Abstract
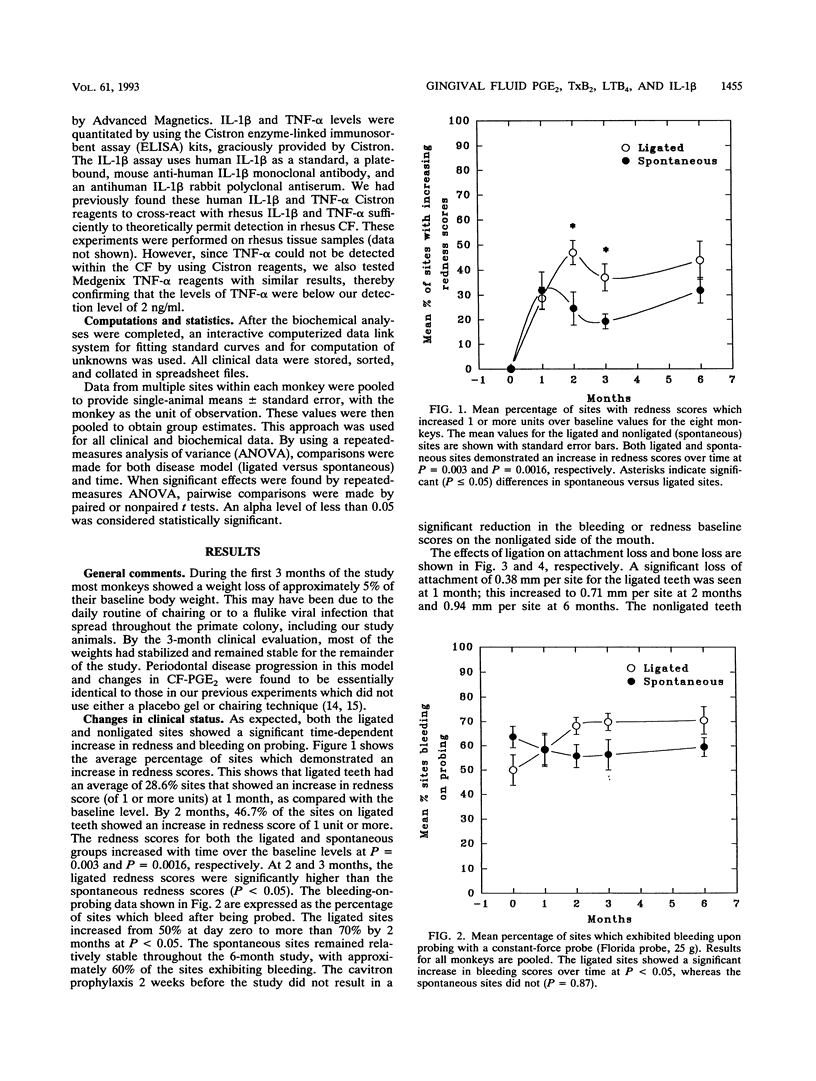
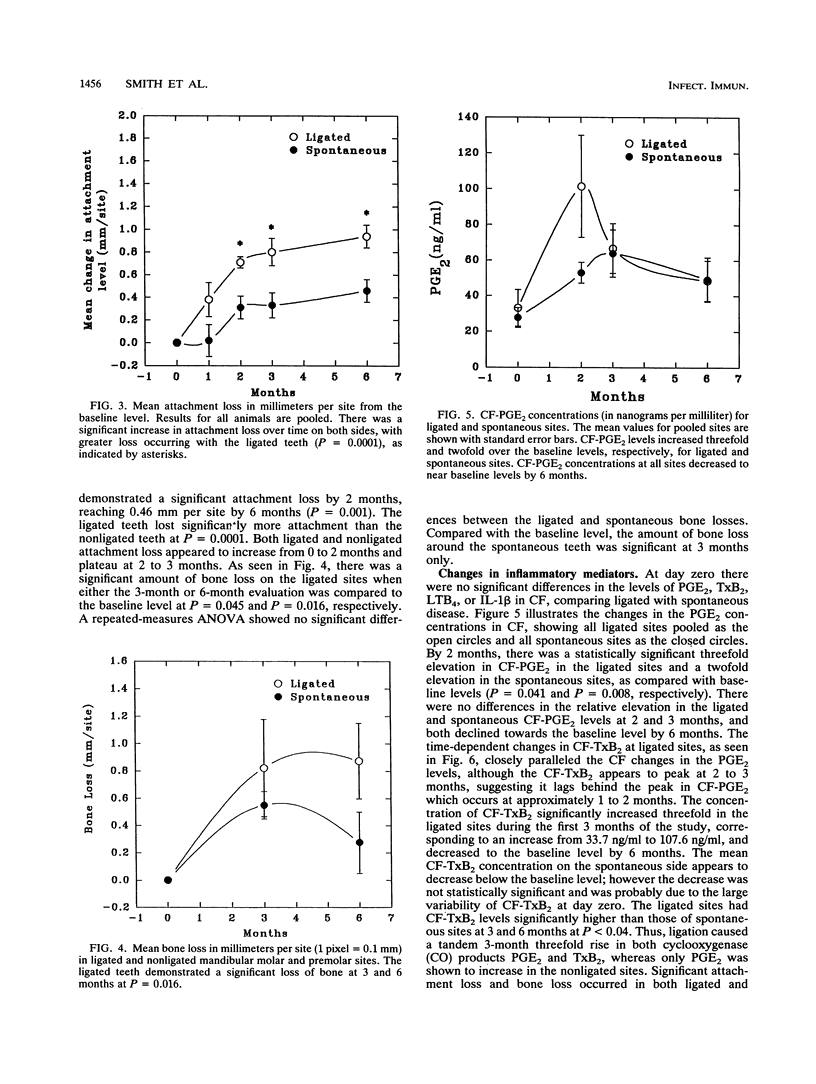
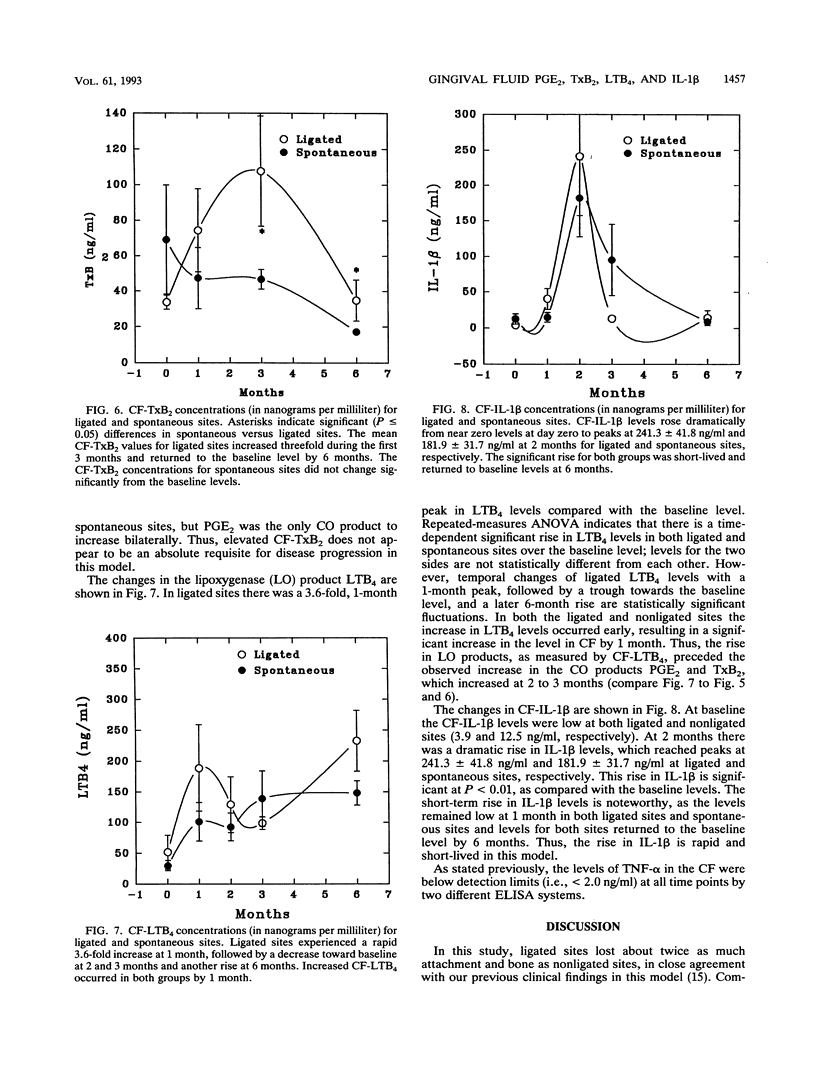
Ligature-induced periodontitis was monitored for 6 months in eight Macaca mulatta monkeys to examine clinical status, radiographic bone level, and crevicular fluid (CF) levels of prostaglandin E2 (PGE2), thromboxane B2 (TxB2), interleukin-1 beta (IL-1 beta), tumor necrosis factor alpha, and leukotriene B4 (LTB4). A split-mouth design was used, with eight ligated teeth and eight contralateral nonligated teeth which develop soft-chow-promoted (spontaneous) disease. Ligated sites experienced an average attachment loss of 0.94 mm per site and linear bone loss of 0.88 mm per site, with spontaneous-periodontitis sites experiencing approximately half the loss of ligated sites. The CF mediator levels showed increased levels of PGE2 and TxB2 at the ligated sites, as compared with the spontaneous sites, with no significant contralateral differences in the IL-1 beta or LTB4 responses. The concentrations of LTB4 in CF reached an early threefold peak over the baseline level at 1 month. By 2 months there was a statistically significant threefold elevation in CF-PGE2 in the ligated sites and a twofold elevation in the spontaneous sites as compared to the baseline level (P = 0.041 and 0.008, respectively). The monocyte product IL-1 beta increased sharply at 2 months and returned to the baseline level by 6 months at both ligated and nonligated sites. Tumor necrosis factor alpha in CF was below the limit of detection at all sites throughout the experiment (i.e., < 2 ng/ml). The selective elevation of both PGE2 and TxB2 in ligated sites, compared with levels in spontaneous sites, in the presence of similar levels of LTB4 and IL-1 beta provides further evidence that these molecules regulate the magnitude of the tissue-destructive response in progressive periodontitis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreizen S., Levy B. M. Monkey models in dental research. J Med Primatol. 1977;6(3):133–144. doi: 10.1159/000459735. [DOI] [PubMed] [Google Scholar]
- Goodson J. M. Diagnosis of periodontitis by physical measurement: interpretation from episodic disease hypothesis. J Periodontol. 1992 Apr;63(4 Suppl):373–382. doi: 10.1902/jop.1992.63.4s.373. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Jeffcoat M. K., Page R., Reddy M., Wannawisute A., Waite P., Palcanis K., Cogen R., Williams R. C., Basch C. Use of digital radiography to demonstrate the potential of naproxen as an adjunct in the treatment of rapidly progressive periodontitis. J Periodontal Res. 1991 Sep;26(5):415–421. doi: 10.1111/j.1600-0765.1991.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Jeffcoat M. K., Reddy M. S., Webber R. L., Williams R. C., Ruttimann U. E. Extraoral control of geometry for digital subtraction radiography. J Periodontal Res. 1987 Sep;22(5):396–402. doi: 10.1111/j.1600-0765.1987.tb01605.x. [DOI] [PubMed] [Google Scholar]
- Levy B. M. The nonhuman primate as an analogue for the study of periodontal disease. J Dent Res. 1971 Mar-Apr;50(2):246–253. doi: 10.1177/00220345710500021501. [DOI] [PubMed] [Google Scholar]
- Nuki K., Soskolne W. A., Raisz L. G., Kornman K. S., Alander C. Bone resorbing activity of gingiva from beagle dogs following metronidazole and indomethacin therapy. J Periodontal Res. 1981 Mar;16(2):205–212. doi: 10.1111/j.1600-0765.1981.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S., Braswell L. D., Loos A. S., Johnson H. G., Hall C. M., McClure H., Orkin J. L., Strobert E. A., Green M. D., Odle B. M. Effects of flurbiprofen on the progression of periodontitis in Macaca mulatta. J Periodontal Res. 1987 Nov;22(6):473–481. doi: 10.1111/j.1600-0765.1987.tb02058.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S., Odle B. M., Braswell L. D., Johnson H. G., Hall C. M., McClure H., Orkin J. L., Strobert E. A., Green M. D. Changes in cyclooxygenase metabolites in experimental periodontitis in Macaca mulatta. J Periodontal Res. 1989 Jan;24(1):63–74. doi: 10.1111/j.1600-0765.1989.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S., Odle B. M., Van Dyke T. E. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986 Mar;21(2):101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Offenbacher S., Williams R. C., Jeffcoat M. K., Howell T. H., Odle B. M., Smith M. A., Hall C. M., Johnson H. G., Goldhaber P. Effects of NSAIDs on beagle crevicular cyclooxygenase metabolites and periodontal bone loss. J Periodontal Res. 1992 May;27(3):207–213. doi: 10.1111/j.1600-0765.1992.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Page R. C., Simpson D. M., Ammons W. F., Schectman L. R. Host response in chronic periodontal disease. J Periodontal Res. 1972;7(4):283–296. doi: 10.1111/j.1600-0765.1972.tb01717.x. [DOI] [PubMed] [Google Scholar]
- Page R. C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res. 1991 May;26(3 Pt 2):230–242. doi: 10.1111/j.1600-0765.1991.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Ruttimann U. E., Webber R. L., Schmidt E. A robust digital method for film contrast correction in subtraction radiography. J Periodontal Res. 1986 Sep;21(5):486–495. doi: 10.1111/j.1600-0765.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Stashenko P., Fujiyoshi P., Obernesser M. S., Prostak L., Haffajee A. D., Socransky S. S. Levels of interleukin 1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991 Aug;18(7):548–554. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Stashenko P., Jandinski J. J., Fujiyoshi P., Rynar J., Socransky S. S. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991 Aug;62(8):504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jeffcoat M. K., Howell T. H., Reddy M. S., Johnson H. G., Hall C. M., Goldhaber P. Ibuprofen: an inhibitor of alveolar bone resorption in beagles. J Periodontal Res. 1988 Jul;23(4):225–229. doi: 10.1111/j.1600-0765.1988.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jeffcoat M. K., Kaplan M. L., Goldhaber P., Johnson H. G., Wechter W. J. Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science. 1985 Feb 8;227(4687):640–642. doi: 10.1126/science.3969553. [DOI] [PubMed] [Google Scholar]


