Abstract
In this review, we address the question, central to cognition, of whether nonhuman animals such as rats are capable of extracting and extending information from a given learning situation to a new learning situation without generalizing through a physical dimension of the stimuli. This capacity underlies abstraction, which is a hallmark of human cognition and necessary for complex information processing such as language acquisition. We selectively review recent experiments with rats in which systematic changes in information processing of new stimuli are observed after training with different stimuli. These results strongly suggest that this capacity is present in rats. We also review two articles in which clear limitations to this capacity are detected. We conclude that, within specified limits, rats are capable of using prior experience when faced with a learning situation that involves new stimuli. We interpret this ability as a rudimentary form of abstraction. In the face of these provocative results, new theories of learning should be designed to account for these findings.
Keywords: Abstraction, Rule learning, Transfer, Nonlinearity, Rats
Introduction
In the short fictitious story “Funes the memorious,” Jorge Luis Borges (1942) described Ireneo Funes, a South American man who had unlimited memory. Borges offered several creative examples of the unlimited capacity of this character’s memory, and nearing the end of the story concludes: “Without effort, he had learned English, French, Portuguese, Latin. I suspect, nevertheless, that he was not very capable of thought. To think is to forget a difference, to generalize, to abstract. In the overly replete world of Funes there were nothing but details, almost contiguous details.” Funes’ memories were so vivid (i.e., eidetic) that remembered events were as though actually perceived, so that “It was not only difficult for him to understand that the generic term dog embraced so many unlike specimens of differing sizes and different forms; he was disturbed by the fact that a dog at three-fourteen (seen in profile) should have the same name as the dog at three-fifteen (seen from the front).” Hence, Borges’ conclusion was that Funes was not able to generalize, to abstract.
In essence, this description does not differ much from some observations made by Oliver Sacks (1970), who has described several cases of extreme memory. For example, in chapter 22, he describes Martin A and notes that, “if a page of an encyclopedia or newspaper was read to him, or a map of Asia’s rivers or New York’s subway shown to him, it was recorded instantly, in his eidetic memory”. Indeed, Sacks later notes that “there seemed little or no emotion in such memories—no more emotion than there is in a street map of New York—nor did they connect, or ramify, or get generalized, in any way”. Thus, both fictitious and clinical descriptions agree that the capacity to store information alone does not in itself provide the basis for much thought. In order to think, one must be able to flexibly use these memories, to generalize. These descriptions also agree with modern views in the cognitive sciences. For example, Gentner (2003) has proposed that, “the ability to draw abstractions from particulars—to generalize experience and store regularities across vastly different cases” is critical for high-order information processing in humans.
The ability to extend abstract structure to new instances is one of the basic hallmarks of human cognition (Marcus 2001). By abstraction, we mean that prior experience modifies in a systematic fashion the way subjects learn or encode a new experience, but importantly, this occurs in the absence of stimulus generalization. This capacity has also been claimed to play a pivotal role in language acquisition. Some theorists (e.g., Pinker 1991) have argued that successful language acquisition necessitates both associative processes like those proposed by connectionist models (e.g., McClelland and Rumelhart 1986) and rule-like processes presumably performed by genetically determined computational modules (e.g., Chomsky and Halle 1990). Thus, one area of research in psycholinguistics is aimed at understanding how humans are so good at discriminating phonetic forms given that language across speakers is highly variable (Kraljic et al. 2008).
As noted by Hauser et al. (2002), an important question in cognition is whether the ability to extract and generalize abstract information distinguishes humans from other animals. This question is important for two main reasons. First, it is important because research in basic learning and memory has used nonhuman animals, such as rodents, pigeons, and even insects (Bouton 2007). The reasons for this seem obvious. Basic learning theorists are concerned with the fundamental mechanisms underlying information processing and attempt to isolate these mechanisms from the influence of genetic variation (using subjects drawn from a pool of subjects of a given outbred strain) and prior experience (by preventing differential exposure to stimulation from the environment that may be related to the learning experience in question). If the ability to abstract and generalize abstract information is unique to humans and that capacity facilitated our adapting to a vast range of environments (Gentner 2003), there would be little of direct value to humans in studying cognition in nonhuman animals. Another way to formulate this issue is to ask: when we study basic learning processes in nonhuman species, are we only studying subjects like Ireneo Funes who could not generalize prior experiences (in his case in the form of eidetic memories) to new situations?
The second reason why the question of whether animals can use prior information and generalize it to new situations is important has a theoretical basis. For many years, theories of learning only computed changes for a given cue (e.g., a tone) regardless of the co-presentation of other cues (e.g., Bush and Mosteller 1951). For these theories, it did not make a difference in the amount of behavioral control acquired by a cue (e.g., a tone) if a second stimulus (e.g., a flashing light) is presented during training. This early position is refuted by phenomena such as overshadowing and blocking, both of which are instances of reduced behavioral control by the target stimulus due to the co-presentation during training of cues either of higher salience (overshadowing; Pavlov 1927) or that have previously undergone excitatory training (blocking; Kamin 1968). With the observation of blocking by Kamin (1968) and rediscovery of Pavlov’s (1927) overshadowing phenomenon (see below), theories of learning began to explain differential changes in behavioral control of a target cue as a result of co-presentation of nontarget cues on a given trial. The resultant idea of total error correction that was embraced by many theories provided a good account for the observations of early cue competition phenomena (Pearce 1987; Pearce and Hall 1980; Rescorla and Wagner 1972). However, these theories assumed that changes in associative strength would occur only if the target cue was presented on a given trial. This assumption was challenged by observations of changes in behavioral control by cues that did not receive further training. For example, Kaufman and Bolles (1981) observed recovery from the overshadowing deficit as a result of extinction of the overshadowing cue without any further training of the overshadowed cue. This observation and many others with rats and humans (e.g., Shanks 1985) suggested that absent cues could also undergo changes in behavioral control, as long as another cue that had previously been paired with the absent target cue received further training. Models like the comparator hypothesis (Miller and Matzel 1988; Stout and Miller 2007) diverged from other models in accounting these phenomena, by assuming that competition occurred at the time of testing, and therefore changes in behavioral control by a target could happen without further direct learning concerning the target. The uniqueness of the comparator hypothesis in accounting for these effects (now well known as retrospective revaluation effects) did not last long. Soon after the development of the comparator hypothesis, other researchers modified models focused on acquisition processes by assuming that learning could also happen to an absent cue, provided that a cue that was associated with it was further trained. These revised models assumed that changes in associative strength of absent but associated cues should always be opposite in direction to the changes by the associate cues that were present (Dickinson and Burke 1996; Van Hamme and Wasserman 1994). Thus, in the last 30 years, we have seen associative theory moving towards recognizing and accounting not only for direct cue competition between cues trained together, but also for changes in behavioral control by absent target cues when a companion cue is further reinforced or extinguished following completion of training of the compound of the target and the companion cues (Dickinson and Burke 1996; Miller and Matzel 1988; Van Hamme and Wasserman 1994). Nevertheless, none of these theories has incorporated a mechanism by which prior experience with stimuli dissimilar to the target cue or any companion of the target can change how subjects subsequently process information concerning the target. Generalization along a physical dimension of the stimuli (i.e., similarity) is one such mechanism, but in the examples that we outline below, this is not a plausible explanation. Thus, if we accept the idea that animals such as rats can extract information beyond the physical dimensions of the stimuli and apply it to a new situation, theories of learning will have to be modified to account for these phenomena.
This review is concerned with the question of whether nonhuman animals such as pigeons and rats are capable of using, in a novel situation, information previously acquired with different stimuli, when there is no principled rule to explain it through conventional stimulus generalization along a physical dimension (Spence 1937). We will argue that such capacity is present in nonhuman animals such as pigeons and rats and can be understood as a rudimentary form of what in humans is called abstraction. We will finally discuss the theoretical implications of these provocative findings, and contend that associative theories of learning currently being developed for the application to nonhuman animals have to incorporate a way to account for the effects of prior experience in novel learning situations. Not only will this advancement place associative learning theories closer to the domain of human cognition, it also will have more ecological validity in that our representations of different experiences are not isolated units stored in segregated memory nodes, but rather experiences that shape the way we subsequently perceive and deal with the world.
The effect of prior experience upon new physically unrelated learning
That humans are capable of using prior information and transferring it to new experiences is beyond question, although the degree of such transfer is subject to argument. Rule learning is observed at ages as early as 7 months (Marcus et al. 1999). It is a hallmark of human cognition and is necessary for language acquisition (Pinker 1991). Moreover, rule learning has been observed in monkeys (cotton-top tamarins; Hauser et al. 2002) in an experiment using stimuli and procedures similar to those used with human infants (Marcus et al. 1999). Hauser et al. used a habituation–discrimination procedure in which subjects were repeatedly presented with strings of consonant–vowel tokens (e.g., ta-la-la) in the form of ABB (or AAB) patterns. After the monkeys achieved a habituation criterion, they were tested through presentation of different stimuli but with the same pattern and a different pattern, with observing behavior towards the speaker being scored when the pattern was presented. Most subjects responded to the new pattern, suggesting they had learned the pattern with which they were presented during habituation. Thus, although cotton-top tamarins are not capable of learning language, they seem to be capable of abstracting information from a prior experience and using that information to modify their behavior in new situations. Pigeons also appear capable of learning abstract concepts and using these concepts to process new stimuli. For example, pigeons master same-different discriminations, transpose relations (e.g., bigger than), and even learn relations between relations (e.g., Cook and Wasserman 2007). Moreover, even honey bees can master same-different discriminations (Giurfa et al. 2001). These studies have been extensively reviewed recently (e.g., Lazareva and Wasserman 2008), so we will not discuss them here. In these studies, subjects either receive “warm-up” blocks of training with the original training trials before being tested with new stimuli or are tested with the new exemplars while responding for the previously trained stimuli. In contrast, here we review two-stage studies in which prior experience transferred after a long delay to new experiences and changed the way subjects processed new information. This suggests learning can have long-term consequences upon subsequent information processing. Thus, the question is whether nonhuman animals, such as rats, are capable of using previously acquired ways of processing information while learning new information involving stimuli that are dissimilar to the prior learning situation.
We should note that at least two different forms of rule learning have been distinguished (Gómez and Gerken 2000). One form is pattern-based in which the stimuli used are formed by presenting stimuli in repeating forms such ABA (as described above), which can be viewed in terms of relational operations between the stimuli in the sequence. A second, category-based rule-like learning, also relies on structural relations but between abstract functional roles. For example, instead of using artificial strings of consonant–vowel tokens (e.g., ta-la-la), the strings are composed of abstract forms such as noun–verb–noun. We will return to this distinction when we discuss the theoretical implications of the studies presented here, as some authors have proposed that only the second form is characteristic of human language and thought (Penn et al. 2008). In this section, we review experiments from six papers that used two-stage transfer designs in which the effects of learning during the first stage was assessed during a delayed second stage with physically dissimilar stimuli. By physically dissimilar, we mean that the stimuli were highly discriminated by the subjects (and thus the results cannot be explained in terms of stimulus generalization) and sometimes of different modalities. Four of these papers have successfully demonstrated that prior experience influences new learning (Alvarado and Rudy 1992; Beckers et al. 2006; Murphy et al. 2008; Urcelay and Miller 2009). Although we will argue that these demonstrations mean that rats have the long-term ability to generalize in some rudimentary form across different experiences (e.g., abstraction), we will also discuss one paper that failed to show such effects (Williams and Braker 2002) and a second paper that shows that this capacity in rats is constrained because it does not easily generalize as situations become more different (Wheeler et al. 2008).
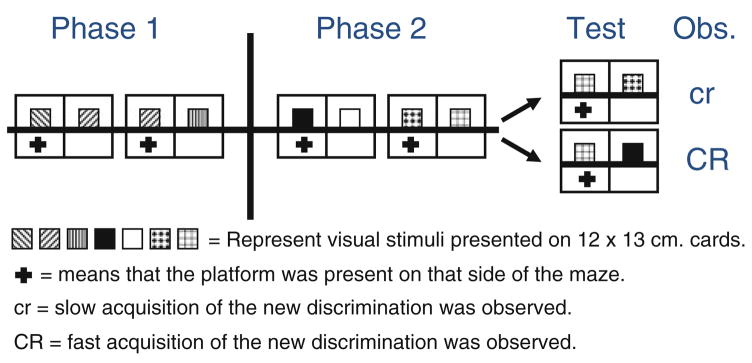
Alvarado and Rudy (1992) conducted a series of experiments in which they investigated the use of elemental and configural strategies by rats in solving a simple version of the transverse patterning task. By elemental strategies, we mean that a compound of two stimuli is broken down by animals into its constitutive elements each of which can enter into an association with the outcome, whereas with configural strategies, animals seem to treat the compound itself as a new stimulus different from its constitutive elements. In this simple version of the task, subjects have to solve two discriminations formed from only three stimuli (A, B, and C). Each stimulus is presented simultaneously with one other stimulus in each of the two discriminations and subjects are reinforced for choosing a given stimulus in one of the two cases and not in the other (A+ vs. B−, and B+ vs. C−, where + indicates reinforcement and—nonreinforcement). Presumably, this task can be solved by the animal configuring the two cues constituting each pair. For example, given a compound of A and B, respond to A. Alternatively, responding can be based on the rates of reinforcement of each individual stimulus, because A has a higher rate of reinforcement than B (100 vs. 50%) and B has a higher rate of reinforcement than C (50 vs. 0%). In other words, by processing each stimulus of a pair separately, subjects can successfully solve the discrimination. In Experiment 1, Alvarado and Rudy used a water-escape version of the Lashley jumping-stand task and trained subjects either on the above-mentioned discrimination or in an unrelated discrimination (A+ vs. B−, D+ vs. C−). In this task, subjects had to swim towards a hidden platform located in one of two partitions of a water maze. To determine in which partition the platform was hidden, pairs of visual stimuli were suspended from rods on each side of the partition. The stimuli were constructed from square cards (12 cm × 13 cm) with distinctive patterns on them. Then all subjects were trained in a reversed discrimination (C+ vs. A−). If subjects were solving both the related (A+ vs. B−, B+ vs. C−) and the unrelated problems (A+ vs. B−, D+ vs. C−) elementally, then they should have solved the reversal (C+ vs. A−) equally fast because the histories of reinforcement of A and C were the same for these groups. If animals solved the patterning problem (A+ vs. B−, B+ vs. C−) using a configural strategy, then they should solve the reversal faster relative to subjects that received training with the unrelated problem, because C+ vs. A− is a new combination (i.e., configuration). The data suggested that animals were solving this task (A+ vs. B−, B+ vs. C−) using a configural strategy. After Alvarado and Rudy established that the simpler version was solved in a configural manner, in Experiment 3, they initially trained all their subjects to solve (A+ vs. B−, B+ vs. C−) discriminations and subsequently presented subjects with two new discriminations (O+ vs. P−, Q+ vs. R−). At test, they reversed contingencies for two cues belonging to the same pair as in training (R+ vs. Q−; which can be solved elementally or configurally) or presented cues from different pairs (R+ vs. O−; potentially a new configuration). They observed that prior configural training increased learning about the new configuration but did not affect learning about the reversal (see Fig. 1 for design and stimuli identity). In other words, they observed that prior configural training facilitated learning of a new configural problem. These findings are problematic for contemporary associative models of learning because these models assume that representations are fixed, regardless of whether the model’s basic assumptions are elemental or configural (Shanks 2005).
Fig. 1.
Schematic representation of Experiment 3 by Alvarado and Rudy (1992). Rats were trained in a water-escape version of the Lashley jumping-stand task. Rats had to choose one of the two sides based on patterns that were hanging 2 cm above the water surface. Configural training during Phase 1 increased transfer to a new configuration (but not to the reversal of a pattern) during the test. Each phase was commenced 24 h after the end of the previous phase
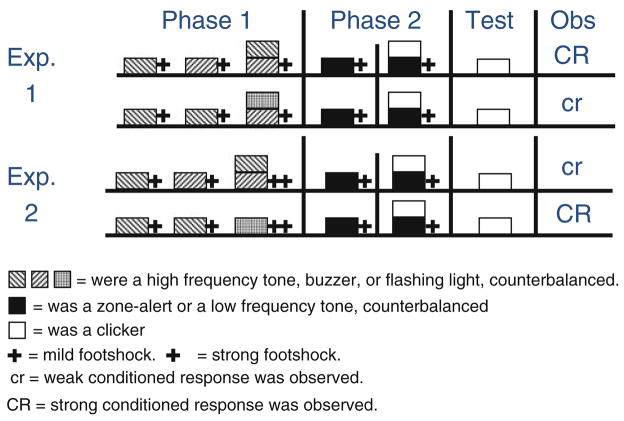
In a recent series of experiments, Beckers et al. (2006) tested whether prior subadditive training and submaximal training would affect the degree to which they saw blocking in a fear conditioned bar-press suppression preparation (see Fig. 2 for the design of their studies and the visual and auditory stimuli employed). As it was already mentioned, blocking is the decreased responding observed to a cue (X) when it is trained in the presence of a second cue (AX+) that has previously been trained (A+; Kamin 1968). Some explanations of blocking in humans emphasize the importance of inferential processes that can be stated in the form of propositional knowledge (e.g., De Houwer et al. 2005). Within this framework, blocking results from an inferential process that does not assign any predictive potential to the blocked cue because the blocking cue had undergone prior training and thus was a better predictor of the outcome. Because this explanation posits that subjects assume causal additivity of the predictive value of cues (A+ and X+ imply AX++), it makes the prediction that prior subadditive training (C+, D+, CD+) should decrease the blocking effect even though different cues are used for blocking training, because it contradicts the initial assumption of additivity. This effect has been documented in humans (e.g., Beckers et al. 2005). Beckers et al. (2006, Experiment 1) investigated whether subadditive pretraining with different cues would likewise affect the magnitude of the blocking effect in rats, in which the basic blocking effect is also readily obtained. That is, prior to the blocking training, they presented subjects with information contradicting linear combinatorial effects of cues in compound. In other words, they presented trials in which one cue was reinforced (C+), trials in which a second cue was reinforced (D+), and trials in which a compound of the two cues was reinforced with an outcome of similar intensity (CD+). Other groups received irrelevant pretraining in which there was no subadditive relationship between the two cues (C+, C+, DE+, i.e., a control condition). After this pre-training, they conducted simple blocking treatment with different cues (A+ followed by AX+) and observed reliable blocking in the control condition lacking the CD+ trials but not in the subadditive condition, thereby supporting the view that blocking results from elemental (i.e., linear) processing (see Fig. 2, top panel). The authors explained their results in terms of inferential reasoning processes, and although it is difficult to appreciate how rats can solve problems using logic inferences, it is clear from the results that subadditive (i.e., nonlinear) pre-training with a different set of cues decreased the size of the blocking effect. Overall, this suggests that rats, like humans, are sensitive to prior training and use that information in subsequent training with a different set of cues.
Fig. 2.
Schematic representation of Experiments 1 and 2 by Beckers et al. (2006). They used rats in a Pavlovian fear-conditioning preparation. In Experiment 1, subadditive pretraining (1 + 1 = 1; top group) but not a control treatment (bottom group) decreased the size of the blocking effect with different stimuli. In Experiment 2, additive pretraining (1 + 1 = 2; top group) increased blocking with nonrelated stimuli during Phase 2. Note that during Phase 1, they used both auditory and visual stimuli, but during Phase 2 blocking treatment, they only used auditory stimuli. Consequently, any transfer observed from the prior training cannot be explained by generalization along a stimulus dimension. Each phase of training was commenced 24 h after the end of the previous phase, and the test was administered at least 72 h after training
A second prediction that stems from the inferential explanation of blocking is that prior additive pretraining should increase the blocking effect when blocking treatment is administered with parameters that typically yield weak blocking. In order to test this prediction, Beckers et al. (2006; Experiment 2) administered additive pre-training (C+, D+, CD++) or irrelevant pretraining (C+, C+, E++) and subsequently trained all animals in a blocking procedure with parameters that ordinarily yield weak or no blocking. They observed reliable blocking after additive pretraining but not after irrelevant pretraining, again indicating that prior experience with different cues reliably influenced the blocking effect (see Fig. 2, bottom panel). Taken together, these demonstrations show that blocking, an effect widely observed in nonhuman and human animals, is also sensitive to prior training in both human and rats, suggesting that both species are able to apply knowledge acquired in phase 1 to a different set of cues in phase 2.
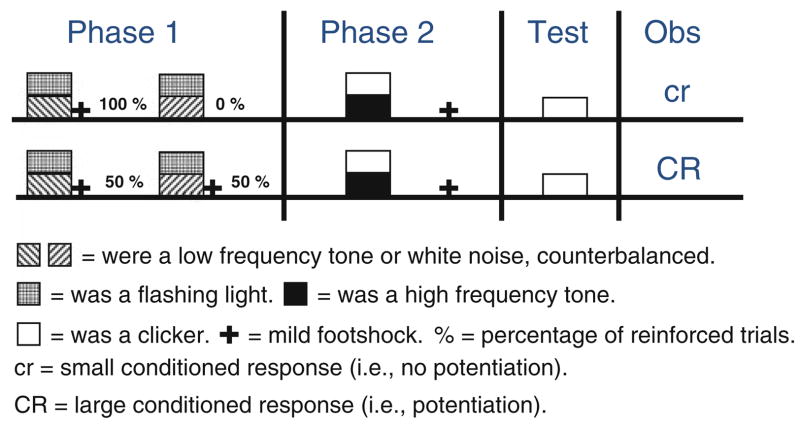
Next, we will describe a recent experiment conducted in our laboratory that assessed diverse explanations of an effect called potentiation, which we observed in fear conditioning of rats with auditory cues (Urcelay and Miller 2009; Experiment 3). Potentiation is a paradoxical effect that results in enhanced acquired behavioral control of a (usually low salience) cue when it is trained in the presence of a second (usually more salient) cue. That is, reinforcing an AX compound (i.e., AX+) results in more acquired behavioral control by X than when it is trained alone (X+). Potentiation is paradoxical because most typically AX+ trials result in weaker responding to X than do X+ trials, an effect called overshadowing. There are at least two explanations of potentiation. One assumes that subjects encode all cues separately and associate them. In other words, a chain consisting of the association between X and A (i.e., within-compound association) and the association between A and the US (due to A’s higher salience) summates with responding due to the X-US association to support the potentiated responding that is observed to X (Durlach and Rescorla 1980). This is a process akin to second-order conditioning. Alternatively, potentiation has been explained as resulting from subjects encoding the AX compound during training as a unique configuration and responding at the time of test of X as if they retrieved the entire configuration (Kucharski and Spear 1985; Rescorla 1981). Importantly, the within-compound account assumes elemental processing of stimuli, whereas the configural account, as the name implies, assumes the simultaneously presented cues as configured. Urcelay and Miller attempted to dissociate these two explanations by administering prior training that was intended to bias subjects towards elemental encoding. They reasoned that if during training of compound cues subjects encode a unitary representation of the compound, training them to assess the potential of each cue separately (i.e., elementally) should decrease the potentiation effect. They administered relative stimulus validity pretraining (BY+, CY−; Wagner et al. 1968) or an irrelevant control treatment (control; BY±, CY±, where ± indicates 50% partial reinforcement) and subsequently administered compound cue training with different cues (AX+). The reasoning was that in relative validity pre-training, subjects have to attend to only one cue (B in the example above) of each compound to determine if a compound will be followed by reinforcement, whereas in the control condition there is no cue that unambiguously informs subjects regarding reinforcement. Consistent with a configural interpretation of potentiation, Urcelay and Miller observed less potentiation after relative validity pretraining than after control pretraining (see Fig. 3). Seemingly, subjects learned during the pretraining that the compound was made of two elements, and this prior experience decreased the potentiation effect that was observed in the control condition, in which subjects presumably were configuring. Of note, in that experiment Urcelay and Miller also assessed the effect of relative validity pretraining (or control) on elemental trace conditioning and found that prior relative validity pretraining increased conditioned responding to a cue trained alone (X+). That is, they found that prior relative validity pre-training decreased responding to X after compound trainings (AX+) but increased responding to X when it was trained alone, which suggests that the effects of prior training in rats can decrease or increase learning depending on the specific circumstances of training.
Fig. 3.
Schematic representation of Experiment 3 by Urcelay and Miller (2009). They used rats in a Pavlovian fear-conditioning preparation. They administered relative validity pretraining using auditory and visual stimuli and subsequently assessed potentiation where the delayed reinforcement (20 s) of a compound resulted in enhanced behavioral control by one of the elements. Relative validity pretraining (i.e., an elemental problem) decreased subsequent configural learning to different stimuli. Again, note that during Phase 1, they used both auditory and visual stimuli, but during Phase 2, they only used auditory stimuli, so any transfer observed from the Phase 1 training cannot be explained by generalization along a stimulus dimension. Each phase of training was commenced 24 h after the end of the previous phase, and the test was administered at least 72 h after training
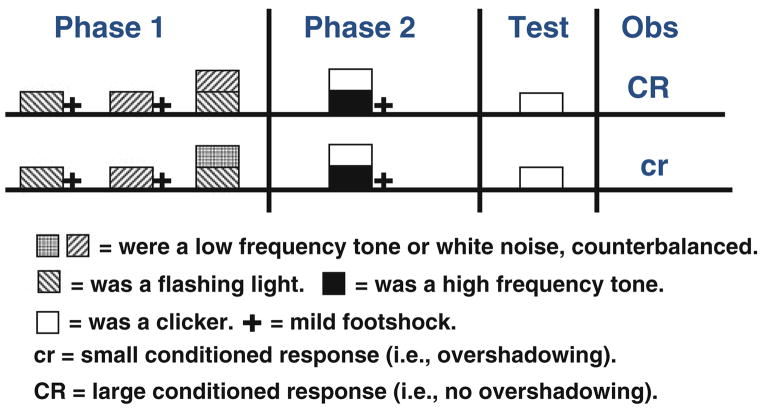
In another experiment, Urcelay and Miller (2009, Experiment 4) asked if a different type of pretraining could bias the subjects to process subsequent patterns of stimuli configurally (as opposed to elementally as in their Experiment 3). They hypothesized that negative patterning (B+, Y+, BY−), which presumably promotes configural encoding, would decrease subsequent overshadowing. Overshadowing is thought to be the result of elemental encoding of the different parts of the compound. Thus, subjects had to learn in Phase 1 that the compound of the two cues is different from the sum of each cue separately; that is, there is a nonlinear relationship between the cues in their association with the outcome. If rats can generalize across tasks involving different cues and overshadowing results from elemental encoding of the compound, prior exposure to negative patterning should decrease the overshadowing deficit that develops during Phase 2. Consistent with this explanation, they observed that prior negative patterning training, but not control training, aimed at equating exposure to the stimuli and reinforcer without any explicitly nonlinear solution (B+, Y+, BC−), decreased the overshadowing deficit, that is, increased conditioned responding (see Fig. 4). Thus, these two experiments found that phenomena that presumably result from configural (i.e., potentiation) and elemental (i.e., overshadowing) encoding disappear if prior to this training subjects experience training with different cues that biases subjects towards the opposite kind of encoding.
Fig. 4.
Schematic representation of Experiment 4 by Urcelay and Miller (2009), who used rats in a Pavlovian fear-conditioning preparation. They administered negative patterning pretraining (which is solved using a configural [nonlinear; 1 + 1 = 0] solution) using auditory and visual stimuli and subsequently overshadowing in which the immediate reinforcement of a compound typically results in decreased behavioral control by one of the elements. Negative patterning pretraining (i.e., a configural problem) decreased subsequent elemental learning to different stimuli. The use of stimuli of both auditory and visual modalities during Phase 1 and only auditory stimuli during Phase 2 precludes any interpretation of the results in terms of stimulus generalization. Each phase of training was commenced 24 h after the end of the previous phase, and the test was administered at least 72 h after training
The results mentioned earlier all support the view that rats are capable of transferring previously learned abstract relationships to novel situations. One argument against this interpretation is that the data may not reflect transfer of abstract structure but rather result from stimuli generalization. Although generalization is an appealing explanation that associative models of learning often invoke as a basis for some prior-experience effects (e.g., McLaren and Mackintosh 2000 e.g., McLaren and Mackintosh 2002), we saw that prior experience could modulate new learning in both directions. That is, in the experiments by Urcelay and Miller (2009), relative validity pretraining decreased responding to X when it was trained in the presence of A, but increased responding to X when it was trained alone. This challenges simple generalization accounts because simple generalization to the target due to the prior treatment should have been similar, regardless of the new training conditions, and it was not. Similarly, prior relative validity pretraining decreased responding to the target after compound training but increased it after elemental training. Moreover, for the McLaren and Mackintosh model to be able to account for transfer based on generalization, the model assumes by default 25% generalization between any two cues, which in practice implies that a cow and a thimble look 25% alike for any perceiver. Perception and storage capacity do a far better job than that. Ultimately, the model does not account for the fact that in these experiments, we were able to systematically decrease (Experiment 3) and increase (Experiment 4) responding to a target with prior training using different stimuli.
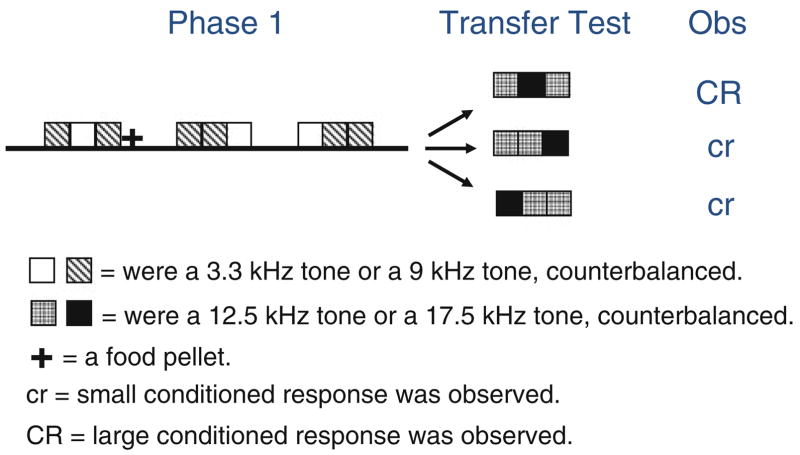
A recent set of data by Murphy et al. (2008) also suggests that rats are capable of learning rules and transferring them to novel stimuli without any possibility that generalization on a stimulus dimension can account for the behavior. In a first experiment, they exposed rats to a pattern of light on (A) and off (B; counterbalanced) in the form of ABA, AAB, or BAA. After the target sequence, subjects received food pellets as reinforcement (e.g., ABA+), whereas after the two alternative sequences, they did not (e.g., AAB− or BAA−). They observed that subjects spent more time searching for food in the food hopper after the pattern that was consistently reinforced than after the alternative patterns. This suggests that rats are capable of discriminating the patterns although they are made with the same stimuli. Presumably, it was the pattern of the three stimuli (including the position in the sequence) that informed subjects about reinforcement, although it is unclear how that pattern was represented (see Corballis 2009; Mondragón et al. 2009; and ‘Theoretical implications’ below for a discussion). In a second experiment, they first administered similar training to that just described but using two auditory pure tones (and twice the amount of training). In a second phase, they tested subjects with patterns made with two different auditory tones C and D (all stimuli were counterbalanced). They observed more searching behavior when the patterns were consistent with the pattern reinforced during Phase 1 (see Fig. 5). That is, they observed that training a pattern with stimuli A and B influenced behavior (i.e., transferred) to a pattern made with stimuli C and D. Notably, what distinguished the patterns was the sequence (CDC vs. DCC, or CCD), not the physical identity of the new stimuli (stimuli identity was counterbalanced within groups). This demonstration of rule learning using strings of auditory stimuli is a clear example of pattern-based generalization between different sets of stimuli, as defined by Gómez and Gerken (2000), where relations between stimuli are perceptually bound. However, it is clear that the three examples presented earlier cannot be categorized under the umbrella of ‘pattern-based’ rule learning, as different types of trials were administered during pretraining, and in some cases (e.g., Urcelay and Miller 2009), the types of trials administered during training were completely different from those administered during target training (e.g., B+, Y+, BY− during pretraining followed by AX+ during target training). We will return to this distinction later.
Fig. 5.
Schematic representation of Experiment 2 by Murphy et al. (2008) using rats in a Pavlovian appetitive preparation. They reinforced one pattern (i.e., ABA) but not two other patterns (i.e., AAB or BAA) of auditory stimuli and tested subsequently on similar patterns but made of different auditory stimuli (i.e., CDC vs. CCD or DCC) and saw better conditioned performance to the similar pattern (i.e., CDC) than to the alternative patterns (i.e., CCD or DCC). Because the stimuli identities during both Phases 1 and 2 were fully counterbalanced, these results cannot be interpreted in terms of stimulus generalization. The transfer test was administered at least 24 h after the end of training
Overall, we have presented several examples of experiments conducted with rats as subjects in which an effect of prior training upon subsequent learning was observed. These demonstrations suggest that the effects can increase or decrease learning to a target depending on the circumstances of testing (and on the pretraining regimen). Although these experiments have been conducted while testing various phenomena with diverse preparations and different theoretical explanations of the phenomena, they all support the idea that rats are capable of using information acquired with different stimuli and transferring that information to a new situation. However, as we will see in the next section, these long-term effects (at least in rats) are constrained by several changes between the conditions of pretraining and those of target training.
Limits on generalization of the effects of prior training
We have just reviewed evidence suggesting that rats are capable of using information from previous experience and transferring it to a learning situation involving new stimuli. In this section, we will discuss a paper that investigated the limits to such transfer and another paper in which such transfer was not observed.
As a follow-up to the effect reported by Beckers et al. (2006), Wheeler et al. (2008) conducted a series of three experiments investigating the boundaries for transfer of the subadditive pretraining effect on blocking. That is, they tested the effect of training C+, D+, CD+ upon subsequent blocking treatment (A+ then AX+). In Experiment 1, they replicated the effect seen by Beckers et al. (2006) in that subadditive pretraining decreased the blocking effect. Moreover, in that experiment, they observed that if the context in which subadditive pretraining was administered differed from that where blocking training was conducted, the effect disappeared. In other words, no effect of subadditive pretraining was observed after a change in context from pretraining to blocking training. In Experiment 2, they assessed whether a retention interval (21 days) between subadditive pretraining and blocking training would diminish the effect. With a one-day retention interval, they again replicated the basic finding, whereas after 21 days, they observed no effect of pretraining on blocking. This is consistent with the view that contexts can be indexed along both physical and temporal dimensions (Bouton 1993), in that a change in physical context (Experiment 1) or temporal context (retention interval, Experiment 2) diminished the effect of subadditive pretraining. Moreover, in Experiment 3, they observed that the effect of subadditive pretraining disappeared if they used cues of different duration during sub-additive pretraining and during blocking treatment. Specifically, they used stimuli of short (15 s) or long (60 s) duration and found that the subadditive pretraining was effective when the duration of the cues was similar during pretraining and blocking training, but not when the cues were of different duration. Overall, these experiments replicate the findings by Beckers et al. (2006), but also suggest that prior experiences transfer to new experiences only when the two tasks share similar spatiotemporal contexts, at least in rats.
Williams and Braker (2002) failed to find an effect of conceptually relevant training but with different stimuli. They reported two appetitive conditioning experiments, one in which rats had to emit a lever press during the presentation of a Pavlovian signal for food, and a second that used nose poking as a dependent measure. Because in Experiment 2 they did not use transfer tests to assess new stimuli, we will focus on Experiment 1. They administered training that had a so-called linear solution (i.e., elemental; C+, D+, E−, CD+, DE− [we later speak to the nonlinear relationship C+, D+, CD+ embedded here]) or nonlinear solution (i.e., configural; C+, D+, E−, CD−, DE+) and orthogonally administered to half of these groups either A+, B+ or A+, B−. Critical to their assessment of transfer from elemental or configural pretraining were tests of the AB compound. They expected, similar to their findings in humans (Williams and Braker 1999), that elemental pre-training would result in more responding to the AB compound when B was reinforced than when it was not reinforced, which is what they observed. In other words, elemental pretraining should have encouraged subjects to adopt a linear summation rule when responding to the AB. However, after configural pretraining, subjects should have treated the AB compound as different from its elements, so Williams and Braker expected no differences in responding to the AB compound regardless of B training. Thus, because the compound should have been treated (after the configural pretraining) as a new configuration, no linear summation was expected in this group. Contrary to their expectations (and to what was observed in humans), responding to the AB compound was higher when B was previously reinforced than when it was not. One aspect of this experiment that may have prevented them from observing their expected results was that configural training was never complete. Thus, it may be possible that the lack of transfer was due to incomplete configural training. Consistent with this argument are data from William’s laboratory using humans (Mehta and Williams 2002), in which they observed that the final level of learning on the pretraining task was a strong determinant of transfer. Therefore, it is possible that the lack of transfer was due to insufficient learning during Phase 1. Moreover, they began training with cues of 30-s duration and progressively decreased the duration to 5 s. Although they administered substantial training with the latter duration, Wheeler et al. (2008) have observed that the effect of prior training disappears when the duration of the cues is different, which may be a reason why Williams and Braker failed to find an effect. Regardless of these a posteriori reasons, this experiment suggests that in rats, there are several limits to the effect of prior training.
In summary, these studies demonstrate that through principled ways such as changes in physical, temporal contexts, or duration of stimuli (Wheeler et al. 2008), or through simple failures to find an effect of prior experience perhaps due to insufficient pretraining (Williams and Braker 2002), that there are limits to the generality of these transfer effects in rats. This is not surprising because ultimately successful learning must be the result of a balance between generalization and discrimination between experiences. Too much generalization has the risk of overriding a current learning experience, whereas too much discrimination would impede the appropriate use of prior experiences. What these results suggest is that the capacity to generalize information in rats may be much more limited than that observed in humans. In support of this argument is the observation of retrospective subadditive treatment that Beckers et al. (2005) saw in humans by switching the order of phases of subadditive training and conventional blocking treatment (Experiment 4) that was not observed in rats (Beckers et al. 2006; unpublished observations).
Theoretical implications
In the previous section, we have summarized several experiments that successfully demonstrated an effect of prior learning upon new learning with distinctively different physical cues. Moreover, because these demonstrations cannot be accounted for, by generalization across physical dimensions of the stimuli, they can be viewed as a simple form of generalization of abstract structure to new situations. As mentioned in the introduction, the capacity of extending and generalizing abstract structure to new situations is sometimes viewed as a hallmark of human cognition (Marcus 2001). The data we summarized suggest that rats are also capable of extracting information from a prior experience and using it in a subsequent situation. However, from the data we reviewed, it is not clear if prior experience changes the way subjects encode new information (favored by Melchers et al. 2008) or the way they retrieve it (favored by Beckers et al. 2005) or both. Although we believe this question is critical to the understanding of human and nonhuman cognition, further experiments will have to be designed to assess these two possibilities. We now will discuss the implications of abstraction for associative theories of learning.
In the introduction, we briefly summarized how theories of learning were modified to account for situations that involve multiple cues (i.e., cue competition phenomena) and later modified again to account for learning about absent stimuli (i.e., retrospective revaluation effects). However, none of the contemporary theories of learning accounts for the effects of prior experience with a different set of cues that we have described here. Taken to the extreme, one may argue that learning theorists in the last 100 years have assumed that their subjects were like Ireneo Funes, in that prior information did not relate to (or alter) new experiences unless there were direct or mediated (i.e., higher order) associations between elements of the experiences. In other words, most associative theories of learning compute changes of associative strength, but prior experiences with distinctly different cues have no influence in these computations (Mackintosh 1975; Miller and Matzel 1988; Pearce 1987; Pearce and Hall 1980; Rescorla and Wagner 1972; Wagner 1981). Thus, given the evidence reviewed earlier, we believe that the next challenge for associative learning theory will be to account for the effect of prior experiences without assuming that prior experience changes new learning through a process of generalization between stimuli along any physical dimension. Clearly, the next challenge is to determine how prior experiences influence new learning. In contrast, there are a number of models, primarily connectionist, of human concept formation and category learning that allow transfer of prior learning (e.g., Nosofsky et al. 1994). However, these models have rarely been applied to data from nonhuman subjects. This is one arena in which students of animal learning might look for viable accounts of behavior in nonhumans that is indicative of abstract rule learning.
One possibility for associative learning theories is to consider a second process (independent of the changes in associative strength) that accounts for the prior experience effect through a rule-like learning mechanism. This is the path that many cognitive models have taken in order to accommodate a number of findings in linguistics (e.g., Pinker 1991) and analogical inference and relational generalization (e.g., Hummel and Holyoak 2003), to name a few areas, but we think that this alternative provides gains in explanatory power at the expense of parsimony. An alternative approach is to assume that subjects are capable of flexibly processing information in an elemental or configural manner depending on many circumstances such as task demands, prior experience, stimuli characteristics, and verbal instructions (in the case of humans). Another way of stating this is to posit that the significance of combinations of elements relative to when the elements are presented alone can be either nonlinear (C+, D+, DC−) or linear (C+, D+, CD++), and that subjects develop context-specific biases concerning the degree to which they will process cues nonlinearly. Such an assumption was entertained by Williams and colleagues in their research with humans, where they found a strong effect of prior experience upon new learning that could not be explained in terms of stimulus generalization (Williams and Braker 1999; Williams et al. 1994).
Recently, the assumption of representational flexibility has been revived in a review of data that pose challenges to contemporary associative theories of learning (Melchers et al. 2008). This view has the merit of being able to account for some of these effects we reviewed without the assumption of simultaneous dual processes. For example, it explains the data by Alvarado and Rudy (1992) by assuming that the prior configural pretraining facilitated later learning of a new configural problem. A similar analysis accounts for the data of Murphy et al. (2008). Prior extensive training of a given pattern-based configuration (e.g., ABA) transferred to similar configurations (CDC) but not dissimilar configurations (CCD). Applied to the experiments by Urcelay and Miller (2009; Experiment 3), the assumption is that if during potentiation treatment subjects are encoding the compound as a unique configuration, prior elemental pretraining (BY+, CY−, a linear relationship) should decrease this type of encoding, decreasing potentiation. Moreover, this view also can explain instances of elemental (i.e., linear) processing in which stimuli seem to compete with each other (e.g., overshadowing) and allow for the prediction that prior configural training (B+, Y+, BY−, a nonlinear relationship) should decrease this type of phenomenon. This prediction was recently confirmed in Experiment 4 of Urcelay and Miller (2009). One could argue that the data of Beckers et al. (2006) is problematic for this view. They administered a pretreatment (C+, D+, CD+) somewhat similar to that used by Williams and Braker (1999; C+, D+, CD+, E−, DE−), but found that it decreased blocking. In contrast, Williams and Braker hypothesized that such treatment should encourage their subjects to treat cues elementally, which should have enhanced blocking. However, the relationship between elements and compounds in the treatment administered by Beckers et al. (2006) was unambiguously nonlinear, in which case blocking should have been attenuated, as it was. Williams and Braker administered a more complex treatment that had this nonlinear component, but also other cues that had a clear linear relationship (D+, E−, DE−).
In this view, elemental and configural encoding can be seen as extremes of a continuum on which one can manipulate stimuli attributes (through changes along physical dimensions), task demands, or even subjects (by instructions or prior experience) and explain a wide range of phenomena in the literature. Obviously, there is a long way to go in testing these assumptions, but at least this view offers a mechanism through which prior experience might influence new learning. As we have seen, effects of prior experience with physical dissimilar stimuli have now been observed in several rat experiments, suggesting that this ability is present in nonhuman species and should be taken seriously. These findings also have important theoretical implications because the issue of whether representational flexibility can be seen in nonhuman animals (i.e., rats) is also a concern for basic associative learning theorists (Livesey and Harris 2008; Wagner and Vogel 2008). The experiments reviewed here strongly suggest that these capacities are indeed present in rats and that learning theories need to seriously embrace this corpus of phenomena.
In the introduction, we noted that at least two forms of rule-like learning have been distinguished in the literature (Gómez and Gerken 2000). One form is based on higher order perceptually bound stimuli (such as ta-la-la) and the second is based on structural relations between abstract forms (such as noun–verb–noun). These have been called pattern-based and category-based rules, respectively. This distinction is relevant because it has been proposed that only the second kind of rule learning is critical for human thought and language (Penn et al. 2008). The study by Murphy et al. (2008) that we discussed earlier clearly falls into the first category, as they used strings of tones that could potentially be bound by higher order perceptual processes. However, it is difficult to see how higher order perceptual binding can explain the transfer effects that were observed by Urcelay and Miller (2009). For example, Urcelay and Miller (Experiment 3) administered relative validity pretraining (BY+, CY−) in which two compounds that shared a common element were differentially reinforced. In the second phase, they trained a new compound AX that was followed by reinforcement and found decreased responding to X at test in comparison with a group that did not receive relative validity pretraining. If the transfer was mediated by perceptually bound relations between a compound trained in Phase 1 and that trained in Phase 2, it is not clear which compound (BY+ or CY−) was “chosen” by the subjects. One may arbitrarily argue that the nonreinforced compound transferred to the new compound and that is why subjects responded less to the target at test. However, in Experiment 4, Urcelay and Miller administered negative patterning pretraining (B+, Y+, BY−) followed by reinforced training of AX and observed more responding at test by X. Thus, even if we arbitrarily choose a post hoc explanation for Experiment 3 based on higher order perceptually based generalization, this explanation would predict the opposite result in Experiment 4. This obviously does not mean that in these experiments, rats were performing abstract rule-like learning as the one humans do with noun–verb–nouns, but it clearly suggests that a pattern-based rule is insufficient to explain the pattern of results.
Conclusions
In summary, we have reviewed a number of two-stage transfer experiments that show that rats’ learning and memory is sensitive to prior experiences, and we also reviewed evidence suggesting that there are limits to this capacity (at least in rats). The ability to generalize across situations, to abstract beyond the physical dimensions of stimuli, is widely recognized as an ability that is present in humans. Here, we showed that this ability is seemingly also present in nonhuman animals (rats; and also pigeons, see above), which in turn suggests that the way these capacities are manifest across phylogeny may not be discontinuous. In other words, we believe that, the cognitive capacities observed in different species are not qualitatively different, but rather quantitatively different (contrary to Penn et al. 2008; Penn and Povinelli 2007). Indeed, we devoted a section of this article to review studies that suggest that the degree to which prior experience transfers to new experiences is constrained in rats, as it is also constrained in infants (Bovet et al. 2005). We also recognized the difficulty of associative learning theories in dealing with these effects, and we suggested a possible solution based on representational flexibility. It is our hope that further empirical data will show the generality of these effects in nonhumans, so that these effects might be viewed as a serious challenge to contemporary models of learning and promote the development of more appropriate models. Just as associative learning theories had to accommodate cue competition phenomena first and retrospective revaluation later, it is probably time to start looking at the effects of prior experience in order to more closely model human cognition. After all, the great mass of the human population does not process information like Ireneo Funes did, but rather are able to generalize, including on abstract dimensions.
Acknowledgments
National Institute of Mental Health Grant 33881 (RRM) supported the preparation of this review. The authors thank Eric Curtis, Jeremie Jozefowiez, Kenneth J. Kurtz, Mario Laborda, Bridget McConnell, Gonzalo Miguez, Cody Polack, and James Witnauer for their comments on an earlier version of this manuscript. We also wish to thank Derek C. Penn and three anonymous reviewers for insightful comments that have largely improved this manuscript.
Footnotes
Part of the analysis presented here was submitted by the first author in partial fulfillment of the requirements for his doctoral dissertation at the State University of New York at Binghamton.
Contributor Information
Gonzalo P. Urcelay, Email: gu203@cam.ac.uk, Behavioural and Clinical Neuroscience Institute and Department of Experimental Psychology, University of Cambridge, Downing St, Cambridge CB2 3EB, UK
Ralph R. Miller, Email: rmiller@binghamton.edu, Department of Psychology, SUNY-Binghamton, Binghamton, NY 13902-6000, USA
References
- Alvarado MC, Rudy JW. Some properties of configural learning: an investigation of the transverse-patterning problem. J Exp Psych: Anim Behav Process. 1992;18:145–153. doi: 10.1037//0097-7403.18.2.145. [DOI] [PubMed] [Google Scholar]
- Beckers T, De Houwer J, Pineño O, Miller RR. Outcome additivity and outcome maximality influence cue competition in human causal learning. J Exp Psych: Learn Mem Cogn. 2005;31:238–249. doi: 10.1037/0278-7393.31.2.238. [DOI] [PubMed] [Google Scholar]
- Beckers T, Miller RR, De Houwer J, Urushihara K. Reasoning rats: forward blocking in Pavlovian animal conditioning is sensitive to constraints of causal inference. J Exp Psych: Gen. 2006;135:92–102. doi: 10.1037/0096-3445.135.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges JL. Fictions. Grove Press; NY: 1942. Funes the memorious; pp. 107–115. [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psych Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and behavior: a contemporary synthesis. Sinauer Associates, Inc; Sunderland: 2007. [Google Scholar]
- Bovet D, Vauclair J, Blaye A. Categorization and abstraction abilities in 3-year-old children: a comparison with monkey data. Anim Cogn. 2005;8:53–59. doi: 10.1007/s10071-004-0226-y. [DOI] [PubMed] [Google Scholar]
- Bush RR, Mosteller F. A mathematical model for simple learning. Psych Rev. 1951;58:313–323. doi: 10.1037/h0054388. [DOI] [PubMed] [Google Scholar]
- Chomsky N, Halle M. The sound pattern of english. MIT Press; Cambridge: 1990. [Google Scholar]
- Cook RG, Wasserman EA. Learning and transfer of relational matching-to-sample by pigeons. Psychon Bull Rev. 2007;14:1107–1114. doi: 10.3758/bf03193099. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Do rats learn rules? Anim Behav. 2009;78:e1–e2. [Google Scholar]
- De Houwer J, Vandorpe S, Beckers T. On the role of controlled cognitive processes in human associative learning. In: Wills AJ, editor. New directions in human associative learning. Lawrence Erlbaum Associates; Mahwah: 2005. pp. 41–63. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgments. Q J Exp Psych. 1996;36A:29–50. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Durlach PJ, Rescorla RA. Potentiation rather than overshadowing in flavor-aversion learning: an analysis in terms of within-compound associations. J Exp Psych: Anim Behav Process. 1980;6:175–187. [PubMed] [Google Scholar]
- Gentner D. Why we’re so smart. In: Gentner D, Goldin-Meadow S, editors. Language in mind: advances in the study of language and thought. MIT Press; Cambridge: 2003. pp. 195–235. [Google Scholar]
- Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature. 2001;410:930–933. doi: 10.1038/35073582. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Gerken L. Infant artificial language learning and language acquisition. Trends Cog Sci. 2000;4:178–186. doi: 10.1016/s1364-6613(00)01467-4. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Weiss D, Marcus G. Rule learning by cotton-top tamarins. Cogn. 2002;86:B15–B22. doi: 10.1016/s0010-0277(02)00139-7. [DOI] [PubMed] [Google Scholar]
- Hummel JE, Holyoak KJ. A symbolic-connectionist theory of relational inference and generalization. Psych Rev. 2003;110:220–264. doi: 10.1037/0033-295x.110.2.220. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. ‘Attention like” processes in classical conditioning. In: Jones MR, editor. Miami symposium on the prediction of behavior: aversive stimulation. University of Miami Press; Miami: 1968. pp. 9–31. [Google Scholar]
- Kaufman MA, Bolles RC. A nonassociative aspect of overshadowing. Bull Psychon Soc. 1981;18:318–320. [Google Scholar]
- Kraljic T, Brennan SE, Samuel AG. Accommodating variation: dialects, idiolects, and speech processing. Cogn. 2008;107:54–81. doi: 10.1016/j.cognition.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski D, Spear NE. Potentiation and overshadowing in preweanling and adult rats. J Exp Psych: Anim Behav Process. 1985;11:15–34. doi: 10.1037//0097-7403.11.1.15. [DOI] [PubMed] [Google Scholar]
- Lazareva OE, Wasserman EA. Categories and concepts in animals. In: Menzel R, Byrne J, editors. Learning theory and behavior. Vol. 1 of Learning and memory: a comprehensive reference. Vol. 4. Elsevier; Oxford: 2008. pp. 197–226. [Google Scholar]
- Livesey EJ, Harris JA. What are flexible representations?: commentary on Melchers, Shanks, and Lachnit. Behav Process. 2008;77:437–439. doi: 10.1016/j.beproc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Marcus GF. The algebraic mind: integrating connectionism and cognitive science. MIT Press; Cambridge: 2001. [Google Scholar]
- Marcus GF, Vijayan S, Bandi Rao S, Vishton PM. Rule learning by seven-month-old infants. Science. 1999;283:77–80. doi: 10.1126/science.283.5398.77. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE, editors. Parallel distributed processing: explorations in the microstructure of cognition. Vol. 1. MIT Press; Cambridge: 1986. [DOI] [PubMed] [Google Scholar]
- McLaren IPL, Mackintosh NJ. An elemental model of associative learning: I. Latent inhibition and perceptual learning. Anim Learn Behav. 2000;28:211–246. [Google Scholar]
- McLaren IPL, Mackintosh NJ. Associative learning and elemental representation: II. Generalization and discrimination. Anim Learn Behav. 2002;30:177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- Melchers KG, Shanks DR, Lachnit H. Stimulus coding in human associative learning: flexible representations of parts and wholes. Behav Process. 2008;77:413–427. doi: 10.1016/j.beproc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: a response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Academic Press; Orlando: 1988. pp. 51–92. [Google Scholar]
- Mondragón E, Murphy RA, Murphy VA. Rats do learn XYX rules. Anim Behav. 2009;78:e3–e4. [Google Scholar]
- Murphy RA, Mondragón E, Murphy VA. Rule learning in rats. Science. 2008;319:1849–1851. doi: 10.1126/science.1151564. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Palmeri TJ, McKinley SC. Rule-plus-exception model of classification learning. Psych Rev. 1994;101:53–79. doi: 10.1037/0033-295x.101.1.53. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. Oxford University Press; London: 1927. [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psych Rev. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psych Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- Penn DC, Povinelli DJ. Causal cognition in human and nonhuman animals: a comparative, critical review. Ann Rev Psych. 2007;58:97–118. doi: 10.1146/annurev.psych.58.110405.085555. [DOI] [PubMed] [Google Scholar]
- Penn DC, Holyoak KJ, Povinelli DJ. Darwin’s mistake: explaining the discontinuity between human and nonhuman minds. Behav Brain Sc. 2008;31:109–178. doi: 10.1017/S0140525X08003543. [DOI] [PubMed] [Google Scholar]
- Pinker S. Rules of language. Science. 1991;253:530–535. doi: 10.1126/science.1857983. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Simultaneous associations. In: Harzem P, Zeiler MD, editors. Predictability, correlation, and contiguity. Wiley; New York: 1981. pp. 47–80. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black WF, Prokasy AH, editors. Classical conditioning II: current research and theory. Appleton Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Sacks O. The man who mistook his wife for a hat. Harper & Row; NY: 1970. A walking groove; pp. 187–194. [Google Scholar]
- Shanks DR. Connectionist models of basic human learning processes. In: Houghton G, editor. Connectionist models in cognitive psychology. Psych. Press; Hove: 2005. pp. 45–82. [Google Scholar]
- Spence KW. The differential response in animals to stimuli varying within a single dimension. Psych Rev. 1937;44:430–444. [Google Scholar]
- Stout SC, Miller RR. Sometimes-competing retrieval (SOCR): a formalization of the comparator hypothesis. Psych Rev. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Urcelay GP, Miller RR. Potentiation and overshadowing in Pavlovian fear conditioning. J Exp Psych: Anim Behav Process. 2009;35:340–356. doi: 10.1037/a0014350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: the role of nonpresentation of compound stimulus elements of nonpresentation of compound stimulus elements. Learn Motiv. 1994;25:127–151. [Google Scholar]
- Wagner AR, Vogel EH. Configural and elemental processing in associative learning: commentary on Melchers, Shanks, and Lachnit. Behav Process. 2008;77:446–450. doi: 10.1016/j.beproc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. J Exp Psych. 1968;76:171–180. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Beckers T, Miller RR. The effect of subadditive pretraining on blocking: limits on generalization. Learn Behav. 2008;36:341–351. doi: 10.3758/LB.36.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Braker DS. Influence of past experience on the coding of compound stimuli. J Exp Psych: Anim Behav Process. 1999;25:461–474. [Google Scholar]
- Williams DA, Braker DS. Input coding in animal and human associative learning. Behav Process. 2002;57:149–161. doi: 10.1016/s0376-6357(02)00011-6. [DOI] [PubMed] [Google Scholar]
- Williams DA, Sagness KE, McPhee JE. Configural and elemental strategies in predictive learning. J Exp Psych: Learn Mem Cogn. 1994;20:694–709. [Google Scholar]