Abstract
The circumventricular organs (CVO) lack a well-formed blood-brain barrier and produce superoxide (O2•−) in response to angiotensin II and other hypertensive stimuli. This increase in central O2•− has been implicated in regulation of blood pressure. The extracellular superoxide dismutase (SOD3) is highly expressed in cells associated with CVO, and particularly with tanycytes lining this region. To understand the role of SOD3 in the CVO in blood pressure regulation, we performed intracerebroventricular (ICV) injection an adenovirus encoding Cre-recombinase (AdCre, 5×108 particles/ml) in mice with loxP sites flanking the SOD3 coding region (SOD3loxp/loxp mice). An adenovirus encoding red-fluorescent protein (AdRFP) was injected as a control. Deletion of CVO SOD3 increased baseline blood pressure modestly and markedly augmented the hypertensive response to low-dose angiotensin II (140 ng/kg/day), while ICV injection of AdRFP had minimal effects on these parameters. AdCre treated mice exhibited increased sympathetic modulation of heart rate and blood pressure variability, increased vascular superoxide production and T cell activation as characterized by increased circulating CD69+/CD3+ cells. Deletion of CVO SOD3 also markedly increased vascular T cell and leukocyte infiltration caused by angiotensin II. We conclude that SOD3 in the CVO plays a critical role in regulation of blood pressure and its loss promotes T cell activation and vascular inflammation, in part by modulating sympathetic outflow. These findings provide insight into how central signals produce vascular inflammation in response to hypertensive stimuli such as angiotensin II.
Keywords: Superoxide dismutase, circumventricular organ, hypertension, T cell
INTRODUCTION
The superoxide dismutases (SOD) are antioxidant enzymes that catalyze dismutation of superoxide anion (O2•−) into hydrogen peroxide and oxygen. There are three isozymes of SOD in mammals, including the cytoplasmic Cu/Zn-SOD (SOD1), the mitochondrial SOD (SOD2) and the extracellular SOD (ecSOD or SOD3).1 Considerable evidence supports the view that O2•− plays a critical role in the pathogenesis of hypertension. For example, reducing tissue O2•− by administration of membrane targeted forms of SOD, SOD mimetics (e.g. tempol), or deletion of the gene encoding for the NADPH oxidase subunit p47phox reduces blood pressure in several experimental models of hypertension.2–4 In addition, embryonic deletion of SOD3, which would be expected to increase extracellular O2•−, augments hypertension in response to angiotensin II or DOCA-salt challenge.5, 6 While these studies support a role for SOD3 and oxidative stress in the genesis of hypertension, they do not address potential sites or mechanisms by which deletion of SOD3 augments hypertension. Potential sites where this enzyme could modulate blood pressure include the vasculature, the kidney and the brain. In addition to oxidative stress, recent studies from our laboratory and others have also suggested an important role for peripheral T-lymphocyte activation and vascular inflammation in the development of angiotensin II-induced hypertension. The relationship between oxidative events in sites such as the central nervous system, T cell activation and vascular inflammation remain poorly understood.
The purpose of the present studies was to explore the hypothesis that defects in brain SOD3 might participate in the development of hypertension and immune cell activation evoked by angiotensin II. This was accomplished by utilizing SOD3loxp/loxp mice with loxP sites flanking the SOD3 coding region enabling targeted deletion of this gene upon exposure to Cre-recombinase. By intracerebroventricular injection of an adenovirus encoding Cre-recombinase, we were able to selectively delete SOD3 in CNS circumventricular structures and study the role of this protein in regulation of blood pressure and peripheral immune activation.
MATERIALS AND METHODS
Mice previously created in our laboratory,7 with loxP sites flanking the SOD3 coding region, were used in these studies. To delete CNS SOD3 we performed intracerebroventricular injections (ICV) of an adenovirus encoding Cre-recombinase (AdCre) and employed an adenovirus encoding red fluorescent protein (AdRFP) as a control. Measurements of blood pressure, T cell activation and vascular O2•− production were performed as previously described.8 For detailed Material and Methods, please see http://hyper.ahajournals.org.
RESULTS
CNS Expression of AdRFP and AdCre and effect on SOD3
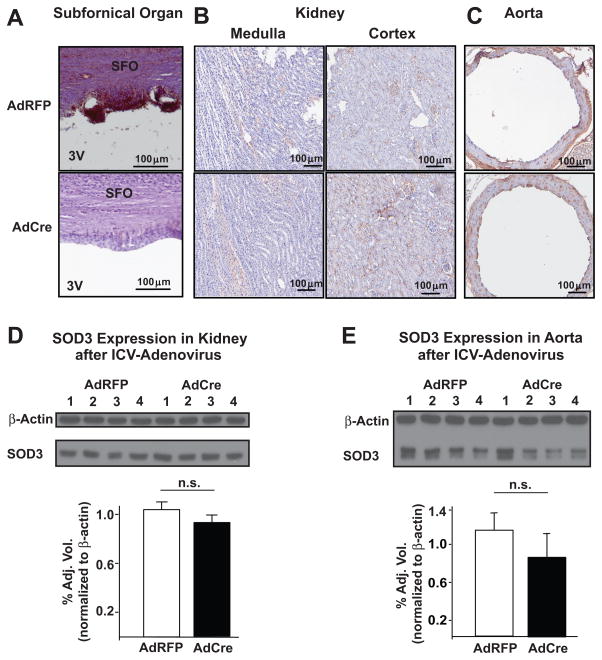
To determine the distribution of adenovirus following ICV injections, we analyzed RFP expression by fluorescence at various times after AdRFP injection. We found that RFP was expressed at the highest levels 7 days following AdRFP injection (data not shown). RFP expressing cells were typically located within approximately 50 μm of the ventricular wall (Figure S1A). RFP labeled cells were most abundant in CVO and particularly in the subfornical organ (SFO; Figure S1A). Occasional RFP-labeled cells were observed in areas adjacent to the SFO (Figure S1B and S1C), but not in circumventricular tissues far distant from the SFO (Figure S1D and S1E). ICV injection of AdCre also led to expression of Cre-recombinase in the SFO (Figure S1F). Seven days following ICV injection of AdCre, SOD3 immunostaining was no longer detectable in the SFO or in other cells lining the 3rd ventricle (Figure 1A). In contrast to the results obtained with ICV-AdCre, injection of AdRFP had no effect on SOD3 levels in this region (Figure 1A). Because of these preliminary findings, we began infusion of either angiotensin II or buffer at least 2 weeks following ICV-injection and used AdRFP as a control.
Figure 1.
Effect of ICV AdCre and AdRFP on SOD3 protein in various organs. Panels A–C show immunostaining for SOD3 (brown staining) in the subfornical organ (Panel A) the kidneys (Panel B) and the aortas (Panel C) of mice injected with either AdRFP or AdCre. Panels D and E show Western blots for SOD3 in kidney and aortas with b-actin as a loading control. Adjacent to each Western blot are mean densitometric analyses presented as a bar graph (n = 4 for each, n.s. = not significant).
To confirm that ICV injections of AdCre specifically deleted SOD3 in the CNS, we performed immunostaining and western analysis for SOD3 in kidneys and aortas of AdRFP and AdCre mice. The results showed no differences in SOD3 expression in these tissues (Figure 1B–E). In other experiments we injected an identical volume of AdCre intraperitoneally. Immunostaining and western analysis indicated that peripherally-administered AdCre had no effect on either renal or aortic levels of SOD3 (Figure S2). Moreover, Western blots revealed no expression of Cre-recombinase in aortas seven days following ICV injection of AdCre (data not shown). Taken together, these results indicated that ICV injections of AdCre specifically deleted SOD3 in the CNS, with no detectable action at peripheral sites.
Effect of CNS SOD3 deletion on blood pressure and heart rate
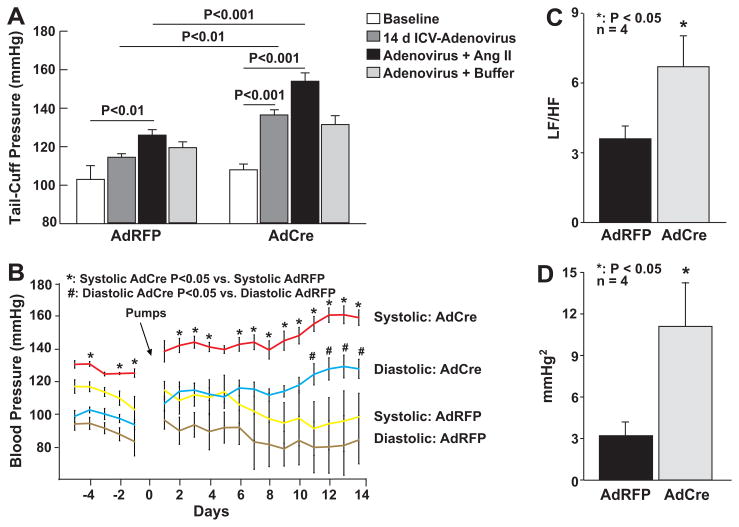
ICV injection of AdRFP in SOD3loxp/loxp mice did not affect baseline blood pressure measured 7–14 days as determined by either tail-cuff measurements or radiotelemetry monitoring in SOD3loxp/loxp mice (Figure 2A and 2B). Moreover, two-week infusion of low-dose of angiotensin II raised blood pressure minimally in these animals. In contrast to the effects of AdRFP, CNS SOD3 deletion with AdCre caused a 21 ± 2 mmHg increase in blood pressure at baseline and markedly augmented the hypertensive response to angiotensin II as measured using the tail cuff method (Figure 2A). Radiotelemetry also confirmed these effects of CNS SOD3 deletion (Figure 2B). Two-way analysis of variance indicated that the effect of angiotensin II was significantly greater in mice that received AdCre versus AdRFP (p = 0.03). Heart rate was similar in AdCre injected mice compared to AdRFP injected mice (Figure S3).
Figure 2.
Effect of SOD3 deletion in the circumventricular structure on hemodynamics. Mice received ICV injections of either AdCre or AdRFP and blood pressure was measured by tail cuff (Panel A) or radiotelemetry (Panel B). Heart rate (Panel C) and systolic blood pressure (Panel D) variability was analyzed using Hemolab software package (LF/HF = Low Frequency/High Frequency).
Effects of CNS SOD3 deletion on sympathetic modulation of heart rate and systolic pressure
Power spectral analysis identifies oscillations in heart rate and blood pressure that are modulated by inputs from the renin-angiotensin system, sympathetic and parasympathetic neurons and locally released vasoactive factors such as nitric oxide. As an example, sympathetic outflow modulates low frequency oscillations (0.015 to 0.6 Hz), while parasympathetic tone affects both low and high (0.2 to 5 Hz) oscillations of heart rate. Thus the ratio of low to high frequency heart rate variability reflects sympathetic cardiovascular modulation. This ratio was increased twofold following deletion of SOD3 in the CNS (Figure 2C), in keeping with an increase in sympathetic outflow. Likewise, absolute values of low frequency blood pressure oscillations provide indirect assessment of sympathetic outflow. These low frequency blood pressure oscillations were three-fold higher in mice following AdCre as compared to AdRFP injection (Figure 2D). Taken together, these data indicate that SOD3 in the CVO plays an important role in modulating sympathetic outflow.
Effect of CNS SOD3 deletion on reactive oxygen species
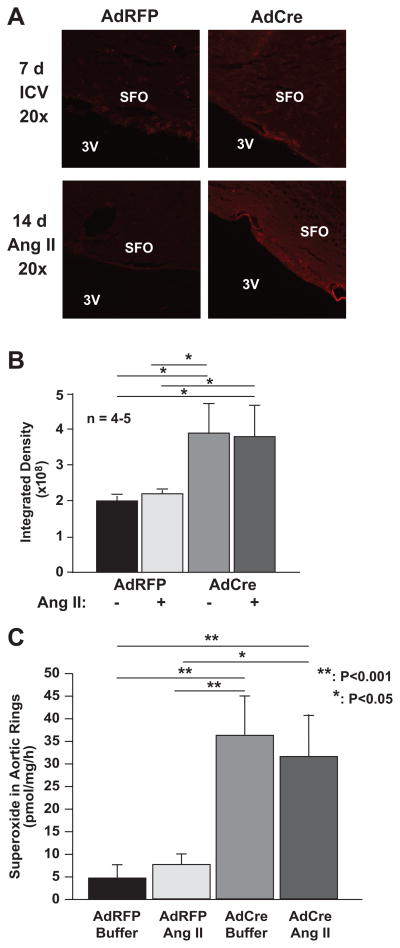
Protein nitrotyrosines are markers of endogenous production of reactive oxygen and nitrogen species.9 Protein nitrotyrosines were not detected after AdRFP injection but were prevalent in cells surrounding the subfornical organ following SOD3 deletion (Figure 3A). These were most obvious in cells immediately adjacent to the 3rd ventricle. Of note, infusion of angiotensin II did not further increase protein nitrotyrosines in the CVO (Figure 3B).
Figure 3.
Indices of oxidative stress as detected by nitrotyrosine staining and superoxide detection using electron spin resonance. Mice underwent ICV injections of either AdCre or AdRFP and subsequent infusion of angiotensin II as in figure 2. Panel A shows immunostaining for nitrotyrosine in the subfornical organ (SFO) and adjacent structures in the 3rd ventricle (3V). Panel B shows quantification of pixel intensities from experiments depicted in A (n = 4–5). Panel C shows quantification of vascular superoxide production as detected by ESR and the spin probe CAT1-H (n = 6).
Increased sympathetic outflow in response to SOD3 deletion in the CVO could alter vascular O2•− production. To examine this possibility, we examined aortic O2•− levels using ESR and the spin probe CAT-1H. As evident in figure 3C, CNS SOD3 deletion markedly increased vascular O2•− production as compared to AdRFP injected mice. Of interest, infusion of low-dose angiotensin II did not increase vascular O2•− further in either AdCre or AdRFP injected mice.
Effect of CNS SOD3 deletion on peripheral inflammatory markers
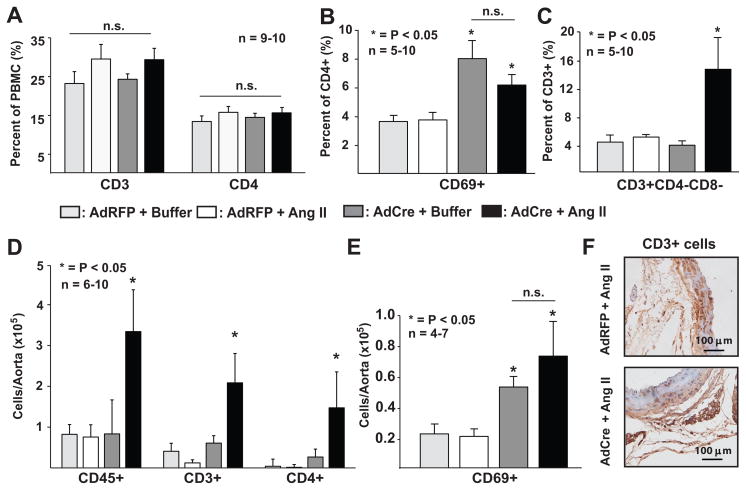
Previous studies have shown that T lymphocytes play a key role in the genesis of angiotensin II-induced hypertension.8 In these previous experiments, angiotensin II infusion increased circulating CD44high and CD69+ T cells and promoted vascular accumulation of inflammatory cells, particularly in the perivascular fat. To determine if CNS SOD3 mediates these effects, we measured markers of peripheral inflammation following deletion of CNS SOD3 with and without peripheral infusion of a low-dose of angiotensin II. Central injection of either AdRFP or AdCre had no effect on the composition of peripheral blood mononuclear cells (PBMC, Figure 4A). Likewise, ICV injection of AdRFP had no effect on the percentage of CD69+/CD4+ cells either at baseline or in response to angiotensin II (Figure 4B). In contrast, injection of AdCre increased in the percentage circulating CD69+/CD4+ cells (Figure 4B) even in the absence of angiotensin II infusion, indicating that deletion of CNS SOD3 by itself is sufficient to stimulate CD4+ cell activation. Angiotensin II caused no further increase in CD4+CD69+ cells following central SOD3 deletion. In contrast to CD69+/CD4+ cells, the percentage of CD3+CD4−CD8−/CD3+ cells was only increased when both Ang II was administered and CNS SOD3 was deleted (Figure 4C).
Figure 4.
Role of circumventricular SOD3 in T cell activation and vascular infiltration of inflammatory cells. Mice were treated as in figure 2 and 14 days following either buffer or angiotensin II infusion (140 ng/kg/min) FACS was employed for analysis of peripheral blood and vascular cells. Panel A shows the percent of total peripheral blood mononuclear cells for circulating CD3+ cells or percent of CD3+ that are CD4+. Panel B shows the percent of CD4+ cells expressing the early activation marker CD69. Panel C shows the percent of total T cells (CD3+) that are double negative for both CD4 and CD8. Panel D shows FACS analysis of single cell suspensions of aortic homogenates. Panel E shows the total number of CD4+/CD69+ cells per aorta. Panel F shows immunostaining for CD3+ cells in aortas and perivascular fat mice treated with either ICV AdRFP or angiotensin II or AdCre and angiotensin II for two weeks (PBMC: Peripheral Blood Mononuclear Cells, n.s. = not significant).
In aortic tissue, inflammatory CD45+, CD3+, and CD69+ cells were markedly increased by peripheral angiotensin infusion only when central SOD3 had been deleted by prior ICV injection of AdCre (Figure 4D). Neither angiotensin infusion in AdRFP-treated mice, nor deletion of CNS SOD3 alone increased the prevalence of these inflammatory cells in aortic tissue (Figure 4D). Similar to peripheral blood, CD69+ cells in the aorta were increased by central deletion of SOD3 regardless of whether angiotensin II was infused or not (Figure 4E). Confirmatory immunostaining of aortic tissue for CD3+ cells revealed dense, infiltrating, accumulations of CD3+ cells in perivascular regions adjacent to the aorta (Figure 4F).
Functional enabling of inflammatory lymphocytes is dependent on the expression of cell surface adhesion molecules and pro-inflammatory cytokines. In mice infused for 14 days with Ang II, deletion of CNS SOD3 by prior ICV injection of AdCre significantly increased aortic expression of mRNA for the adhesion molecule ICAM-1 but not VCAM-1 (Figure S4 A and B). Additionally, AdCre treatment combined with Ang II infusion increased mRNA expression for the cytokine IL-17A (Figure S4C) without significantly affecting RANTES mRNA levels (Figure S4D). Thus, increasing oxidant stress in CNS circumventricular cells by deletion of SOD3 increased peripheral T cell activation while homing of these cells to vascular tissues was dependent on both central SOD3 deletion and angiotensin II infusion.
DISCUSSION
In this study, we found that endogenous SOD3 located in circumventricular cells critically modulates blood pressure and heart rate at baseline and in response to angiotensin II. Deletion of SOD3 in this region modestly elevated blood pressure at baseline and markedly enhanced the hypertensive response to a low dose angiotensin II, which usually has minimal effect on blood pressure in mice. CVO SOD3 deletion also enhanced O2•− production in peripheral vessels and promoted T cell activation and vascular infiltration. SOD3 in the central nervous system has been implicated in learning and memory,10, 11 however our current findings illustrate a previously unknown role of this protein in central regulation of hemodynamics.
Our current findings support the concept that reactive oxygen species (ROS) in the CNS plays an important role in the regulation of blood pressure and the genesis of hypertension. The current results are in accord with those of Zimmerman, et al who reported an attenuated pressor response to centrally administered angiotensin II as well as prevention of hypertension caused by peripheral angiotensin II after ICV injections of an adenovirus encoding for intracellular superoxide dismutase.12, 13 Taken together, these studies suggest that increasing CNS ROS by deleting SOD augments hypertensive responses to angiotensin II, whereas decreasing CNS ROS by enhancing SOD action reduces these responses.
Interestingly, Zimmerman et al has reported that in contrast to SOD1, augmenting SOD3 levels by ICV adenoviral delivery had no effect on angiotensin-induced hypertension.13 A logical conclusion from their study is that the extracellular superoxide dismutase and by inference extracellular O2•− has no role in modulating central signaling. It should be recognized, however, that cells lining the 3rd ventricle express large amounts of SOD3 under normal conditions,14 and that augmenting these already high levels might have little additional effect on O2•− scavenging. Our present study demonstrates that endogenous SOD3 clearly has a role in hemodynamic modulation, both at baseline and in response to angiotensin II.
The precise location within the CNS where generation of ROS influences central cardiovascular regulation is not known. Several lines of evidence suggest that CVO, and the SFO in particular, might be likely sites of action. The CVO, which are located adjacent to the cerebral ventricles and lack a blood brain barrier, are the CNS structures most often implicated in development of hypertension and mediation of the central actions of angiotensin II. These organs include the organum vasculosum of the lamina terminalis (OVLT), the area postrema (AP), and the SFO.15 Lesions that disrupt the anteroventral third ventricle (AV3V), which includes the OVLT, prevents development of many forms of experimental hypertension in rodents.16, 17 In addition, circumventricular cells mediate virtually all of the central actions of angiotensin II; including drinking behavior, vasopressin secretion, and sympathetic outflow.18 In the present studies, ICV administration of adenovirus produced RFP-labeled cells only within 50–100 μm of the cerebral ventricles with prominent occurrence of RFP-positive cells and deletion of SOD3 in the SFO. Immunohistochemical studies have identified SOD3 positive cells throughout the CNS, with a particularly concentrated distribution associated with tanycytes abutting the ventral third cerebral ventricle.14 In addition, Zimmerman, et al reported that peripheral infusions of angiotensin II produced marked elevation in O2•− production specifically in the SFO.12 Collectively, these observations suggest that ROS production in circumventricular structures, perhaps specifically the SFO, could be important for cardiovascular regulation and the development of angiotensin-induced hypertension.19, 20, 21 While we observed a large amount of SOD3 in the vicinity of the SFO, our study does not exclude a role for other circumventricular organs in modulation of blood pressure or inflammatory cell activation.
Analysis of heart rate and systolic pressure variability provided additional insight into how central SOD3 deletion affected sympathetic cardiovascular regulation. The increased ratio of low to high frequency heart rate variability and the absolute increase in systolic pressure variability are compatible with an increase in sympathetic outflow following central SOD3 deletion. These findings are in accord with the above-mentioned role of CVO in modulating sympathetic outflow. In many cells, including cardiac myocytes, vascular smooth muscle cells and neuronal cells, ROS enhance calcium transients. Calcium sequestration is inhibited and calcium release in enhanced by O2•− and other ROS.22 In keeping with this, angiotensin II activates the NADPH oxidase in neurons and enhances inward calcium flux in these cells in a O2•− -dependent fashion.23 At first glance, it would seem unlikely that scavenging extracellular O2•− would affect such intracellular signaling events. Scavenging extracellular O2•− would reduce the formation of peroxynitrite, which can diffuse inside cells and have myriad effects. For example, peroxynitrite enhances calcium inward current and mitochondrial calcium release in a variety of cells.24 Moreover, a recent study has shown that SOD3 deletion leads to partial inactivation of SOD1 in the heart of mice exposed to transaortic constriction, thus leading to an increase in intracellular O2•− and alteration of gene expression.25 Likewise, alterations of extracellular redox state can have profound effects on intracellular signaling and gene expression, likely via modifying cell surface proteins that convey signals to the cytoplasm.26 Loss of the extracellular SOD3 could therefore have important effects on intracellular signaling events in the CVO. In the present study, we observed increased nitrotyrosine staining following SOD3 in the CVO. While nitrotyrosines can be formed by other mechanisms, this finding is compatible with increased formation of peroxynitrite resulting from SOD3 loss.
Related to the above, an increase in extracellular O2•− caused by SOD3 deletion could reduce NO signaling in the CVO via the rapid, diffusion limited reaction between O2•− and NO. NO has sympathoinhibitory effects in neurons of the SFO,27 and its loss could further increase sympathetic outflow, in keeping with the effect of CVO SOD3 deletion on heart rate and blood pressure variability. The precise contribution of NO loss vs. excessive O2•− remains undefined.
An interesting finding in the current study is that deletion of SOD3 in periventricular cells increased the number of circulating T cells expressing the early activation marker CD69 and vascular O2•− production at baseline, and increased the number of circulating double negative (CD4−/CD8−) T cells following low dose angiotensin II infusion. These results are in keeping with studies by Ganta et al indicating that angiotensin II can promote immune activation via increasing sympathetic nerve firing.28 The superimposition of angiotensin II markedly augmented the vascular inflammation following central SOD3 deletion and led to severe hypertension. Consistent with this, we also found that the vascular levels ICAM-1 mRNA was markedly upregulated by deletion of SOD3 in the CVO. Our data do not allow an understanding of whether these phenomena are the cause or consequence of the severe hypertension that occurred upon angiotensin II infusion. Prior studies showing that hypertension is blunted in the absence of T cells or by T cell suppression support a causative role of inflammation in hypertension 8, 29.
The abundant expression of IL-17A mRNA in the vessel indicates the presence of CD4+ TH17 cells.30 TH17 cells develop independently of TH1 or TH2 CD4+ cells in response to signals such as IL-23, TGFβ and IL-6.31 It has recently been recognized that IL-17 contributes to a variety of autoimmune diseases including psoriasis, rheumatoid arthritis, experimental allergic encephalitis and inflammatory bowel diseases.31 Interestingly, plasma levels of IL-17 and circulating TH17 cells are elevated in humans with unstable angina and myocardial infarction.32 The precise role of the double negative T cells in hypertension is unclear, but in other conditions, double negative T cells secrete high levels of IFN-γ and promote local inflammation.33, 34 We also observed an increase in aortic CD45+ cells, which represent not only T cells but also other inflammatory cells such as macrophages. As evident in figure 4, approximately one half of the total CD45+ cells were T cells. The presence of these cells further emphasizes the importance of interactions between T cells and macrophages, which often co-exist in an inflammatory milieu. Taken together, these data strongly indicate that a central perturbation such as deletion of SOD3 from periventricular cells can markedly enhance peripheral inflammatory responses.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by NIH Grants P01HL58000, R01HL39006, P01HL58000 and a VA Merit Grant. Heinrich E. Lob is supported through an American Heart Association Postdoctoral Fellowship Grant 0825345E.
Footnotes
Disclosure: None
Perspectives
Previous studies from our laboratory and others have shown that mice with embryonic deletion of SOD3 have normal blood pressure at baseline, but demonstrate augmented hypertension in response to either angiotensin II or DOCA-salt challenge.5, 6 While these studies show that SOD3 has an important role in blood pressure regulation, they do not demonstrate the specific organs or cells in which O2•− scavenging is important. Our current results indicate that the CNS, and in particular the CVO, are likely sites where SOD3 modulates blood pressure. Moreover, our finding that deletion of CVO SOD3 enhances vascular O2•− level demonstrates that there is an important interplay between central and peripheral regulation of O2•−. It is likely that alterations of central neuronal firing caused by deletion of SOD3 in the CVO enhances neurohumoral stimuli that promote vascular O2•− production, and possibly production of reactive oxygen species in other organs such as the kidney, leading to the hypertensive state. Finally, this study provides new evidence that central oxidative stress can causes peripheral T cell activation and vascular inflammation, which further augments hypertension and the target-organ damage caused by this disease.
LITERATURE CITED
- 1.Beyer W, Imlay J, Fridovich I. Superoxide dismutases. Progress in nucleic acid research and molecular biology. 1991;40:221–253. doi: 10.1016/s0079-6603(08)60843-0. [DOI] [PubMed] [Google Scholar]
- 2.Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991;88:10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 4.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 5.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 6.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–481. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- 7.Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol. 2008;173:915–926. doi: 10.2353/ajpath.2008.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 10.Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26:3933–3941. doi: 10.1523/JNEUROSCI.5566-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, Crapo JD. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28:381–390. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 14.Oury TD, Card JP, Klann E. Localization of extracellular superoxide dismutase in adult mouse brain. Brain research. 1999;850:96–103. doi: 10.1016/s0006-8993(99)02103-4. [DOI] [PubMed] [Google Scholar]
- 15.Whyte DG, Johnson AK. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (AV3V) during acute heat stress in the rat. Clin Exp Pharmacol Physiol. 2005;32:457–461. doi: 10.1111/j.1440-1681.2005.04211.x. [DOI] [PubMed] [Google Scholar]
- 16.Brody MJ. Central nervous system and mechanisms of hypertension. Clin Physiol Biochem. 1988;6:230–239. [PubMed] [Google Scholar]
- 17.Gordon FJ, Haywood JR, Brody MJ, Johnson AK. Effect of lesions of the anteroventral third ventricle (AV3V) on the development of hypertension in spontaneously hypertensive rats. Hypertension. 1982;4:387–393. doi: 10.1161/01.hyp.4.3.387. [DOI] [PubMed] [Google Scholar]
- 18.Akera T, Ku DD, Brody TM, Manian AA. Inotropic action of hydroxylated chlorpromazine metabolites and related compounds. Biochem Pharmacol. 1978;27:995–998. doi: 10.1016/0006-2952(78)90431-8. [DOI] [PubMed] [Google Scholar]
- 19.Kirby RF, Johnson AK. Regulation of sodium and body fluid homeostasis during development: implications for the pathogenesis of hypertension. Experientia. 1992;48:345–351. doi: 10.1007/BF01923428. [DOI] [PubMed] [Google Scholar]
- 20.Barnes KL, Ferrario CM. Angiotensin and CNS regulation of blood pressure. Clin Exp Hypertens. 1980;2:465–477. doi: 10.3109/10641968009037125. [DOI] [PubMed] [Google Scholar]
- 21.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 22.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2005;289:F1012–1019. doi: 10.1152/ajprenal.00144.2005. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca(2+)-activated K(+) channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H1883–1890. doi: 10.1152/ajpheart.2000.278.6.H1883. [DOI] [PubMed] [Google Scholar]
- 25.Lu Z, Xu X, Hu X, Zhu G, Zhang P, van Deel ED, French JP, Fassett JT, Oury TD, Bache RJ, Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 27.Rauch M, Schmid HA, deVente J, Simon E. Electrophysiological and immunocytochemical evidence for a cGMP-mediated inhibition of subfornical organ neurons by nitric oxide. J Neurosci. 1997;17:363–371. doi: 10.1523/JNEUROSCI.17-01-00363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–1691. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 29.Bravo Y, Quiroz Y, Ferrebuz A, Vaziri ND, Rodriguez-Iturbe B. Mycophenolate mofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am J Physiol Renal Physiol. 2007;293:F616–623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 30.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 31.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Kadena T, Matsuzaki G, Fujise S, Kishihara K, Takimoto H, Sasaki M, Beppu M, Nakamura S, Nomoto K. TCR alpha beta+ CD4− CD8− T cells differentiate extrathymically in an lck-independent manner and participate in early response against Listeria monocytogenes infection through interferon-gamma production. Immunology. 1997;91:511–519. doi: 10.1046/j.1365-2567.1997.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks EG, Wirt DP, Goldblum RM, Vaidya S, Asuncion MT, Patterson JC, Ware CF, Klimpel GR. Double-negative (CD4− CD8−) T cells with an alpha/beta T cell receptor. Non-MHC-restricted cytolytic activity and lymphokine production. J Immunol. 1990;144:4507–4512. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.