Abstract
Objective:
To evaluate the possible association between maternal smoking during pregnancy (MSDP) and offspring outcomes of birth weight, pre-term birth, remediation, low scholastic achievement, regular smoking, attention deficit hyperactivity disorder (ADHD) and conduct problems (CD) while controlling for similar behaviors in parents.
Methods:
Using telephone interviews, data were collected, in 2001 and 2004, as a part of two U.S. offspring-of-twins projects. Fathers, who were twins participating in the Vietnam Era Twin Registry, their female spouse and their offspring were interviewed – information on 1342 unique pregnancies in mothers with a history of regular smoking was utilized for these analyses. The association between MSDP and birth weight, pre-term birth, remediation, low scholastic achievement, regular smoking, attention deficit hyperactivity disorder and conduct disorder while controlling for similar behaviors in parents, was examined using regression.
Results:
MSDP was associated with decreased birth weight, low scholastic achievement, regular smoking and ADHD. However, the association between MSDP and offspring ADHD was explained by maternal ADHD. MSDP was also associated with earlier age of offspring initiation of smoking and onset of regular smoking.
Conclusions:
MSDP may influence certain offspring outcomes via mechanisms that are independent from genetic risk attributable to comorbid conditions. Assisting expecting mothers with their smoking cessation efforts will likely provide widespread health benefits to both mother and offspring.
INTRODUCTION
In 2006, 17% of women giving birth reported cigarette smoking during pregnancy during their last trimester (Centers for Disease Control (CDC), 2004; PRAMS, 2007). While persistent cigarette smoking itself is one of the leading contributors to preventable death (Centers for Disease Control (CDC), 2002), maternal smoking during pregnancy (MSDP) has in and of itself been implicated as a potential risk factor associated with multiple offspring outcomes such as low birth weight, conduct disorder (CD), attention deficit hyperactivity disorder (ADHD), offspring smoking and cognitive dysfunction (Knopik, 2009).
Low birth weight and other birth outcomes
There is considerable evidence supporting the hypothesis that offspring of mothers who smoke during their pregnancies have impaired fetal growth, pre-term birth and lower birth weight infants (Conter et al., 1995; D'Onofrio et al., 2003; Knopik et al., 2005; Bada et al., 2005; Salihu et al., 2005; Kyrklund-Blomberg et al., 2005; Stroud et al., 2009; McCowan et al., 2009; Thiriez et al., 2009), which, in turn, are correlated with a host of neuropsychological developmental delays. Of these, the association between MSDP and low birth weight appears to account for many of the associated outcomes, replicating consistently in prospective and retrospective studies.
Cognitive dysfunction and impaired learning
Some studies report impaired learning in those prenatally exposed to cigarette smoke, although results are equivocal (Bauman et al., 1991; Eskenazi and Trupin, 1995; Cornelius et al., 2001; Batstra et al., 2003; Mortensen et al., 2005; Lambe et al., 2006). Using an array of laboratory tasks, Cornelius et al., (Cornelius et al., 2001) found support for an association between MSDP and deficits in multiple aspects of learning and memory. In another study, MSDP was associated, via familial factors, with poor school performance (Lambe et al., 2006).
Externalizing behaviors
While the mechanism underlying their association is still under investigation, several studies report higher rates of externalizing behaviors, including attention deficit hyperactivity disorder (ADHD), conduct disorder (CD) as well as higher impulsivity and other problem behaviors in the offspring of mothers who smoked during their pregnancy (Milberger et al., 1996; Fergusson et al., 1998; Wakschlag et al., 2002; Batstra et al., 2003; Maughan et al., 2004; Knopik et al., 2005; Wakschlag et al., 2006; Cornelius et al., 2007; D'Onofrio et al., 2008; Knopik et al., 2009). Two recent studies have investigated the familial links between MSDP and externalizing disorders (Knopik et al., 2006; D'Onofrio et al., 2008) – broadly, these studies suggest that familial factors partially contribute to the association between MSDP and externalizing behavior, although the mechanisms are unclear. Multiple studies also report the association between MSDP and externalizing behaviors to be explained by maternal problem behaviors (Orlebeke et al., 1999; Silberg et al., 2003; Maughan et al., 2004).
Offspring smoking:
There is growing evidence that prenatal exposure to cigarette smoking increases the likelihood of cigarette smoking in the offspring (Cornelius et al., 2000; Cornelius et al., 2005). Given the high correlation between maternal nicotine dependence and MSDP (Agrawal et al., 2008; O'Callaghan et al., 2009), these youth represent a particularly vulnerable group of individuals with both increased genetic (inherited often from both parents who are smokers) and environmental risk (attributable to MSDP and potentially, via exposure to parents who smoke) risk.
In this study, using data on 1342 unique pregnancies, with offspring aged 12-32, their fathers and mothers, we investigate the association between MSDP and offspring outcomes of low birth-weight, pre-term birth, remediation, low scholastic achievement, ADHD, CD problems and regular smoking in the offspring. We highlight the importance of controlling for the influence of parental behaviors in studies of MSDP and offspring outcomes and, as a secondary analysis, also test for potential genetic and environmental links that may contribute to the relationship between MSDP and offspring outcomes.
METHODS AND MATERIALS
Sample
Offspring, with their biological mother and father, constituted the analytic sample. In 2001 and 2004 respectively, data collection was initiated from two offspring-of-twins (OOT) studies, which aimed to examine outcomes in the children of VETR twin fathers, who were members of the Vietnam Era Twin Registry (VETR) (Goldberg et al., 1987; Eisen et al., 1987; Henderson et al., 1990), which is a U.S. registry of male like-sex twin pairs in which both co-twins served in the military during the Vietnam Era (1965- 1975). Father twin pairs who (a) were concordant or discordant for alcohol dependence (AD, Project 1), or (b) were concordant or discordant for illicit drug dependence (DD, Project 2), along with (c) unaffected control twin pairs, were selected. Spouses and offspring of twin fathers were invited to participate. Fathers had already been interviewed by telephone, in 1992, with the Diagnostic Interview Schedule (DIS) (Robins et al., 1996). An adaptation of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA)(Bucholz et al., 1994) was used to collect data from mothers and offspring. For both projects, biological mothers or custodial mothers (for e.g. step mothers) were eligible to participate if twins provided permission to contact them. Offspring were eligible to participate if the VETR twin father and biological and/or custodial mothers gave permission to contact them (In Project 2, permission was granted by either VETR twin father or mother). Parents provided written consent for their minor aged offspring to be interviewed. Due to the nature of data collection, there was some overlap of subjects (N=249) across the two studies – for these subjects, the more recently conducted Project 2 data were used. Descriptions of survey contents and response rates have been previously published (Jacob et al., 2003; Scherrer et al., 2004; Duncan et al., 2008).
Baseline data were used to create the database for the present study. Maternal data and offspring data were necessary to conduct these analyses and hence, any participants missing these components were excluded from the analyses. Additionally, to exclude spurious effects of a custodial mother reporting on MSDP or outcomes of her adoptive child, we excluded 9% of the mothers who were not the biological parent of the child. This yielded a sample size of 1741 offspring with maternal and paternal data – however, the key predictor, MSDP, was only assessed in the 1342 pregnancies (some offspring are related to each other, thus this total refers to 1342 unique pregnancies) in 1,122 mothers who previously reported smoking 100 or more cigarettes in a lifetime - this was our final sample.
Measures
Offspring Outcomes
Several outcomes were examined in the offspring: Birth weight (BW); Preterm birth (PREM); Remediation (i.e. taking remedial classes/a speech/language therapist, REM); Conduct problems (CD); ADHD (ADHD); Low scholastic achievement (EDU) and Regular smoking (REGSMK).
Definitions for each measure are listed in Table 1.
TABLE 1.
Prevalence of offspring outcomes, paternal and maternal measures in 1,342 offspring of 1,122 mothers from two U.S. offspring of twins studies conducted in 2001 and 2004.
| Measure | Abbreviation | % |
|---|---|---|
| Maternal smoking during pregnancy | MSDP | 14.2 |
| Self-reported whether they had smoked during the 1st, 2nd and the 3rd trimester of pregnancy. | ||
| Maternal drinking during pregnancy | MDDP | 12.3 |
| Self-reported whether they had consumed alcoholic beverages even on one day after knowing they were pregnant (during any pregnancy). |
||
| Maternal heavy smoking | MHSI | 16.7 |
| A score of 4 or more on the Heaviness of Smoking Inventory (H.S.I.) (Heatherton et al., 1989) indicating risk for nicotine dependence (Chabrol et al., 2005). |
||
| Maternal current smoking | MCUR | 15.5 |
| Self-reported smoking at time of interview. | ||
| Maternal lifetime drug use | MDRG | 43.3 |
| Self-reported use of cannabis, cocaine, stimulants, sedatives, opiates, heroin, psychedelics, inhalants or hallucinogens. |
||
| Maternal alcohol dependence | MAD | 8.6 |
| Meeting criteria for lifetime DSM-IV alcohol dependence based on self-reported symptoms | ||
| Maternal attention deficit hyperactivity disorder | MADHD | 7.8 |
| Self-reported meeting criteria for ADHD prior to age 7 years. | ||
| Maternal conduct problems | MCDP | 22.8 |
| Self-reported, endorsing one or more items from a conduct problems behavior checklist. | ||
| Paternal DSM-IIIR nicotine dependence | PND | 47.4 |
| Meeting criteria for nicotine dependence from self-reported symptoms. | ||
| Paternal alcohol dependence | PAD | Grp 1: 31.7, Grp 2: 11.6, Grp 3: 13.2 |
| High-risk sampling. | ||
| Paternal drug dependence | PDD | Grp 1: 69.1, Grp 2: 5.4, Grp 3: 6.8 |
| High-risk sampling. | ||
| Paternal current smoking | PCUR | 24.6 |
| Self-reported smoking at time of interview. | ||
| Paternal conduct disorder | PCD | 9.4 |
| Meeting criteria for DSM-IIIR conduct disorder based on self-reported symptoms. | ||
| Offspring birth weight | BW* | Mean: 121.9 ounces (SE 0.6) ** |
| Maternal report of birth weight (in ounces). | ||
| Offspring premature birth | PREM | 7.9 |
| Maternal report that the offspring was born 3 or more weeks prior to their due date. | ||
| Offspring attention deficit hyperactivity disorder problem | ADHD | 10.5 |
| Maternal endorsement of 6 or more DSM-IV inattention or hyperactivity/impulsivity symptoms (with or without impairment). |
||
| Offspring conduct disorder (3+ symptoms) | CD | 13.5 |
| Self-report of 3 or more DSM-IV symptoms of conduct disorder. | ||
| Offspring regular smoking | REGSMK | 18.5 |
| Self-report of having smoked 21 or more cigarettes over the lifetime and smoking three or more times per week for a minimum of three weeks. |
||
| Offspring remediation/remedial classes | REM | 20.3 |
| Maternal report of offspring using a speech/language therapist or using remedial/special education. | ||
| Offspring low scholastic achievement | EDU | 16.4 |
| Self-report of consistently receiving C's or lower grades in school. |
4.5% of the offspring had a birthweight that was less than 88 ounces (approximately 2496 grams).
121.9 ounces ≈ 3, 456 grams
Maternal Covariates
The primary maternal predictor was maternal smoking during pregnancy (MSDP). Mothers self-reported whether they had smoked during the 1st, 2nd and the 3rd trimester of pregnancy, only if they had previously reported smoking a 100 or more cigarettes in their lifetime (N=1,342 pregnancies in 1,122 biological mothers). Due to the distribution of the data (85.8% didn't report MSDP, 2.4% reporting 1st trimester only, 2% reporting 1st or 2nd trimester but not 3rd and 10.3% reporting MSDP into the 3rd trimester) a dichotomous measure reflecting any MSDP was coded. The prevalence of MSDP (14.2%) is comparable to U.S. population estimates (Centers for Disease Control (CDC), 2004). Secondary analyses used multi-level variables that excluded any smoking in the 1st trimester that may have occurred prior to knowledge of pregnancy.
Other covariates were Maternal drinking during pregnancy (MDDP); Maternal Heavy Smoking (MHSI); Maternal Current smoking (MCUR); Maternal Drug Use (MDRG); Maternal Alcohol Dependence (MAD); Maternal ADHD (MADHD) and Maternal CD problems (MCDP). These are defined in Table 1. Data on maternal drug use during (any) pregnancy were available but rates were very low (1.9%) and hence, we did not include this measure in the current study.
Paternal Covariates
Covariates from the father included Paternal Lifetime Nicotine Dependence (PND); Paternal Current Smoking (PCUR) and Paternal conduct disorder (PCD), each defined in Table 1. As the original studies selected father twins on the basis of their alcohol or drug dependence status, sample selection bias was controlled by adjusting for the ascertainment strategy, using the 4-group design(Jacob et al., 2003). According to this design, fathers, who were the VETR twin pairs, were designated as:
Group 1 if the father of the offspring had alcohol dependence (AD) or drug dependence (DD) – the offspring in this instance is at high genetic and environmental risk.
Group 2 if the father of the offspring was unaffected but his identical co-twin (who shares 100% of his genes identical-by-descent) had a diagnosis of AD or DD – the offspring here is at high genetic risk but low environmental risk.
Group 3 if the father of the offspring was unaffected but his fraternal co-twin (who shares 50% of his genes identical-by-descent) had a diagnosis of AD or DD – the offspring is at moderate genetic but low environmental risk.
Group 4, where irrespective of zygosity, both the father and his cotwin are unaffected – the offspring is at low genetic and environmental risk.
Two 4-group design variable sets, one each for AD (PAD) and for DD (PDD), were included as covariates representing paternal alcohol and drug dependence and to control for sampling design. Ethnicity (Caucasian – 94.4%), paternal (63.8%) and maternal (70.5%) education representing 13 or more years of educational attainment were used as covariates.
Statistical analyses
A series of logistic regression models were fitted to the data. The offspring outcome was predicted by MSDP as well as the remaining maternal and paternal covariates – all analyses controlled for PAD, PDD as well as ethnicity and parental education. In instances where an offspring outcome was highly correlated with another outcome measure (for e.g. offspring smoking correlated with offspring ADHD), the mediating influence of the correlated measure was tested in a subsequent model.
For any offspring outcome (except birth weight and pre-term birth) that was significantly associated with MSDP in the multivariate models, we also fit Kaplan-Meier survival curves to examine whether MSDP also influenced age at onset of the outcome (for e.g. if MSDP associated with regular smoking in the offspring, is it also associated with earlier onset of regular smoking). All analyses were done in STATA with a robust variance estimator that allowed for clustered observations (i.e. adjusting standard errors when related offspring/siblings are treated as independent observations).
RESULTS
Correlation between measures
MSDP was negatively correlated with birth weight and positively with regular smoking in the offspring (Table S1-S3). Correlations between MSDP and ADHD or CDP were modest, however correlations between offspring ADHD and maternal ADHD (MADHD) and maternal CD (MCDP) were higher. MSDP as well as mother's H.S.I. (MHSI) and current smoking (MCUR) was also correlated with nicotine dependence and current smoking in the father, suggesting positive assortative mating (i.e. tendency for smokers to marry smokers). Finally, CD, ADHD, regular smoking (REGSMK) and low scholastic achievement (EDU) were correlated with each other in the offspring, likely representing a general predisposition to externalizing behaviors. EDU was also correlated parental education.
Association between MSDP and offspring outcomes
In univariate models (Table 2) and after adjusting for PAD, PDD, ethnicity and parental education, MSDP was associated with decreasing birth weight, ADHD, low scholastic achievement and with regular smoking in the offspring. In multivariate models, controlling for all maternal and paternal covariates, MSDP had a sustained influence on decreasing birth weight and offspring's regular smoking. The effects of MSDP on offspring ADHD was attributable to mother's self-reported ADHD, maternal CDP and with low paternal education. Maternal ADHD, maternal low education and paternal current smoking were associated with low scholastic achievement. MDDP was not associated with these offspring outcomes.
TABLE 2.
Univariate and multivariate association between MSDP and offspring outcomes in 1,342 offspring from two U.S. offspring of twins studies conducted in 2001 and 2004.
| Birth weight* (BW) |
Preterm birth (PREM) |
Attention Deficit Hyperactivity Disorder (ADHD) |
Conduct problems (CD) |
Regular smoking (REGSMK) |
Remediation (REM) |
Low Scholastic Achievement (EDU) |
|
|---|---|---|---|---|---|---|---|
| Univariate | |||||||
| MSDP | −7.72 [−11.13, −4.31] |
1.38 [0.76, 2.49] |
1.74 [1.10, 2.75] |
1.27 [0.82, 1.96] |
2.25 [1.54, 3.27] |
1.16 [0.79, 1.72] |
1.51 [1.01-2.25] |
| Adjusting for ethnicity, parental education and study design | |||||||
| MSDP | −8.00 [−11.46,−4.55] |
1.14 [0.79, 1.65] |
1.53 [1.00,2.35] |
1.18 [0.76, 1.18] |
2.09 [1.43, 3.07] |
1.20 [0.81, 1.77] |
1.53 [1.02, 2.30] |
| Adjusting for all maternal and paternal covariates | |||||||
| MSDP | −7.63 [−11.12,−4.14] |
- | 1.74 [1.18, 2.58] |
- | |||
| MDDP | - | - | - | - | |||
| HSI | - | - | - | - | |||
| MCUR | - | - | - | - | |||
| MDRG | - | - | 1.74 [1.26, 2.39] |
- | |||
| MAD | - | - | - | - | |||
| MADHD | - | 4.10 [2.50, 6.72] |
- | 2.03 [1.31, 3.16] |
|||
| MCD | - | 1.29 [1.10, 1.52] |
- | - | |||
| PND | - | - | 1.76 [1.28, 2.42] |
1.41 [1.01, 1.97] |
|||
| PAD | - | - | - | - | |||
| PDD | - | - | - | - | |||
| PCUR | - | - | - | - | |||
| PASPD | - | - | - | - | |||
Continuous measure – coefficient shown.
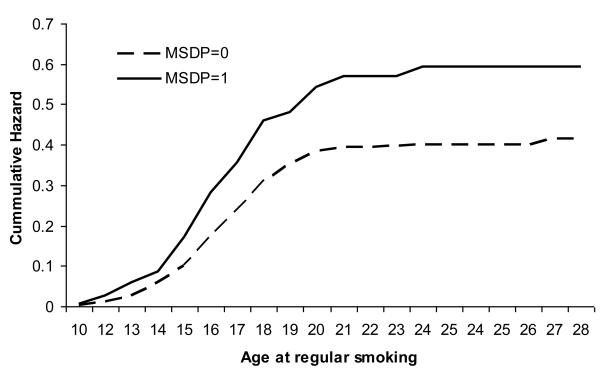
Kaplan-Meier curves for survivor function were tested for initiation of cigarette smoking and regular smoking (Figure 1). MSDP was also associated with earlier onset of regular smoking (hazard ratio of 1.48, 95% C.I. [1.13-1.94]) in the offspring.
FIGURE 1.
Nelson-Aelan curves representing hazards of regular smoking at various ages of onset in 1342 offspring of mothers reporting maternal smoking during pregnancy (MSDP) and those without MSDP from two U.S. offspring of twins studies conducted in 2001 & 2004.
DISCUSSION
Previous studies have attempted to disentangle the etiology of the relationship between MSDP and smoking as well as externalizing behaviors in the offspring (see Knopik, 2009 for a review). While, using a subset of these data, Knopik et al (2009) recently showed univariate associations between ADHD problems and MSDP, a preponderance of studies, including ours, support the hypothesis that for externalizing behaviors, such as ADHD and CD, transmission of risk may be attributable to a latent predisposition to non-normative behaviors (Silberg et al., 2003; Maughan et al., 2004; Knopik et al., 2005; Knopik et al., 2006; D'Onofrio et al., 2008) – in other words, women with a predisposition to non-normative/problem behaviors tend to also smoke during their pregnancy and their offspring are at increased risk for externalizing psychopathology via a direct transmission of genetic risk from mother (and father) to offspring. A recent study found no differences in rates of externalizing behaviors between siblings discordant for MSDP also alluding to the possible role of family environment that may jointly increase likelihood of MSDP as well as later externalizing behaviors in the offspring (D'Onofrio et al., 2008) – the potential role of parental behaviors or general permissive parenting may be explored in this context.
The literature surrounding the impact of MSDP on birth weight and other birth complications is quite robust. We validate those findings here - offspring whose mothers reported MSDP, on average, weighed 215.5 grams (7.6 ounces) less at birth when compared to offspring whose mothers may have been regular smokers but did not report MSDP. Animal studies demonstrate the effects of nicotine on fetal and placental development – a common hypothesis here posits that nicotine exerts anorexigenic effects on both mother and fetus (Perkins et al., 1994; Ernst et al., 2001) exacerbated by hypoxia related to respiratory difficulties in the fetus (Abel, 1980; Byrd and Howard, 1995; Cnattingius, 2004). Some studies have also considered the possible mediating role of lower maternal weight gain, lower maternal BMI and premature birth – however, the effect of MSDP on low birth weight appears to be independent and direct.
After adjusting for maternal and paternal covariates, low scholastic achievement, via self-reported school grade was not associated with MSDP, suggesting that other familial factors that correlate with both MSDP and offspring scholastic achievement may be at play. While early studies (Butler and Goldstein, 1973; Fogelman, 1980; Fogelman and Manor, 1988)demonstrated strong associations between MSDP and scholastic achievement, recently, Lambe et al., (2006) have reported that MSDP during the first but not the second pregnancy is associated with low scholastic achievement in the second offspring. Additionally, consistent with our finding, another study (Braun et al., 2009) reported that maternal education attenuated the relationship between MSDP and intellectual abilities, further reinforcing the role of correlated familial factors.
The findings of the present study should be viewed with some limitations in mind. First, the parent studies were designed to study outcomes in the offspring of twin pairs - while this can be particularly useful in disentangling the role of genetic factors from environmental influences, we were limited in our ability to do this due to (a) reduced power and (b) because the fathers, but not the mothers, were twins. Thus, a formal test of genetic influences of MSDP on offspring outcome is not possible (Eaves et al., 2005). Additionally, the fathers were members of the VETS – this, as well as strategies for contacting mothers and offspring, may influence the generalizability of our findings. Second, reports of MSDP were retrospective and cross-sectional and may have been influenced by recall bias or other personal factors. While studies show high reliability in self-reported smoking during pregnancy (Heath et al., 2003; Reich et al., 2003; Pickett et al., 2009), recent increases in dissemination of information on adverse consequences of MSDP on offspring may have discouraged some mothers from disclosing it. Third, maternal gestational weight was not available on all participants and hence could not be used as a control. Fourth, maternal nicotine dependence was assessed using the H.S.I and DSM-based nicotine dependence measures were not available. Finally, as the mother often reported on the offspring and herself (e.g. ADHD), rater bias may have influenced our findings to some extent.
MSDP increases neonatal intensive care unit admissions by 20%(Adams et al., 2002). There continues to be a clear need for health care professionals to counsel expectant mothers (and where possible, fathers/spouses/partners of expectant mothers) regarding the adverse consequences of smoking during their pregnancy and offer behavioral counseling or safe pharmacological aids. Not only does MSDP, directly and indirectly, influence the well-being of the offspring (from fetal stages through young adulthood) but also has key health consequences for the expecting mother. As articulated in the core objectives of Healthy People 2010 (Healthy People 2010, 2000), reducing risk behaviors and increasing education surrounding prenatal health, will be vital in attaining better quality of life for mothers, their offspring and consequently, families in general.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by grants DA020810, DA18660, DA14363, DA18267 and DA019951, DA23668 & DA19951 from the National Institute on Drug Abuse (NIDA), grants AA11667, AA11822, AA007580, and AA11998 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and from a Merit Review Grant (Jacob) from the Department of Veterans Affairs Medical Research Service. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the VET Registry. Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense, National Personnel Records Center, National Archives and Records Administration, the Internal Revenue Service, National Opinion Research Center, National Research Council, National Academy of Sciences, and the Institute for Survey Research at Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Smoking during pregnancy: a review of effects on growth and development of offspring. Hum Biol. 1980;52:593–625. [PubMed] [Google Scholar]
- Adams EK, Miller VP, Ernst C, Nishimura BK, Melvin C, Merritt R. Neonatal health care costs related to smoking during pregnancy. Health Econ. 2002;11:193–206. doi: 10.1002/hec.660. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, Heath AC, Madden PA. Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine Tob Res. 2008;10:567–578. doi: 10.1080/14622200801978672. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester BM, Gard CC, Wright LL, Lagasse L, Higgins R. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol. 2005;25:631–637. doi: 10.1038/sj.jp.7211378. [DOI] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Bauman KE, Flewelling RL, LaPrelle J. Parental cigarette smoking and cognitive performance of children. Health Psychol. 1991;10:282–288. doi: 10.1037//0278-6133.10.4.282. [DOI] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Kalkbrenner A, Zimmerman J, Nicholas JS. The effect of maternal smoking during pregnancy on intellectual disabilities among 8-year-old children. Paediatr. Perinat. Epidemiol. 2009;23:482–491. doi: 10.1111/j.1365-3016.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret RJ, Cloninger RC, Dinwiddie SH, Hesselbrock V, Nurnberger JI, Reich T, Schmidt I, Schuckit MA. A New, Semi-Structured Psychiatric Interview For Use In Genetic Linkage Studies. J. Stud. Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Butler NR, Goldstein H. Smoking in pregnancy and subsequent child development. Br Med J. 1973;4:573–575. doi: 10.1136/bmj.4.5892.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd RS, Howard CR. Children's passive and prenatal exposure to cigarette smoke. Pediatr Ann. 1995;24:640–645. doi: 10.3928/0090-4481-19951201-07. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Annual smoking-attributable mortality, years of potential life lost, and economic costs-United States. MMWR. 2002;51:300–303. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Smoking During Pregnancy - United States, 1990-2002. MMWR. 2004;53:911–915. [PubMed] [Google Scholar]
- Chabrol H, Niezborala M, Chastan E, de LJ. Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers. Addict Behav. 2005;30:1474–1477. doi: 10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–40. doi: 10.1080/14622200410001669187. S125-S140. [DOI] [PubMed] [Google Scholar]
- Conter V, Cortinovis I, Rogari P, Riva L. Weight growth in infants born to mothers who smoked during pregnancy. BMJ. 1995;310:768–771. doi: 10.1136/bmj.310.6982.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, DeGenna N, Day NL. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9:739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol. Teratol. 2005;27:667–676. doi: 10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. J Dev Behav Pediatr. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Turkheimer EN, Eaves LJ, Corey LA, Berg K, Solaas MH, Emery RE. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. J Child Psychol Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Grant JD, Heath AC, Nelson EC, Jacob T, Bucholz KK. The association between cannabis abuse and dependence and childhood physical and sexual abuse: evidence from an offspring of twins design. Addiction. 2008;103:990–997. doi: 10.1111/j.1360-0443.2008.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Maes HH. Revisiting the children of twins: can they be used to resolve the environmental effects of dyadic parental treatment on child behavior? Twin Res Hum Genet. 2005;8:283–290. doi: 10.1375/1832427054936736. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. II. Effects on neurodevelopment at age 5 years. Am J Epidemiol. 1995;142:S19–S29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Fogelman K. Smoking in pregnancy and subsequent development of the child. Child Care Health Dev. 1980;6:233–249. doi: 10.1111/j.1365-2214.1980.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Fogelman KR, Manor O. Smoking in pregnancy and development into early adulthood. BMJ. 1988;297:1233–1236. doi: 10.1136/bmj.297.6658.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, True W, Eisen S, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: ascertainment bias. Acta Genet Med Gemellol (Roma) 1987;36:67–78. doi: 10.1017/s0001566000004608. [DOI] [PubMed] [Google Scholar]
- Healthy People 2010 . Understanding and Improving Health. U.S. Government Printing Office; Washington, D.C.: 2000. [Google Scholar]
- Heath AC, Knopik VS, Madden PA, Neuman RJ, Lynskey MJ, Slutske WS, Jacob T, Martin NG. Accuracy of mothers' retrospective reports of smoking during pregnancy: comparison with twin sister informant ratings. Twin Res. 2003;6:297–301. doi: 10.1375/136905203322296656. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Validity of the Fagerstrom test for nicotine dependence and of the Heaviness of Smoking Index among relatively light smokers. Br. J. Addict. 1989;84:791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, Scherrer J, Fu Q. Genetic and environmental effects on offspring alcoholism: new insights using an offspring-of-twins design. Arch Gen Psychiatry. 2003;60:1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol. 2009;34:1–36. doi: 10.1080/87565640802564366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Jacob T, Slutske WS, Bucholz KK, Madden PA, Waldron M, Martin NG. Maternal alcohol use disorder and offspring ADHD: disentangling genetic and environmental effects using a children-of-twins design. Psychol Med. 2006:1–11. doi: 10.1017/S0033291706007884. 1-11. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Jacob T, Haber JR, Swenson LP, Howell DN. Paternal alcoholism and offspring ADHD problems: a children of twins design. Twin Res Hum Genet. 2009;12:53–62. doi: 10.1375/twin.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Sparrow EP, Madden PA, Bucholz KK, Hudziak JJ, Reich W, Slutske WS, Grant JD, McLaughlin TL, Todorov A, Todd RD, Heath AC. Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: a female twin study. Psychol Med. 2005;35:625–635. doi: 10.1017/s0033291704004155. [DOI] [PubMed] [Google Scholar]
- Kyrklund-Blomberg NB, Granath F, Cnattingius S. Maternal smoking and causes of very preterm birth. Acta Obstet Gynecol Scand. 2005;84:572–577. doi: 10.1111/j.0001-6349.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrang A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, Moss-Morris R, North RA. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338:b1081. doi: 10.1136/bmj.b1081. doi: 10.1136/bmj.b1081.,b1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. A dose-response relationship between maternal smoking during late pregnancy and adult intelligence in male offspring. Paediatr. Perinat. Epidemiol. 2005;19:4–11. doi: 10.1111/j.1365-3016.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- O'Callaghan FV, Al MA, O'Callaghan M, Alati R, Najman JM, Williams GM, Bor W. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults - a birth cohort study. Aust N Z J Public Health. 2009;33:371–377. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Child behavior problems increased by maternal smoking during pregnancy. Arch Environ. Health. 1999;54:15–19. doi: 10.1080/00039899909602231. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, DiMarco A, Fonte C. Acute effects of tobacco smoking on hunger and eating in male and female smokers. Appetite. 1994;22:149–158. doi: 10.1006/appe.1994.1014. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Dukic V, Kasza K, Niessner M, Wright RJ, Wakschlag LS. The complex enterprise of modelling prenatal exposure to cigarettes: what is ‘enough’? Paediatr. Perinat. Epidemiol. 2009;23:160–170. doi: 10.1111/j.1365-3016.2008.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRAMS 2006 North Carolina Pregnancy Risk Assessment Monitoring System Survey Results. 2007 http://www.schs.state.nc.us/SCHS/prams/2006/SMK53L_A.html.
- Reich W, Todd RD, Joyner CA, Neuman RJ, Heath AC. Reliability and stability of mothers' reports about their pregnancies with twins. Twin Res. 2003;6:85–88. doi: 10.1375/136905203321536209. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz K, Compton W. DIS-IV. Saint Louis: 1996. [Google Scholar]
- Salihu HM, Aliyu MH, Kirby RS. In utero nicotine exposure and fetal growth inhibition among twins. Am J Perinatol. 2005;22:421–427. doi: 10.1055/s-2005-915219. [DOI] [PubMed] [Google Scholar]
- Scherrer JF, Waterman BM, Heath AC, Bucholz KK, True WR, Jacob T. Are substance use, abuse and dependence associated with study participation? Predictors of offspring nonparticipation in a twin-family study. J Stud Alcohol. 2004;65:140–144. doi: 10.15288/jsa.2004.65.140. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys' conduct disturbance: an examination of the causal hypothesis. Biol Psychiatry. 2003;53:130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Goodwin MS, Shenassa E, Buka S, Niaura R, Rosenblith JF, Lipsitt LP. Maternal Smoking During Pregnancy and Neonatal Behavior: A Large-Scale Community Study. Pediatrics. 2009;123:e842–e848. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriez G, Bouhaddi M, Mourot L, Nobili F, Fortrat JO, Menget A, Franco P, Regnard J. Heart rate variability in preterm infants and maternal smoking during pregnancy. Clin Auton. Res. 2009 doi: 10.1007/s10286-009-0003-8. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J Am Acad Child Adolesc Psychiatry. 2006;45:461–467. doi: 10.1097/01.chi.0000198597.53572.3e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.