Abstract
Cranial bones and sutures are mechanically loaded during mastication. Their response to masticatory strain, however, is largely unknown, especially in the context of age change. Using strain gages, this study investigated masticatory strain in the posterior interfrontal and the anterior interparietal sutures and their adjacent bones in 3- and 7-month-old miniature swine (Sus scrofa). Double-fluorochrome labeling of these animals and an additional 5-month group was used to reveal suture and bone growth as well as features of suture morphology and fusion. With increasing age, the posterior interfrontal suture strain decreased in magnitude and changed in pattern from pure compression to both compression and tension, whereas the interparietal suture remained in tension and the magnitude increased unless the suture was fused. Morphologically, the posterior interfrontal suture was highly interdigitated at 3 months and then lost interdigitation ectocranially in older pigs, whereas the anterior interparietal suture remained butt-ended. Mineralization apposition rate (MAR) decreased with age in both sutures and was unrelated to strain. Bone mineralization was most vigorous on the ectocranial surface of the frontal and the parietal bones. Unlike the sutures, with age bone strain remained constant while bone MARs significantly increased and were correlated with bone thickness. Fusion had occurred in the interparietal suture of some pigs. In all cases fusion was ectocranial rather than endocranial. Fusion appeared to be associated with increased suture strain and enhanced bone growth on the ectocranial surface. Collectively, these results indicate that age is an important factor for strain and growth of the cranium.
Keywords: cranial suture, masticatory strain, skull growth, pig
Physiologically, braincase sutures are subject to two major mechanical loads. One is the force caused by brain expansion and the other results from masticatory muscle contraction. The former is a static load and no technique is yet available to measure it on living animals. In comparison, masticatory strain is dynamic and easily measured using strain gages. In a previous study we found that during chewing the posterior interfrontal suture was consistently compressed, whereas the adjacent anterior interparietal suture was tensed (Herring and Teng, 2000). However, age may change the masticatory mechanics. In the previous study (Herring and Teng, 2000), the oldest animal in the sample (over 5 months of age) demonstrated high and paradoxical suture strain, suggesting the possibility of age-associated strain change in these sutures. In the current study, these two sutures were revisited in two age groups, 3 months and 7 months, to examine strain in relation to age.
How might masticatory strain influence cranial sutures? As growth sites, sutures are expected to respond readily to epigenetic factors (Herring, 1993; Opperman, 2000) including mechanical stimuli. Unlike long bones, in which strain magnitude (Rubin and Lanyon, 1985; Turner et al., 1994), rate (O’Connor et al., 1982), history (Carter, 1987) and circumferential gradients (Gross et al., 1997; Judex et al., 1997) are all important for adaptation, the polarity of strain, i.e., compression vs tension, has traditionally been considered the key factor for sutures (Herring, 1993). Using orthodontic appliances, a body of studies suggests that suture growth is promoted by tension and retarded by compression (Ten Cate et al., 1977; Persing et al., 1986; Miyawaki and Forbes, 1987; Wagemans et al., 1988; Kokich, 1992; Teng et al., 1996; Parr et al., 1997; Sasaki et al., 2002). Masticatory strain can be compressive or tensile or both, depending on the suture (Herring and Mucci, 1991; Rafferty and Herring, 1999). Surprisingly, the precept “compression-resorption, tension-formation” is not always followed. In the pig, no evident retardation of bone formation was associated with the highly compressed nasofrontal suture (Rafferty and Herring, 1999), suggesting that compression does not necessarily inhibit suture growth. The effect on cranial sutures is still unknown. In the current study, we hypothesize that cranial suture growth is related to masticatory strain polarity and that the compressed interfrontal suture grows more slowly than the tensed interparietal suture.
The contribution of cranial sutures to the overall growth of the braincase has long been an issue of argument. Based on observation of 3-month-old pigs, Brash (1934) concluded that braincase growth is due to ectocranial apposition and endocranial resorption without any contribution from the sutures. In contrast, Mednick and Washburn (1956) found that cranial sutures were critical for the growth of the skull in infant pigs (1–8 weeks). Evidently, this discrepancy is due to the different ages of animals. Given the age-associated decrease of growth as reported in rat cranial sutures (Ten Cate et al., 1977), one can hypothesize that at a certain age, braincase growth shifts from the sutures to the bone surfaces, leading to thickening rather than widening of the bones. These two early papers (Brash, 1934; Mednick and Washburn, 1956) were qualitative and did not include animals older than 3 months. Thus this hypothesis was not rigorously tested. In this study, we quantified growth at sutures and at the surfaces of adjacent bones in pigs aged 3, 5 and 7 months.
Another aspect of cranial sutures is fusion. Investigations in rodents show that the dura mater plays a significant role in governing the patency or fusion of cranial sutures (Opperman, 2000; Warren et al., 2001). However, suture fusion in rats/mice is limited to the posterior interfrontal, which fuses shortly after weaning (Mehrara et al., 1999). This differs from the situation in most larger mammals including humans, in which sutures fuse gradually with age. In particular, the rodent model is inappropriate for investigating the influence of local mechanical environment, since the only suture to fuse does so at a very early age. In this sense, the pig is a better model. Interestingly, unlike rodents which have a fused posterior interfrontal and patent interparietal suture, the pig’s interparietal suture fuses distinctly earlier than the posterior interfrontal suture (Herring, 1972). Observations on about 50 dry skulls of known age in our collection suggested that the interparietal suture fuses at about 7 months of age, while the posterior interfrontal suture remains patent for some months longer. In this study, strains were observed before and after the presumed time of suture fusion. We expected that suture fusion would lower suture strain while strains on adjacent bones should increase because of the loss of compliant soft tissue. Finally, because premature cranial suture fusion (craniosynostosis) is associated with increased bone formation at suture margins (De Pollack et al., 1996), we investigated whether normally fusing sutures have a similar history.
MATERIALS AND METHODS
Animals
A total of 14 Hanford miniature pigs (Charles River Labs; now available from Sinclair Research Farms, Columbia, MO) were used. At the time of sacrifice, 4 pigs were approximately 3 months old, 4 pigs 5 months old, and 6 pigs 7 months old. All animals received vital staining with double fluorochromes. Strain measurements of the cranial sutures and bones were performed as a terminal experiment on the 3-month and the 7-month groups. A few days before the terminal experiment, baseline electromyography (EMG) was performed on each pig to verify its normal masticatory function. All animal procedures were approved by the University of Washington Animal Care Committee.
Double Fluorochrome Labeling
Seven days prior to the terminal experiment, calcein (Sigma, St. Louis, MO) was injected intravenously (12.5mg/Kg calcein dissolved in sterile 0.9% saline to make a 5mg/ml solution, neutralized with 1N NaOH and filtered at 0.22μm). The second fluorochrome, alizarin complexone (Sigma), was given 5 days later following the same procedures.
Terminal Experiment
On the day of the terminal experiment, pigs were anesthetized with halothane and nitrous oxide. An incision parallel to the sagittal axis was made on the skull about 1 cm to the right of the midline. The periosteum was reflected, exposing a window of the frontal and parietal bones and the interparietal and interfrontal sutures. Bone surfaces were prepared as described elsewhere (Rafferty and Herring, 1999). Single-element strain gages (EP-08-125BT-120; Measurements Group, Raleigh, NC) were placed over the interparietal and the interfrontal sutures at locations 0.5–1 cm away from bregma and perpendicular to the sutures. The tabs of the gages were glued to bone, while the suture itself was shielded from the glue by a narrow (1–2 mm) strip of Teflon tape. Stacked 45° rosette gages (SK-06-030WR-120, Measurements Group) were glued to the right parietal and frontal bones at locations 0.5–1 cm away from both the midline and the coronal suture (Fig. 1). The incisions were closed and infiltrated with local anesthetic (2% procaine). Fine-wire electromyography electrodes were then placed percutaneously in the bilateral masseter and temporalis as described elsewhere (Herring and Mucci, 1991).
Fig. 1.
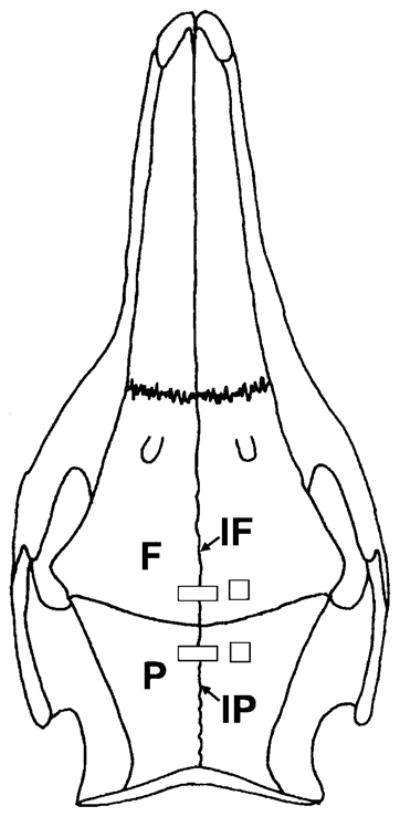
Schematic illustration of strain gage placement. Single-element (rectangle) strain gages were glued to the posterior interfrontal (IF) and the anterior interparietal (IP) sutures. The middle of the strain gage was positioned right above the suture and the suture was shielded from glue by placing a small strip of Teflon tape underneath the strain gage. Stacked rosette strain gages (square) were bonded to the right frontal (F) and parietal (P) bones.
Strain signals were led to Measurements Group Model 2120A strain gage conditioners and amplifiers, while EMG signals were led to high-impedance probes (model 7HIP5G: Grass Instruments, Quincy, MA) and amplifiers (Grass, 7P3C). Amplified strain and EMG signals were digitized (MP100, Biopac Systems, Santa Barbara, CA) and input into a Power Macintosh running Acknowledge III (Biopac Systems).
Prior to removal of anesthetic, an analgesic (Ketorolac 1mg/Kg) was injected intramuscularly. Pigs were allowed to awaken and were fed their normal food (pig chow pellets) while strain and EMG data were collected at 500 Hz for about 15 minutes. They were then reanesthetized for euthanasia. Intracardiac (for the 3-month pigs) or carotid (for the 5-month and 7-month pigs) perfusion was performed with 3 liters of heparinized saline followed by 3 liters of fixative (Prefer, Anatech LTD, Battle Creek, MI). The skinned whole head was immersed in Prefer.
Strain Data Analysis
The magnitude of strain during the power stroke of mastication was obtained by subtracting baseline strain from the peak value. The strain value after each power stroke was used for this calculation, because of baseline wander. Negative strains indicate compression and positive strains indicate tension. The maximum shear strain (sum of the absolute values of principal compressive and tensile strains) at the power stroke was used to reflect peak masticatory strain for the bones (Hylander and Johnson, 1989). EMG recordings of the masseter and temporalis muscles were used to determine the chewing side and to define the power stroke. At least 3 good chewing sequences, each containing about 20 chewing cycles, were analyzed and averaged per individual.
Specimen Harvest and Processing
Bone specimens including the interparietal or the interfrontal suture were removed from the fixed skull at or close to their respective suture gage locations, cleaned completely of attached soft tissues and stored in 70% ethanol.
Specimens were dehydrated in an ethanol series and infiltrated with Micro-bed embedding resin solution (EMS, Fort Washington, PA). Ultraviolet light (365 nm) was used to cure the resin to complete the embedding procedure. Embedded specimens were attached to plastic stubs with epoxy and sectioned in the coronal plane at a thickness of 10–20 μm using a circular saw microtome (Leica SP1600, Germany). 8–10 serial sections were collected for each specimen. Due to the thickness (300 μm) of the saw blade, adjacent sections were 300 μm apart. Sections were let dry flat at 37°C and then were mounted on slides with DPX mounting medium (Fluka, Switzerland).
Image Capture and Analysis
Sections were viewed with a fluorescent light microscope (Nikon Eclipse E400, Japan). As calcein fluoresces green and alizarin complexone red, the same field was separately captured under green and red fluorescent light using a Spot camera (Spot Image, Chantilly, VA). The magnification of all images was held constant. The two images were then merged to one file. For the 3-month and 5-month groups, ectocranial and endocranial images were captured and analyzed separately. For the larger 7-month-old pigs, ectocranial, middle and endocranial images were captured and analyzed separately. Images were also captured from the ectocranial bone surfaces near the suture. To measure the thickness of bone, low-power images containing the full ectocranial-endocranial distance were captured under all-fluorescence. 4–5 sections for every suture or bone surface were measured and averaged.
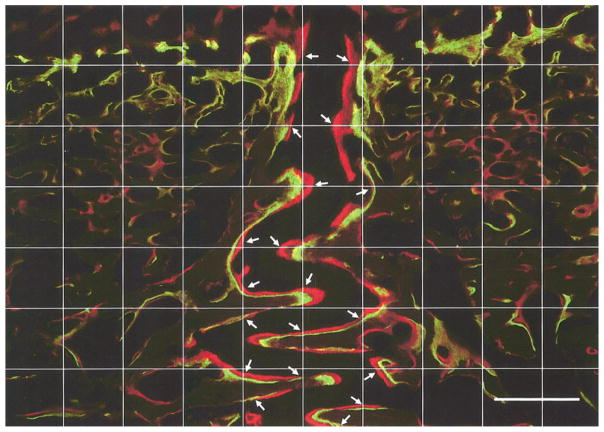
Image analysis was carried out using the software MetaVue (Universal Imaging Corp, Downingtown, PA). Image files with merged double-labels were measured for mineral apposition rate (MAR), defined as the average distance between the centers of the two labels divided by 5 days (time interval between injections). In order to obtain an objective measurement, a square test grid consisting of 11 vertical and 8 horizontal lines (including edges of the image) was superimposed on each image. Intersections of suture margins to test lines were measured; depending on the alignment of suture structure, either horizontal or vertical test lines were used (Fig. 2). The density of the grid was chosen after a pilot study demonstrating its accuracy and efficiency. Each image of an unfused suture resulted in 14–30 measurements, which were averaged. Images from the fusing interparietal sutures had fewer double-labeled surfaces and produced only about 10 measurements for each image.
Fig. 2.
An 11 × 8 (including edges) test grid superimposed on a double-labeled image (interfrontal suture from #302). Measurements of MAR were made at intersections of the grid with suture margins (arrows). Either vertical or horizontal lines were used depending on the alignment of the suture. In places where an intersection did not hit a double-label, the closest double-labeled area above or to the right of the test line was measured. Measurement was perpendicular to the suture surface and was from the center of the green label to the center of the red label. Calibration bar, 500 μm.
To reflect the level of continuous mineralization along the whole suture margin, double-label linear percentage (DLP), defined as the length of double-labeled suture margin divided by the total length of suture margin, was measured.
Suture width was obtained by dividing the area of the suture space by the average length of two suture margins.
Apposition on the ectocranial surfaces appeared as broad bands of color. The bone-surface mineral apposition rate (BMAR) was obtained by dividing the band area (from the front of the green label to the front of the red label) by the average length of the two fronts and then by 5 days.
Bone thickness was defined as the linear distance from the ectocranial surface to the endocranial surface parallel to the sagittal plane. Measurements were performed at 2.5 mm and 5 mm from the suture on each side and averaged. Some 7-month-old pigs had parietal bones so thick that capturing an image was impossible. Therefore, measurements were performed on sample blocks using an electronic digital caliper.
RESULTS
Suture Ectocranial Appearance
In ectocranial view, the interfrontal suture was patent in all animals, appearing as a clear opening along the midline between the frontal bones. The interfrontal sutures could be characterized as straight or slightly interdigitated according to Herring (1972). Three members (#304, #306, #307) of the 7-month group and one (#301) of the 5-month group had fused interparietal sutures, defined as an obliterated (complete fusion) or disappearing (partly fused) suture line. Fused interparietal sutures were individually variable in appearance. The entire in terparietal sutures of #304 and #307 were fused, while in #306 the suture was fused posteriorly but unfused anteriorly as it approached the coronal suture. Pig #301 was not examined prior to sectioning. The unfused interparietal sutures except one were indistinguishable from the interfrontal suture. This individual (#303) displayed an interparietal groove containing the suture, which had entrapped the periosteum.
Suture and Bone Strain
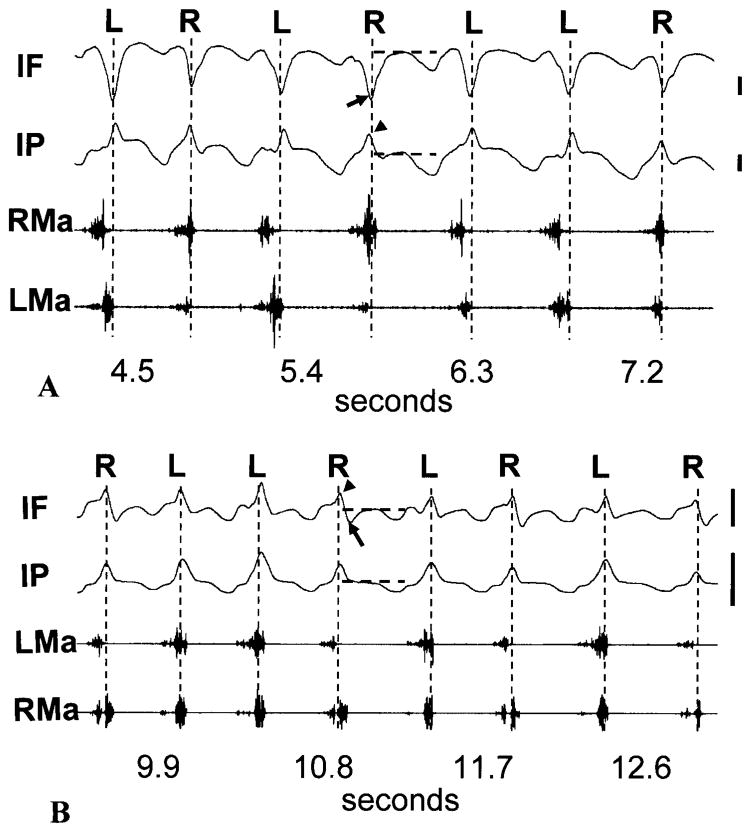
No strain data were obtained from pig #322, which refused food, or from the bone gages of #303, which malfunctioned. Typical suture strain recordings are shown in Fig. 3. The peak strains coincided with the power stroke of mastication. In 3-month-old animals, consistent with previous work (Herring and Teng, 2000), the posterior interfrontal suture was compressed (arrow, Fig. 3A) while the interparietal suture was tensed (arrow head, Fig. 3A). The tensile pattern in the interparietal suture did not change with age, but the interfrontal suture of 7-month-old pigs all had bi- or trimodal power stroke strains in which the tensile strain (arrow head, Fig. 3B) often exceeded the compressive strain (arrow, Fig. 3B).
Fig. 3.
Sutural strain and masseter activity during mastication. An approximate baseline (neutral) strain was assessed for each power stroke of strain channels. The power strokes are indicated by vertical broken lines. For one power stroke in each tracing, the baselines are shown as horizontal broken lines. Compared to baseline, in 3-month-old pigs (#299, A), interfrontal suture (IF) power stroke strains were compressive (arrow), while those of the interparietal suture (IP) were tensile (arrowhead); in 7-month-old pigs (#307, B), the interparietal suture strain was still tensile (arrowhead) but the interfrontal suture demonstrated a tensile peak (arrowhead) followed by a smaller compression at each power stroke. Chewing side was identified by late activity in the ipsilateral masseter and/or contralateral temporalis compared to the contralateral masseter and ipsilateral temporalis. RMa, right masseter; LMa, left masseter; R: right chew; L, left chew. Scale bar, 200 microstrain.
As anticipated, these midline sutures showed no consistent differences related to chewing side, although some individuals demonstrated right-left variations. Therefore, peak strains regardless of chewing side were averaged for Table 1. After Bonferroni correction for multiple t-tests, there were no significant differences between the strain magnitudes of the interfrontal and interparietal sutures. There were age differences, however. The absolute inter-frontal strain (tension minus compression) decreased with age (2-sample t-test, p=0.006), while the interparietal strains became variable. This interparietal strain variability in the 7-month-old pigs was related to fusion. Fused sutures (#304, #306, #307) had strains comparable to those of the 3-month group, whereas unfused sutures (#303, #321) had significantly higher strains (2-sample t-test, p=0.009) than those of the 3-month group. In summary, with increasing age, posterior interfrontal suture strain became more complex in pattern but decreased in magnitude, whereas the interparietal suture strain pattern remained constant while its magnitude increased unless the suture was fused.
TABLE 1.
Peak suture and bone shear strains (mean με ± SD) during mastication
| IF1 |
|||||
|---|---|---|---|---|---|
| Animal | Compression | Tension | IP2 | Frontal bone | Parietal bone |
| 3-month | |||||
| 297 | −522 ± 175 | NA4 | 107 ± 33 | 136 ± 22 | 46 ± 10 |
| 298 | −151 ± 34 | NA4 | 75 ± 27 | 52 ± 26 | 25 ± 11 |
| 299 | −690 ± 193 | NA4 | 247 ± 68 | 100 ± 30 | 43 ± 19 |
| 300 | −582 ± 213 | NA4 | 62 ± 38 | 87 ± 31 | 29 ± 12 |
| Mean ± SD | −486 ± 234 | 123 ± 85 | 94 ± 35 | 36 ± 11 | |
| 7-month | |||||
| 303 | −216 ± 48 | 64 ± 22 | 466 ± 110 | ND5 | ND5 |
| 321 | −41 ± 7 | 89 ± 18 | 391 ± 81 | 79 ± 24 | 25 ± 6 |
| 322 | ND5 | ND5 | ND5 | ND5 | ND5 |
| 3043 | −167 ± 17 | 148 ± 20 | 22 ± 5 | 122 ± 33 | 64 ± 33 |
| 3063 | −107 ± 11 | 123 ± 101 | 181 ± 24 | 60 ± 17 | 55 ± 30 |
| 3073 | −59 ± 26 | 95 ± 20 | 88 ± 7 | 58 ± 13 | 27 ± 11 |
| Mean ± SD | −118 ± 74 | 104 ± 33 | 230 ± 192 | 78 ± 30 | 43 ± 20 |
IF, interfrontal suture;
IP, interparietal suture;
fused interparietal sutures;
NA, no tension;
ND, no data.
Compared to suture strains, bone strains were low. As reported previously (Herring and Teng, 2000), alternation of chewing side changed the direction of principal strain (not shown). The magnitude of strain, however, was not different between chewing sides. Shear strains were averaged and are shown in Table 1. Neither the frontal nor the parietal bone strains changed between 3 months and 7 months of age. However, over the combined sample, the parietal bone was less strained than the frontal bone (paired t-test with the 3-month and 7-month groups combined, p=0.002).
Internal Suture Morphology
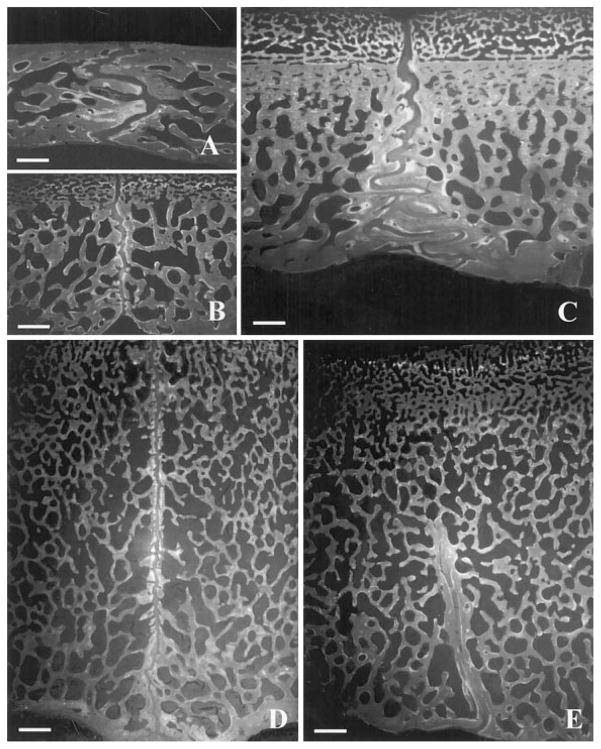
Seen in coronal section, the internal surfaces of the interparietal sutures of all animals were flat or slightly irregular (Fig 4B, 4D). However, coronal sections of the interfrontal suture showed a high level of internal interdigitation. In the 3-month-old pigs, the entire interfrontal suture was interdigitated (Fig. 4A), but in the 5- and 7-month-old pigs only the endocranial section remained interdigitated while the ectocranial section was relatively flat (Fig. 4C). The collagen arrangement in the flat and interdigitated parts of the sutures was also different as reported elsewhere (Herring and Rafferty, 2000; Herring and Teng, 2000). Specifically, in flat areas, numerous Sharpey’s fibers were inserted perpendicularly into the bone fronts, whereas in the interdigitated sections, collagen fibers were generally arranged obliquely relative to the suture edges (not shown).
Fig. 4.
Suture internal morphology. For each coronal section, the ectocranial surface is toward the top. A) Interfrontal and B) interparietal suture from 3-month-old pig #298. The former was interdigitated while the latter was relatively butt-ended. C) Interfrontal and D) interparietal suture from 7-month-old pig #321. The interparietal suture was butt-ended throughout the entire suture, whereas the interfrontal suture remained interdigitated endocranially but became butt-ended ectocranially. E) Interparietal suture of 7-month-old pig #304 shows that fusion was ectocranial. The endocranial section remained patent. To scale: calibration bar, 1000 μm.
Histological sections also confirmed interparietal suture fusion in subjects #301, #304, #306 and #307, and lack of fusion in the other pigs. Surprisingly, fusion was limited to the ectocranial side in every case, with the fused portion varying among individuals. The endocranial side was always patent (Fig 4E). Thus the interparietal suture in the pig initiates fusion from the ectocranial side rather than from the dura mater side.
Bone Growth at Suture Margins and Suture Width
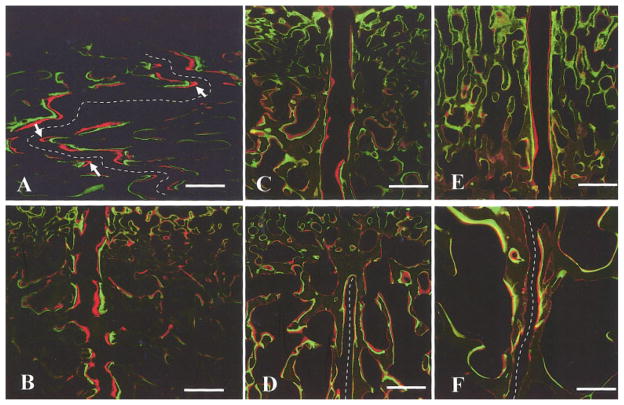
Most sutural bone margins were double-labeled. Data are presented in Table 2 and typical suture images are shown in Fig. 5. In interdigitated interfrontal sutures, the zones of new mineralization were wider at the tips of interdigitation than at the flat fronts, suggesting faster growth (Arrows, Fig. 5A). Although a few subjects showed differences in ectocranial versus endocranial MAR and DLP, there was no general pattern, so regions were combined. Average MAR decreased with age in both the interfrontal (Fig. 5A, C) and the interparietal sutures (Fig. 5B, D; Table 2, ANOVA, p<0.001 and p=0.007, respectively). Compared to that of the interfrontal suture, MAR in the interparietal suture was smaller (paired t-test with all subjects included, p=0.001), indicating slower sutural growth at the interparietal suture. Fusion of the interparietal suture tended to decrease MAR (2-sample t-test between fused and unfused interparietal suture MARs of the 7-month group, p=0.05). DLP was constant with age in the interfrontal suture, whereas it tended to decrease in the interparietal suture (ANOVA, p=0.084) (Table 2). This change probably relates to fusion, as subjects having fused interparietal sutures (#301, #304, #306, #307) generally had lower DLPs.
TABLE 2.
Sutural growth and width
| Animal | MAR1-IF2 (μm/day) | MAR1-IP3 (μm/day) | DLP4-IF2 (%) | DLP4-IP3 (%) | IF2 width (μm) |
IP3 width (μm) |
||
|---|---|---|---|---|---|---|---|---|
| Ectocranial | Endocranial | Ectocranial | Endocranial | |||||
| 3-month | ||||||||
| 297 | 12.0 | 9.6 | 45.0 | 63.3 | 191.3 | 179.7 | 204.6 | 192.4 |
| 298 | 9.0 | 4.6 | 70.0 | 70.2 | 207.2 | 197.4 | 154.3 | 153.9 |
| 299 | 11.4 | 10.2 | 65.1 | 61.6 | 230.7 | 214.3 | 242.9 | 193.6 |
| 300 | 12.7 | 10.8 | 63.6 | 65.8 | 212.4 | 204.3 | 256.4 | 206.5 |
| Mean ± SD | 11.3 ± 1.6 | 8.8 ± 2.8 | 60.9 ± 17.7 | 65.2 ± 4.9 | 210.4 ± 16.2 | 198.9 ± 14.6 | 214.6 ± 45.8 | 186.6 ± 22.7 |
| 5-month | ||||||||
| 293 | 8.1 | 7.2 | 61.2 | 61.5 | 221.5 | 171.2 | 207.6 | 169.4 |
| 294 | 6.4 | 4.9 | 46.7 | 37.6 | 238.5 | 193.2 | 180.3 | 167.8 |
| 302 | 7.1 | 7.3 | 62.0 | 68.8 | 217.1 | 156.3 | 272.4 | 173.4 |
| 3015 | 5.3 | 5.2 | 42.4 | 30.0 | 227.5 | 146.5 | NA6 | 117.0 |
| Mean ± SD | 6.7 ± 1.2 | 6.2 ± 1.3 | 53.1 ± 10.3 | 49.5 ± 16.9 | 226.1 ± 9.3 | 166.8 ± 20.3 | 220.1 ± 47.3 | 156.9 ± 26.7 |
| 7-month | ||||||||
| 303 | 5.3 | 5.3 | 55.8 | 55.9 | 249.5 | 140.1 | 203.2 | 169.4 |
| 321 | 6.0 | 5.5 | 56.0 | 60.8 | 223.4 | 130.1 | 193.4 | 128.8 |
| 322 | 5.7 | 4.0 | 65.6 | 35.1 | 251.5 | 212.9 | 235.5 | 121.3 |
| 3045 | 5.1 | 4.0 | 59.1 | 35.1 | 249.6 | 156.6 | NA6 | 130.9 |
| 3065 | 5.6 | 3.0 | 66.1 | 47.8 | 240.5 | 166.6 | NA6 | 117.1 |
| 3075 | 4.5 | 2.5 | 55.1 | 22.5 | 202.2 | 114.9 | NA6 | 92.1 |
| Mean ± SD | 5.4 ± 0.5 | 4.1 ± 1.2 | 59.6 ± 5.0 | 42.9 ± 14.5 | 236.1 ± 19.6 | 153.5 ± 34.4 | 210.7 ± 22.0 | 126.6 ± 25.2 |
| ANOVA: p | 30.001 | 0.007 | 0.391 | 0.084 | 0.095 | 0.065 | 0.961 | 0.011 |
MAR, mineral apposition rate;
IF, interfrontal suture;
IP, interparietal suture;
DLP, double-label linear percentage;
fused interparietal sutures;
NA, no ectocranial suture.
Fig. 5.
Mineralization at suture margins and suture width. A) Interfrontal and B) interparietal suture of a 3-month-old pig (#297); C) interfrontal and D) interparietal suture of a 7-month-old pig (#306). Suture mineral apposition rate (MAR) was greater in younger animals. E) Ectocranial and F) endocranial regions of the unfused interparietal suture of a 7-month-old pig (#322). The average width was greater on the ectocranial side. Arrows in (A) indicate high growth rate at the tips of interdigitations. For clarity, the suture space in (A), (D) and (F) are marked by broken lines. To scale: calibration bar, 500 μm.
As shown in Table 2, suture width was greater on the ectocranial side than on the endocranial side in both the interfrontal (paired t-test with all subjects included, p<0.001) and the interparietal (p=0.003) sutures. In some 7-month-old pigs, a middle region was also measured and the width was intermediate (not shown), suggesting that the sutures gradually narrow ectocranially to endocranially. Interestingly, except for the ectocranial part of the interparietal suture, all suture sites showed a tendency to change width with age (Table 2). First, the endocranial width of both sutures decreased with age, although only that of the interparietal suture was significant. If the outlying #322 was excluded, ANOVA of the endocranial width of the interfrontal suture reached a p-value of 0.01. Second, the ectocranial width of the interfrontal suture tended to increase with age. Fusion of the ectocranial part of the interparietal suture did not significantly affect its endocranial width (2-sample t-test between fused and un-fused interparietal sutures of the 7-month group, p=0.23), although this comparison had little power.
Relationship between Suture Growth and Strain
Pearson correlation coefficients were calculated between growth indices and sutural strain magnitude. For the 7-month-old pigs, the sum of the absolute value of the compressive and tensile strains in the interfrontal suture was used for this analysis. A significantly positive correlation was found in the interfrontal suture (r=0.804, p=0.009). To test whether this correlation was an artifact of age changes, correlations were calculated separately with age groups. Neither the 3-month nor the 7-month group showed a significant correlation between MAR and strain magnitude. Therefore, the correlation of the sample as a whole was simply due to age changes in both MAR and strain. For the interparietal suture, neither the combined nor the separate samples yielded a significant correlation. Furthermore, neither DLP nor sutural width was correlated with strain magnitude.
Ectocranial Growth and Bone Thickness
While substantial bone growth was observed on the ectocranial surfaces of the frontal and the parietal bone, no bone formation was found on the endocranial surfaces, except occasionally next to the sutures of some 3-month-old pigs. Bone mineral apposition rate at the ectocranial surface increased with age for both the frontal and the parietal bones (Table 3, Fig. 6), with the latter growing more (paired t-test with all subjects included, p<0.001). Similarly, although bones thickened significantly with age (Table 3), the parietal bone was significantly thicker than the frontal (paired t-test with all subjects included, p<0.001). Ectocranial bone mineral apposition rate was significantly correlated with bone thickness (frontal bone, r=0.828, p<0.001; parietal bone, r=0.682, p=0.01), but not with bone strain (frontal bone, r=−0.30, p=0.47; parietal bone, r=0.48, p=0.27).
TABLE 3.
Bone thickness and ectocranial growth rate
| Bone thickness (mm) |
BMAR1 (μm/day) |
|||
|---|---|---|---|---|
| Animal | frontal | parietal | frontal | parietal |
| 3-month | ||||
| 297 | 3.4 | 5.3 | 20.5 | 118.7 |
| 298 | 3.9 | 6.4 | 4.7 | 62.6 |
| 299 | 4.7 | 9.5 | 19.8 | 82.1 |
| 300 | 4.6 | 9.4 | 28.7 | 54.4 |
| Mean ± SD | 4.2 ± 0.6 | 7.7 ± 2.1 | 18.4 ± 10.0 | 79.5 ± 28.7 |
| 5-month | ||||
| 293 | 4.3 | 5.3 | 51.1 | 98.3 |
| 294 | 9.5 | 10.5 | 101.7 | 149.8 |
| 302 | 9.1 | 16.5 | 64.6 | 127.8 |
| 3011 | 11.9 | 13.7 | 128.5 | 170.6 |
| Mean ± SD | 8.7 ± 3.2 | 11.5 ± 4.8 | 86.5 ± 35.2 | 136.6 ± 31.0 |
| 7-month | ||||
| 303 | 15.3 | 15.6 | 134.1 | 165.7 |
| 321 | 10.0 | 13.0 | 123.6 | 144.3 |
| 322 | 10.5 | 14.3 | 71.2 | 110.8 |
| 3042 | 8.7 | 12.2 | 55.1 | 116.0 |
| 3062 | 12.2 | 17.9 | 111.1 | 197.0 |
| 3072 | 14.5 | 17.0 | 69.4 | ND3 |
| Mean ± SD | 11.9 ± 2.6 | 15.0 ± 2.2 | 94.1 ± 32.9 | 146.8 ± 35.8 |
| ANOVA:p | 0.002 | 0.013 | 0.005 | 0.027 |
BMAR, bone-surface mineral apposition rate,
fused interparietal sutures;
ND: no data.
Fig. 6.
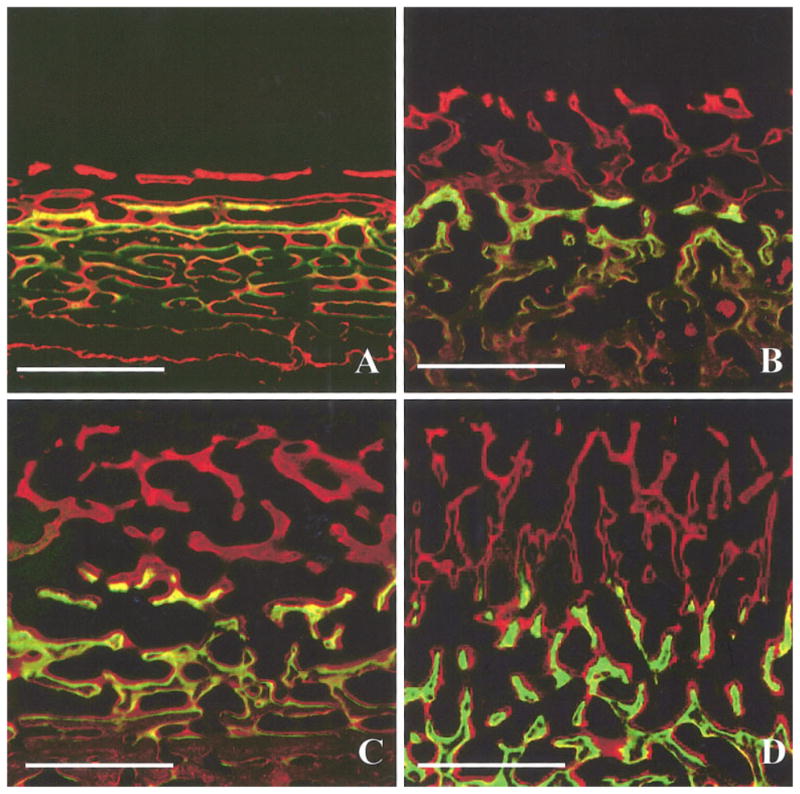
Mineralization at the ectocranial surfaces. A) Frontal and B) parietal bone of a 3-month-old pig (#299); C) frontal and D) parietal bone of a 7-month-old pig (#321). In the younger animals, frontal bone apposition was laminar and parallel to the bone surface, whereas parietal apposition was plexiform. In the older animals both bones showed plexiform apposition. The older pigs grew faster than the younger pigs and the parietal bone grew faster than the frontal bone of the same age. To scale: calibration bar, 500 μm.
DISCUSSION
Suture Strain
Masticatory strain changes with age in both the inter-frontal and the interparietal sutures, but not in the same way. The interfrontal suture showed a decreased overall strain magnitude with an added tensile peak during the power stroke. The interparietal suture was tensed at all ages, but strain increased in the absence of fusion. The outlying pig from the previous study (Herring and Teng, 2000) is thus explained. This animal was older than the rest of the sample and showed the strain pattern typical of the 7-month-old animals in the present study.
The compressive strain seen in the posterior interfrontal suture of 3-month-old pigs is actually part of a more complex pattern with tension more anteriorly (Rafferty and Herring, 1999; Herring and Teng, 2000). Overall, the frontal bones appear to rotate around a neutral axis situated between the posterior and middle thirds of the suture. This masticatory strain has been shown to be caused by contraction of the masseter muscles (Herring and Teng, 2000). The age effects of diminished compression and added tension in the posterior interfrontal suture suggest that the neutral axis of frontal bone rotation has moved posteriorly toward bregma. Thus the strain gage location, squarely in the compressed region of the 3-month-old pigs, would have been located near the neutral axis of the 7-month-old animals. Such a relocation of the neutral axis could come about either by a relative increase in masseter muscle strength or by a physical change in the position of the sutures caused by unequal bone growth.
For interparietal suture strain, the age effect was in magnitude only, but the situation is complicated by fusion. Strain increased in the absence of fusion, but in the presence of fusion strain did not decrease. Because the fused sutures of the older animals must have been stiffer than the unfused sutures of the younger animals, loads must have been substantially larger in the older animals. Thus, both the fused (undiminished strain despite increased stiffness) and the unfused (high strain) data indicate that the interparietal sutures of older pigs bear larger loads. Tension in this suture is a result of temporalis muscle contraction (Herring and Teng, 2000), and therefore it is reasonable to attribute the increased tension to stronger temporalis muscles.
Suture Internal Morphology
Suture morphology has long been suggested to reflect local biomechanical environment (Moss, 1957). Relatively simple at birth, many mammalion sutures gain interdigitation during growth. According to Jaslow’s impact-loading experiment on sheep skulls, sutural interdigitation is related to the potential for energy absorption via a mechanism of increased surface area leading to increased collagen fibers (1990). Using a finite element model, Borke and coworkers (2003) pointed out that highly complex sutures can relieve tensile and shear stress. Nevertheless, in vivo studies on pig sutures (Herring and Mucci, 1991; Rafferty and Herring, 1999; Herring and Teng, 2000) suggest that interdigitation is not associated with magnitude of strain, but with masticatory compression, whereas butt-ended morphology is associated with tension, again regardless of magnitude. Interdigitation facilitates an oblique arrangement of collagen fibers, which is a good compression-resistance mechanism (Herring and Rafferty, 2000). The current study adds to this evidence. In 3-month pigs, the compressed posterior interfrontal suture is highly interdigitated with oblique fibers, whereas the tensed interparietal suture is butt-ended with perpendicular fibers. Interdigitation evidently arose from differential growth along the suture margins, as mineralization at the tips of interdigitation appeared to be faster than other areas (Fig 5A, arrows). While the anterior interparietal suture remained butt-ended, with growth the posterior interfrontal suture lost interdigitation ectocranially. Provisionally, we ascribe this striking change in morphology to the addition of tension to this suture’s strain pattern.
Sutural Growth
Methodology
Double-labeling vital stains have been extensively used to study growing long bones (Iwaniec and Crenshaw, 1998; Lerner et al., 1998) and occasionally sutures (Engström et al., 1986; Parr et al., 1997; Rafferty and Herring, 1999). The five-day interval produced good separation of the labels in the younger pigs, but in the slower growing sutures of older pigs, the labels were often superimposed. MAR was intended to reveal the average growth rate along the entire suture, while DLP was to reveal the relative growth activity of the suture. In contrast to the relative unambiguousness of DLP, one frequently mentioned problem of MAR measurement is the subjective determination of the center positions of diffuse labels. Our sections were thin (10–20 μm) and most images had distinct labels; the determination of the center position was easy and clear. A more difficult problem is that the suture margins do not grow equally at all locations, especially in interdigitated sutures. Our approach was to use a grid to determine the measuring sites objectively and to make enough measurements to assure consistent average values.
Age changes in MAR
Not surprisingly, MAR decreased with age in both the interparietal and the interfrontal sutures, as has been reported in rats (Ten Cate et al., 1977). It is usually assumed that growth of braincase sutures is a function of tension arising from brain growth and that these sutures cease to grow when the brain reaches adult size (Moss, 1954). The overall growth rate of Hanford swine does not begin to decrease until after the age of 8 months (Bustad et al., 1966). In domestic pigs, the brain grows rapidly at 1–2 months after birth and slows down thereafter (Pond et al., 2000). The Hanford minipigs maintain a low but constant growth rate in brain weight from 30 to 176 days after birth (Fig. 1 in Friedman et al., 1994). The continued but decreased rate of sutural mineralization in the period of 3–7 months, therefore, consistent with the notion that sutural growth follows brain growth.
MAR and masticatory strain
As in our previous study of facial sutures, we found that masticatory compression does not retard suture growth (Rafferty and Herring, 1999). In fact, in this study, the compressed posterior interfrontal suture generally had a higher MAR than the tensed interparietal suture. This supports a recent finding that cyclic (but probably not constant) compression is osteogenic at sutures (Kopher and Mao, 2003). Strain magnitude and MAR were not correlated in either suture except insofar as both are affected by age.
Suture width
Sutural width has been correlated with increased growth rates and strains in the presence of extrinsically applied loads (Mao, 2002; Kopher and Mao, 2003). However, whereas MAR did not differ on ectocranial and endocranial sides, sutural width did, especially in the older pigs. Thus, sutural width was not correlated with growth rate under normal in vivo loading. It may be that the uneven suture width is a byproduct of the rapid mineral apposition on the curved ectocranial bone surfaces. Another possibility is that suture width reflects strain rather than growth rate itself. Strain levels may differ between the ectocranial and the endocranial side. We have no data on strain from the endocranial side of the suture in living animals. However, given the great thickness of the bones (especially in older pigs), it is reasonable to speculate that the endocranial side is less influenced by masticatory strain. Instead, it would be more influenced by the (presumably smaller) strain of brain expansion (Mednick and Washburn, 1956).
Bone Strain, Thickness and Growth
Strain magnitude and orientation of the frontal and the parietal bones were the same as in our previous report (Herring and Teng, 2000). In contrast to sutural strains, bone strains did not change with age. The substantial increase of bone thickness with age evidently compensates for the stronger muscle activity in the older pigs, keeping strain level constant. The frontal and the parietal bones are mainly strained by the masseter and the temporalis, respectively (Herring and Teng, 2000). As the masseter and the temporalis muscles in the pig have similar physiological cross sections, about 30% of the jaw muscle total (Herring, 1985), it is reasonable to assume that they load these bones equally during mastication. The higher strain observed on the frontal bone therefore may be ascribed to its lesser thickness relative to the parietal bone.
Apposition on the bone surface was much faster than on sutures (Table 3). However, in contrast to Mednick and Washburn’s (1956) observations on 1–8 week pigs, we did not find endocranial apposition. Therefore, by the age of 2–3 months, the endocranial surface no longer contributes to bone thickening. Instead, ectocranial bone apposition is solely responsible for this growth, as also indicated by the strong correlation of BMAR with bone thickness. BMAR of both bones significantly increased with age.
Surface bone strain, being constant, was of course not correlated with BMAR and thickness, which increased with age. In fact, the parietal bone was less strained than the frontal bone but grew faster. Therefore, it is unlikely that the impetus for cranial bone thickening arises from increasing surface strain. It is also unlikely that the osteogenic signal arises from the dura mater, because the growing surface is ectocranial, 4–15 mm away from the dura. We conclude that the osteogenic signal must arise from a superficial tissue, possibly the periosteum itself.
Suture Fusion
Unexpectedly, the fused interparietal sutures were closed only ectocranially. Endocranially, although sutural width was narrower, they were still patent (and growing). This contrasts with data on both humans (Todd and Lyon, 1924) and murid rodents (Bradley et al., 1996) indicating that synostosis begins endocranially. The pig situation could be explained by inhibiting signals from the dura mater (Opperman et al., 1995) but is not consistent with a dural trigger of fusion (Greenwald et al., 2000). Thickening of the cranium could decrease the diffusion of osteoin-hibitory signals to the ectocranial side, allowing fusion to be initiated. It is also conceivable that dural signaling is not involved, and that fusion is triggered ectocranially by the periosteum. The periosteum does not inhibit suture fusion in rats (Opperman et al., 1994) and in pigs it is clearly extremely osteogenic.
Mechanical factors could also be involved in the ectocranial initiation of interparietal suture fusion. The effectors of suture strain are the muscles, which are located ectocranially. With age, ectocranial interparietal suture strain rises (#303, #321, table 1). However, due to the thickening of the cranium, the endocranial side would be less strained or even compressed. The high tensile strain would be expected to promote bone formation on the ectocranial side, perhaps triggering suture fusion. Endocranially, the strain patterns are probably less osteogenic. Once the suture is fused, less strain is exerted, explaining the relatively low MAR and DLP in the remaining patent part as shown in Table 2. Alternatively, the fusion may just be the result of rapid ectocranial bone apposition, incidentally sealing the suture on the ectocranial surface, leaving the endocranial section as it was. Study of cellular osteogenetic activity along the suture margins and the contribution of the dura mater and the periosteum will help us understand this issue better.
Overview
Returning to the questions posed in the Introduction, we first found that masticatory strain did change with increasing age, but only in the sutures. Parietal and frontal bone strain remained constant. Second, neither the polarity (compression versus tension) nor the magnitude of sutural strain was correlated with sutural growth rate. Polarity did, however, predict suture internal morphology, including age changes. Third, the quantification of growth rates confirmed the notion that as pigs age, enlargement of the braincase shifts from sutural to ectocranial surface apposition. Nevertheless, sutural growth certainly does not cease at 3 months of age in the pig. Fourth, fusion of the interparietal suture did lower suture strain magnitude (in the context of an age increase), but the expected increase in parietal bone strain did not occur. The reason for this probably relates to the rapid thickening of the bone, which would stiffen it and lower strain levels. Finally, suture fusion was not related to increased apposition rate at the suture margins; in fact this rate was decreased. Instead, interparietal suture fusion started from the ectocranial surface in slow-growing sutures. Taken as a whole, these findings point to an important role for the periosteum in determining not only braincase growth, but also suture fusion.
Acknowledgments
Grant sponsor: NIDCR; Grant number: DE 08513.
The authors thank Dr. Kathy Rafferty for help with animal experiments, Dr. Pannee Ochareon for technical advice, and Ms Patricia Emry for help with histology.
LITERATURE CITED
- Borke JL, Zhang G, Yu JC, Isales CM. Mechanical environment and suture complexity. J Dent Res. 2003;82:A1505. [Google Scholar]
- Bradley JP, Levine JP, Blewett C, Krummel T, McCarthy JG, Longaker MT. Studies in cranial suture biology: in vitro cranial suture fusion. Cleft Palate Craniofac J. 1996;33:150–156. doi: 10.1597/1545-1569_1996_033_0150_sicsbv_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Brash JC. Some problems in the growth and developmental mechanics of bone. Edinburgh Med J NS. 1934;41:305–387. [PMC free article] [PubMed] [Google Scholar]
- Bustad LK, Horstman VG, England DC. Development of Han-ford miniature swine. In: Bustad LK, McClellan RO, Burns MP, editors. Swine in biomedical research. Seattle: Frayn Printing Co; 1966. pp. 769–774. [Google Scholar]
- Carter DR. Mechanical loading history and skeletal biology. J Biomech. 1987;20:1095–1109. doi: 10.1016/0021-9290(87)90027-3. [DOI] [PubMed] [Google Scholar]
- De Pollack C, Renier D, Hott M, Marie PJ. Increased bone formation and osteoblastic cell phenotype in premature cranial suture ossification (craniosynostosis) J Bone Miner Res. 1996;11:401–407. doi: 10.1002/jbmr.5650110314. [DOI] [PubMed] [Google Scholar]
- Engström C, Kiliaridis S, Thilander B. The relationship between masticatory function and craniofacial morphology. II. A histological study in the growing rat fed a soft diet. Eur J Orthod. 1986;8:271–279. doi: 10.1093/ejo/8.4.271. [DOI] [PubMed] [Google Scholar]
- Friedman L, Gaines DW, Newell RF, Sager AO, Matthews RN, Braunberg RC. Body and organ growth of the developing Hormel-Hanford strain of male miniature swine. Lab Anim. 1994;28:376–379. doi: 10.1258/002367794780745083. [DOI] [PubMed] [Google Scholar]
- Greenwald JA, Mehrara BJ, Spector JA, Warren SM, Crisera FE, Fagenholz PJ, Bouletreau PJ, Longaker MT. Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency. J Bone Miner Res. 2000;15:2000. doi: 10.1359/jbmr.2000.15.12.2413. [DOI] [PubMed] [Google Scholar]
- Gross TS, Edwards JL, McLeod KJ, Rubin CT. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997;12:982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- Herring SW. Sutures—a tool in functional cranial analysis. Acta Anat (Basel) 1972;83:222–247. doi: 10.1159/000143860. [DOI] [PubMed] [Google Scholar]
- Herring SW. Morphological correlates of masticatory patterns in peccaries and pigs. J Mamm. 1985;66:603–617. [Google Scholar]
- Herring SW. Epigenetic and functional influences on skull growth. In: Hanken J, Hall BK, editors. The Skull. Chicago: Univ. of Chicago Press; 1993. pp. 153–206. [Google Scholar]
- Herring SW, Mucci RJ. In vivo strain in cranial sutures: the zygomatic arch. J Morphol. 1991;207:225–239. doi: 10.1002/jmor.1052070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SW, Rafferty KL. Cranial and facial sutures: functional loading in relation to growth and morphology. In: Davidovitch Z, Mah J, editors. Biological Mechanisms of Tooth Eruption, Resorption and Replacement by Implants. Boston, MA: EBSCO Media; 2000. pp. 269–276. [Google Scholar]
- Herring SW, Teng S. Strain in the braincase and its sutures during function. Am J Phys Anthrop. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR. The relationship between masseter force and masseter electromyogram during mastication in the monkey Macaca fascicularis. Arch Oral Biol. 1989;34:713–722. doi: 10.1016/0003-9969(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Crenshaw TD. Distribution of mineralization indices of modeling and remodeling over eight months in middiaphyseal cross sections of femurs from adult swine. Anat Rec. 1998;250:136–145. doi: 10.1002/(SICI)1097-0185(199802)250:2<136::AID-AR2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jaslow CR. Mechanical properties of cranial sutures. J Biomech. 1990;23:313–321. doi: 10.1016/0021-9290(90)90059-c. [DOI] [PubMed] [Google Scholar]
- Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res. 1997;12:1737–1745. doi: 10.1359/jbmr.1997.12.10.1737. [DOI] [PubMed] [Google Scholar]
- Kokich VG. Sutural response to orthopedic forces. In: Mc-Namara JA, editor. Bone Biodynamics in Orthodontic and Orthopedic Treatment. Ann Arbor: University of Michigan; 1992. pp. 173–188. [Google Scholar]
- Kopher RA, Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003;18:521–528. doi: 10.1359/jbmr.2003.18.3.521. [DOI] [PubMed] [Google Scholar]
- Lerner AL, Kuhn JL, Hollister SJ. Are regional variations in bone growth related to mechanical stress and strain parameters? J Biomech. 1998;31:327–335. doi: 10.1016/s0021-9290(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Mao JJ. Mechanobiology of craniofacial sutures. J Dent Res. 2002;81:810–816. doi: 10.1177/154405910208101203. [DOI] [PubMed] [Google Scholar]
- Mednick LW, Washburn SL. The role of the sutures in the growth of the brain case of the infant pig. Am J Phys Anthropol. 1956;14:175–191. doi: 10.1002/ajpa.1330140215. [DOI] [PubMed] [Google Scholar]
- Mehrara BJ, Greenwald J, Chin GS, Dudziak M, Sagrioglu J, Stein-brech DS, Saadeh PB, Gittes GK, Longaker MT. Regional differentiation of rat cranial suture-derived dural cells is dependent on association with fusing and patent cranial sutures. Plast Reconstr Surg. 1999;104:1003–1013. doi: 10.1097/00006534-199909040-00016. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Forbes DP. The morphologic and biochemical effects of tensile force application to the interparietal suture of the Sprague-Dawley rat. Am J Orthod Dentofacial Orthop. 1987;92:123–133. doi: 10.1016/0889-5406(87)90367-2. [DOI] [PubMed] [Google Scholar]
- Moss M. Growth of the calvaria in the rat: the determination of osseous morphology. Am J Anat. 1954;94:333–361. doi: 10.1002/aja.1000940302. [DOI] [PubMed] [Google Scholar]
- Moss ML. Experimental alteration of sutural area morphology. Anat Rec. 1957;127:569–590. doi: 10.1002/ar.1091270307. [DOI] [PubMed] [Google Scholar]
- O’Connor JA, Lanyon LE, MacFie H. The influence of strain rate on adaptive bone remodeling. J Biomech. 1982;15:767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Min Res. 1995;10:1978–1987. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Persing JA, Sheen R, Ogle RC. In the absence of periosteum, transplanted fetal and neonatal rat coronal sutures resist osseous obliteration. J Craniofac Surg. 1994;5:327–332. doi: 10.1097/00001665-199411000-00012. [DOI] [PubMed] [Google Scholar]
- Parr JA, Garetto LP, Wohlford ME, Arbuckle GR, Roberts WE. Sutural expansion using rigidly integrated endosseous implants: an experimental study in rabbits. Angle Orthod. 1997;67:283–290. doi: 10.1043/0003-3219(1997)067<0283:SEURIE>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Persing JA, Babler WJ, Nagorsky MJ, Edgerton MT, Jane JA. Skull expansion in experimental craniosynostosis. Plast Reconst Surg. 1986;78:594–603. doi: 10.1097/00006534-198611000-00006. [DOI] [PubMed] [Google Scholar]
- Pond WG, Boleman SL, Fiorotto ML, Ho H, Knabe DA, Mersmann HJ, Savell JW, Su DR. Perinatal ontogeny of brain growth in the domestic pig. Proc Soc Exp Biol Med. 2000;223:102–108. doi: 10.1177/153537020022300114. [DOI] [PubMed] [Google Scholar]
- Rafferty KL, Herring SW. Craniofacial sutures: morphology, growth and in vivo masticatory strains. J Morphol. 1999;242:167–179. doi: 10.1002/(SICI)1097-4687(199911)242:2<167::AID-JMOR8>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcif Tissue Int. 1985;37:411–417. doi: 10.1007/BF02553711. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sugiyama H, Tanaka E, Sugiyama M. Effects of sutural distraction osteogenesis applied to rat maxillary complex on craniofacial growth. J Oral Maxillofac Surg. 2002;60:667–675. doi: 10.1053/joms.2002.33117. [DOI] [PubMed] [Google Scholar]
- Ten Cate AR, Freeman E, Dickinson JB. Sutural development: structure and its response to rapid expansion. Am J Orthod. 1977;71:622–636. doi: 10.1016/0002-9416(77)90279-2. [DOI] [PubMed] [Google Scholar]
- Teng S, Herring SW, Ferrari CS. Use of a bite-opening appliance in the miniature pig: masticatory function and sutural strain. In: Davidovitch Z, editor. The Biological Mechanisms of Tooth Eruption, Resorption and Replacement by Implants. Birmingham, AL: EBSCO Media; 1996. pp. 413–421. [Google Scholar]
- Todd TW, Lyon DW. Endocranial suture closure, its progress and age relationship: Part I. Adult males of white stock. Am J Phys Anthropol. 1924;7:325–384. [Google Scholar]
- Turner CH, Forwood MR, Rho J-Y, Yoshikawa T. Mechanical loading thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:87–97. doi: 10.1002/jbmr.5650090113. [DOI] [PubMed] [Google Scholar]
- Wagemans PAHM, van de Velde JP, Kuijpers-Jagtman AM. Sutures and forces: a review. Am J Orthod Dentofacial Orthop. 1988;94:129–141. doi: 10.1016/0889-5406(88)90361-7. [DOI] [PubMed] [Google Scholar]
- Warren SM, Greenwald JA, Spector JA, Bouletreau P, Mehrara BJ, Longaker MT. New developments in cranial suture research. Plast Reconstr Surg. 2001;107:523–540. doi: 10.1097/00006534-200102000-00034. [DOI] [PubMed] [Google Scholar]