Abstract
Dendritic spines are now known to be subject to use-dependent plasticity that affects both their structure and their numbers. Recently, it has been demonstrated that a use-dependent increase in the density of dendritic spines occurs in both excitatory and inhibitory synapses in the mouse barrel cortex. Furthermore, it has been shown that although this increase in the density of spines and excitatory synapses is transient, the increase in spinous inhibitory synapses is long-lasting. These findings lend further support to the hypothesis that synapses in the mature cortex are subject to continual use-dependent plasticity.
Despite the existence of contradictory data from over four decades of research, many neuroscientists and neuroscience textbooks adhere to the dogma that neuronal circuits remain static in the mature CNS. Indeed, the pre-eminent cartographer of brain circuits, Ramón y Cajal, is often erroneously cited as an originator and adherent to this dogma. This is despite the fact that Cajal posited, almost 100 years ago, that to explain the acquisition of new skills in adults ‘…it is necessary to admit…the establishment of other new pathways, by means of branching and progressive growth of the terminal dendritic and axonal arborizations’ [1]. Since then, the capacity of the mature cerebral cortex to form new synapses (synaptogenesis) has been repeatedly demonstrated (see [2–4]). Synaptogenesis in the mature neocortex is now known to occur in response to central and peripheral lesions, and even following innocuous changes in neuronal activity patterns. Particularly exciting are findings that behavioral manipulations, such as cognitive or motor training, or exposure to novel environments, are associated with cortical synaptogenesis (see [2–4]). These findings provide support for the postulate that, in the mature neocortex, synaptic rearrangement is a continual process, reflecting and responsible for use-dependent functional plasticity. Many of these studies focus on synapses associated with dendritic spines (the small protrusions along the dendrites of many types of neurons).
Dendritic spines
Synapses involving dendritic spines are of particular interest for several reasons. In the cerebral cortex (and many other brain regions) ~75% of the cells have dendrites that bear a high density of spines. In the cerebral cortex, spiny neurons are most often excitatory, and include pyramidal and spiny stellate cells [5]. Furthermore, the majority of synapses in the cortex are excitatory, and most of these are formed on the spines of other excitatory neurons [5]. The small size of dendritic spines, and the fact that they are connected to their parent dendrite by a very thin neck, endows these structures with additional interesting properties [6]. The large input-resistant characteristic of dendritic spines results in large voltage changes in the postsynaptic cell, even in response to small synaptic inputs. Limited diffusion through the thin spine necks, isolates changes in calcium dynamics and other biochemical processes to the individual spines. Thus, signal-transduction cascades can occur in individual spines without affecting the rest of the neuron, and the spines can be isolated from events occurring in their parent dendrites. Voltage-dependent conductances localized to dendritic spines further enrich the computational capabilities of these structures [7]. Finally, the ability of spines to alter their shape, for example, the diameter or length of their necks, provides a powerful mechanism for synaptic plasticity in spinous synapses [6].
Spine plasticity and synaptogenesis
Interest in the computational complexity of dendritic spines significantly increased following reports that spines – and their associated synapses – can change their shape and/or number in a use-dependent fashion. For example, in the hippocampus of the female rat, dendritic spines undergo rapid and continual turnover during every estrous cycle [8]. Several recent studies have shown that the number and structure of dendritic spines is altered following the induction of long-term potentiation, and that these anatomical and electrophysiological changes are causally related (see [6]).
The region of the rodent somatosensory cortex known as the barrel cortex [9], is a favored model for studying plasticity of spines and synapses. Importantly, the barrel cortex contains, in its fourth layer, anatomically defined representations of individual whiskers on the rodent’s snout (see [10]). Several research groups take advantage of this organization because it enables the manipulation of activity experienced by specific whiskers, and examination of the consequences in individual, identified cortical barrels.
Inhibitory synapses with dendritic spines
Most inhibitory synapses in the cortex are formed on dendritic shafts and cell bodies. By contrast, the vast majority of excitatory synapses are formed with spines, each of which usually receiving a single excitatory synapse. A small proportion (<5%) of spines form two synapses, the second of which is most often associated with an inhibitory axon terminal. Similar to their excitatory counterparts, these inhibitory spinous synapses are uniquely positioned to regulate postsynaptic responses. For example, because inhibitory spinous synapses are often located on the thin spine necks, they probably exert potent inhibition, thereby completely shunting the influences of the excitatory synapses that share the same spine. Therefore, use-dependent alterations in the number of inhibitory spinous synapses might have an even greater effect than quantitatively similar increases or decreases in the number of excitatory spinous synapses.
Evidence for use-dependent plasticity in inhibitory spinous synapses is scant. In their seminal studies, Micheva and Beaulieu have shown that the density of inhibitory spinous synapses in layer IV barrels is reduced following postnatal whisker removal [11]. Most recently, Welker and colleagues have provided compelling evidence that the density of synapses actually increases following repetitive stimulation of a whisker [12]. Furthermore, they provide intriguing data on the time course of this plasticity, as well as its potential functional correlates.
Welker’s group examined the consequence of repetitive stimulation of a single whisker on the density and proportion of synapses in both the stimulated cortical barrel and adjacent barrels. Twenty-four hours after repetitive stimulation, the spine density in the corresponding barrel was significantly higher (~26%) compared with an adjacent, un-stimulated barrel, or in barrels of unstimulated animals. The total density of synapses with these spines increased, but the density of synapses with dendritic shafts remained unchanged. Although both excitatory and inhibitory spinous synapses increased in density, the observed increase in excitatory synapses was relatively modest (12%) compared with the nearly fourfold increase in inhibitory spinous synapses. The vast majority of these newly formed inhibitory synapses occurred on spines that have a second, excitatory synapse, resulting in an approximately threefold increase in the number of dually innervated spines.
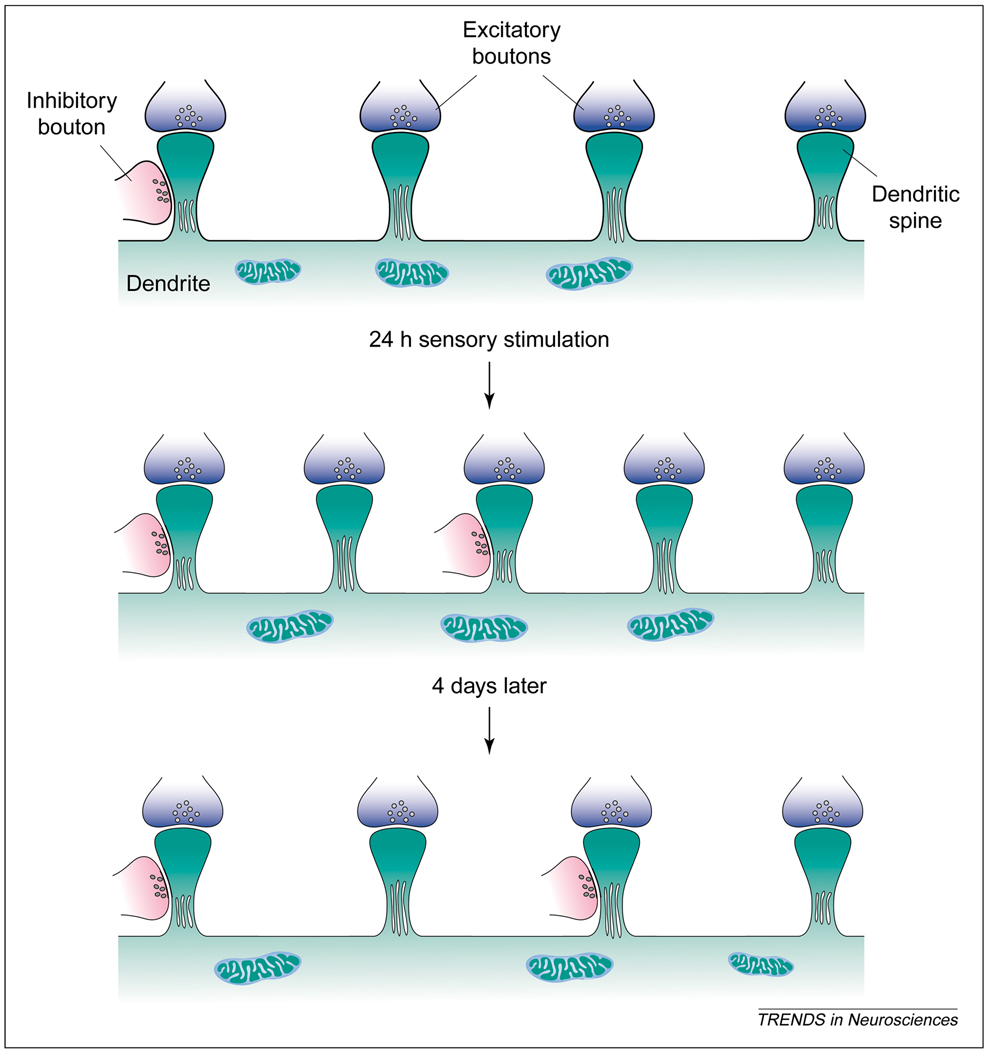
A striking finding is that some, but not all, of these changes are transitory. Four days after repetitive stimulation, both the total spine density and the density of excitatory synapses returned to control levels. By contrast, the density of inhibitory spinous synapses and the density of dually innervated spines remained significantly higher. These changes are summarized in Fig. 1.
Fig. 1.
A summary of the changes in synaptic organization following repetitive whisker stimulation. Immediately following 24 h of repetitive stimulation of a single whisker, the densities of dendritic spines, and of excitatory and inhibitory synapses with these spines, significantly increase. Four days later, total spine density returns to control levels, but the increased density of inhibitory spinous synapses remains unchanged. Reproduced with permission from Ref [12].
Such dramatic and long-lasting changes in inhibitory spinous synapses are probably reflected in the response properties of barrel neurons. Indeed, Knott et al. show that immediately following 24 h of repetitive stimulation of a whisker, cells in the corresponding barrel respond to stimulation with a reduced number of spikes. Four days later, these electrophysiological changes are largely reversed, and there is a significant increase in the late component of the responses (50–100 msec post-stimulus) [12]. It is difficult to correlate these electrophysiological responses directly with changes in the density of inhibitory synapses. For example, whisker stimulation potently and rapidly activates inhibitory neurons in the barrels, suggesting that early-onset responses are affected [13,14]. In particular, both immediately after and four days following the chronic stimulation, when spines are three-times more likely to receive inhibitory synapses, it is surprising that there is no change in the magnitude of short-latency responses (3–12 msec post-stimulus) to whisker deflections. Nevertheless, the findings clearly indicate that use-dependent changes in the incidence of inhibitory spinous synapses are reflected in the response properties of barrel neurons.
Concluding remarks
The study by Welker et al. Contains several significant and novel findings. Although use-dependent changes in spinous synapses were previously reported to occur following whisker removal [11], this is the first demonstration of an increase in these synapses following relatively innocuous, enhanced whisker activation. Of potentially greater significance is the finding that the density of both dendritic spines and excitatory synapses returns to control levels four days after chronic stimulation. This finding lends additional support to the hypothesis that cortical synapses, particularly those involving dendritic spines, are continually formed and removed in response to changes in neural activity patterns. Long-term changes in inhibitory spinous synapses are also intriguing; they indicate that these synapses might be subject to different modification rules.
Cortical synapses are subject to relatively rapid and complex turnover patterns, which raises another important experimental consideration. Essentially, all studies of whisker-to-barrel, and other sensory systems, make use of animals raised and kept in relative sensory deprivation. The findings of Welker et al., as well as previous studies demonstrating dramatic use-dependent effects on synaptic organization (see [4]) and response properties [15], raise provocative and potentially difficult issues regarding the use of housed rodents in neuroscience research. It is possible that studies using rodents reared and kept in cages, especially in isolation, examine cortical circuits in which the numbers and proportions of various classes of synapses have been strongly affected by the peculiar and unnatural conditions of their environments [15].
Acknowledgements
I thank D.J. Simons and E.L. White for their comments on earlier versions of this manuscript. Our research is supported by grants PHS:NINDS NS-35360 and NS-31078.
References
- 1.Cajal SRY. Anatomicophysiological considerations on the cerebrum. In: DeFelipe J, Jones EG, editors. Cajal on the Cerebral Cortex. An Annotated Transalation of the Complete Writings. Oxford University Press; 1911. pp. 465–490. [Google Scholar]
- 2.Flohr H. Post-Lesion Neural Plasticity. Springer-Verlag; 1988. [Google Scholar]
- 3.Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral Cortex. Development and Maturaton of Cerebral Cortex. Vol. 7. Plenum Press; 1988. pp. 391–440. [Google Scholar]
- 4.Weiler IJ, et al. Morphogenesis in memory formation: synaptic and cellular mechanisms. Behav. Brain Res. 1995;66:1–6. doi: 10.1016/0166-4328(94)00116-w. [DOI] [PubMed] [Google Scholar]
- 5.White EL. Cortical Circuits: Synaptic Organization of the Cerebral Cortex – Structure, Function and Theory. Birkhäuser; 1989. [Google Scholar]
- 6.Nimchinsky EA, et al. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 7.Segev I, Rall W. Computational study of an excitable dendritic spine. J. Neurophysiol. 1988;60:499–523. doi: 10.1152/jn.1988.60.2.499. [DOI] [PubMed] [Google Scholar]
- 8.McEwen BS, et al. Oestrogens and the structural and functional plasticity of neurons: implications for memory, ageing and neurodegenerative processes. In: Bock GR, Goode JA, editors. Non-reproductive Actions of Sex Steroids. Vol. 191. John Wiley & Sons; 1995. pp. 52–66. [DOI] [PubMed] [Google Scholar]
- 9.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 10.Keller A. Synaptic organization of the barrel cortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex: the Barrel Cortex of Rodents. Vol. 11. Plenum Press; 1995. pp. 221–262. [Google Scholar]
- 11.Micheva KD, Beaulieu C. An anatomical substrate for experience-dependent plasticity of the rat barrel field cortex. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knott GW, et al. Formation of dendritic spines with GABAergic synapses by whisker stimulation in induced adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 13.Swadlow HA, et al. Sharp, local synchrony among putative feed-forward inhibitory interneurons of rabbit somatosensory cortex. J. Neurophysiol. 1998;79:567–582. doi: 10.1152/jn.1998.79.2.567. [DOI] [PubMed] [Google Scholar]
- 14.Pinto DJ, et al. Circuit dynamics and coding strategies in rodent somatosensory cortex. J. Neurophysiol. 2000;83:1158–1166. doi: 10.1152/jn.2000.83.3.1158. [DOI] [PubMed] [Google Scholar]
- 15.Polley DB, et al. Two directions of plasticity in the sensory-deprived adult cortex. Neuron. 1999;24:623–637. doi: 10.1016/s0896-6273(00)81117-4. [DOI] [PubMed] [Google Scholar]