31(23):1429-1435 [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Braunwald E, Moyé LA, et al. SAVE Investigators Effect of captopril on morbidity and mortality in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. N Engl J Med. 1992;327(10):669-677 [DOI] [PubMed] [Google Scholar]
- 33.SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302 [DOI] [PubMed] [Google Scholar]
- 34.Acute Infarction Ramipril Efficacy (AIRE) Study Investigators Effect of ramipril on mortality and morbidity on survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993;342(8875):821-828 [PubMed] [Google Scholar]
- 35.CIBIS-II Investigators and Committees The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353(9146):9-13 [PubMed] [Google Scholar]
- 36.MERIT-HF Study Group Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353(9169):2001-2007 [PubMed] [Google Scholar]
- 37.Packer M, Coats AJ, Fowler MB, et al. Carvedilol Prospective Randomized Cumulative Survival Study Group Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651-1658 [DOI] [PubMed] [Google Scholar]
- 38.Vaughn DE. Fibrinolytic balance, the renin-angiotensin system and atherosclerotic disease. Eur Heart J. 1998;19(suppl G):G9-G12 [PubMed] [Google Scholar]
- 39.Garg R, Yusuf S, Collaborative Group on ACE Inhibitor Trials Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure [published correction appears in JAMA 1995;274(6):462] JAMA 1995;273(18):1450-1456 [PubMed] [Google Scholar]
- 40.Bristow MR. β-Adrenergic receptor blockade in chronic heart failure. Circulation 2000;101(5):558-569 [DOI] [PubMed] [Google Scholar]
- 41.Kazi D, Deswal A. Role and optimal dosing of angiotensin-converting enzyme inhibitors in heart failure. Cardiol Clin. 2008;26(1):1-14, v [DOI] [PubMed] [Google Scholar]
- 42.Xamoterol in Severe Heart Failure Study Group Xamoterol in severe heart failure [published correction appears in Lancet. 1990;336(8713):698] Lancet 1990;336(8706):1-6 [PubMed] [Google Scholar]
- 43.Beta-Blocker Evaluation and Survival Trial Investigators A trial of beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344(22):1659-1667 [DOI] [PubMed] [Google Scholar]
- 44.Willenheimer R, Van Veldhuisen DJ, Silke B, et al. CIBIS III Investigators Effect of survival and hospitalization on initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol study (CIBIS) III. Circulation 2005;112(16):2426-2435 [DOI] [PubMed] [Google Scholar]
- 45.Bristow MR, Gilbert EM, Abraham WT, et al. MOCHA Investigators Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 1996;94(11):2807-2816 [DOI] [PubMed] [Google Scholar]
- 46.Packer M, Poole-Wilson PA, Armstrong PW, et al. ATLAS Study Group Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. Circulation 1999;100(23):2312-2318 [DOI] [PubMed] [Google Scholar]
- 47.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L, CONSENSUS Trial Study Group Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation 1990;82(5):1730-1736 [DOI] [PubMed] [Google Scholar]
- 48.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000;101(6):594-597 [DOI] [PubMed] [Google Scholar]
- 49.Pitt B, Zannad F, Remme WJ, et al. Randomized Spironolactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709-717 [DOI] [PubMed] [Google Scholar]
- 50.Pitt B, Remme W, Zannad F, et al. Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction [published correction appears in N Engl J Med. 2003;348(22):2271] N Engl J Med. 2003;348(14):1309-1321 [DOI] [PubMed] [Google Scholar]
- 51.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351(6):543-551 [DOI] [PubMed] [Google Scholar]
- 52.Roig E, Perez-Villa F, Morales M, et al. Clinical implications of increased plasma angiotensin II despite ACE inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21(1):53-57 [DOI] [PubMed] [Google Scholar]
- 53.Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet 2000;355(9204):637-645 [DOI] [PubMed] [Google Scholar]
- 54.Lee VC, Rhew DC, Dylan M, Badamgarav E, Braunstein GD, Weingarten SR. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high risk acute myocardial infarction [published correction appears in Ann Intern Med. 2005;42(5):391. Dosage error in article text] Ann Intern Med. 2004;141(9):693-704 [DOI] [PubMed] [Google Scholar]
- 55.Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345(23):1667-1675 [DOI] [PubMed] [Google Scholar]
- 56.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan in Acute Myocardial Infarction Trial Investigators Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [published correction appears in N Engl J Med. 2004;350(2):203] N Engl J Med. 2003;349(20):1893-1906 [DOI] [PubMed] [Google Scholar]
- 57.Ferdinand KC. Isosorbide dinitrate and hydralazine hydrochloride: a review of efficacy and safety. Expert Rev Cardiovasc Ther. 2005;3(6):993-1001 [DOI] [PubMed] [Google Scholar]
- 58.Ignarro LJ, Lippton H, Edwards JC, et al. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218(3):739-749 [PubMed] [Google Scholar]
- 59.Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure: results of the Veterans Administration Cooperative Study. N Engl J Med. 1986;314(24):1547-1552 [DOI] [PubMed] [Google Scholar]
- 60.Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic heart failure. N Engl J Med. 1991;325(5):303-310 [DOI] [PubMed] [Google Scholar]
- 61.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure [published correction appears in N Engl J Med. 2005;352(12):1276] N Engl J Med. 2004;351(20):2049-2057 [DOI] [PubMed] [Google Scholar]
- 62.Gheorghiade M, Adams KF, Jr, Colucci WS. Digoxin in the management of cardiovascular disorders. Circulation 2004;109(24):2959-2964 [DOI] [PubMed] [Google Scholar]
- 63.Gheorgiade M, Ferguson D. Digoxin: a neurohormonal modulator in heart failure? Circulation 1991;84(5):2181-2186 [DOI] [PubMed] [Google Scholar]
- 64.Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533 [DOI] [PubMed] [Google Scholar]
- 65.Rathore SS, Wang Y, Krumholz HM. Sex based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347(18):1403-1411 [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto K, Ikeda U, Furuhashi K, Irokawa M, Nakayama T, Shimada K. The coagulation system is activated in idiopathic cardiomyopathy. J Am Coll Cardiol. 1995;25(7):1634-1640 [DOI] [PubMed] [Google Scholar]
- 67.Nguyen KN, Aursnes I, Kjekshus J. Interaction between enalapril and aspirin on mortality after acute myocardial infarction: subgroup analysis of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). Am J Cardiol. 1997;79(2):115-119 [DOI] [PubMed] [Google Scholar]
- 68.Massie BM, Collins JF, Ammon SE, et al. WATCH Trial Investigators Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation 2009;119(12):1616-1624 [DOI] [PubMed] [Google Scholar]
- 69.Packer M, O'Conner CM, Ghali JK, et al. Failure Prospective Randomized Amlodipine Survival Evaluation Study Group Effect of amlodipine on morbidity and mortality in severe chronic heart failure. N Engl J Med. 1996;335(15):1107-1114 [DOI] [PubMed] [Google Scholar]
- 70.Udelson JE, DeAbate CA, Berk M, et al. Effects of amlodipine on exercise tolerance, quality of life, and left ventricular function in patients with heart failure from left ventricular systolic dysfunction. Am Heart J. 2000;139(3):503-510 [DOI] [PubMed] [Google Scholar]
- 71.Cohn JN, Ziesche S, Smith R, et al. Vasodilator-Heart failure Trial (V-HeFT) Study Group Effect of the calcium antagonist felodipine as supplementary vasodilator therapy in patients with chronic heart failure treated with enalapril: V-HeFT III. Circulation 1997;96(3):856-863 [DOI] [PubMed] [Google Scholar]
- 72.Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S, Adverse Experience Committee. Multicenter Diltiazem Postinfarction Research Group Diltiazem increases late-onset congestive heart failure in postinfarction patients with early reduction in ejection fraction. Circulation 1991;83(1):52-60 [DOI] [PubMed] [Google Scholar]
- 73.Goldsmith SR, Gheorghiade M. Vasopressin antagonism and heart failure. J Am Coll Cardiol. 2005;46(10):1785-1791 [DOI] [PubMed] [Google Scholar]
- 74.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297(12):1319-1331 [DOI] [PubMed] [Google Scholar]
- 75.Anand I, McMurray J, Cohn JN, et al. EARTH Investigators Long-term effects of darusentan on the left ventricular remodelling and clinical outcomes in the endothelinA receptor antagonist trial in heart failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364(9431):347-354 [DOI] [PubMed] [Google Scholar]
- 76.Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002;106(8):920-926 [DOI] [PubMed] [Google Scholar]
- 77.Califf RM, Adams KF, McKenna WJ, et al. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1997;134(1):44-54 [DOI] [PubMed] [Google Scholar]
- 78.Chung ES, Packer M, Lo KH, et al. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107(25):3133-3140 [DOI] [PubMed] [Google Scholar]
- 79.Mann DL, McMurray JJ, Packer M, et al. Target anticytokine therapy in patients with chronic heart failure: the results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation 2004;109(13):1594-1602 [DOI] [PubMed] [Google Scholar]
- 80.Cohn JN, Pfeffer MA, Rouleau J, et al. Adverse mortality effect of central sympathetic inhibition with sustained-release moxonidine in patients with heart failure (MOXCON). Eur J Heart Fail. 2003;5(5):659-667 [DOI] [PubMed] [Google Scholar]
- 81.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279-285 [DOI] [PubMed] [Google Scholar]
- 82.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631 [DOI] [PubMed] [Google Scholar]
- 83.Bradley TD, Logan AG, Kimoff RJ, et al. CANPAP Investigators Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025-2033 [DOI] [PubMed] [Google Scholar]
- 84.Roy D, Talajic M, Nattel S, et al. Atrial Fibrillation and Congestive Heart Failure Investigators Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667-2677 [DOI] [PubMed] [Google Scholar]
- 85.Køber L, Torp-Pedersen C, McMurray JJV, et al. Dronedarone Study Group Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358(25):2667-2677 [DOI] [PubMed] [Google Scholar]
- 86.Khan MN, Jaïs P, Cummings J, et al. PABA-CHF Investigators Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359(17):1778-1785 [DOI] [PubMed] [Google Scholar]
- 87.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1439-1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301(14):1451-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation 2008;117(21):e350-e408 [DOI] [PubMed] [Google Scholar]
- 90.Auricchio A, Kloss M, Trautmann SI, Rodner S, Klein H. Exercise performance following cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. Am J Cardiol. 2002;89(2):198-203 [DOI] [PubMed] [Google Scholar]
- 91.Cleland JG, Daubert JC, Erdmann E, et al. Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization therapy on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539-1549 [DOI] [PubMed] [Google Scholar]
- 92.McAlister FA, Eskowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 2007;297(22):2502-2514 [DOI] [PubMed] [Google Scholar]
- 93.Beshai JF, Grimm RA, Nagueh SF, et al. RethinQ Study Investigators Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357(24):2461-2471 [DOI] [PubMed] [Google Scholar]
- 94.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117(20):2608-2616 [DOI] [PubMed] [Google Scholar]
- 95.Saxon LA. Sudden cardiac death: epidemiology and temporal trends. Rev Cardiovasc Med. 2005;6(suppl 2):S12-S20 [PubMed] [Google Scholar]
- 96.Moss AJ, Zareba W, Hall WJ, et al. Multicenter Automatic Defibrillator Implantation Trial II Investigators Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883 [DOI] [PubMed] [Google Scholar]
- 97.Bardy GH, Lee KL, Mark DB, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators Amiodarone or an implantable cardio-defibrillator for congestive heart failure [published correction appears in N Engl J Med. 2005;352(20):2146] N Engl J Med. 2005;352(3):225-237 [DOI] [PubMed] [Google Scholar]
- 98.Chow T, Kereiakes DJ, Onufer J, et al. MASTER Trial Investigators Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? the MASTER (Microvolt T wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52(20):1607-1615 [DOI] [PubMed] [Google Scholar]
- 99.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39(7):1151-1158 [DOI] [PubMed] [Google Scholar]
- 100.Diodato MD, Moon MR, Pasque MK, et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78(3):794-799 [DOI] [PubMed] [Google Scholar]
- 101.Jones RH, Velazquez EJ, Michler RE, et al. STICH Hypothesis 2 Investigators Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360(17):1705-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. 2002;162(15):1689-1694 [DOI] [PubMed] [Google Scholar]
- 103.Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137(2):352-360 [DOI] [PubMed] [Google Scholar]
- 104.Adams KF, Jr, Fonarow GC, Emerman CL, et al. ADHERE Scientific Advisory Committee and Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure Registry (ADHERE). Am Heart J. 2005;149(2):209-216 [DOI] [PubMed] [Google Scholar]
- 105.Binanay C, Califf RM, Hasselblad V, et al. ESCAPE Investigators and ESCAPE Study Coordinators Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294(13):1625-1633 [DOI] [PubMed] [Google Scholar]
- 106.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Biscardin WJ, ADHERE Scientific Advisory Committee, Study Group, and Investigators Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293(5):572-580 [DOI] [PubMed] [Google Scholar]
- 107.Peacock WF, IV, De Marco T, Fonarow GC, et al. ADHERE Investigators Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358(20):2117-2126 [DOI] [PubMed] [Google Scholar]
- 108.Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC. Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med. 1985;102(3):314-318 [DOI] [PubMed] [Google Scholar]
- 109.Dormans TP, van Meyel JJ, Gerlag PG, Tan Y, Russel FG, Smits P. Diuretic efficacy of high dose furosemide in severe heart failure: bolus injection versus continuous infusion. J Am Coll Cardiol. 1996;28(2):376-382 [DOI] [PubMed] [Google Scholar]
- 110.Mouallem M, Brif I, Mayan H, Farfel Z. Prolonged therapy by the combination of furosemide and thiazides in refractory heart failure and other fluid retaining conditions. Int J Cardiol. 1995;50(2):89-94 [DOI] [PubMed] [Google Scholar]
- 111.Mehta RH, Rogers JG, Hasselblad V, et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial Investigators Association of weight change with subsequent outcomes in patients hospitalized with acute decompensated heart failure. Am J Cardiol. 2009;103(1):76-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown JR, Uber PA, Mehra MR. The progressive cardiorenal syndrome in heart failure: mechanisms and therapeutic insights. Curr Treat Options Cardiovasc Med. 2008;10(4):342-348 [DOI] [PubMed] [Google Scholar]
- 113.Geisberg C, Butler J. Addressing the challenges of cardiorenal syndrome. Cleve Clin J Med. 2006;73(5):485-491 [DOI] [PubMed] [Google Scholar]
- 114.Weinfeld MS, Chertow GW, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J. 1999;138(2, pt 1):285-290 [DOI] [PubMed] [Google Scholar]
- 115.Wencker D. Acute cardio-renal syndrome: progression from congestive heart failure to congestive kidney failure. Curr Heart Fail Rep. 2007;4(3):134-138 [DOI] [PubMed] [Google Scholar]
- 116.Mullens W, Abrahams Z, Skouri HN, et al. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51(3):300-306 [DOI] [PubMed] [Google Scholar]
- 117.Costanzo MR, Guglin ME, Saltzberg MT, et al. UNLOAD Trial Investigators Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure [published correction appears in J Am Coll Cardiol. 2007;49(10):1136] J Am Coll Cardiol. 2007;49(6):675-683 [DOI] [PubMed] [Google Scholar]
- 118.Mills RM, LeJemtel TH, Horton DP, et al. Natrecor study group Sustained hemodynamic effects of an infusion of nesiritide (human b-type natriuretic peptide) in heart failure: a randomized, double-blind placebo-controlled clinical trial. J Am Coll Cardiol. 1999;34(1):155-162 [DOI] [PubMed] [Google Scholar]
- 119.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure [published correction appears in Circulation. 2005;111(17):2274] Circulation 2005;111(12):1487-1491 [DOI] [PubMed] [Google Scholar]
- 120.O'Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1, pt 1):78-86 [DOI] [PubMed] [Google Scholar]
- 121.Cuffe MS, Califf RM, Adams KF, Jr, et al. Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Investigators Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002;287(12):1541-1547 [DOI] [PubMed] [Google Scholar]
- 122.Buckley MJ, Leinbach RC, Kastor JA, et al. Hemodynamic evaluation of intra-aortic balloon pumping in man. Circulation 1970;41(5)(suppl):II130-II136 [DOI] [PubMed] [Google Scholar]
- 123.Dilley RB, Ross J, Jr, Bernstein EF. Serial hemodynamics during intra-aortic balloon counterpulsation for cardiogenic shock. Circulation 1973;48(1)(suppl):III99-III104 [DOI] [PubMed] [Google Scholar]
- 124.Miller LW, Pagani FD, Russel SD, et al. HeartMate II Clinical Investigators Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357(9):885-896 [DOI] [PubMed] [Google Scholar]
- 125.Lietz K, Long JW, Kfoury AG, et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation 2007;116(5):497-505 [DOI] [PubMed] [Google Scholar]
- 126.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289(2):194-202 [DOI] [PubMed] [Google Scholar]
- 127.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved systolic function. J Am Coll Cardiol. 2003;41(2):217-223 [DOI] [PubMed] [Google Scholar]
- 128.Senni M, Redfield MM. Heart failure with preserved systolic function: a different natural history? J Am Coll Cardiol. 2001;38(5):1277-1282 [DOI] [PubMed] [Google Scholar]
- 129.Tan YT, Wenzelburger F, Lee E, et al. The pathophysiology of heart failure with normal ejection fraction; exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54(1):36-46 [DOI] [PubMed] [Google Scholar]
- 130.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure with preserved systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065-1072 [DOI] [PubMed] [Google Scholar]
- 131.Lubien E, DeMaria A, Krishnaswamy P, et al. Utility of B-natriuretic peptide in detecting diastolic dysfunction: comparison with Doppler velocity recordings [published correction appears in Circulation. 2002;106(3):387] Circulation 2002;105(5):595-601 [DOI] [PubMed] [Google Scholar]
- 132.Klingbeil AU, Schneider M, Martus P, Messerli FH, Schmieder RE. A meta-analysis of the effects of treatment of left ventriclular mass in essential hypertension. Am J Med. 2003;115(1):41-46 [DOI] [PubMed] [Google Scholar]
- 133.Solomon SD, Janardhanan R, Verma A, et al. Valsartan In Diastolic Dysfunction (VALIDD) Investigators Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet 2007;369(9579):2079-2087 [DOI] [PubMed] [Google Scholar]
- 134.Yusuf S, Pfeffer MA, Swedberg K, et al. CHARM Investigators and Committees Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362(9386):777-781 [DOI] [PubMed] [Google Scholar]
- 135.Massie BM, Carson PE, McMurray JJ, et al. I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359(23):2456-2467 [DOI] [PubMed] [Google Scholar]
- 136.Ahmed A, Rich MW, Fleg Jl, et al. Effect of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation 2006;114(5):397-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hernandez AF, Hammil BG, O'Connor CM, et al. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) registry. J Am Coll Cardiol. 2009;53(2):184-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goldberg LR, Jessup M. A time to be born and a time to die [editorial]. Circulation 2007;116(4):360-362 [DOI] [PubMed] [Google Scholar]
- 139.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997;95(12):2660-2667 [DOI] [PubMed] [Google Scholar]
- 140.Kolling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving β-blockers. J Heart Lung Transplant. 2004;23(12):1414-1422 [DOI] [PubMed] [Google Scholar]
- 141.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113(11):1424-1433 [DOI] [PubMed] [Google Scholar]
- 142.Mozaffarian D, Anker SK, Anand I, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation 2007;116(4):392-398 [DOI] [PubMed] [Google Scholar]
- 143.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260-266 [DOI] [PubMed] [Google Scholar]
- 144.Fonarow GC, Abraham WT, Albert NM, et al. OPTIMIZE-HF Investigators and Hospitals Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Arch Intern Med. 2007;167(14):1493-1502 [DOI] [PubMed] [Google Scholar]
- 145.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991;83(3):778-786 [DOI] [PubMed] [Google Scholar]
- 146.Taylor DO, Edwards LB, Aurora P, et al. Registry of the International Society of Heart and Lung Transplantation: twenty-fifth official adult heart transplant report—2008. J Heart Lung Transplant. 2008;27(9):943-956 [DOI] [PubMed] [Google Scholar]
- 147.Morgan JA, John R, Weinberg AD, et al. Long-term results of cardiac transplantation in patients 65 years of age and older: a comparative analysis. Ann Thorac Surg. 2003;76(6):1982-1987 [DOI] [PubMed] [Google Scholar]
- 148.Kriz W. Adenosine and ATP: traffic regulators in the kidney. J Clin Invest. 2004;114(5):611-613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Givertz MM, Massie BM, Fields TK, et al. The effects of KW-3902, an adenosine A1-receptor antagonist on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50(16):1551-1560 [DOI] [PubMed] [Google Scholar]
- 150.Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomized amlodipine survival evaluation (PRAISE). J Am Coll Cardiol. 2003;41(11):1933-1939 [DOI] [PubMed] [Google Scholar]
- 151.Adams KF, Jr, Patterson JH, Orem RM, et al. STAMINA-HFP Registry Investigators Prospective assessment of the occurrence of anemia in patients with heart failure: results from the Study of Anemia in a Heart Failure Population (STAMINA-HFP) Registry. Am Heart J. 2009;157(5):926-932 [DOI] [PubMed] [Google Scholar]
- 152.Parissis JT, Kourea K, Andreadou I, et al. Effects of Darbepoetin Alfa on plasma mediators of oxidative and nitrosative stress in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2009;103(8):1134-1138 [DOI] [PubMed] [Google Scholar]
- 153.Gissi-HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372(9645):1223-1230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.