Abstract
Background
Recent data have shown that HTLV-1 is prevalent among HIV positive patients in Mozambique, although the impact of HTLV-1 infection on HIV disease progression remains controversial. Our aim was to determine the phenotypic profile of T lymphocytes subsets among Mozambican patients co-infected by HIV and HTLV-1.
Methods
We enrolled 29 patients co-infected by HTLV-1 and HIV (co-infected), 59 patients mono-infected by HIV (HIV) and 16 healthy controls (HC), respectively.
For phenotypic analysis, cells were stained with the following fluorochrome-labeled anti-human monoclonal antibodies CD4-APC, CD8-PerCP, CD25-PE, CD62L-FITC, CD45RA-FITC. CD45RO-PE, CD38-PE; being analysed by four-colour flow cytometry.
Results
We initially found that CD4+ T cell counts were significantly higher in co-infected, as compared to HIV groups. Moreover, CD4+ T Lymphocytes from co-infected patients presented significantly higher levels of CD45RO and CD25, but lower levels of CD45RA and CD62L, strongly indicating that CD4+ T cells are more activated under HTLV-1 plus HIV co-infection.
Conclusion
Our data indicate that HTLV-1/HIV co-infected patients progress with higher CD4+ T cell counts and higher levels of activation markers. In this context, it is conceivable that in co-infected individuals, these higher levels of activation may account for a faster progression to AIDS.
Background
Infection by the Human Immunodeficiency Virus (HIV) has been considered a serious infectious disease particularly in Southern Africa, which harbors more than 2/3 of all worldwide cases of HIV [1].
The emergence of several co-pathogens has aggravated this scenario in resource-limited settings [2,3]. Human T-lymphotropic virus type 1 (HTLV-1) has been implicated as a frequent co-pathogen in areas or groups where both viruses are prevalent [4,5]. In the southern Africa region where HIV is highly prevalent [6], the prevalence of co-infection by HTLV-1 and HIV varies among countries and in several places have been reported be higher than 10% [7-9]. A recent study conducted in Mozambique reported a co-infection prevalence rate of 4.5% among HAART naïve HIV positive individuals. Thus, it is conceivable that the impact of chronic infection by HTLV-1 on HIV disease progression is a relevant issue in AIDS research. Nevertheless, such an issue remains controversial, and published data are conflicting[10-12]. If in one hand it was initially postulated that co-infected patients progress faster to AIDS[13], further studies reported contradictory results[10,14].
The influence of HTLV-1 on HIV disease progression has been tightly linked, not only to several molecular events [15,16] but to its potential to induce high levels immune activation [17]. Although the mechanisms by which chronic activation induced by HTLV-1 could potentially affect the progression to AIDS are not completely understood, we have learned from the natural history of HIV infection that chronic activation of the immune system takes part and triggers a number of cellular and molecular pathways related to CD4+ T cell loss and immune deregulation [18-22]. In this context, HTLV-1 per se induces a strong immune activation [23,24] that has been associated to immunosuppression, unresponsiveness and immune deregulation [25,26]. How the immune system behaves in the presence of both HIV and HTLV-1 remains to be clarified.
The concern regarding the clinical outcome as a result of co-infection by HTLV-1 and HIV has gained a special relevance in recent years, in face of the growing body of evidence showing that: a) co-infection is prevalent in several geographical regions in Southern Africa [27,28]; b) we and others showed that co-infected patients present stable CD4+ T lymphocytosis irrespective of their progression to AIDS, which by certain extent could mask the immunosuppression with consequent inappropriate therapeutic decisions in terms of the initiation of Highly Active Anti Retroviral Therapy (HAART) and prophylaxis for opportunistic infections [29,30].
The situation has being aggravated by the fact that neither cure nor effective treatment is yet available for HTLV infection [31] in such a way that clinicians are unable to control the effects exerted by the virus.
Previous studies have enrolled patients with different HAART experiences and some authors believe that this may partially explain the divergence of results obtained [11]. Other studies were based on in vitro manipulation [15] or used simian models [32]. In addition, no study has been so far conducted in Africa, the region carrying the greatest burden of HIV disease and where the epidemiology of HIV and other diseases is quite different [1].
Altogether, these data raise the need to define to what extent HTLV-1 impacts the clinical progression to AIDS in an African setting. We evaluated herein HTLV-1 and HIV co-infected HAART naïve adult patients, in terms of T cell phenotype, further correlating the expression of activation markers and HIV-1 viral load. We showed that co-infected patients progress with higher levels of CD4+ T cells expressing activation markers and a massive loss of naïve cells, thus suggesting that co-infected patients progress faster to AIDS.
Methods
Study design and subjects
A case control study was conducted with participants consisting of three sets, namely, individuals co-infected by HIV and HTLV-1 (Co-infected), HIV mono-infected patients (HIV) and healthy controls (HC). Co-infected and HIV groups were recruited from an ongoing cohort of HIV infected patients followed in the Alto Maé Health Centre, in the city of Maputo, Mozambique. In the period between March and June 2006, 724 HIV 1/2 infected patients were invited to participate and 704 (97.4%) accepted to be part of this study. They were all screened for HTLV-1 infection and 32 patients (4.5%, 32/704) were founded to be co-infected by HTLV-1 and HIV. Three patients with a positive HTLV-1 antibody test did not return to collect their result and were excluded of the study. Co-infected were matched at a ratio 1:2 with HIV mono-infected by age, sex and HIV clinical stage system as defined by WHO [33]. Co-infected and HIV mono-infected were matched without prior knowledge of CD4+T cell counts results.
Healthy controls were not matched by age and sex as we did with co-infected and HIV-mono-infected, since they were recruited on a consecutive basis from the routine blood donors at the blood bank of Maputo Central Hospital. In addition, most of blood donors are males and younger as demonstrated by two previous studies conducted at the same Blood Bank ([34,35]) Informed consent to participate in the study was requested to all participants, and the study was approved by the National Bioethics Committee in Mozambique and by the Sydney University Ethics Committee, Australia.
Physical and neurological examination was performed by two medical doctors blinded for the HTLV-1 status. Socio-demographic data, sexual/reproductive history and clinical data were also recorded from each participant.
The study population consisted of 59 HIV, 29 co-infected and 16 healthy controls individuals. All HIV and co-infected individuals were naïve to HAART. The median age was 40 years (IQR, 34 - 48 years) for co-infected individuals, 41 years (IQR, 32 - 47) for HIV patients and 32 (IQR, 29 - 38) for HC, respectively. There was a predominance of the female gender among HIV and co-infected individuals (86,3% and 86.4% respectively) and predominance of the male gender among HC (80%; see table 1). Almost half of co-infected and HIV patients were classified as stage 2 for HIV clinical disease (58.4% and 56.0% for co-infected and HIV patients respectively) based on the WHO criteria [33]. None of the co-infected or HIV patients was at stage 4 of HIV clinical disease (table 1). HAM/TSP or ATL cases were not detected in subjects of any groups.
Table 1.
General characteristics of HIV/HTLV-1 co-infected, HIV mono infected and healthy Mozambican subjects
| General Features | HIV/HTLV-1 (n = 29) |
HIV (n = 59) |
HC (n = 16) |
p value |
|---|---|---|---|---|
| Age, years | ||||
| Median | 40.0 | 41.0 | 32.0 | |
| IQR | 34.0 - 48.0 | 32.0 - 47.0 | 29.0 - 38.0 | 0.123* |
| Gender | ||||
| Male | 4 (13.8%) | 8 (13.6%) | 12 (80%) | |
| Female | 25 (86.3%) | 51 (86.4%) | 3 (20%) | 0.000** |
| HIV clinical stage*** | ||||
| I | 7 (24.1%) | 14 (23.7%) | ||
| II | 17 (58.4%) | 33 (56.0%) | ||
| III | 5 (17.2%) | 12 (20.3%) | 0.587** |
* One Way Anova; ** Chi square; ***as defined by WHO[30];
HIV/HTLV-1, HIV/HTLV-1 co-infected; HIV, HIV mono-infected; HC, healthy controls
Co-infected and mono-infected were comparable regarding clinical presentation of opportunistic diseases (table 2).
Table 2.
Clinical presentation of study groups
| Clinical presentation | HIV/HTLV-1, n(%) (n = 29) |
HIV, n(%) (n = 59) |
p value* |
|---|---|---|---|
| Assymptomatic | 7 (24.1) | 14 (23.7) | |
| Papular pruritic eruptions | 3 (10.3) | 14 (23.7) | |
| Dermatitis | 2 (6.9) | 2 (3.4) | |
| Seborrhoeic dermatitis | 2 (6.9) | 0 (0.0) | |
| Folliculitis | 0 (0.0) | 1 (1.7) | |
| Herpes zoster < 5 years | 3 (10.3) | 4 (6.8) | 0.570 |
| TInea capitis | 5 (17.2) | 8 (13.6) | |
| Candidiasis | 3 (10.3) | 4 (6.8) | |
| Tuberculosis < 1 year | 1 (3.5) | 4 (6.8) | |
| Weight loss > 10% | 3 (10.3) | 7 (11.9) | |
| Chronic diarrhoea | 0 (0.0) | 1 (1.7) |
* Fisher chi square test
Blood samples
Ten milliliters of venous whole blood were requested from each volunteer. The blood was collected aseptically into a 5 ml vacuum tube with K3EDTA and a 5 ml vacuum tube for serum separation (Becton-Dickinson Vacutainer Systems, USA). Blood specimens were delivered at the laboratory within four hours of collection.
HIV Serology
All patients enrolled in this study were screened for anti-HIV 1/2 antibodies at the Voluntary Counseling and Testing (VCT) services of the Mozambican Health Center. Patients were tested for HIV according to the Mozambican National protocol consisting of a sequential algorithm of two immunochromatographic rapid tests. All individuals were first screened using the Determine HIV-1/2 test (Abbott Laboratories, Japan). All specimens reactive on the screening assay were further tested using the Uni-Gold HIV test (Trinity Biotech, Ireland). Individuals reactive on both assays were considered positive for HIV-1/2 infection.
HTLV Serology
All samples were screened for anti-HTLV-1+2 antibodies using the qualitative EIA Murex HTLV-1 + 2 (Murex Biotech Limited, UK). Specimens reactive on the EIA were confirmed by a Western blot assay (HTLV BLOT 2.4, Genelabs® Diagnostics, Switzerland). All patients with reactivity to antigens encoded by the GAG gene (p19 with or without p24) and to two antigens encoded by the ENV gene (GD21 and rgp46-I) were considered to be infected by HTLV-1 according to the instructions provided by the manufacturer. All HTLV positive samples in our study population were typed as HTLV-1 by Western blot.
T cell immunophenotyping
Phenotypic analysis of circulating T lymphocytes was performed through four colour flow cytometry on fresh EDTA-anticoagulated whole blood using a FACSCalibur™ flow cytometer (Becton-Dickinson Biosciences, USA). T cells counts were obtained based on a lyse-no wash protocol using CellQuest Software for acquisition (BD Biosciences). Cells were stained with the following fluorescent-labeled anti-human monoclonal antibodies CD4-APC, CD8-PerCP, CD25-PE, CD62L-FITC, CD45RA-FITC. CD45RO-PE, CD38-PE (all from BD Biosciences). Analyses were performed using the Summit software v 4.3.2, 2006 (Dako Cytomation, Inc., Fort Collins, USA).
HIV-1 subtyping
Samples from co-infected and mono-infected individuals were sequenced for HIV-1 subtyping. A 297-bp fragment encompassing full sequence of the protease gene was amplified using nested PCR. The outer primers PRT15F 5' TGAAAGATTGTACTGAGAGACAGG 3'/K2R 5' GTCAATGACATACAGAAGTTAGTGGGAAAA 3' were used in first-round PCR, and DP10F 5' CAACTCCCTATCAGAAGCAGGAGAAG 3'/RVP3R 5'-CCATACAATACTCCAGTATTTGCC-3' were used as inner primers during the second-round of nested PCR. The conditions for both rounds of PCR were described previously [36]. Amplified DNA was quantified, purified and sequenced under PCR conditions described elsewhere [37], using the primers from the second-round PCR. The genetic subtypes were determined using the amino acid sequences of the protease genes, deduced from the nucleic acid sequences, and were compared to a subtype B consensus sequence from the Stanford HIV Protease Sequence database http://hivdb.stanford.edu/hiv/.
The analysis of the Mozambique sequence with HIV Drug Resistance database showed that all samples sequenced (20 mono-infected and 24 co-infected) were subtype C in protease gene with profile of similarity of 98% compared with sequence subtype C from the Stanford HIV Protease Sequence database.
Stool samples
Stools were collected into a sterile, wide mouth, leak-proof container with a tight fitting lid containing a preservative solution. Stools were kept at room temperature until delivery at the study setting within six hours of collection. Small amounts of stool specimens were placed on microscope slides and mixed with 0.9% NaCl solution to prepare wet mount smears. Slides were then examined under a light microscope to screen for cysts of Giardia lamblia, Entamoeba hystolitica, Entamoeba coli and Balantidium coli, ova of Ascaris lumbricoides, Trichuris trichiura and Ancylostoma duodenale and larvae of Strongyloides stercolaris.
Statistical analysis
Data was analyzed using the statistics package STATA 9.0 (College Station, Texas: StataCorp, USA, 2005).
Taking into consideration that the major goal of our study was to compare activation markers between co-infected versus HIV mono-infected patients, two sample comparison means with a ratio 1:2 (case:control) was used to determine the required sample size in these groups. Due to lack of information regarding comparison of activation markers between these groups, we calculated a sample size enough to detect a least a difference of 10% in the mean of these cells frequencies with a standard deviation of 14 at a significance level of 5%.
The Mann-Whitney test and the One Way Anova trend test were used to compare the differences among numerical variables in the three groups. Associations between categorical variables were determined using the Pearson Chi-square test, the Fisher exact test or the Chi-square trend test, as appropriated.
Results
HIV/HTLV-1 co-infected individuals present higher and stable CD4+ T cell counts
We first showed that HIV/HTLV-1 co-infected individuals exhibited a higher absolute and relative CD4+ T cell counts (median: 525 cells/mm3, versus 274 cells/mm3, p = 0.000 and 24.9% versus 15.9%, p = 0.000, see table 3) and a higher CD4+/CD8+ T cell ratio (0.5 versus 0.30, p = 0.004), when compared with the HIV mono-infected group. Moreover, CD4+ T lymphocytosis in co-infected individuals was stable irrespective of their HIV clinical stage, thus contrasting with the gradual loss of CD4+ T cells from HIV clinical stage I through stage IV seen in the mono-infected group (figure 1). Both groups were similar regarding CD8+ T cell absolute counts but co-infected individuals presented lower CD8+ T cell relative counts (p = 0.505 and p = 0.009, respectively; see table 3). These patterns were the same in both males and females (data not shown).
Table 3.
Distribution of T cell subsets in peripheral blood from HIV/HTLV-1 co-infected, HIV mono-infected and healthy Mozambican subjects
| T cell subsets and viral load | HIV/HTLV-1 (n = 29) |
HIV (n = 59) |
HC (n = 16) |
|---|---|---|---|
| CD4+ T cell counts (cells/mm3) | |||
| Median | 525a | 274 | 472 |
| IQR | 310 - 827 | 183 - 436 | 412 - 775 |
| CD4+ T cell counts (%) | |||
| Median | 24.9 a, b | 15.9 | 45.0 |
| IQR | 19.0 - 32.7 | 9.4 - 21.0 | 37.0 - 48.0 |
| CD8+ T cell counts, cells/mm3 | |||
| Median | 1002b | 937 | 302 |
| IQR | 649 - 1090 | 606 - 1358 | 190 - 391 |
| CD8+ T cell counts, % | |||
| Median | 46.8a, b | 54.2 | 23.0 |
| IQR | 36.2 - 53.0 | 42.7 - 61.3 | 21.0 - 29.0 |
| CD4+ T/CD8+ T ratio | |||
| Median | 0.5a, b | 0.3 | 1.8 |
| IQR | 0.3 - 0.8 | 0.2 - 0.4 | 1.2 - 2.5 |
| CD8+ CD38+ T cells (MFI) | |||
| Median | 341.5 | 279.9 | 209.7 |
| IQR | 285.1 - 367.1 | 225.4 - 373.3 | 209.7 - 225.4 |
| HIV-1 RNA viral load (copies/mL) | |||
| Median | 56,385 | 37,573 | |
| IQR | 14,749 - 277,570 | 11,322-176,837 |
One ANOVA corrected by Bonferoni was used to compare groups.
HIV/HTLV-1, HIV/HTLV-1 co-infected; HIV, HIV mono-infected; HC, healthy controls; MFI, Median Fluorescence Intensity
a p < 0.05 comparison co-infected versus HIV mono-infected
b p < 0.05 comparison co-infected versus healthy controls
Figure 1.
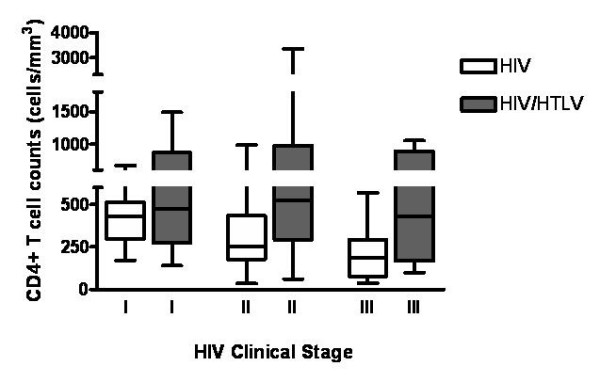
Absolute CD4+ T cell counts in different HIV clinical stages, among different HTLV status. There is a consistent and stable high absolute CD4 T cell count in co-infected individuals, contrasting with the progressive decrease of this cell subset in the monoinfected group, related to the progression of HIV disease. p value for Anova trend test was 0.000 and 0.945 for HIV/HTLV-1 and HIV groups respectively.
Higher proportions of CD4+ T cells bearing an activated phenotype in HIV/HTLV-1 co-infected individuals
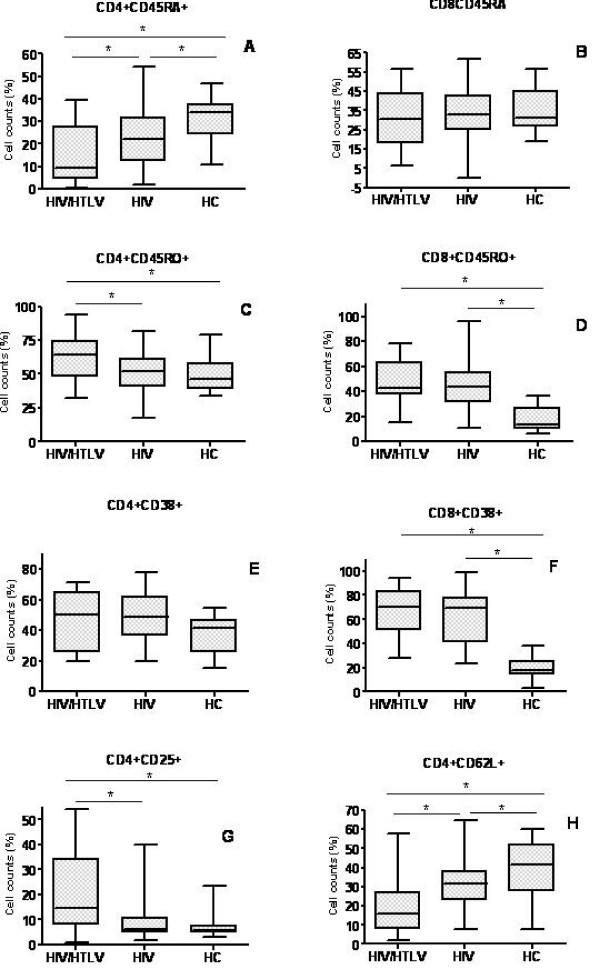
The data described above, on the differences in CD4+ T cell counts between co-infected versus HIV patients, pointed to a differential activation state of T cells in these patients. In fact, when compared to HIV or to HC participants, co-infected individuals presented significantly higher membrane levels of CD25 and CD45RO on CD4+ T cells (0.007 and 0.040 respectively, figure 2C and 2G). Also the density of CD38 molecules on the surface of CD8+ T cells was higher in co-infected when compared to HIV and HC participants, although not statistically significant, (figure 3 and table 3). The relative numbers of CD8+CD38+ and CD8+CD45RO+ cells in co-infected although statistical significantly higher than HC (p = 0.000 for both CD8+CD38+ and CD8+CD45RO+ respectively, figures 2D and 2F) was only slightly higher than HIV individuals (median: 51.3%, versus 41.2%, p = 0.652 for CD8+CD38+ and 37.4% versus 31.0%, p = 0.512 for CD8+CD45RO+ respectively, figures 2D and 2F).
Figure 2.
Distribution of T cell subsets in HIV/HTLV-1 co-infected, HIV mono-infected and healthy Mozambican subjects. Co-infected patients presented higher CD4+CD45RO+ (memory cells, C), CD8+CD38+ (activated cells, E) and lower CD4+CD45RA+ (naïve cells, A) and CD4+CD62L+ (naïve cells, H). *p < 0.05, as ascertained by One Way Anova. HIV/HTLV = coinfected, HIV = mono-infected by HIV and HC = healthy controls.
Figure 3.
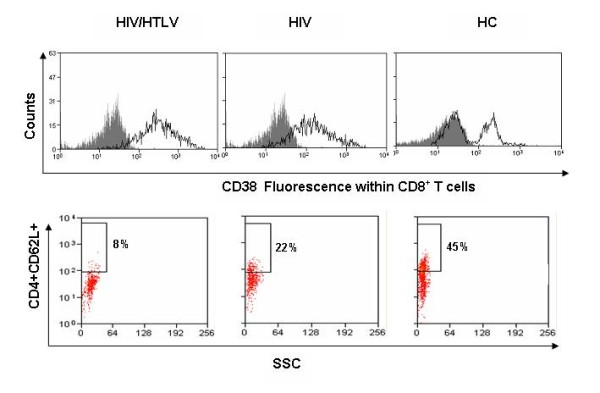
Activated and naïve T cell profiles HIV/HTLV-1 co-infected, HIV mono-infected and healthy Mozambican subjects. Dot plots show the typical phenotype of activated (upper panels) and naïve T lymphocytes (lower panel). Upper panels reveal that co-infected patients presented with higher density of CD38 molecules on T CD8+ T cells. Co-infected patients presented lower frequency of CD4+CD62L+ cells when compared with HIV positive patients and health controls (lower panel).
All three groups were similar regarding the expression of CD38 on CD4+ T cells (figure 2E).
Naïve cells were phenotyped for the expression of CD45RA and CD62L on CD4+ and CD8+ T cells respectively. Co-infected and HIV individuals exhibited lower levels of CD45RA (p = 0.023 and 0.037 respectively) and CD62L (p = 0.026 and 0.041 respectively) on CD4+ T lymphocytes, when compared to HC, Such a loss of naïve cells was more pronounced in the co-infected group (figures 2A, H and 3).
Of interest, stool evaluation revealed no significant differences in helminthic or protozoan loads among the three groups of patients (data not shown), indicating that the relative role of parasites in inducing lymphocyte activation is likely similar in the various groups.
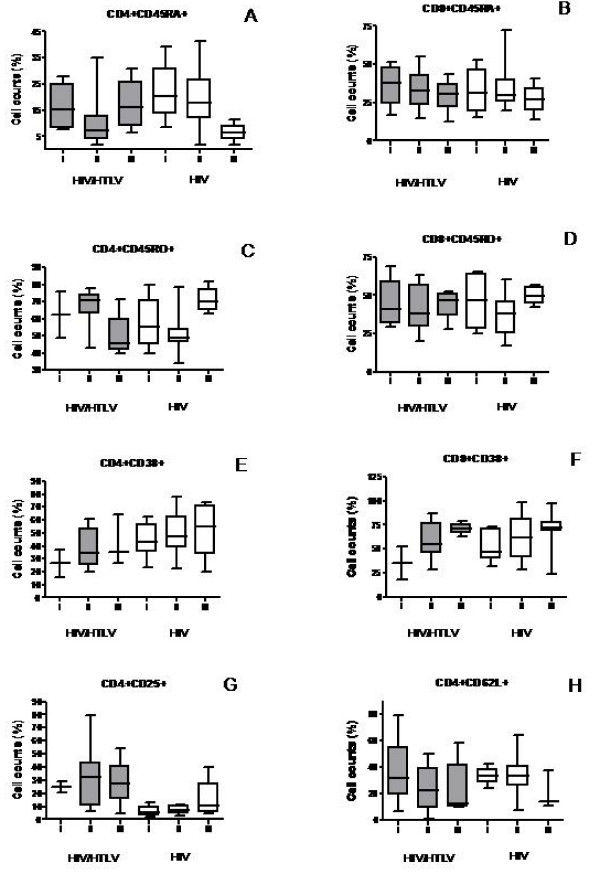
Activation markers on T cells correlate with HIV clinical stage
The small sample size of our study was a limitation to assess the changes in the activation by HIV clinical stage. Therefore, although a statistical analysis was not possible to perform, we stratified the analysis of these subsets by HIV clinical stage in an intent to define a pattern (figure 4). Our data found that the relative numbers of CD8+CD45RO+ (figure 4D) T cells remained unchanged from HIV clinical stages I to III. By contrast, there was an increase in the expression of CD38 in both CD4+ and CD8+ T cell subsets (figures. 4E-F) and a decrease of CD4+CD62L+ and CD8+CD45RA+ (figure 4B) subsets from clinical stage I through III in both groups. The frequency of CD4+CD45RO+ (figure 4A) CD4+CD25+ (figure 4G) subsets was higher from stage I through III among HIV mono infected patients. Rather unexpectedly, among co-infected patients the frequencies of these subsets were lower in the clinical stage III when compared to clinical stage II. Similarly in respect to the frequency of the CD4+CD45RA+ subset (figure 4A) there was an decrease from stage I through stage III among HIV monoinfected patients. Surprisingly, among co-infected patients, the frequency of cells in the clinical stage III was higher than in the clinical stage II. It is possible that the small sample size in each clinical stage may explain these unexpected findings
Figure 4.
Changes in the subsets of T cells in the distinct HIV clinical stages. CD8+CD45RO+ (D) remained unchanged in the various HIV clinical stages in both groups. CD38+ on CD4+ and CD8+ T cells (E and F), CD4+CD25+ and CD4+CD45RO+ subsets were increased from clinical stage I through III in both groups. CD4+CD45RA+ (A), CD8+CD45RA+ (B) and CD4+CD62L+ (H) decreased from stage I through III. Co-infected patients in the clinical stage III for CD4+CD25+ and CD4+CD45RO+ and CD4+CD45RA+ did not follow the pattern observed in HIV mono-infected individuals. I = HIV Clinical Stage I, II = HIV Clinical Stage I, III = HIV Clinical Stage III.
HIV-1 viral load positively correlates with the enhancement of T cell activation markers in both co-infected and HIV groups
Although weak, there was an inverse correlation between the proportions of CD4+CD45RA+ naïve cells and HIV-1 viral load. Co-infected group appeared to present a better correlation when compared to HIV group (r = -0.224 versus -0.204 respectively, figures 5A-B), but the difference was not statistically significant.
Figure 5.
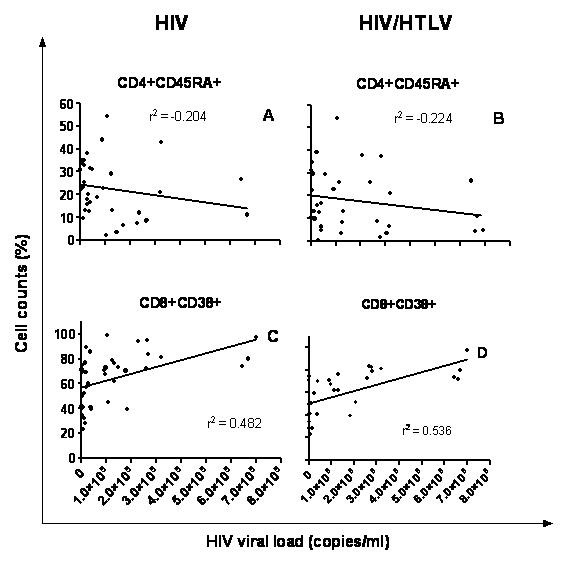
Correlation between naïve CD4 (CD4+CD45RA+) or activated CD8 (CD8+CD38+) T lymphocytes with HIV-1 viral Load. Panels A and B depict a negative correlation between CD4+CD45RA+ (naive cells) and HIV-1 viral load in both co-infected and HIV-1 subjects. By contrast, lower panels show a positive correlation between CD8+ CD38 (activated cells, C and D) and HIV-1 viral load in both co-infected and HIV-1 subjects.
In contrast, there was a positive correlation between the proportions of CD8+CD38+ cells and HIV-1 viral load. Again co-infected group presented a slightly higher correlation, but such a difference was not statistically significant (r = 0.536 versus 0.482 respectively, figures 5C-D).
Discussion
HIV and HTLV-1 have emerged as common co-pathogens especially in areas or groups where both viruses are circulating [5,9,38,39]. Nevertheless, the impact of HTLV-1 on HIV disease progression is still a matter of debate with controversial results [10-14,30,40]. Here, for the first time we conducted a case control study in an African setting, aiming to determine the impact of HTLV-1 infection on HIV disease. In fact, to our knowledge, the present study is pioneer in the region, since it was conducted in HAART naïve patients, on a well controlled cross-sectional basis.
Previous studies were conducted mainly in South and North Americas where the epidemiology of HIV infection and other diseases is quite different from that seen in sub-Saharan African countries [29,30].
We found no evidence of HTLV-2 in our study population. This is in keeping with two recent studies conducted among blood donors in Maputo city[34,35] and suggest that only HTLV-1 (but not HTLV-2) circulates in Mozambique.
As expected, co-infected individuals presented a stable CD4+ T lymphocytosis irrespective of their progression to AIDS, contrasting with the depletion of CD4+ T cells counts observed among HIV patients over time. To date, it is well established that cell immortalization and transformation induced by Tax and Rex proteins encoded by HTLV-1 genes constitute major events related to uncontrolled CD4+ T cell growth and proliferation [41,42].
The intriguing progression to AIDS in the presence of normal or high levels of CD4+T cells counts suggest these to be functionally altered. Consensus exists that both HIV [43,44] and HTLV [24,25,45] separately induce functional modifications on T cells populations, characterized among others by a decrease of naïve populations and higher levels of cell activation when compared with uninfected individuals.
Here we found that co-infected individuals presented markedly lower expression of CD45RA+ (a phenotypic marker of naïve T lymphocytes) on CD4+ T cells. Naïve cells are considered the first cells to be depleted in the presence of immune activation [46,47] and represent one of the hallmarks of HIV infection [48]. The magnitude and impact exerted by naïve T cells erosion on HIV disease progression remain to be defined. Although not fully understood, there is a consensus that for both HIV and HTLV-1, the loss of naïve cells has been linked, among others, to, (i) a homeostatic mechanism to replenish the cells being killed (ii) a massive recruitment of naïve cells, partially imposed by the mechanisms driving the activation and (iii) the impairment of T cell production [19,20,46-49].
In our study, the erosion of the naïve compartment was further confirmed by evaluating the expression of CD62L, another marker for naïve T lymphocytes, usually lost upon activation. As expected, there was a dramatic loss of CD4+CD62L+ lymphocytes in the co-infected group, when compared to HIV mono-infected and HC groups. Importantly, these differences were further confirmed when we compared the groups in terms of naive cell absolute counts (data not shown) arguing against an indirect effect of higher percent counts of memory cells. Whether there is an impairment of T cell production, if they are dying faster or if more cells being recruited from the naïve T cell pool into activated/memory cell compartment remain to be determined.
Not surprisingly, this loss of naïve cells in co-infected individuals was accompanied by higher frequencies of memory and activated cells as measured by CD45RO+ (memory), CD38+ and CD25+ (activated) cell markers. In fact, co-infected individuals presented with higher proportions of CD45RO+ on CD4+ T cells when compared to the HIV and HC groups. These findings are in agreement with previously data [45,46,50,51], Similarly, the relative number of CD4+CD25+ cells seen in co-infected patients was higher than what was found in HIV and HC individuals. It is conceivable that the increase of CD4+CD25+ cells is a consequence of the virus-driven induction of IL-2/IL-2 receptor expression by tax, as previously reported [12,23,52]. Interestingly, the frequency of CD38+cells within the CD8+ T cell compartment but not in CD4+ T cells was increased in co-infected and HIV when compared to HC. This is in keeping with the results showed in a case-control study conducted among HAM/TSP patients [24]. Although we did not find differences in the frequency of CD38+ cells, either in CD8+ or CD4+ T cells, we found that co-infected patients presented higher expression of CD38 in CD8+ T cells (as ascertained by Median Fluorescence Intensity measurements) when compared to HIV patients. Nevertheless differences were not statically significant.
Noteworthy, increased expression of CD38 on the surface of CD8+ T cells have long been considered an even better prognostic predictor of progression to AIDS and response to HAART than HIV viral load itself [53,54]. This is relevant due to the fact that such parameter is being proposed to be included in clinical settings to monitor HIV disease progression [55].
It is now widely accepted that the presence of chronic activation is a major factor influencing the pathogenesis of HIV in Africa [56]. HTLV-1 is a strong activator of immune system. Immune activation and exaggerated immune response has been demonstrated to be the main pathogenetic mechanism involved in the HTLV-1 associated inflammatory syndromes[24,57-59]. The immunodominant Tax protein encoded by HTLV transactivates and modulates a large number of genes playing a key role in triggering several pathways leading to cell activation[60-62]. Available data demonstrate that a large proportion of asymptomatic carries progress with high levels of immune activation[63].
On the basis of the patients' age and their HAART naïve status, we believe that HTLV-1 infection preceded HIV infection. Considering that individuals chronically infected by HTLV progress with immune activation, it is conceivable that these patients acquire HIV infection in a pre-activated immune milieu, and the presence of immune hyper activation not only turns them more susceptible to acquire HIV, but also to progress faster to a poor prognosis.
Cases and controls were matched by age and clinical stage (WHO) so that to be comparable in terms of clinical presentation (see table 2), Clinical staging system is performed on the basis of patient's clinical presentation. This information is important when interpreting the differences in the activation markers between these groups. Another aspect deserving discussion is the helminthic infection as a factor involved in immune activation, particularly in Southern Africa [3,64]. Accordingly, a differential presence of parasitic infection in our patients could bias our results. However, this does not seem to be the case since in all groups evaluated, the degree of protozoan and helminthic infections were similar. Of note, all samples sequenced in both groups were founded to be HIV subtype C, ruling out any linkage between HIV subtype in mono and co-infected groups, and immunological/clinical behavior.
Conclusion
In conclusion, although HIV/HTLV-1 co-infected individuals quantitatively maintain a normal or high CD4+ T cells counts, these cells are likely functionally altered presenting with a dramatic decrease of naïve cells and higher activation patterns. Yet, if these changes account for a faster progression to AIDS remains to be determined.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ESG participated in the study design, data collection, data analysis, and writing the manuscript. NBB participated in the study design, data collection, data analysis and writing the manuscript. DRB participated in the study design, sample processing and data analysis. WiS participated in the study design, data analysis and writing the manuscript. SDSB participated in the study design, data analysis and writing the manuscript. CMA and AT participated in HIV subtyping and writing the manuscript. IVJ participated in the study design, data collection, data analysis and writing the manuscript. None of the authors have any financial or personal interest in the World Bank Quick Impact Fund. All authors have read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Eduardo Samo Gudo, Email: esamogudo@yahoo.com.br.
Nilesh B Bhatt, Email: nbhatt.mz@gmail.com.
Dulce Ramalho Bila, Email: dulcebila@hotmail.cmo.
Celina Monteiro Abreu, Email: celydion@yahoo.com.
Amílcar Tanuri, Email: atanuri@biologia.ufrj.br.
Wilson Savino, Email: savino@fiocruz.br.
Suse Dayse Silva-Barbosa, Email: susebarbosa@hotmail.com.
Ilesh V Jani, Email: ivjani@email.com.
Acknowledgements
We thank the efforts of the entire field-work team in facilitating the completion of this investigation specially the staff members of the Alto Maé HIV Outpatient Clinic and the staff of the Department of Immunology at the Mozambican National Institute of Health. This work was supported by funds from the World Bank Quick Impact Fund, the Mozambican Ministry of Science and Technology, AVERT, UK. the Oswaldo Cruz Foundation, CNPq/Pro-África Program and CAPES/PEC-PG Program (Brazil).
This manuscript was presented in part at X International Symposium on HTLV in Brazil, held in June 2008, Rio de Janeiro, Brazil.
References
- 2007. JUNPoHAUaWHOW. AIDS epidemic update: December 2007. AIDS epidemic update: December 2007. 2007.
- Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 2001;14(4):753–777. doi: 10.1128/CMR.14.4.753-777.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17(4):1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Koito A, Takatsuki K, Ikematsu S, Matsuda J, Mori H, Fukui M, Akashi K, Matsumoto K. Frequent infection with human T-cell lymphotropic virus type I in patients with AIDS but not in carriers of human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1989;2(3):272–276. [PubMed] [Google Scholar]
- de Araujo AC, Casseb JS, Neitzert E, de Souza ML, Mammano F, Del Mistro A, De Rossi A, Chieco-Bianchi L. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sao Paulo, Brazil. Eur J Epidemiol. 1994;10(2):165–171. doi: 10.1007/BF01730366. [DOI] [PubMed] [Google Scholar]
- WHO U Unicef. Towards Universal access. Scaling up priority HIV/AIDS interventions in the health sectorsector: progress report 2008. Geneva: WHO; 2008. [Google Scholar]
- Olaleye DO, Ekweozor CC, Sheng Z, Rasheed S. Evidence of serological cross-reactivities with human immunodeficiency virus types 1 and 2 and human T-lymphotropic virus types I and II in sera of pregnant women in Ibadan, Nigeria. Int J Epidemiol. 1995;24(1):198–203. doi: 10.1093/ije/24.1.198. [DOI] [PubMed] [Google Scholar]
- Adjei AA, Adiku TK, Kumi PF, Domfeh AB. Human T-lymphotropic type-1 virus specific antibody detected in sera of HIV/AIDS patients in Ghana. Jpn J Infect Dis. 2003;56(2):57–59. [PubMed] [Google Scholar]
- Hishida O, Ayisi NK, Aidoo M, Brandful J, Ampofo W, Osei-Kwasi M, Ido E, Igarashi T, Takehisa J, Miura T. Serological survey of HIV-1, HIV-2 and human T-cell leukemia virus type 1 for suspected AIDS cases in Ghana. Aids. 1994;8(9):1257–1261. doi: 10.1097/00002030-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Harrison LH, Schechter M. Coinfection with HTLV-I and HIV: increase in HTLV-I-related outcomes but not accelerated HIV disease progression? AIDS Patient Care STDS. 1998;12(8):619–623. doi: 10.1089/apc.1998.12.619. [DOI] [PubMed] [Google Scholar]
- Brites C, Oliveira AS, Netto EM. Coinfection with HIV and human T lymphotropic virus type 1: what is the real impact on HIV disease? Clin Infect Dis. 2005;40(2):329–330. doi: 10.1086/426690. [DOI] [PubMed] [Google Scholar]
- Casoli C, Pilotti E, Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007;9(3):140–149. [PubMed] [Google Scholar]
- Bartholomew C, Blattner W, Cleghorn F. Progression to AIDS in homosexual men co-infected with HIV and HTLV-I in Trinidad. Lancet. 1987;2(8573):1469. doi: 10.1016/S0140-6736(87)91172-X. [DOI] [PubMed] [Google Scholar]
- Harrison LH, Quinn TC, Schechter M. Human T cell lymphotropic virus type I does not increase human immunodeficiency virus viral load in vivo. J Infect Dis. 1997;175(2):438–440. doi: 10.1093/infdis/175.2.438. [DOI] [PubMed] [Google Scholar]
- Moriuchi H, Moriuchi M, Fauci AS. Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1. J Exp Med. 1998;187(10):1689–1697. doi: 10.1084/jem.187.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca A, Mufti GJ. Co-infection with HTLV-I/II and HIV-1. Lancet. 1990;336(8711):383. [PubMed] [Google Scholar]
- Casseb J, Hong MA, Salomao S, Duarte AJ, Gallo D, Hendry RM. Coinfection with human immunodeficiency virus and human T-cell lymphotropic virus type I: reciprocal activation with clinical and immunologic consequences. Clin Infect Dis. 1997;25(5):1259–1260. doi: 10.1086/516969. [DOI] [PubMed] [Google Scholar]
- Ascher MS, Sheppard HW. AIDS as immune system activation. II. The panergic imnesia hypothesis. J Acquir Immune Defic Syndr. 1990;3(2):177–191. [PubMed] [Google Scholar]
- Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. Aids. 2008;22(4):439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. Aids. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- Leng Q, Bentwich Z, Magen E, Kalinkovich A, Borkow G. CTLA-4 upregulation during HIV infection: association with anergy and possible target for therapeutic intervention. Aids. 2002;16(4):519–529. doi: 10.1097/00002030-200203080-00002. [DOI] [PubMed] [Google Scholar]
- Hunt PW. Role of immune activation in HIV pathogenesis. Curr HIV/AIDS Rep. 2007;4(1):42–47. doi: 10.1007/s11904-007-0007-8. [DOI] [PubMed] [Google Scholar]
- Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24(39):6035–6046. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- Al-Fahim A, Cabre P, Kastrukoff L, Dorovini-Zis K, Oger J. Blood mononuclear cells in patients with HTLV-I-associated myelopathy: lymphocytes are highly activated and adhesion to endothelial cells is increased. Cell Immunol. 1999;198(1):1–10. doi: 10.1006/cimm.1999.1580. [DOI] [PubMed] [Google Scholar]
- Yasunaga J, Sakai T, Nosaka K, Etoh K, Tamiya S, Koga S, Mita S, Uchino M, Mitsuya H, Matsuoka M. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood. 2001;97(10):3177–3183. doi: 10.1182/blood.V97.10.3177. [DOI] [PubMed] [Google Scholar]
- Mascarenhas RE, Brodskyn C, Barbosa G, Clarencio J, Andrade-Filho AS, Figueiroa F, Galvao-Castro B, Grassi F. Peripheral blood mononuclear cells from individuals infected with human T-cell lymphotropic virus type 1 have a reduced capacity to respond to recall antigens. Clin Vaccine Immunol. 2006;13(5):547–552. doi: 10.1128/CVI.13.5.547-552.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MB, Parker SP, Crewe-Brown HH, McIntyre J, Cubitt WD. Seroepidemiology of HTLV-I in relation to that of HIV-1 in the Gauteng region, South Africa, using dried blood spots on filter papers. Epidemiol Infect. 1996;117(2):343–348. doi: 10.1017/S0950268800001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melbye M, Poulsen AG, Gallo D, Pedersen JB, Biggar RJ, Larsen O, Dias F, Aaby P. HTLV-1 infection in a population-based cohort of older persons in Guinea-Bissau, West Africa: risk factors and impact on survival. Int J Cancer. 1998;76(3):293–298. doi: 10.1002/(SICI)1097-0215(19980504)76:3<293::AID-IJC1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Schechter M, Harrison LH, Halsey NA, Trade G, Santino M, Moulton LH, Quinn TC. Coinfection with human T-cell lymphotropic virus type I and HIV in Brazil. Impact on markers of HIV disease progression. Jama. 1994;271(5):353–357. doi: 10.1001/jama.271.5.353. [DOI] [PubMed] [Google Scholar]
- Beilke MA, Theall KP, O'Brien M, Clayton JL, Benjamin SM, Winsor EL, Kissinger PJ. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis. 2004;39(2):256–263. doi: 10.1086/422146. [DOI] [PubMed] [Google Scholar]
- Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7(4):266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- Fultz PN, McGinn T, Davis IC, Romano JW, Li Y. Coinfection of macaques with simian immunodeficiency virus and simian T cell leukemia virus type I: effects on virus burdens and disease progression. J Infect Dis. 1999;179(3):600–611. doi: 10.1086/314627. [DOI] [PubMed] [Google Scholar]
- WHO HAP. ANTIRETROVIRAL THERAPY FOR HIV INFECTION IN ADULTS AND ADOLESCENTS: Recommendations for a public health approach. 2006. pp. 13–16. [PubMed]
- Cunha L, Plouzeau C, Ingrand P, Gudo JP, Ingrand I, Mondlane J, Beauchant M, Agius G. Use of replacement blood donors to study the epidemiology of major blood-borne viruses in the general population of Maputo, Mozambique. J Med Virol. 2007;79(12):1832–1840. doi: 10.1002/jmv.21010. [DOI] [PubMed] [Google Scholar]
- Gudo ES, Abreu CM, Mussa T, do Rosario Augusto A, Otsuki K, Chambo E, Amade N, Tanuri A, Ferreira OC Jr, Jani IV. Serologic and molecular typing of human T-lymphotropic virus among blood donors in Maputo City, Mozambique. Transfusion. 2009;49(6):1146–50. doi: 10.1111/j.1537-2995.2009.02100.x. [DOI] [PubMed] [Google Scholar]
- Dumans AT, Soares MA, Pieniazek D, Kalish ML, De Vroey V, Hertogs K, Tanuri A. Prevalence of protease and reverse transcriptase drug resistance mutations over time in drug-naive human immunodeficiency virus type 1-positive individuals in Rio de Janeiro, Brazil. Antimicrob Agents Chemother. 2002;46(9):3075–3079. doi: 10.1128/AAC.46.9.3075-3079.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MA, De Oliveira T, Brindeiro RM, Diaz RS, Sabino EC, Brigido L, Pires IL, Morgado MG, Dantas MC, Barreira D. A specific subtype C of human immunodeficiency virus type 1 circulates in Brazil. Aids. 2003;17(1):11–21. doi: 10.1097/00002030-200301030-00004. [DOI] [PubMed] [Google Scholar]
- Holmgren B, da Silva Z, Larsen O, Vastrup P, Andersson S, Aaby P. Dual infections with HIV-1, HIV-2 and HTLV-I are more common in older women than in men in Guinea-Bissau. Aids. 2003;17(2):241–253. doi: 10.1097/00002030-200301240-00015. [DOI] [PubMed] [Google Scholar]
- Ryst E van der, Joubert G, Smith MS, Terblanche M, Mollentze F, Pretorius AM. HTLV-I infection in the Free State region of South Africa: a sero-epidemiologic study. Cent Afr J Med. 1996;42(3):65–68. [PubMed] [Google Scholar]
- Brites C, Alencar R, Gusmao R, Pedroso C, Netto EM, Pedral-Sampaio D, Badaro R. Co-infection with HTLV-1 is associated with a shorter survival time for HIV-1-infected patients in Bahia, Brazil. Aids. 2001;15(15):2053–2055. doi: 10.1097/00002030-200110190-00023. [DOI] [PubMed] [Google Scholar]
- Marriott SJ, Semmes OJ. Impact of HTLV-I Tax on cell cycle progression and the cellular DNA damage repair response. Oncogene. 2005;24(39):5986–5995. doi: 10.1038/sj.onc.1208976. [DOI] [PubMed] [Google Scholar]
- Grassmann* Ralph, Aboud2 Mordechai, Jeang3 Kuan-Teh. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- Ginaldi L, De Martinis M, D'Ostilio A, Di Gennaro A, Marini L, Profeta V, Quaglino D. Activated naive and memory CD4+ and CD8+ subsets in different stages of HIV infection. Pathobiology. 1997;65(2):91–99. doi: 10.1159/000164109. [DOI] [PubMed] [Google Scholar]
- Xie J, Qiu ZF, Li TS, Han Y, Zuo LY, Ma XJ, Liu ZY, Wang AX. [Characteristics of immunophenotypic alterations in 263 HIV/AIDS patients] Zhonghua Yi Xue Za Zhi. 2006;86(14):965–969. [PubMed] [Google Scholar]
- Mukae H, Kohno S, Morikawa N, Kadota J, Matsukura S, Hara K. Increase in T-cells bearing CD25 in bronchoalveolar lavage fluid from HAM/TSP patients and HTLV-I carriers. Microbiol Immunol. 1994;38(1):55–62. doi: 10.1111/j.1348-0421.1994.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8(4):319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- Douek DC, Betts MR, Hill BJ, Little SJ, Lempicki R, Metcalf JA, Casazza J, Yoder C, Adelsberger JW, Stevens RA. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167(11):6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- Di Mascio M, Sereti I, Matthews LT, Natarajan V, Adelsberger J, Lempicki R, Yoder C, Jones E, Chow C, Metcalf JA. Naive T-cell dynamics in human immunodeficiency virus type 1 infection: effects of highly active antiretroviral therapy provide insights into the mechanisms of naive T-cell depletion. J Virol. 2006;80(6):2665–2674. doi: 10.1128/JVI.80.6.2665-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Buckler-White A, Buckler C, Imamichi H, Goeken RM, Lee WR, Lafont BA, Byrum R, Lane HC. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in Simian immunodeficiency virus-infected macaques. J Virol. 2007;81(2):893–902. doi: 10.1128/JVI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah I, Fukumori LM, Montanheiro P, Vergara MP, Smid J, Duarte AJ, Penalva de, Oliveira AC, Casseb J. Patterns of in vitro lymphoproliferative responses among HTLV-1-infected subjects: upregulation by HTLV-1 during HIV-1 co-infection. Scand J Immunol. 2007;65(6):577–580. doi: 10.1111/j.1365-3083.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Murakami T, Hattori T, Maeda Y, Matsushita S, Kannagi M, Sagawa K, Takatsuki K. Immunological and virological status of a hemophiliac infected with human T cell lymphotropic virus type 1 and human immunodeficiency virus type 1, and results of therapy. Int J Hematol. 1991;54(1):85–90. [PubMed] [Google Scholar]
- Fukushima N, Nishiura Y, Nakamura T, Kohno S, Eguchi K. Blockade of IL-2 receptor suppresses HTLV-I and IFN-gamma expression in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. Intern Med. 2007;46(7):347–351. doi: 10.2169/internalmedicine.46.6118. [DOI] [PubMed] [Google Scholar]
- Holub M, Beran O, Kalanin J, Hnykova J, Spala J, Rozsypal H. [CD38 antigen as a marker for immunological follow-up in HIV-positive patients.] Klin Mikrobiol Infekc Lek. 2004;10(5):229–235. [PubMed] [Google Scholar]
- Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- Coetzee LM, Tay SS, Lawrie D, Janossy G, Glencross DK. From research tool to routine test: CD38 monitoring in HIV patients. Cytometry B Clin Cytom. 2009;76(6):375–84. doi: 10.1002/cyto.b.20478. [DOI] [PubMed] [Google Scholar]
- Bentwich Z, Kalinkovich A, Weisman Z, Grossman Z. Immune activation in the context of HIV infection. Clin Exp Immunol. 1998;111(1):1–2. doi: 10.1046/j.1365-2249.1998.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Higashiyama Y, Kadota J, Mukae H, Yanagihara K, Tomono K, Kohno S. Elevated levels of soluble adhesion molecules in sera and BAL fluid of individuals infected with human T-cell lymphotropic virus type 1. Chest. 2000;118(6):1754–1761. doi: 10.1378/chest.118.6.1754. [DOI] [PubMed] [Google Scholar]
- Taylor GP. Pathogenesis and treatment of HTLV-I associated myelopathy. Sex Transm Infect. 1998;74(5):316–322. doi: 10.1136/sti.74.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Grenade L, Manns A, Fletcher V, Derm D, Carberry C, Hanchard B, Maloney EM, Cranston B, Williams NP, Wilks R. Clinical, pathologic, and immunologic features of human T-lymphotrophic virus type I-associated infective dermatitis in children. Arch Dermatol. 1998;134(4):439–444. doi: 10.1001/archderm.134.4.439. [DOI] [PubMed] [Google Scholar]
- Copeland KF, Heeney JL. T helper cell activation and human retroviral pathogenesis. Microbiol Rev. 1996;60(4):722–742. doi: 10.1128/mr.60.4.722-742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashanchi F, Brady JN. Transcriptional and post-transcriptional gene regulation of HTLV-1. Oncogene. 2005;24(39):5938–5951. doi: 10.1038/sj.onc.1208973. [DOI] [PubMed] [Google Scholar]
- Boxus M, Twizere JC, Legros S, Dewulf JF, Kettmann R, Willems L. The HTLV-1 Tax interactome. Retrovirology. 2008;5:76. doi: 10.1186/1742-4690-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SB, Porto AF, Muniz AL, de Jesus AR, Magalhaes E, Melo A, Dutra WO, Gollob KJ, Carvalho EM. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkow G, Bentwich Z. HIV and helminth co-infection: is deworming necessary? Parasite Immunol. 2006;28(11):605–612. doi: 10.1111/j.1365-3024.2006.00918.x. [DOI] [PubMed] [Google Scholar]