Abstract
The Vaccinia virus gene A35R (Copenhagen designation) is highly conserved in mammalian-tropic poxviruses and is an important virulence factor, but its function was unknown. We show herein that A35 does not affect viral infectivity, apoptosis induction, or replication; however, we found that A35 significantly inhibited MHC class II-restricted antigen presentation, immune priming of T lymphocytes, and subsequent chemokine and cytokine synthesis. A35 localized to endosomes and reduced the amount of a model antigenic peptide displayed in the cleft of class II MHC. In addition, A35 decreased VV specific T cell responses in vivo. Thus, this is the first report identifying a function for the A35 protein in virulence as well as the first report identifying a VV gene that inhibits peptide antigen presentation.
Keywords: poxvirus, vaccinia, macrophage, antigen presentation, cytokine, apoptosis, MHC
INTRODUCTION
Poxviruses are large, double-stranded DNA viruses (~200 kb) that are capable of infecting a wide variety of animals (Moss, 2001). The most infamous poxvirus is Variola virus, the causative agent of smallpox. Smallpox is estimated to have killed over 500 million people in the 20th century before it was declared eradicated by the World Health Organization in 1980 (Mahalingam, Damon, and Lidbury, 2004). Smallpox now presents itself as a danger in biowarfare and bioterrorism. Other poxviruses can also cause significant disease in humans. In 2003, a Monkeypox outbreak occurred in the United States, infecting over 70 people (Chen et al., 2005). Fortunately, this was an attenuated strain, and there were no human fatalities (Chen et al., 2005). A widespread monkeypox outbreak would be more difficult to contain than smallpox, due to the fact that monkeypox can replicate and persist in animal reservoirs. Other emerging zoonotic poxviruses include buffalopox in Asia (Kolhapure et al., 1997), tanapox in Africa, Europe and the U.S. (Dhar et al., 2004; Stich et al., 2002), cowpox in humans and primates (causing widespread fatalities) (Martina et al., 2006; Matz-Rensing et al., 2006; Steinborn, Essbauer, and Marsch, 2003), and Cantagalo in South America (Damaso et al., 2000). Molluscum contagiosum virus is commonly seen in children and AIDS patients, accounts for nearly 300,000 doctor visits each year in the US (Molino, Fleischer, and Feldman, 2004), and is emerging as a sexually transmitted disease.
The eradication of smallpox was the result of the most successful vaccination program in history. Routine vaccination was stopped in most countries in the early 1970’s due to the poor safety record of the vaccine, live Vaccinia virus (VV). Vaccination is contraindicated for many individuals, including those who have inflammatory skin conditions, such as eczema, and those that are immunocompromised or pregnant (Lederman et al., 2009). In a recent vaccine trial, it was reported that 1 out of every 450 of those vaccinated had a serious adverse vaccine reaction (Casey et al., 2005), and vaccine trials were stopped after reports of increased adverse cardiac events following vaccination (Eckart et al., 2004; Upfal and Cinti, 2004). In recent years, attenuated and/or non-replicating VV strains have been increasingly employed to reduce risks, and many VV strains are being used as a platform in the development of vaccines for other diseases, such as HIV, malaria, and cancer (Dunachie et al., 2006; Schell et al., 2009; Stober et al., 2007; Viner et al., 2006). Thus it is crucial to elucidate the virulence mechanisms in VV and particularly those that interfere with the development of a robust immune response. An increased understanding of poxvirus pathogenesis will aid both in antiviral drug development and in the design of improved vaccines.
While VV elicits a strong immune response, it also encodes a plethora of immunosuppressive genes and many of unknown function (Upton et al., 2003a). Gene 158 of Vaccinia virus Western Reserve (WR) strain, commonly referred to as the A35 gene by the VV Copenhagen designation, is highly conserved in mammalian-tropic poxviruses and vaccine strains (Roper, 2006; Upton et al., 2003b), but has no similarity to non-poxvirus proteins, giving no clues as to its function. A mutant A35 deletion virus (A35Δ) replicated normally in several tissue culture cell lines, but was highly attenuated (100–1000 fold) in the intranasal and intraperitoneal mouse challenge models. A rescue virus was constructed that returned the wild type A35 gene to the mutant, and this virus construct restored virulence, confirming that the viral phenotype mapped to the A35 locus. While it is difficult to compare the virulence of various gene deletion mutants because many have not been tested in the intranasal model measuring weight loss (intracranial inoculations or measures of viral titers in organs have often been performed instead), available data in the i.n. model indicate that the A35Δ virus is more attenuated than the A46R deletion mutant (Stack et al., 2005), similar in virulence to the IL-18 binding protein knock out virus (Reading and Smith, 2003), slightly less attenuated than the TK knock out (Lee et al., 1992), and less attenuated than the E3L deletion, which causes a 1000 fold attenuation (Vijaysri et al., 2008). The A35Δ virus produced similar quantities of the various morphogenic forms of virus compared to the parental wild type WR virus, with similar kinetics, and virus particles were similarly infectious on a per particle basis (Roper, 2006). We showed that this gene encodes a non-glycosylated, non-secreted 23 kDa protein expressed early during the virus life cycle, but its mechanism of action was unknown. Here, we show that VV A35 gene product blocks immune priming of T lymphocytes by interfering with MHC class II-restricted antigen presentation.
MATERIALS AND METHODS
Cells and virus
VV Western Reserve (WR) strain and A35Δ mutant virus stocks were propagated using BS-C-1 cells as previously described (Roper, 2006). For titrations of VV, BS-C-1 monolayers were fixed and stained with 0.1% crystal violet in 20% ethanol. All cells were grown in media containing 10% fetal bovine serum. CD4+ RsL-11 T cell clones were derived and maintained as previously described (Mannie and Norris, 2001). CTLL-2 (ATCC # TIB-214) were maintained in RPMI supplemented with 0.4% IL-2-containing baculovirus supernatant (Mannie and Norris, 2001). RAW 264.7 (ATCC # TIB-71), a mouse macrophage cell line, was maintained in DMEM. The mouse B cell line 1153 (kind gift from Janice Blum) was maintained in RPMI (Li et al., 2005).
Cell tropism replication
The cell type of interest was split into two groups of equal number for infection. One group was infected with WR and the other with A35Δ. The cells were infected for 20 h at MOI=1 followed by three freeze/thaw cycles to release newly formed virus particles. Replication was measured by titering the cell lysates on a monolayer of BS-C-1 cells and counting the plaques that formed 40 hours later. For early replication in mouse organs, groups of mice were intranasally challenged with WR (Roper, 2006) and A35Δ at 104 pfu/mouse, and nose, lungs, blood, brain and spleen were harvested on day 1, 2 and 3 (6 mice on each day) post challenge. Organs were freeze/thawed three times, homogenized, sonicated, and titered as above.
Peritoneal macrophage isolation and antigen presentation assays
Lewis rats (bred and maintained at the AAALAC-certified Brody School of Medicine animal care facility at East Carolina University) were injected intraperitoneally with 200 μg of inactivated C. parvum in 5 ml of HBSS. Two to three days later, the rats were sacrificed and peritoneal exudate cells (PEC) were harvested by washing the peritoneum in cold HBSS. PEC (>95% CD45+ hematopoietic lineage) infected for 5 h with either WR or A35Δ, and placed into 96-well plates. PEC were incubated with 50 nM guinea pig myelin basic protein (GPMBP) for 30 minutes, followed by addition of 25,000 Lewis rat CD4+ RsL.11 clones specific for GPMBP (Mannie and Norris, 2001). The antigen presentation plates were incubated for 24–48 h at 37°C, 5.0% CO2. After incubation, 50 μl of supernatants were transferred into an empty 96-well plate and frozen for the following assays. All animal experiments were in compliance with NIH guidelines.
CTLL IL-2 Bioassay
IL-2 was measured using previously described methods (Mannie and Norris, 2001). Briefly, 10,000 CTLL clones were washed, resuspended in RPMI, and added to the collected supernatants. The plates were incubated for 48 h at 37°C, 5.0% CO2, followed by the addition of 10 μl of MTS/PMS (2.0 mg/mL MTS, Promega; and 0.1 mg/mL PMS, Sigma). The absorbance was read at 492 nm. Media only was used to define the background control level and known IL-2 containing supernatants were used as positive control.
NO measurement
50 μl of the harvested supernatants were transferred into a separate 96-well plate followed by the addition of 50 μl of Griess Reagent (1% sulfanilamide–0.1% N-[1-naphthy] ethylenediamine in 2.5% phosphoric acid) into each well. The absorbance was read at 540 nm (Campos-Neto et al., 1998).
Cytokine measurement
50 ul of the harvested supernatants were analyzed using the LincoPlex 24 Rat Cytokine/Chemokine Luminex Bead Immunoassay Kit according to the manufacturer’s instructions (Linco Research). The supernatants were incubated with a panel of anti-cytokine Abs immobilized on Luminex beads (Bio-Rad Laboratories). The following cytokines were analyzed: IL-1α, IL-1β, IL-2, IL-6, IL-17, IL-18, MIP-1α, GM-CSF, IFN-α, growth regulated oncogene alpha/keratinocyte attractant (GRO/KC), RANTES, TNF-α, MCP-1, eotaxin, G-CSF, IL-4, IL-9, IL-13, IL-5, and IL-10. Reagents for IFNγ were not available at the time. Samples were run according to the manufacturer’s instructions (Bio-Rad) and analyzed on the BioPlex protein array reader (Bio-Rad) in the Duke University Human Vaccine Institute Immune Reconstitution Core Facility (Durham, NC).
RsL-11 stimulation assay
CD4+ RsL.11 T lymphocytes were washed and resuspended in RPMI. The cells were infected for 3–4 h, plated in a 96-well format, and stimulated with PEC pulsed with 50 nM GPMBP, 25 ug/ml Con A (Sigma), or 100 nM PMA/2 uM ionomycin (Sigma). The plates were incubated at 37°, 5% CO2. 50 ul of the harvested supernatants were collected at 20- and 40-h and assayed for IL-2 using the CTLL bioassay already described.
Metabolism/survival assays
Cells of interest were infected with VV (MOI=2). Metabolism was measured by the addition of 10 ul MTS/PMS 4–24 hpi, and analyzed similarly to the method described above for the CTLL IL-2 Bioassay. Absorbance was read at 492 nm at various times post infection. Tetrazolium salts such as MTS are reduced to colored formazan products during cellular respiration. As only live cells respire, the MTS assay can be used to measure cell survival.
Flow cytometry
Cells were infected for 4 hours and then washed in cold PBS containing 1% heat-inactivated FBS and 0.1% sodium azide. 3 × 105 cells were stained with an anti-MHC II concentrated supernatant (Y3P, AF6120, and MKS4), OX1 anti-CD45, or human anti VV (Cangene VIG) for 1 h on ice, washed once, and then incubated with a FITC-conjugated goat anti-mouse IgG (Southern Biotech) or anti human-PE. For measurement of apoptosis, cells were infected and then stained with annexinV-FITC/propidium iodide (PI) (BD Pharmingen) per the manufacturer’s instructions. Expression was measured using a FACScan (Becton Dickinson) and analyzed with Cell Quest software.
Peptide-MHC association
The Y-Ae antibody specifically detects a complex of peptide 52–68 from I-EdMHC class II bound in the cleft of MHC class II I-A b (Itano et al., 2003; Rudensky et al., 1991). This complex is formed in mice that express both alleles. Spleens were harvested from B10.A-H2^i5 H2-T18^a/(5R)SgSnJ mice (Jackson Labs); C57Bl/6 mice were used as a negative control. Unfractionated splenocytes were infected with sucrose gradient purified virus for 3 h (MOI=10) and then incubated with biotin-conjugated Y-Ae (eBioscience). Cells were washed and incubated with streptavidin-RPE (Southern Biotech). Goat polyclonal IgG antibody to CLIP (P-15, Santa Cruz) and donkey anti-goat IgG FITC (Jackson Labs) were used to measure CLIP peptide bound in MHC class II on the surface of cells. Samples were analyzed using a FACScan equipped with Cell Quest software.
Immunofluorescence microscopy
PEC were harvested as described above and grown on coverslips for 4 h. The cells were then infected at an MOI=10 for 2 hours, fixed and permeabilized in 3% paraformadehyde and 0.1% Triton X-100, respectively, and then stained with a rabbit anti-A35 antibody 1:4000 (Roper, 2006) and one of the following polyclonal goat anti-endosome antibodies (Santa Cruz Biotechnology) at 1:80 diluted in 0.1% Triton X-100/PBS: Lamp-1 (C-20), Rab 7 (A-16), Rab 5B (C-12), or RhoB (M-19). The coverslips were then washed and incubated with Alexa Fluor 633 (Invitrogen) and a FITC-conjugated anti-rabbit IgG (Sigma) for 45 m, followed by a final round of washes. The cells were visualized with a Zeiss confocal microscope and pictures were taken with the 100x objective.
One-step growth curve
A one-step growth curve was performed as previously described (Roper, 2006).
IFNγ Elispot
To enumerate cells secreting IFNγ, 96-well ELISA plates (Immulon H2B Thermo Electron) were coated overnight with rat anti-mouse IFN-γ (1 mg/ml Pharmingen). Plates were washed with blocking buffer (PBS 1% FBS) before adding murine splenocytes in RPMI 1640. Stimulation of splenocytes was achieved by the addition of virus (MOI=2) followed by incubation for 40 h at 37°C. Plates were then washed and incubated with 0.4 ul biotinylated rat anti-mouse IFN-γ (Pharmingen 0.5 mg/ml). Plates were washed and incubated with streptavidin AP for 1 hr at 37°C. Plates were developed with agarose/BCIP/AMP buffer mixture. Spots were counted using a dissection microscope.
Statistical analysis
Experiments were repeated at least 3 times and representative data are shown. A two-tailed Student’s t test was used to compare groups. p values < 0.05 were considered significant.
RESULTS
A35 effects on replication
We have shown previously that the A35Δ virus is attenuated in a mouse challenge model, but that the mutant virus replicated and spread similar to the WR parental strain in 8 cell lines (RK-13, HeLa, CV-1, BS-C-1, A549, MRC-5, TK-, BHK-21) derived from human, rabbit, monkey and hamster (Roper, 2006). Still it was possible that the A35Δ was attenuated in vivo because the A35 gene was required for replication in certain cell types or tissues, such as immune cells, neural tissue (since the VV-WR strain is neurovirulent) (Smee and Sidwell, 2004), or heart because VV causes cardiac inflammation (Arness et al., 2004; Eckart et al., 2004). Various cell preparations listed below (or BS-C-1 cells as control permissive cells) (Roper, 2006) were infected with WR or A35Δ mutant at an MOI of 1 for 1 day, and then virus titers were measured. The presence of the A35 gene in WR did not reproducibly enhance viral replication more than 2 fold relative to the A35Δ in any of the cells or tissues tested, including: Lewis Rat Peritoneal Exude Cells (PEC), splenocytes from a young and old rat, rat thymocytes, activated and resting RSL-11 T-cells, R1-T T-cells, rat alveolar Macrophage line NR8383, mouse CTLL, PC-12 neuronal cells (NGF-differentiated and undifferentiated morphologies), Heldtilt Mouse primary brain and heart cell suspensions, human fetal fibroblasts, and HEK (human kidney line) cells (data not shown). We also assessed the effects of A35 on early replication in tissues of mice intranasally challenged with WR and A35Δ at 104 pfu/mouse (Roper, 2006), and found no significant difference in replication in nose, lungs, blood, brain and spleen on days 1, 2 and 3 post challenge. Thus, there was no evidence for A35 involvement in host range or tissue tropism, suggesting that A35 is not required for any steps in replication, morphogenesis, or spread of virus.
A35 inhibits IL-2 and NO production
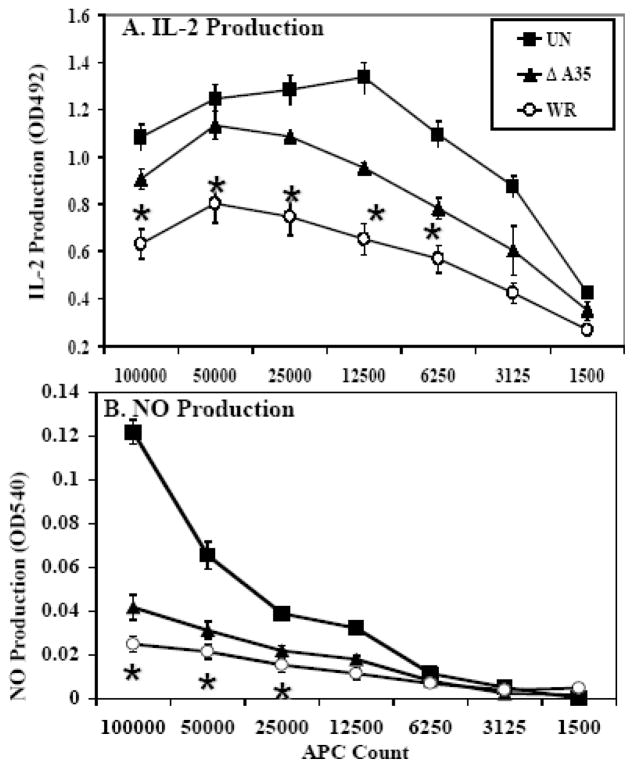
Because the A35 gene was not required for replication but did affect virulence in vivo, we suspected that A35 might be involved in regulating aspects of the host immune response. To test this hypothesis, we used a model in vitro MHC class II-restricted antigen presentation system (Mannie and Norris, 2001) and measured responses of both the T lymphocytes and the macrophage APC. Rats are naturally infected with orthopoxviruses (Martina et al., 2006), and rat peritoneal exude cells (PEC) are a rich source of normal primary macrophage. PEC were infected in triplicate with WR virus or the A35Δ virus, pulsed with antigen (guinea pig myelin basic protein), and incubated in varying numbers with a cognate CD4+ T cell line, RsL.11 (Mannie and Norris, 2001). Supernatants were collected, and IL-2 production was determined as a measure of the T cell response to the antigen presentation. T cells in the uninfected groups were able to secrete more IL-2 than either the T cells in the A35Δ-infected or the WR-infected groups (fig 1A). The WR-infected PECs caused the T-cells to secrete significantly (p < 0.05) less IL-2 than A35Δ-infected cells 15–72 hpi, indicating that A35 down-regulates MHC class II-restricted antigen presentation responses in vitro. A similar A35-mediated down-regulation of IL-2 production was observed when resting PECs and unfractionated rat splenocytes were used as APC (data not shown). No IL-2 was produced in the absence of antigen or either cell type indicating that IL-2 production is a specific response to antigen presentation.
Figure 1. A35 inhibits the amount of antigen presentation-induced IL-2 and NO.
Freshly isolated PEC were isolated from a rat, infected with VV (MOI=2) for 5 hours, pulsed for 30 minutes with 50 nM GPMBP, and then incubated with the cognate CD4+ RsL.11 T cell line. Uninfected PEC served as a control. At 24- to 48-hpi, supernatants (50 ul) were collected and assayed for either IL-2 using the IL-2 dependent cell line CTLL (A) or for NO using Greiss reagent (B). For the CTLL bioassay, media only was used to define the background control level and known IL-2 containing supernatants were used as positive control. IL-2 production was determined as the mean OD value of the experimental group minus the background control level. * p < 0.05
Since production of IL-2 is a measure of the T cell response, we also wanted to measure nitric oxide (NO), which is produced by the macrophage and has been shown to be an important anti-viral defense (Karupiah et al., 1993; MacMicking, Xie, and Nathan, 1997). NO production was measured in supernatants collected from the in vitro antigen presentation system described above. Upon antigen presentation stimulation, uninfected macrophages produced significantly more NO than infected cells, while WR-infected macrophages produced significantly (p < 0.05) less NO than the A35Δ-infected macrophages (fig 1B). NO was not produced in the absence of antigen or either cell type indicating that this is a specific antigen presentation response. These data indicate that A35 inhibits both the T lymphocyte and APC responses to antigen presentation.
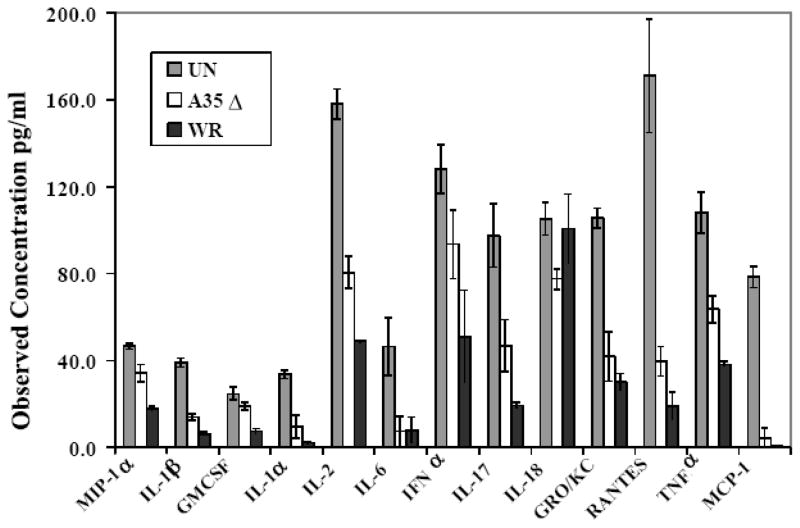
A35 reduces cytokine responses induced by antigen presentation
We next wanted to determine whether A35 specifically reduced the amount of IL-2 and NO produced as the result of antigen presentation (fig 1), or if the presence of A35 in WR broadly affected cytokine responses. Similar to experiments above, PEC were infected in triplicate with WR or A35Δ, pulsed with antigen, and added to RsL.11 T cells. Supernatant cytokines were measured using an antibody-bead-based fluorescent assay. Results showed a broad and significant reduction in multiple cytokines and chemokines (fig 2). The A35Δ virus evoked significantly (p < 0.05) higher levels of MIP1α, IL-1β, GMCSF, IL-1α, IL-2 (confirming our previous IL-2 bioassay results), IFN-α, 1L-17, GROKC, RANTES, and TNFα, (but not IL-18) than WR, suggesting that the presence of A35 inhibits production of these cytokines and chemokines and generally dampens all antigen presentation-induced responses.
Figure 2. A35 globally reduces antigen presentation-induced cytokine synthesis by APC and T cells.
PEC were harvested from Lewis rats, infected with WR or A35Δ virus (MOI=2) for 5 h, pulsed with 50 nM GPMBP, and then co-cultured with the cognate CD4+ RsL.11 T cell line. Supernatants (50 ul) were collected at 24-h post incubation and assayed for the production of cytokines using the LincoPlex 24 Rat Cytokine/Chemokine Luminex Bead Immunoassay Kit. IL-2 and IFNa values were divided by 20 and 100 respectively to fit to scale. IL-6, IL-18, and MCP-1 were not significantly reduced by the presence of the A35 gene in WR, all other differences between WR and A35Δ were p < 0.05.
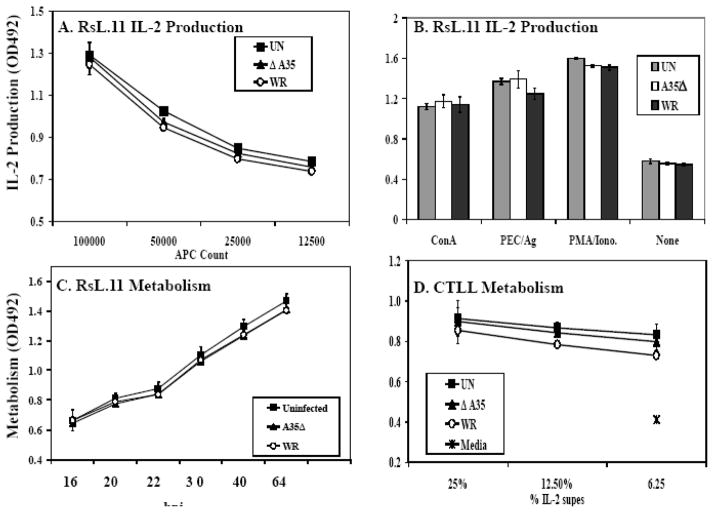
A35 does not affect the RsL.11 or CTLL T cells directly
In the antigen presentation experiments described above, PEC were pre-infected prior to addition of antigen or T cells. However, it remained possible that the T cells also became infected during the co-incubation, as some T cells can be infected by VV (Chahroudi et al., 2005; Sanchez-Puig et al., 2004). To determine if VV and A35 had any direct effects on the responding T cells, we performed a similar assay but with infected T cells and uninfected PEC. We determined if VV and A35 affected the ability of the RsL.11 T cell to produce IL-2. RsL.11 T cells were incubated with either WR or A35Δ for 3 h, washed, and then incubated with a titration of uninfected antigen-pulsed PEC. Supernatants were harvested 24–48 hpi, similar to experiments in fig 1 and 2, and tested for IL-2. Infection with either virus did not significantly affect the response of the T cell compared to uninfected T cells (fig 3A). We also found that VV did not affect RsL.11 responses to mitogen (ConA) or chemical stimulation (PMA/ionomycin) (fig 3B).
Figure 3. A35 does not directly affect T lymphocytes.
(A) RsL.11 T cells were infected with WR or A35Δ virus (MOI= 4) for 4 h and then incubated with decreasing numbers of Ag pulsed PEC. After 24–48 h, supernatants (50 ul) were collected and assayed for IL-2 production using the CTLL IL-2 bioassay. (B) RsL.11 T lymphocytes were infected for 3–4 h and then stimulated with PEC/50 nM GPMBP, 25 ug/ml Con A, or 100 nM PMA/2 uM ionomycin. Supernatants (50 ul) were collected at 20- and 40-h and assayed for IL-2. (C) RsL.11 T cells were infected with VV for 10 h, and growth metabolism was measured by reduction of 10 ul MTS/PMS (D) CTLL cells were infected with VV (MOI=5) for 4 h and then placed in media with varying amounts of an IL-2-containing supernatant. Proliferation was measured by adding 10 ul MTS/PMS.
We next wanted to assess whether RsL.11 T cells were permissive to VV infection. We incubated VV with the known permissive cell line, BS-C-1, and RsL.11 T cells. Levels of infectious virus increased 2 logs in the BS-C-1 culture but did not increase in the RsL.11 T cell culture (data not shown), and infection had no significant effect on cellular metabolism up to 64 hpi (fig 3C). Together these data indicate that the RsL.11 T cells are refractory to VV infection.
To assess viral affects on the CTLL cells used in the IL-2 bioassay, CTLL were incubated with WR or A35Δ for 5 hours and then placed in IL-2-containing media to stimulate their proliferation. IL-2 increased the response of the uninfected CTLL compared to media containing no IL-2, and VV did not significantly alter this response up to 48 hpi (fig 3D). These data suggest that VV (with or without the A35 gene) has little effect on either the RsL.11 or CTLL under these assay conditions and that A35 exerts its influence directly on the APC instead. In order to further test this hypothesis, we performed an experiment using a paraformaldehyde fixed infected human B cell line to present antigen in triplicate as previously described (Li et al., 2005). WR infected (MOI=10) fixed B cells presented 30% as well as uninfected fixed B cells, and significantly less than the A35Δ infected fixed B cells, which presented 68% of the levels of uninfected fixed B cells. These data mirror what we have seen in the rodent system using unfixed antigen presenting cells where A35 potentiates VV inhibition of antigen presentation. Together these data indicate that the APC are directly affected by A35 prior to interaction with T cells. Thus we further explored the effects of A35 on APC.
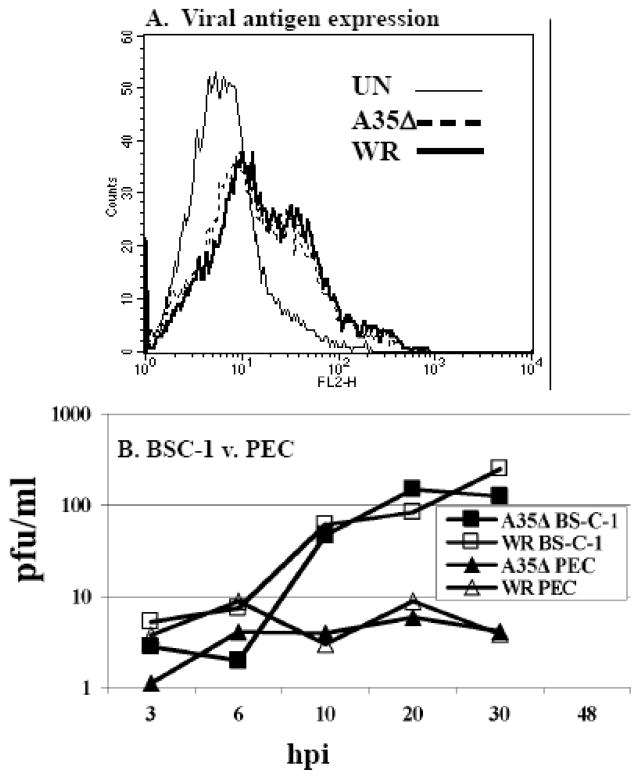
A35Δ is as infectious as WR virus
We had previously shown that A35Δ mutant virus particles were equally infectious on a per particle basis as wild type WR parental virus (Roper, 2006) in BS-C-1 cells, however it was possible that the A35Δ virus infected immune cells (PEC) less well than the wild type, thus allowing WR to be more inhibitory. In order to assess viral infectivity in these cells, PEC were incubated with WR and A35Δ for 10 h, and viral antigens were measured by FACS analysis using a human polyclonal anti-VV hyperimmune serum. In 3 experiments, the A35Δ showed as much viral antigen staining as wild type virus (fig 4A), indicating that the A35Δ is as infectious as wild type virus in these cells and proceeds normally through viral protein expression up to 10 hpi. Uninfected cells stained with anti-VV hyperimmune serum and VV infected cells stained with secondary antibody alone gave comparable negative results.
Figure 4. A35Δ mutant virus is as infectious as WR and replicates equally.
A) PEC were incubated with WR and A35Δ at an MOI of 10 for 10 h, fixed, permeabilized, and incubated with human polyclonal anti-VV hyperimmune sera. Viral antigens were measured by flow cytometry. B) BS-C-1 cells or PEC were infected with either WR or A35Δ mutant virus (MOI=10). Cells were collected at designated times post-infection and cell-associated virus was measured.
A35Δ replicates equal to WR in PECs
We also assessed the replication of both viruses in PEC using a one-step growth curve. We infected PEC and BS-C-1, a known permissive cell line, with WR or A35Δ, and harvested supernatants and cells for titers at various time points. WR and A35Δ were able to replicate similarly in BS-C-1 cells increasing approximately 2 logs by 30 hpi (fig 4A), while neither virus replicated well in PEC. We also measured virus released into supernatants (not shown), and showed that WR and A35Δ had very similar supernatant titers. These data indicate that A35 does not alter viral replication in PEC.
A35 does not induce apoptosis of PEC
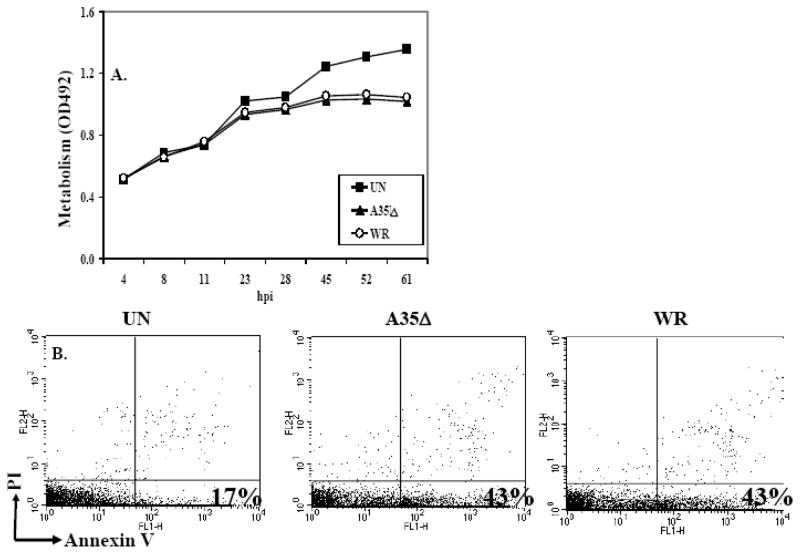
One possible explanation for the A35 mediated reduction in antigen presentation was that A35 might reduce the viability of the APC since VV is known to induce apoptosis in many APC types (Broder et al., 1994; Engelmayer et al.; Humlova et al., 2002; Kastenmuller et al., 2006). To determine this, PEC were infected with WR or A35Δ, and metabolism/viability was measured. While virus infection reduced metabolism/survival of both cell types by 24 hpi, there was no difference in metabolism between the WR- and A35Δ-infected groups, suggesting that A35 does not promote cell death (fig 5A). Similar experiments were carried out in the murine RAW 264.7 macrophage line, and similar results were obtained: VV decreased metabolism of RAW, but A35 had no effect. Apoptosis induction was also measured in PEC using annexin V and propidium iodide staining at 4, 6 and 28 hpi. VV infection induced apoptosis as previously reported, from 17% to 43% in fig 5B, but A35 had no effect at any time point measured, indicating that the A35-induced reduction in antigen presentation was not likely due to the increased induction of cell death.
Figure 5. A35 and apoptosis.
PEC (A) or RAW 264.7 macrophages (B) were infected with A35Δ or VV WR (MOI=2) and metabolism was measured by the reduction of 10 ul MTS/PMS. PEC apoptosis (C) was measured using annexin V and propidium iodide and flow cytometry following a 4 h infection.
A35 does not affect antigen-independent NO production
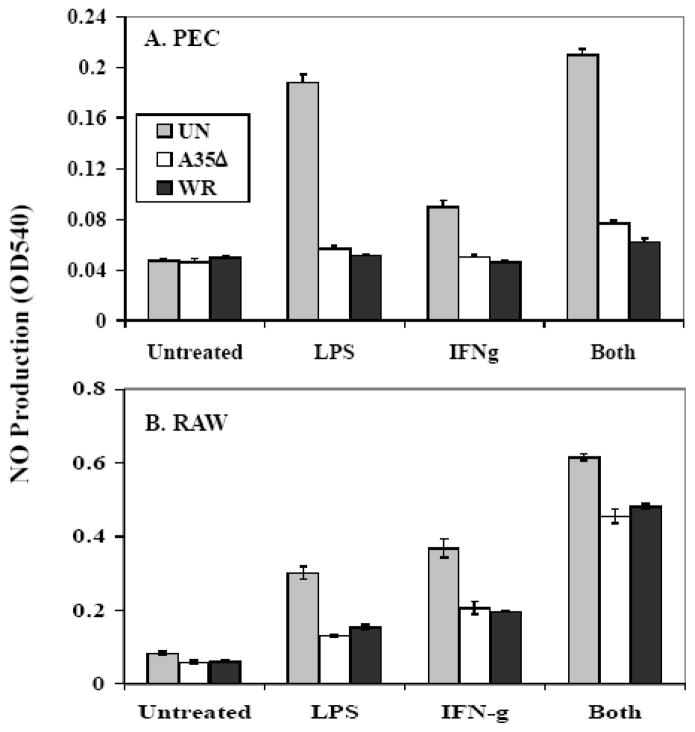
Since A35 acts on APC, and A35 caused an inhibition of NO production by PEC in antigen presentation assays with T lymphocytes (fig 1), we tested the effects of A35 on NO production in response to purified LPS and IFNγ in PEC and the murine RAW 264.7 macrophage cell line. We infected cells for 4 h with either WR or A35Δ, stimulated the cells with IFNγ, LPS, or both, and then measured NO production in supernatants 24 hpi. NO was produced by uninfected PEC and RAW in response to each stimulus (fig 6), and VV infection significantly reduced the amount of NO produced as previously reported (Bellows, Garry, and Jaffe, 2003), however the presence of A35 had no effect on this outcome. We performed this experiment with a higher MOI of 5 and a lower MOI of 1 to assess whether an A35 effect might be seen at lower virus infectious dose, but no effect could be detected. Thus, A35 does not always decrease macrophage NO responses, but inhibits NO production specifically as the result of antigen presentation interactions with T cells (fig 1). These data suggested that A35 specifically blocks the interaction between the PEC and the RsL.11.
Figure 6. A35 does not block antigen-independent NO production.
PEC (A) or RAW 264.7 macrophages (B) were infected at an MOI of 1 for 4 h, and were stimulated with 2 ul LPS, 2 ul, IFN-γ, or a combination. Supernatants (50 ul) were collected at 24–48 h and then assayed for NO production.
A35 and MHC class II and B7.2 expression on APC
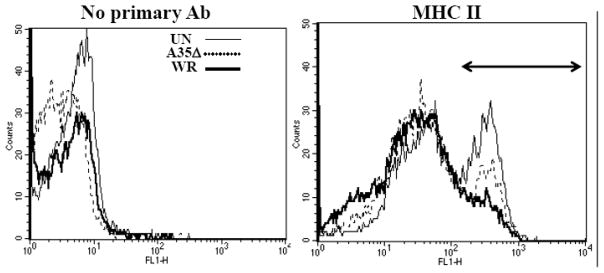
Since data suggested that A35 might block interactions between APC and T cells, we measured the effect of A35 on surface expression of B7.2/CD86 and MHC class II, proteins important in antigen presentation. Several labs have now shown that VV can inhibit MHC class II and CD86 expression on APC in vitro and in vivo under various conditions (Engelmayer et al.; Jenne et al., 2000; Kastenmuller et al., 2006; Li et al., 2005; Yao et al., 2007). PEC were infected for 4 h at an MOI of 10 and analyzed by flow cytometry. Uninfected PECs were 43% bright positive for MHC class II, A35Δ-infected PECs were 39% and WR-infected PEC were 29% (data not shown), indicating that the A35 protein plays a role in inhibiting the highest expression of MHC II on the surface of PECs. We also looked at how A35 affected the MHC II expression on the 1153 murine B cell line. Following a similar approach to that described for the PEC, uninfected 1153 B cells were 38% bright positive (arrow shown) for MHC II while WR-infected 1153 B cells were 15% (fig 7), and A35Δ-infected cells were 23%, indicating that the presence of A35 in WR mildly reduced MHC II surface expression. In the same experiments, there was no difference in CD45 (leukocyte common antigen) expression between the groups, indicating that VV and the presence of the A35 gene mildly but specifically decrease MHC class II on the surface of APC. CD28 stimulation through B7 is required for protection from poxvirus infection (Fang and Sigal, 2006), and B7.2 expression was also measured. PEC and the mouse 1153 B cell line, were infected at an MOI of 5 for 4 hours and incubated with primary antibodies that recognize B7.2/CD86 costimulatory molecules. Surface expression of B7.2 costimulatory molecule was decreased from 16% in uninfected and A35Δ infected cells to 10% in WR infected cells, but infection did not decrease surface expression of CD45. These data suggest that A35 may modestly downregulate surface proteins required for antigen presentation.
Figure 7. A35 decreases MHC class II expression.
1153 B cells were infected with WR or A35Δ virus for 5 h, washed and incubated with no primary antibody, anti-class II MHC, or anti CD45 and then FITC-conjugated secondary and analyzed by flow cytometry. Mean fluorescence intensities (MFI) for MHC class II were: Un 154, A35Δ 111, and WR 77.
A35 decreases the amount of peptide presented in MHC II
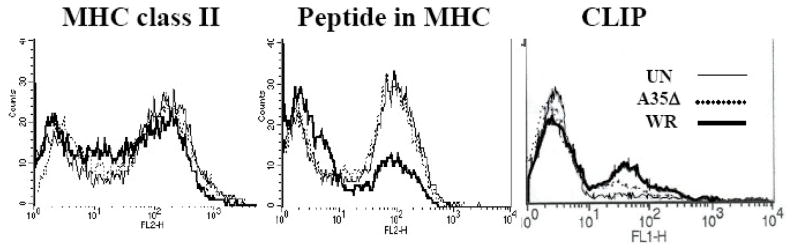
One level of immune regulation, both in homeostasis and infection, lies in controlling the association of peptides with the MHC molecules that present them (Inaba et al., 2000). We next sought to determine whether A35 affected the association of peptides with MHC II on the cell surface. To do this, we used the Y-Ae antibody, which specifically detects peptide 52–68 from I-Ed MHC class II bound in the cleft of mouse MHC class II I-Ab (Itano et al., 2003; Rudensky et al., 1991). MHC class II molecules frequently present self peptides from surface proteins that recirculate through endosomes and are degraded, similar to exogenous antigens that are internalized. The Y-Ae antibody recognizes a major determinant (approximately 12% of MHC molecules) in mice expressing both of these class II molecules (Rudensky et al., 1991; Stimpfling and Richardson, 1965). We measured the amount of peptide bound in the cleft of MHC class II on the surface of uninfected, WR and A35Δ infected spleen cells. As figure 8 shows, uninfected and A35Δ infected cells were 63% positive for class II MHC molecules complexed with this I-E peptide, while infection with WR reduced this to 30% 3 hpi, with only a slight decrease in MHC class II expression. These data indicate that A35 interferes specifically with the loading of this model peptide into the cleft of MHC class II molecules or expression of such molecules on the cell surface. As surface expression of antigenic peptide was decreased by A35 in this model system, we tested whether MHC class II bound to the remnant chaperone invariant chain peptide CLIP was transported to the cell surface. CLIP peptide was detectable on approximately 14% of uninfected cells, 24% of A35Δ infected cells and 33% of WR infected cells (fig 8) suggesting that A35 enhances the binding and/or transport of CLIP peptides and thus may interfere with presentation of a wide variety of naturally occurring MHC class II presented antigenic peptides.
Figure 8. A35 decreases the amount of antigenic peptide associated with MHC class II.
Splenocytes from B10.A-H2^i5 H2-T18^a/(5R)SgSnJ mice (Jackson Labs) were infected for 3 h at an MOI=10, washed, and then incubated with no primary antibody or a biotin-conjugated anti-mouse Ea56–68 peptide bound in the cleft of I-Ab Y-Ae followed by streptavidin-RPE. Samples were analyzed by flow cytometry, and MFI for Y-Ae were: Un 85, A35Δ 76, and WR 36. MFI for CLIP were Un 5.7, A35Δ 8.2, and WR 13.9.
To determine whether A35 affects VV stimulated immune responses to VV antigenic peptides during natural infection, we infected groups of 5 mice with 1000 pfu VV i.n. as previously described (Roper, 2006) and enumerated VV specific IFN γ secreting spleen cells on day 8 post infection. WR infected mice produced significantly fewer IFNγ secreting cells compared to A35Δ infected mice and produced significantly lower levels of VV specific antibody, suggesting that A35 also acts in vivo to suppress immune responses to VV (Rehm et al., 2009a). PBS-vaccinated mice and vaccinated mouse cells without virus stimulation were used as negative controls and produced no signal.
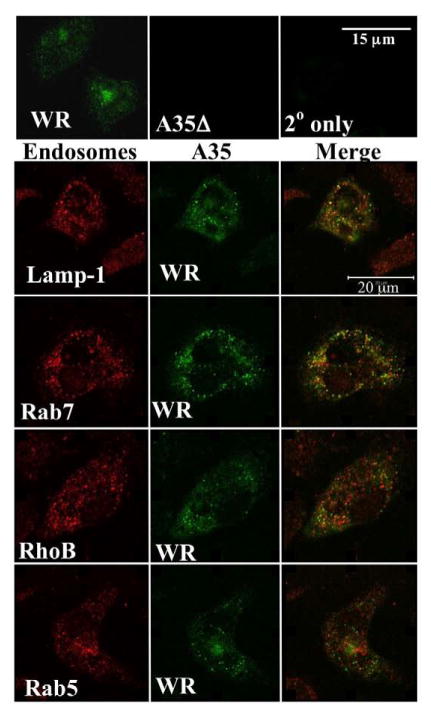
A35 localizes to the endosomes
Since MHC and B7 proteins recycle in endosomes, and MHC class II is loaded with peptides in the endosomes, we determined the intracellular localization of A35 in antigen presenting cells. We had published previously that A35 localizes intracellularly in viral factories in BS-C-1 fibroblast cells, in which there is no A35 phenotype (Roper, 2006). Since our data suggest that A35 functions in APC early after infection, we determined the localization of A35 in PECs at a time point when an A35 phenotype is seen in antigen presentation. PEC were infected with either WR or A35Δ, and A35 was visualized using fluorescent microscopy. The presence of A35 in the WR-infected PEC could be seen as diffuse punctuate staining throughout the cell (fig 9), which is consistent with the staining of endosomal structures, where many aspects of MHC II antigen processing and presentation occur. In order to verify endosomal localization, we co-localized the A35 protein with known endosomal markers, Rab 5 (plasma membrane and early endosomes), Rho B (multivesicular bodies), Rab 7 (late endosomes)), and Lamp-1 (late endosomes and lysosomes) (Chavrier et al., 1990; Feng, Press, and Wandinger-Ness, 1995; Lippincott-Schwartz and Fambrough, 1987) (Robertson et al., 1995). A35 showed partial co-localization with these endosomal markers, although least with Rab5 (fig 9). In each case there were endosomal structures that had both A35 and the marker (yellow colocalization), but also punctate staining for each antibody alone (green or red). Thus, A35 localizes generally throughout the range of endosomes in the cell, but is not found in all endosomes and does not partition to any distinct endosomal type. The presence of A35 in these structures suggests that A35 protein may interact with or affect proteins in endosomes including class II MHC, although further research is required to elucidate with which proteins A35 interacts specifically.
Figure 9. A35 localization in PEC.
PEC were grown on coverslips for 4 h and then infected with WR or A35Δ virus at an MOI=10 for 2 hours. The cells were then fixed and permeabilized, and stained with a rabbit anti-A35 antibody and one of the following: goat anti-LAMP, goat anti-Rab7, goat anti-RhoB, goat anti-Rab7. The A35 protein was visualized with a FITC-conjugated secondary antibody, and the endosomal markers were visualized with Alexa Fluor 633.
DISCUSSION
We showed previously that the VV A35 gene is an important virulence factor, increasing virulence by 100–1000 fold, but its function was unknown. We have shown here that the VV A35 gene product is not required for replication in a variety of cell types and tissues in 6 different mammals. Furthermore, A35 does not control early replication in mouse tissues on days 1–3 post infection before specific immunity develops. However, we have shown that A35 affects the development of adaptive immune responses in vitro, specifically acting on APC. A35 contributes to VV-induced reduction in MHC II antigen presentation (fig 1) and subsequent cytokine synthesis (fig 2) using primary macrophages as APC, even though the mutant virus was able to infect the APC as efficiently as wild type (fig 4). A35 did not affect metabolism, permissiveness, apoptosis of PEC nor NO production induced by immunostimulators in rat PEC or the murine RAW macrophage cell line. These data suggested that A35 did not have a major systemic effect on macrophage, but rather that it specifically interfered with the interaction between APC and T cells since those assays specifically showed an A35-dependent phenotype.
We subsequently found that A35 mildly affected the expression of MHC class II on the surface of the APC (fig 8). While several labs have now shown that VV can inhibit MHC class II and CD86 expression on APC in vitro and in vivo under various conditions, there has been much debate about the circumstances under which this regulation occurs and its significance (Li et al., 2005). Some laboratories have associated the reduction in surface molecules with induction of apoptosis (Kastenmuller et al., 2006), while others have concluded that VV does not block basal B7.2 or class II MHC expression but that it blocks activation-induced B7.2 or class II MHC expression (Engelmayer et al., 1999, Jenne et al., 2000). We have found that early after infection, VV and the A35 gene can mildly decrease expression of these surface markers both in rat PEC and in a murine B cell line. Whether a small decrease in MHC class II levels will have physiologic significance is unknown, however it is known that MHC class II antigen presentation is crucial to protection from poxvirus infection. In studies with knockout mice, it was found that the absence of MHC class I molecules or CD8 T cell response did not diminish protection but that decreases in CD4 or MHC class II expression caused a loss of protective immunity (Wyatt et al., 2004; Xu et al., 2004).
VV genes B1R and H5R have been shown to inhibit Cd1d mediated non-classical lipid antigen presentation, although the importance of this function was difficult to ascertain because of the pleiotropic effects of these genes (Webb et al., 2006). Several laboratories have shown that VV also blocks MHC class II antigen presentation in a number of assays using different APC, model antigens (exogenous, virus encoded and cellular endogenous), and response measures (Rehm et al., 2009). VV-infected primary Langerhans cells were deficient in presenting KLH peptide to a cognate T cell line, as measured by production of IFNγ (Deng et al., 2006). Primary rodent bone marrow derived macrophages infected with VV were deficient in presenting lysozyme peptide to T cells, as measured by stimulation of IL-2 production (Mann et al., 2008). Li et al found that VV inhibited MHC class II antigen presentation in in vitro infected human and rodent APC, including B cell lines, dendritic cells, macrophage and fibroblasts presenting several antigens (Li et al., 2005). The accumulation of data indicates that VV generally inhibits MHC class II antigen presentation; however, no viral gene had previously been identified as blocking antigen presentation. We have shown here that A35 interferes with antigen presentation responses to an exogenous model antigen (Fig 1, 2), as well as surface expression of an endogenous peptide in MHC class II (fig 8). We have also found that A35 increases surface CLIP expression in MHC class II suggesting that A35 may inhibit presentation of a range of antigenic peptides. Further, we have found that A35 diminishes anti VV T cell responses and antibody in mice (Rehm et al., 2009a) suggesting that A35 may similarly inhibit presentation of virally expressed VV antigens during infection in vivo.
Our data suggest that VV A35 contributes to viral inhibition of MHC class II antigen presentation by decreasing the expression of antigenic peptides in the cleft of MHC class II on the surface of infected cells. We showed this in the murine system with an MHC derived peptide as the antigen. Dr. Blum’s lab showed that VV decreases the amount of GAD peptide in the cleft of MHC class II in a human B cell line (Li et al., 2005). Together these data suggest that VV inhibition of peptide loading into the cleft is a general mechanism of immune response inhibition independent of the antigen or APC type. The fact that A35 is very highly conserved in mammalian tropic poxviruses suggests that A35 may act similarly in a number of different viruses and host species. Interestingly, fig 8 shows that there is a population of cells in spleens that is unaffected by the presence of VV and A35 gene (MOI of 10). Since splenocytes contain numerous cell types, these data suggest that there is a VV resistant population in the spleen, consistent with other studies showing differing sensitivities to infection among immune cells (Chahroudi et al., 2005; Sanchez-Puig et al., 2004). It will also be important to determine the effects of A35 on lymphocytes in the target organs, especially the lungs.
In these experiments, we showed that A35 inhibited antigen presentation, suggesting that the A35Δ mutant viruses will provide improved vaccines, as well as improved platform vaccines for other infectious diseases and cancer treatment. The A35 gene is conserved in all VV vaccine strains including MVA. While A35 inhibited antigen presentation, it was also clear that the A35 deletion mutant virus blocked antigen presentation when compared to uninfected APC (fig 1, 2). This suggests that there are multiple poxvirus proteins that function independently to decrease antigen presentation. It is important to identify these immunosuppressive genes and test the cognate knockout viruses as improved poxvirus (or poxvirus platform) vaccines.
While we showed that both T cell and PEC cytokine production was reduced subsequent to antigen presentation interactions, our data indicated that it was the APC that were directly affected by VV, and the T cells were indirectly affected via regulation of the APC. A35 had the effect of suppressing the production of numerous cytokines and chemokines, which are crucial in the development of an appropriate immune response. Notably, many of the cytokines that are regulated by VV and A35 are chemotactic factors (MIP-1α, IL-1, TNF-α, GRO/KC, RANTES, and MCP-1), which aid in the migration of immune response cells to sites of inflammation and infection. Interestingly, IL-18 was not significantly reduced by VV and A35 (fig 2). Perhaps this is because IL-18 is stored intracellularly as pro-IL-18 and rapidly released upon stimulation (Gardella et al., 2000). Since IL-18 is an important factor in the induction of anti-viral IFNγ production, its storage for rapid release may be an evolved host mechanism to contravene immunomodulation by viral gene products such as A35. The importance of IL-18 in antiviral defense is underscored by the possession of an IL-18 binding protein encoded by poxviruses (Reading and Smith, 2003).
There are multiple mechanisms by which A35 could decrease MHC II peptide presentation that must be further explored. Future experiments will address with which cellular proteins A35 interacts. Since A35 may affect peptide loading, it is possible that it interacts with invariant chain that chaperones MHC class II proteins, directly with MHC class II itself, or DM molecules that are involved in the exchange of antigenic peptides. Furthermore, we will test various immune responses to the A35Δ mutant virus in comparison to wild type virus in transgenic mouse models to understand which pathways are inhibited by A35. Our hypothesis is that A35 acts through blocking MHC class II antigen presentation, and we predict that anti-viral CD4 responses will be diminished, and that this decrease in T helper cells will diminish development of most downstream anti-viral effector cells (including CD8 cytotoxic T cells, and antibody secreting B cells). Thus far our animal model data are consistent with this hypothesis, showing a reduction in both VV specific antibody and splenic T lymphocyte responses (Rehm et al., 2009a).
Acknowledgments
We wish to acknowledge the financial support of the North Carolina Biotechnology Center and by NIH grant #U54 AI057157 from Southeastern Regional Center of Excellence for Emerging Infections and Biodefense. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCBC. We wish to thank Dr. Mark Mannie for reagents and helpful discussion on the rat antigen presentation system, and Dr. Janice Blum for reagents, the 1153 B cell line, and help with the Priess B cell antigen presentation experiment.
Abbreviations in the paper
- VV
vaccinia virus
- PEC
peritoneal exudate cells
- MOI
multiplicity of infection
- GPMBP
guinea pig myelin basic protein
- hpi
hours post infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, Collins LC, Ludwig SL, Riddle JR, Grabenstein JD, Tornberg DN. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160(7):642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- Bellows CF, Garry RF, Jaffe BM. Vaccinia virus-induced inhibition of nitric oxide production. J Surg Res. 2003;111(1):127–35. doi: 10.1016/s0022-4804(03)00079-9. [DOI] [PubMed] [Google Scholar]
- Broder CC, Kennedy PE, Michaels F, Berger EA. Expression of foreign genes in cultured human primary macrophages using recombinant vaccinia virus vectors. Gene. 1994;142:167–174. doi: 10.1016/0378-1119(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Campos-Neto A, Ovendale P, Bement T, Koppi TA, Fanslow WC, Rossi MA, Alderson MR. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160(5):2037–41. [PubMed] [Google Scholar]
- Casey CG, Iskander JK, Roper MH, Mast EE, Wen XJ, Torok TJ, Chapman LE, Swerdlow DL, Morgan J, Heffelfinger JD, Vitek C, Reef SE, Hasbrouck LM, Damon I, Neff L, Vellozzi C, McCauley M, Strikas RA, Mootrey G. Adverse events associated with smallpox vaccination in the United States, January–October 2003. Jama. 2005;294(21):2734–43. doi: 10.1001/jama.294.21.2734. [DOI] [PubMed] [Google Scholar]
- Chahroudi A, Chavan R, Koyzr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79(16):10397–407. doi: 10.1128/JVI.79.16.10397-10407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Parton RG, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62(2):317–29. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chen N, Li G, Liszewski MK, Atkinson JP, Jahrling PB, Feng Z, Schriewer J, Buck C, Wang C, Lefkowitz EJ, Esposito JJ, Harms T, Damon IK, Roper RL, Upton C, Buller RM. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaso CR, Esposito JJ, Condit RC, Moussatche N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology. 2000;277(2):439–49. doi: 10.1006/viro.2000.0603. [DOI] [PubMed] [Google Scholar]
- Deng L, Dai P, Ding W, Granstein RD, Shuman S. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J Virol. 2006;80(20):9977–87. doi: 10.1128/JVI.00354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar AD, Werchniak AE, Li Y, Brennick JB, Goldsmith CS, Kline R, Damon I, Klaus SN. Tanapox infection in a college student. N Engl J Med. 2004;350(4):361–6. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, Andersen RF, Bejon P, Goonetilleke N, Poulton I, Webster DP, Butcher G, Watkins K, Sinden RE, Levine GL, Richie TL, Schneider J, Kaslow D, Gilbert SC, Carucci DJ, Hill AV. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006;74(10):5933–42. doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, Boyd SY, Murphy JG, Swerdlow DL, Collins LC, Riddle JR, Tornberg DN, Grabenstein JD, Engler RJ. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44(1):201–5. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Engelmayer J, Larsson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J Immunol. 1999;163(12):6762–8. [PubMed] [Google Scholar]
- Fang M, Sigal LJ. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J Immunol. 2006;177(11):8027–36. doi: 10.4049/jimmunol.177.11.8027. [DOI] [PubMed] [Google Scholar]
- Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131(6 Pt 1):1435–52. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Poggi A, Zocchi MR, Rubartelli A. Control of interleukin-18 secretion by dendritic cells: role of calcium influxes. FEBS Lett. 2000;481(3):245–8. doi: 10.1016/s0014-5793(00)02015-9. [DOI] [PubMed] [Google Scholar]
- Humlova Z, Vokurka M, Esteban M, Melkova Z. Vaccinia virus induces apoptosis of infected macrophages. J Gen Virol. 2002;83(Pt 11):2821–32. doi: 10.1099/0022-1317-83-11-2821. [DOI] [PubMed] [Google Scholar]
- Inaba K, Turley S, Iyoda T, Yamaide F, Shimoyama S, Reis e Sousa C, Germain RN, Mellman I, Steinman RM. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191(6):927–36. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, Jenkins MK. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19(1):47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- Jenne L, Hauser C, Arrighi JF, Saurat JH, Hugin AW. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 2000;7(18):1575–83. doi: 10.1038/sj.gt.3301287. [DOI] [PubMed] [Google Scholar]
- Karupiah G, Xie QW, Buller RML, Nathan C, Duarte C, Macmicking JD. Inhibition of viral replication by interferon-gamma induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kastenmuller W, Drexler I, Ludwig H, Erfle V, Peschel C, Bernhard H, Sutter G. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology. 2006;350(2):276–88. doi: 10.1016/j.virol.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Kolhapure RM, Deolankar RP, Tupe CD, Raut CG, Basu A, Dama BM, Pawar SD, Joshi MV, Padbidri VS, Goverdhan MK, Banerjee K. Investigation of buffalopox outbreaks in Maharashtra State during 1992–1996. Indian J Med Res. 1997;106:441–6. [PubMed] [Google Scholar]
- Lee MS, Roos M, McGuigan LC, Smith KA, Cormier N, Cohen LK, Roberts BE, Payne LG. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol. 1992;66:2617–2630. doi: 10.1128/jvi.66.5.2617-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman E, Miramontes R, Openshaw J, Olson VA, Karem KL, Marcinak J, Panares R, Staggs W, Allen D, Weber SG, Vora S, Gerber SI, Hughes CM, Regnery R, Collins L, Diaz PS, Reynolds MG, Damon I. Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: An investigation of potential fomites in the home environment. Vaccine. 2009;27(3):375–7. doi: 10.1016/j.vaccine.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Li P, Wang N, Zhou D, Yee CS, Chang CH, Brutkiewicz RR, Blum JS. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J Immunol. 2005;175(10):6481–8. doi: 10.4049/jimmunol.175.10.6481. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987;49(5):669–77. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 2004;25(12):636–9. doi: 10.1016/j.it.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mann BA, Huang JH, Li P, Chang HC, Slee RB, O’Sullivan A, Anita M, Yeh N, Klemsz MJ, Brutkiewicz RR, Blum JS, Kaplan MH. Vaccinia virus blocks Stat1-dependent and Stat1-independent gene expression induced by type I and type II interferons. J Interferon Cytokine Res. 2008;28(6):367–80. doi: 10.1089/jir.2007.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannie MD, Norris MS. MHC class-II-restricted antigen presentation by myelin basic protein-specific CD4+ T cells causes prolonged desensitization and outgrowth of CD4- responders. Cell Immunol. 2001;212(1):51–62. doi: 10.1006/cimm.2001.1843. [DOI] [PubMed] [Google Scholar]
- Martina BE, van Doornum G, Dorrestein GM, Niesters HG, Stittelaar KJ, Wolters MA, van Bolhuis HG, Osterhaus AD. Cowpox virus transmission from rats to monkeys, the Netherlands. Emerg Infect Dis. 2006;12(6):1005–7. doi: 10.3201/eid1206.051513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz-Rensing K, Ellerbrok H, Ehlers B, Pauli G, Floto A, Alex M, Czerny CP, Kaup FJ. Fatal poxvirus outbreak in a colony of New World monkeys. Vet Pathol. 2006;43(2):212–8. doi: 10.1354/vp.43-2-212. [DOI] [PubMed] [Google Scholar]
- Molino AC, Fleischer AB, Jr, Feldman SR. Patient demographics and utilization of health care services for molluscum contagiosum. Pediatr Dermatol. 2004;21(6):628–32. doi: 10.1111/j.0736-8046.2004.21602.x. [DOI] [PubMed] [Google Scholar]
- Moss B. Poxviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2849–2884. [Google Scholar]
- Reading PC, Smith GL. Vaccinia virus interleukin-18-binding protein promotes virulence by reducing gamma interferon production and natural killer and T-cell activity. J Virol. 2003;77(18):9960–8. doi: 10.1128/JVI.77.18.9960-9968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm KE, Connor RF, Jones GJB, Yimbu K, Mannie MD, Roper RL. Vaccinia Virus Decreases MHC Class II Antigen Presentation, T cell Priming, and Peptide Association with MHC Class II. Immunol. 2009;128:381–92. doi: 10.1111/j.1365-2567.2009.03120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm KE, Jones GJB, Tripp AA, Metcalf MW, Roper RL. The Poxvirus A35 Protein is an Immunoregulator. J Virol. 2009a doi: 10.1128/JVI.01802-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Paterson HF, Adamson P, Hall A, Monaghan P. Ultrastructural localization of ras-related proteins using epitope-tagged plasmids. J Histochem Cytochem. 1995;43(5):471–80. doi: 10.1177/43.5.7537292. [DOI] [PubMed] [Google Scholar]
- Roper RL. Characterization of the Vaccinia Virus A35R Protein and its Role in Virulence. J Virol. 2006;80(1):306–313. doi: 10.1128/JVI.80.1.306-313.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky A, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991;353(6345):660–2. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- Sanchez-Puig JM, Sanchez L, Roy G, Blasco R. Susceptibility of different leukocyte cell types to Vaccinia virus infection. Virol J. 2004;1(1):10. doi: 10.1186/1743-422X-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell J, Rose NF, Fazo N, Marx PA, Hunter M, Ramsburg E, Montefiori D, Earl P, Moss B, Rose JK. Long-term vaccine protection from AIDS and clearance of viral DNA following SHIV89.6P challenge. Vaccine. 2009;27(7):979–86. doi: 10.1016/j.vaccine.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Sidwell RW. Anti-cowpox virus activities of certain adenosine analogs, arabinofuranosyl nucleosides, and 2′-fluoro-arabinofuranosyl nucleosides. Nucleosides Nucleotides Nucleic Acids. 2004;23(1–2):375–83. doi: 10.1081/ncn-120028334. [DOI] [PubMed] [Google Scholar]
- Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201(6):1007–18. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinborn A, Essbauer S, Marsch W. Human cowpox/catpox infection. A potentially unrecognized disease. Dtsch Med Wochenschr. 2003;128(12):607–10. doi: 10.1055/s-2003-38051. [DOI] [PubMed] [Google Scholar]
- Stich A, Meyer H, Kohler B, Fleischer K. Tanapox: first report in a European traveller and identification by PCR. Trans R Soc Trop Med Hyg. 2002;96(2):178–9. doi: 10.1016/s0035-9203(02)90295-6. [DOI] [PubMed] [Google Scholar]
- Stimpfling JH, Richardson A. Recombination within the histocompatibility-2 locus of the mouse. Genetics. 1965;51:831–846. doi: 10.1093/genetics/51.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. Heterologous priming-boosting with DNA and modified vaccinia virus Ankara expressing tryparedoxin peroxidase promotes long-term memory against Leishmania major in susceptible BALB/c Mice. Infect Immun. 2007;75(2):852–60. doi: 10.1128/IAI.01490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upfal MJ, Cinti S. Smallpox vaccination and adverse cardiac events. Emerg Infect Dis. 2004;10(5):961–2. doi: 10.3201/eid1005.030967. discussion 962. [DOI] [PubMed] [Google Scholar]
- Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. Poxvirus orthologous cluster: toward defining the minimum essential poxvirus genome. J Virol. 2003a;77:7590–600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. Poxvirus orthologous clusters: toward defining the minimum essential poxvirus genome. J Virol. 2003b;77(13):7590–600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaysri S, Jentarra G, Heck MC, Mercer AA, McInnes CJ, Jacobs BL. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: intra-nasal vaccination. Vaccine. 2008;26(5):664–76. doi: 10.1016/j.vaccine.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner KM, Girgis N, Kwak H, Isaacs SN. B5-deficient vaccinia virus as a vaccine vector for the expression of a foreign antigen in vaccinia immune animals. Virology. 2006 doi: 10.1016/j.virol.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TJ, Litavecz RA, Khan MA, Du W, Gervay-Hague J, Renukaradhya GJ, Brutkiewicz RR. Inhibition of CD1d1-mediated antigen presentation by the vaccinia virus B1R and H5R molecules. Eur J Immunol. 2006;36(10):2595–600. doi: 10.1002/eji.200636024. [DOI] [PubMed] [Google Scholar]
- Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101(13):4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Li P, Singh P, Thiele AT, Wilkes DS, Renukaradhya GJ, Brutkiewicz RR, Travers JB, Luker GD, Hong SC, Blum JS, Chang CH. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007;246(2):92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172(10):6265–71. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]