Abstract
Objective
Current treatment options for epithelial ovarian cancer are limited and therapeutic development for recurrent and drug-resistant ovarian cancer is an urgent agenda. We investigated the potential use of genetically engineered Vesicular Stomatitis Virus (VSV) to treat ovarian cancer patients who fail to respond to available therapies. Specifically, we examined the toxicity to hosts and specificity of targeting ovarian tumors using a Wv ovarian tumor model.
Methods
We first tested recombinant VSV for oncolytic activity in a panel of human ovarian epithelial cancer, immortalized, and primary ovarian surface epithelial cells in culture. Then, we test VSV oncolytic therapy using the immune competent Wv mice that develop tubular adenomas, benign tumor lesions derived from ovarian surface epithelial cells.
Results
The expression of GFP encoded by the recombinant VSV genome was detected in about 5% of primary ovarian surface epithelial cells (3 lines) up to 30 days without significantly altering the growth pattern of the cells, suggesting the lack of toxicity to the normal ovarian surface epithelial cells. However, VSV-GFP was detected in the majority (around 90%) of cells that are either “immortalized” by SV40 antigen expression or cancer lines. Some variation in killing time courses was observed, but all the transformed cell lines were killed within 3 days.
We found that regardless of the inoculation route (intra bursal, IP, or IV), VSV specifically infected and replicated in the in situ ovarian tumors in the Wv mice without significant activity in any other organs and tissues, and showed no detectable toxicity. The epithelial tumor lesions were greatly reduced in VSV-targeted ovarian tumors in the Wv mice.
Conclusions
VSV oncolytic activity depends on a cell autonomous property distinguishing primary and transformed cells. The efficient oncolytic activity of VSV for the “immortalized” non-tumorigenic ovarian surface epithelial cells suggests that the selective specificity extends from pre-neoplastic to overt cancer cells. The results demonstrated the explicit targeting of ovarian epithelial tumors by VSV in immune com petent, ovarian tumor-bearing mouse models, and further support the utility of VSV as an effective and safe anti-cancer agent.
INTRODUCTION
Epithelial ovarian cancer is a disease with poor prognosis, few early diagnostic markers, and limited treatment options [1-3]. Chem otherapeutic agents based on platinum derivatives have been widely used to treat a broad range of cancers including epithelial ovarian cancer with some success. Currently, a platinum- and taxane-based combination regimen remains standard frontline chemotherapy for ovarian cancer [1-4]. Unfortunately, intrinsic and acquired resistance to cisplatin/taxane has greatly limited the efficacy of the therapy [5]. New agents, such as Gemcitabine, Doxorubicin, and Topotecan that convey anti-cancer activities via different mechanisms, are being evaluated in clinical trials, and some have been adopted for clinical application [5]. Nevertheless, current treatment options are still very limited, and development of resistance to the cytotoxic chemotherapy remains a key problem to be overcome, and most women ultimately die of the disease. Development of additional chemotherapeutic regimens, biological therapeutic agents, and other unique approaches for treatment of ovarian cancer is a high priority.
An idea is to use particular types of viruses as agents to selectively kill cancer cells [6]. These viruses, referred to as oncolytic viruses, are capable of replicating in cancer but not in normal cells [6]. The potential usefulness of Vesicular Stomatitis virus (VSV) as an anti-cancer therapeutic agent has been investigated [7,8]. VSV is a negative-stranded RNA virus that can infect a large range of cell types, through an as-yet-unidentified but likely ubiquitous cell surface receptor(s). VSV replicates in the cytoplasm of infected cells, though the 11-kilobase viral genome does not integrate into the host genome and has no transforming activity [9].
VSV can infect essentially all human cells in culture and undergo robust replication in certain (often cancerous) cells; however, VSV is relatively non-pathologic for humans, likely due to the inability of the VSV to replicate and amplify in humans. While VSV is largely asymptomatic for humans, domestic and farm animals can become non-lethally infected, with symptoms such as lesions in the mucous membranes of the mouth and nose [7-9]. VSV also has been reported to be neuropathic in mice, following intranasal inoculation and subsequent infection and replication in the central nervous system . VSV infection can be cleared through activation of both the innate and adaptive immune responses [7-9]. The interferons are critically important in antiviral innate immunity and are a family of cytokines produced in response to VSV infection.
The selectivity of VSV to replicate and kill malignant, but not normal cells, has been well established in cultured cells and xenografts in mice of human cancer cells [10,11]. VSV has oncolytic activity in a large range of cancer types [12-20], including ovarian cancer cells [11]. Significant progress has been made to understand the mechanisms of VSV oncolytic activity and selectivity [7,21,22]. Neoplastic cells often have defective immune defense, probably involving the dis-regulation of the interferon system, which is an important factor for the ability of VSV to replicate in cancer cells [11,23]. A defective translational control in protein synthesis in cancer cells is another key factor for VSV oncolytic activity [24-27]. Additional mechanisms likely exist to account fully for the cell autonomous properties of sensitivity to VSV, and these are topics of active investigation.
While the efficacy of VSV for cancer therapy has been established in pre-clinical studies, further studying VSV oncolytic therapy in intact immune competent model organisms, rigorous evaluation of safety, and careful documentation of potential toxic side effects may move VSV oncolytic therapy to the next step, a clinical trial in human cancer patients. Here we report a study of VSV targeting of ovarian tumors in a Wv mouse model that is immune competent and spontaneously develop ovarian epithelial tumors. The Wv (white spotting variant) mice have a point mutation in the c-kit gene that reduces the tyrosine kinase activity and affects the development of germ cells [28,29]. The mutant mice live largely a normal lifespan with few developmental and physiological phenotypes, but the mutation results in a greatly reduced number of ovarian germ cells and follicles [28,29]. As a result of follicle depletion, ovarian morphological aging occurs, and ovarian tubular adenomas develop from ovarian surface epithelial cells in nearly 100% of Wv mice by 3-4 months of age [30,31]. These ovarian tumors are benign, but increasingly neoplastic features develop in older mice [30,31].
In this study, we examined the ability of VSV to target the ovarian tumors in Wv mice. The in situ ovarian tumor-bearing mice are anatomically correct and reflect more accurately the accessibility of VSV to tumor cells and the potential toxic side effects of the oncolytic therapy.
MATERIALS and METHODS
VSV preparation and handling
Recombinant VSV of Indiana serotype was prepared in the laboratory as described previously [32]. The GFP transgene was inserted into the pVSV-XN2 plasmid that contains the entire VSV genome, and the expression of GFP can be used to monitor the expression of VSV-GFP genome. Aliquots of VSV stock of 108 pfu/μl were stored at −80°C until use. As controls, aliquots of VSV-GFP were exposed to UV light for 30 minutes. The viruses were thawed slowly on ice and diluted in PBS (for animal injection) or RPMI medium (for cell culture experiments) to obtain a working stock of 105 pfu/μl. VSV was used according to bio-safety procedure in a P2-level safety facility/room, which was previously inspected by the Institutional Biosafety Committee (IBC). The use of VSV-GFP in this project was reviewed and approved by the IBC and IACUC (Institutional Animal Care and Usage Committee) committees at the University of Miami.
Cell culture and VSV oncolytic assay
Primary Human Ovarian Epithelial (HOSE) cells and Human Immortalized Ovarian epithelial (HIO) cells were provided by Dr. Andrew Godwin of Fox Chase Cancer Center, Philadelphia, PA [33]. All human ovarian cancer cells were purchased from ATCC. Recombinant VSV-GFP Indiana serotype was used to study viral cytolytic activity in normal human ovarian cells (HOSE60 and HOSE65), immortalized cells (HIO-80 and HIO-114), and ovarian cancer cell lines (OVCAR3, OVCAR5, OVCAR10 and ES2). Primary HOSE cells commonly can be maintained up to 10 passages in culture [34,35]. Transfection with the SV40 large T antigen (SV40Tag) expression vector to “immortalize” the primary cells can prolong the HIO cell lifespan in culture up to 20-30 passages [33-35].
For in vitro VSV infection assays, cells were seeded at a density of 105 cells/well in 6-well dishes 24 h prior to VSV-GFP infection. The cells were first rinsed twice with phenol red-free and FBS free RPMI medium, and viruses were added in 1 ml of phenol red-free FBS free RPMI medium containing VSV-GFP diluted to the appropriate viral dosage (105, 106, 107, or 108 pfu/ml) and incubated for 2 hours at 37°C. The “infection” medium was replaced by fresh phenol red-free RPMI medium containing 10% FBS for cancer cells, and 15% FBS for HOSE cells. VSV infection and proliferation was monitored by the expression of VSV-encoded GFP using a fluorescence microscope. Cell death was determined by staining with propidium iodide.
Additionally, VSV proliferation was measured by Western blot of the cell lysates for the expression of VSV viral proteins using either VSV antiserum (provided by Dr. Barber's laboratory) or anti-VSV-G-protein (Santa Cruz Biotechnology, Santa Cruz, CA).
Wv ovarian tumor mouse models
The original founder Wv mice were purchased from Jackson Laboratory (Bar Harbor, ME) and a breeding colony was established at Fox Chase Cancer Center for 4 years [31] and at the University of Miami mouse facility for the last 2 years. The mice are maintained by mating between heterozygous mutant Wv mice and housed in micro-isolated cages with free access to autoclaved water and Purina 5001 standard mouse chow in barricaded viral pathogen-free rooms with husbandry provided by specialized animal technicians and monitored by veterinarians on duty.
Generally, the mice are genotyped by the coat color [31]. Wildtypes are evenly brown or black, heterozygous mice are mosaic with a dorsal or ventral white spot/patch on brown/black background, and homozygous mutant mice are all white coat with black eyes. All homozygous Wv mice were examined and confirmed to be non-albino (yellow coat with red eyes). If in the rare occasion albino mice were identified (through loss of eye pigment), the mice were removed from the colonies. Littermates of female Wv/Wv mutants (tumor bearing) and their heterozygous and wildtype littermates (controls, tumor free) were used for experiments. At 4 months of age, all female Wv homozygous mutant mice consistently develop bilateral ovarian tubular adenomas of similar size [31]. The heterozygous and wildtype littermates were used as controls for the specific tumor targeting by VSV. The tumor-bearing Wv mice injected with UV-inactivated VSV were used as controls for auto-fluorescence.
Surgical procedure and viral delivery
Initially, various routes of VSV delivery, intraperitoneal (IP), intravenous (IV), and intrabursal (IB), were tested. Two female mice of around 4 months were used in each experimental group, and the experiments were repeated three times.
For IB injection, a surgical procedure was needed to access the ovary or ovarian tumor. Only one ovary (the left side) in each animal was injected. The mice were first anesthetized by intramuscular injection of a standard mix of 80-100 mg/kg Ketamine-HCL: Ketaset: Xylazine (5-10 mg/kg). The site of injection was shaved and washed with sterile saline and isopropanol. VSV was injected into the ovarian bursa using a syringe with 29-gauge beveled needle under a dissection microscope. The ovary was first exposed via a small dorsal incision, then the needle was inserted, bevel up into the oviduct near the infundibulum and moved toward the bursa. A volume of 10 μl of VSV-GFP (105 pfu/μl in PBS) was injected into the ovarian bursa. The incision was closed using Dissolvable-5.0, and skin was closed with 7-mm staples. Following surgery, the mice were kept warm with a heating pad until awake. The injected mice were kept separated individually and were closely observed on a daily basis for potential infection or discomfort.
The IV (standard tail vain injection) and IP injections were performed using a 27-gauge syringe without anesthesia.
The animals were euthanized 2 weeks later and infused with 10% buffered formalin. The tissues (ovary, uterine horns, bladder, gut, fat, bile duct, pancreas, liver, kidney, adrenal glands, spleen, heart, lungs, brain, eye, skeletal muscle, skin, fur, bedding, urine and stool) were analyzed using a Zeiss (Stereo Discovery V8) stereo-fluorescence dissecting microscope equipped with a GFP 470 filter and digital camera (Axiocam MRC, with Axiovision 4.6 software). Representative images were recorded. The ovaries (or tumors) and selected tissues were collected in 10% buffered formalin and embedded in paraffin wax for further histology analysis. The epithelial lesions were examined following immunostaining for cytokeratin-18 [31].
RESULTS
Susceptibility to oncolytic activity of VSV of “immortalized” ovarian surface epithelial cells in cultures
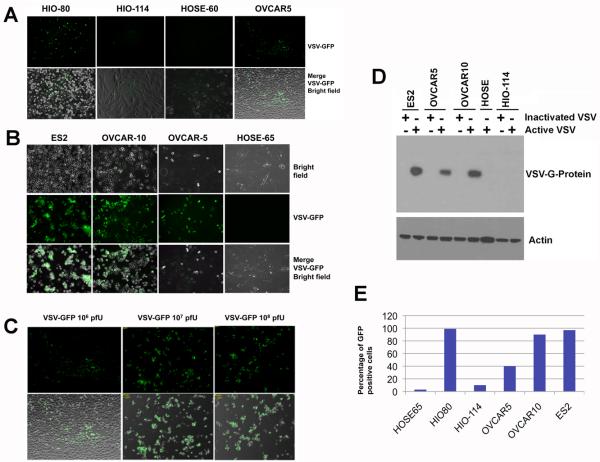
We further characterized the infectivity and replication of VSV in ovarian epithelial and cancer cells in culture using VSV-GFP. The VSV genome was modified by inserting the GFP transgene into the viral genome, and thus the presence of GFP in cells indicates VSV infection and replication [32]. Upon inoculation of VSV-GFP in cultured cells for 24 hours, various degrees of infectivity as indicated by the expression of GFP were observed in a panel of cells (Fig. 1A,B): the majority of ovarian cancer cells NIHOVCAR5, ES2, and NIHOVCAR10 were infected; only a small percentage of primary HOSE cells showed viral replication; all HIO-80 cells showed high degree of viral replication, while only a small percentage of HIO-114 showed viral infection. The infectivity depends on the dosage of VSV, as shown in an example using NIHOVACR5 cells (Fig. 1C). Only 10-20% of the cells expressed GFP 24 hours after the addition of 106 pfu VSV-GFP; however, most NIHOVCAR5 cells were infected when either 107 or 108 pfu of VSV were added. The selective replication of VSV-GFP in cancer cells but not in primary cells or HIO-114 non-tumorigenic “immortalized” cells was also confirmed by assaying VSV-encoded G-protein in cell lysates by Western blot (Fig. 1D). Some small variation in infectivity was observed of various cell lines in repeated experiments over time, and the percentage of infected cells was quantified for a representative experiment, as shown in Fig. 1E.
Fig. 1.
Infection of VSV-GFP in cultured ovarian epithelial and tumor cells. (A) “Immortalized” ovarian surface epithelial cells HIO-80, HIO-114; primary ovarian surface epithelial cell preparation, HOSE-60; and ovarian cancer cells NIH:OVCAR5 in 6-well culture dishes were infected with 106 pfu of VSV-GFP. The cells were observed for GFP expression 24 hours after infection. (B) Ovarian cancer cells ES2, NIH:OVCAR5, NIH:OVCAR10, and primary ovarian surface epithelial HOSE-65 cells in 24-well dishes were infected with 107 pfu of VSV-GFP. The cells were observed for GFP expression 24 hours after infection. (C) Ovarian cancer cells NIH:OVCAR5 in 24-well dishes were infected with increasing titers, 106, 107, and 108 pfu of VSV-GFP. The cells were observed for GFP expression 24 hours after infection. (D) Ovarian cancer cells, ES2, NIH:OVCAR5, NIH:OVCAR10, “immortalized” HIO-114 cells, and primary ovarian surface epithelial preparation HOSE-65 cells in 24-well dishes were infected with 107 pfu of VSV-GFP. The cell lysates were harvested 24 hours after infection for Western blotting analysis of VSV-encoded G-protein, which indicates viral proliferation. (E) The percentage of VSV replicating cells (GFP positive) was quantified by cell counting 24 hours following infection with 107 pfu of VSV-GFP. The value was calculated as an average of 5 images of GFP over-layering on bridge field, and the difference between HOSE cells and cancer cells is large and statistically significant as determined by Student's T-test.
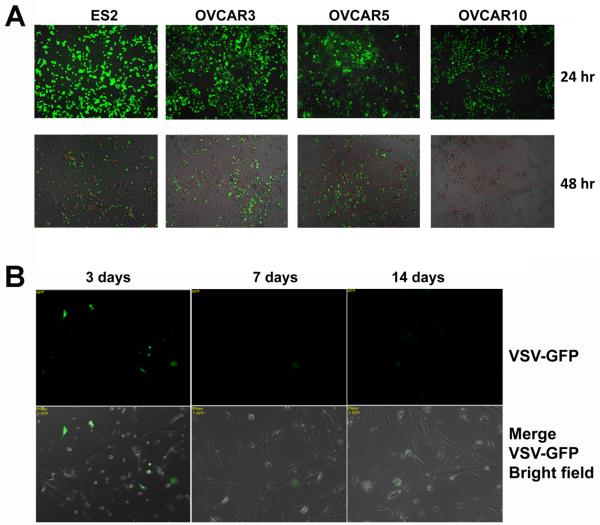
Following VSV infection, the cancer cells rapidly appeared in poor condition, rounded up, and underwent apoptosis. At 24 hours after VSV infection of all cancer lines, a large percentage of cells showed viral infection and replication as indicated by robust signals from expressed GFP, and at 48 hours, extensive cell detachment and death were observed (Fig. 2A). Propidium iodide staining 2 days after VSV infection indicated the majority of the cancer cells in culture had already died (Fig. 2A), and by day 3-post infection, essentially all ovarian cancer cells were detached and dead. UV-inactivated VSV as a control did not affect cell growth (not shown). In HOSE cells, faint and scattered expression of GFP persisted at day 3 and 7, but the GFP signal was essentially absent at day 14 (Fig. 2B), and viable cells were maintained up to 3 weeks. Thus, follow ing exposure to VSV, the HOSE cells survived and showed no detectable phenotype, comparable to controls with the addition of inactivated VSV. Thus, the selectivity and oncolytic activity of VSV was demonstrated in cultured ovarian epithelial and cancer cells. The active viral replication in some lines of HIO cells suggests that oncolytic activity may depend on pre-neoplastic changes in the cells, since HIO cells are not tumorigenic.
Fig. 2.
Oncolytic activity of VSV-GFP in cultured ovarian epithelial and tumor cells. (A) Ovarian cancer cells ES2, NIH:OVCAR5, and A2780 in 24-well dishes were infected with 106 pfu of VSV-GFP. The cells were observed for GFP expression and for PI staining (red) to mark dead cells 2 days after infection. (B) Primary ovarian surface epithelial cell preparation HOSE-65 cells in 24-well dishes were infected with 108 pfu of VSV-GFP and monitored for GFP expression on day 3, 7, and 14 after infection.
Robust infectivity of VSV in ovarian tumors of Wv mice by ovarian inoculation
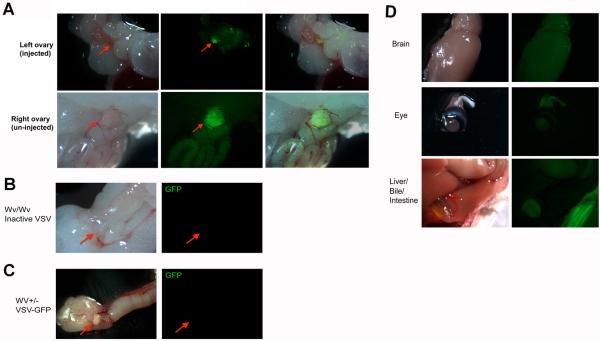
Next, we injected VSV-GFP into the ovarian bursa of 4-month-old wildtype (or Wv +/−) and Wv/Wv mutant mice to examine VSV infection and replication in the Wv ovarian tumors. The Wv ovarian tumor-bearing mice allow us to test VSV infection and treatment of in situ tumors in an immune competent mouse model. By 3 months old, nearly all female Wv/Wv mice develop ovarian tubular adenomas bilaterally. Initially, we delivered VSV-GFP directly into ovarian bursa (IB, intrabursal) by a simple surgical procedure. The virus was injected into the left ovary of each animal, and the right ovary was intended to be a control. Ten days after the virus was injected, ovaries and other organs were collected for analysis. The expression of GFP, as detected under a fluorescence stereomicroscope, indicates the degree of viral infection and replication. As shown in Fig. 3A, an intense GFP signal was detected in the injected left ovary, and surprisingly, GFP expression was equally intense in the non-injected right ovary (Fig. 3A). Thus, the VSV injected in the left ovary apparently accessed the tumor of the right ovary. No GFP signals were detected in ovarian tumors from Wv mice injected with inactivated VSV-GFP (Fig. 3B). Active VSV-GFP also failed to infect and replicate in ovaries of wildtype mice (Fig. 3C). Essentially no significant GFP signal was detected in any other organ examined, including the brain, heart, liver, intestines, kidney, bladder, etc (Fig. 3D).
Fig. 3.
Targeting of VSV following intra ovarian bursa injection in Wv mice. Four-month-old Wv/Wv mice that bear ovarian tumors were injected with 105 pfu of VSV-GFP into the bursa of left ovary. Ten days after injection, the mice were dissected and organs were examined under fluorescence microscopy for GFP signals. (A) The injected left ovary of a representative tumor-bearing Wv mouse shows fluorescence (arrow). The non-injected right ovary from the same mouse also shows GFP fluorescence. As controls, no signals were detected in ovaries in Wv/Wv mice injected with inactivated VSV (B), or in Wv heterozygous (no ovarian tumor) mutant mice (C). (D) Other organs (brain, eye, and liver/bile/intestine are shown) from VSV-GFP-injected mice show no significant GFP signals.
Specific targeting of ovarian tumors in Wv mice by VSV through IP or IV routes
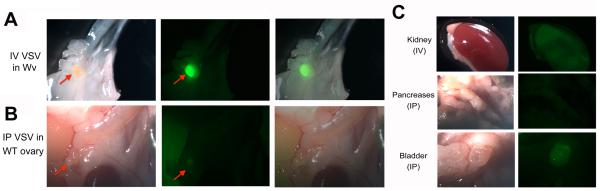
Because the VSV targeted both ovarian tumors when only one side was injected, we next examined the ability of VSV to target Wv ovarian tumors if delivered through intra-peritoneal (IP) or intra-venous (IV) routes. After 10 days, the Wv mice injected with VSV-GFP or inactivated VSV as control were analyzed for GFP expression. Again, robust GFP signals were found only in both ovaries of the VSV-GFP-injected ovarian tumor-bearing animals (Fig. 4A). VSV-GFP injected either by IP or IV routes did not proliferate in ovaries from wildtype mice (Fig. 4B). Other organs were dissected and carefully examined for GFP, and no significant sign of VSV replication was observed in any of the organs (Fig. 4C). Nevertheless, urine and solid wastes found on the bedding of the VSVGFP injected wildtype and Wv mice were found to be intensely fluorescent with GFP, suggesting VSV infection occurred and the viruses were cleared from the animals in tissues other than the ovarian tumors. As control, mock infection with UV-inactivated VSV was performed in parallel in all experiments to ensure the observed GFP signals specifically corresponded to VSV replication. Both IV and IP injection of VSV resulted in very similar targeting of ovarian tumors without significant replication in other tissues, suggesting that the injected VSV accessed cells throughout the entire circulation independent of the route of delivery.
Fig. 4.
Targeting of VSV to ovarian tumors following IP and IV injection in Wv mice. Four-month-old Wv/Wv mice that bear ovarian tumors were injected IP or IV with 105 pfu of VSV-GFP into the control or Wv/Wv mice. Two mice per experimental group were injected. Ten days after injection, the mice were dissected and organs were examined under fluorescence microscopy for GFP signals. (A) The ovary of a representative tumor-bearing Wv mouse injected IV with VSV shows fluorescence indicating VSV proliferation (arrow). (B) The ovaries from wildtype control mice injected IP with VSV-GFP showed no GPF signal. (C) Other organs from VSV-GFP-injected mice by either IP or IV routes show minimal GFP signals.
Effective oncolytic activity of VSV towards ovarian tumors in Wv mice
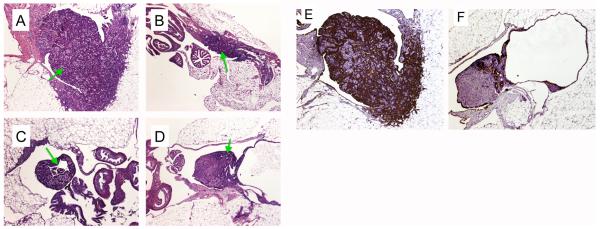
We examined the impact of VSV infection on ovarian tumor histology 10 days after a single dosage of VSV injection. The ovarian tumors were dissected and processed by formaldehyde fixing and paraffin embedding. The blocks were cut to obtain middle sections with the largest area of ovarian or tumor tissues for comparison. In all cases, we observed a substantial reduction in tumor sizes by VSV independent of the route of viral delivering (Fig. 5). As shown in representative examples in Figure 5, compared to an average-size ovarian tumor in 4-month-old Wv mice treated with inactivated VSV (Fig. 5A), the ovarian tumor mass from the VSV-injected Wv mice was reduced approximately 5-fold (Fig. 5B,C,D). Based on the remaining expression of GFP shown in Fig. 3 and Fig. 4, the ovarian tumors still exhibited active VSV infection at the time of tissue harvest. Presumably the VSV treatment might further reduce the tumor sizes in animals examined after a longer period after VSV treatment.
Fig. 5.
Oncolytic activity of VSV towards ovarian tumors in Wv mice. Female Wv mutant mice, two in each experimental group, were injected with VSV or inactivated VSV as control, through IB, IP, and IV routes. Ten days after a single viral injection, the ovarian tissues were harvested for histological analysis. H&E and cytokeratin staining of the sections of ovarian tumors at the widest cross-section of the ovary are shown for comparison. Arrows indicate the ovarian tumors. (A), H&E staining of control, inactivated VSV injected IV; (B), H&E, active VSV injected IV; (C), H&E, active VSV injected IB; (D), H&E, active VSV injected IP; (E), cytokeratin-18 staining of control, inactivated VSV injected IV; (F), cytokeratin-18 staining, active VSV injected IP. The images were taken under a microscope with a 40X magnification and processed identically using Adobe Photoshop.
Additionally, compared to the ovarian tumors from control animals treated with inactivated VSV in which the cytokeratin-positive ovarian epithelial tumor permeated the entire ovary (Fig. 5E), the ovary from VSV treated mice often contains mainly tumor-free ovarian stromal area (Fig. 5F). These results suggest that VSV has robust activity in clearing epithelial tumor lesions.
DISCUSSION
This current study compared the oncolytic activity of VSV in primary human ovarian surface epithelial (HOSE), non-tumorigenic “immortalized” human ovarian surface epithelial (HIO), and ovarian cancer cells in culture [33-35]. Confirming previous reports [10], the differential susceptibility of normal and cancer cells to VSV oncolytic activity is unequivocal. Several ovarian cancer cell lines tested were all sensitive to VSV and the cells died within 3 days following addition of VSV to the cultures. Active VSV replication in these transformed cells was revealed both by the expression of VSV-encoded GFP using immunofluorescence microscopy and by the expression of VSV encoded G-protein by Western blotting. Only a small number of primary ovarian surface epithelial cells were observed to express a low level of VSV-encoded GFP, and the cells maintained a normal growth pattern up to 3 weeks following exposure to VSV. We also observed that some lines of the HIO cells, which may resemble a pre-neoplastic state, are highly susceptible to oncolytic activity of VSV. Thus, it suggests that some subtle changes in cell properties without requirement of a fully neoplastic feature, are sufficient to render a cell type susceptible to killing by VSV. This element of VSV oncolytic selectivity may potentially be used to deal with pre-neoplastic lesions.
Previous reports have demonstrated the ability of VSV to target xenografts of human cancer cells in immune deficient mice [10,11]. The oncolytic activity and the efficacy of VSV as a cancer therapy are well demonstrated in mouse models [7,8], and the important remaining issues are the safety and selectivity. We tested VSV oncolytic therapy further in the immune competent Wv mouse model. The tumors that develop in the Wv mice are epithelial-derived and essentially the counterpart of epithelial proliferation and morphological changes in aging ovaries [31]. These ovarian epithelial tumors are known as tubular adenomas, and are benign, though some malignant features develop in older mice [30]. Using the Wv ovarian tumor models, the current study demonstrated the explicit targeting of VSV to ovarian tumors through various routes of delivery in immune competent mice, without significantly affecting any other organs or showing observable toxicity. The current Wv ovarian tumor model accurately represents the anatomical location of small ovarian tumor lesions and thus the results may be highly relevant to actual therapy in ovarian cancer patients.
The oncolytic virus that is commonly considered and developed is adenovirus, which have been actively investigated in the past decades [6,37,38]. Engineered conditional, replicative competent adenovirus can kill tumor cells but spare normal cells in pre-clinical studies, and the potential of oncolytic adenoviral therapy was regarded with much enthusiasm [37,38]. However, clinical studies of oncolytic adenovirus encountered several difficult barriers including the lack of tumor-specific viral targeting/infection and clearance of the virus by the immune system [37,38]. In humans, the weak tumor cell targeting by adenovirus is a serious shortcoming [37,38]. Although adenovirus can efficiently infect most mammalian cells through specific receptors, the infectivity seems reduced in neoplastic cells, and the majority of adenovirus delivered is sequestered in the liver [37,38]. While oncolytic adenovirus still has good potential and promise, strategies to modify the basic viral structure will be needed to overcome the existing shortcomings.
In comparison, VSV with its specificity for transformed cells and the ability to target tumors without accumulating in other organs may be superior and have great potential as a useful oncolytic agent to treat drug-resistant ovarian cancer. Additional studies of the biology of VSV oncolytic activity such as identification of VSV receptor(s) and its regulation in benign and malignant cells, and rigorous testing in additional ovarian cancer mouse and rat models for safety and efficacy, may bring VSV oncolytic therapy closer for treatment of drug-resistant ovarian cancer patients.
Acknowledgments
The studies were supported by grants R01 CA099471, R01 CA79716, and R01 CA75389 from NCI, NIH, and W81XWH-06-1-0095 from DOD to X.X. Xu, and by grants R01 CA095924 and P01CA128115 from NIH to G.N. Barber. We very much appreciate Dr. Andrew Godwin from Fox Chase Cancer Center (Philadelphia, PA) for providing HIO and HOSE cells used in this research work. We thank Dr. Elizabeth Smith for reading and commenting during the process of preparing the manuscript, and Dr. Marianna Nunez of the Histopathology Core in tissue analysis.
Abbreviations
- GFP
green fluorescence protein
- HOSE cells
human ovarian surface epithelial cells
- HIO cells
Human “immortalized” ovarian surface epithelial cells
- IFN
interferon
- IB
intra-bursal
- IP
intra-peritoneal
- IV
intra-venous
- pfu
plaque forming unit
- PI
propidium iodide
- VSV
vesicular stomatitis virus
- Wv mice
white spotting variant mutant mice
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu XX, Hamilton TC. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 2.Cho KR, Shih IeM. Ovarian Cancer. Annu Rev Pathol. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–28. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–5. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 5.Martin LP, Schilder RJ. Management of recurrent ovarian carcinoma: current status and future directions. Semin Oncol. 2009;36:112–25. doi: 10.1053/j.seminoncol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 7.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17:516–27. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 8.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10(5):210–6. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Wagner RR, Rose JK. Rhabdoviridae: the viruses and their replication. In: Fields BN, Knipe DM, editors. Fields Virology. Lippincott-Raven; 1996. pp. 1121–36. [Google Scholar]
- 10.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75:3474–9. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, Bell JC. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6(7):821–5. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 12.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, Valdes M, Barber G, Vile RG. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 67(6):2840–8. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 13.Ebert O, Shinozaki K, Huang TG, Savontaus MJ, García-Sastre A, Woo SL. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–11. [PubMed] [Google Scholar]
- 14.Ebert O, Harbaran S, Shinozaki K, Woo SL. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005;12:350–8. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 15.Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005;41:196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 16.Hadaschik BA, Zhang K, So AI, Fazli L, Jia W, Bell JC, Gleave ME, Rennie PS. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008;68(12):4506–10. doi: 10.1158/0008-5472.CAN-08-0238. [DOI] [PubMed] [Google Scholar]
- 17.Lun X, Senger DL, Alain T, Oprea A, Parato K, Stojdl D, Lichty B, Power A, Johnston RN, Hamilton M, Parney I, Bell JC, Forsyth PA. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98(21):1546–57. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 18.Césaire R, Olière S, Sharif-Askari E, Loignon M, Lézin A, Olindo S, Panelatti G, Kazanji M, Aloyz R, Panasci L, Bell JC, Hiscott J. Oncolytic activity of vesicular stomatitis virus in primary adult T-cell leukemia. Oncogene. 2006;25(3):349–58. doi: 10.1038/sj.onc.1209055. [DOI] [PubMed] [Google Scholar]
- 19.Lichty BD, Stojdl DF, Taylor RA, Miller L, Frenkel I, Atkins H, Bell JC. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Hum Gene Ther. 2004;15(9):821–31. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 20.Lun X, Senger D, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus on multifocal and invasive gliomas. J Natl Cancer Inst. 2006;98:1546–57. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 21.Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15(4):651–9. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 22.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 23.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77(16):8843–56. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5(1):51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 25.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24(52):7710–9. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 26.Baltzis D, Qu LK, Papadopoulou S, Blais JD, Bell JC, Sonenberg N, Koromilas AE. Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2alpha kinases PERK and PKR. J Virol. 2004;78(23):12747–61. doi: 10.1128/JVI.78.23.12747-12761.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol. 2000;74(20):9580–5. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9(6):1805–13. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990;4(3):390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- 30.Murphy ED. Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells. J Natl Cancer Inst. 1972;48:1283–95. [PubMed] [Google Scholar]
- 31.Yang WL, Cai KQ, Smedberg JL, Smith ER, Klein-Szanto A, Hamilton TC, Xu XX. A reduction of Cox-2 gene dosage counters the menopausal ovarian morphological aging and tumor phenotype in Wv mice. Am J Pathol. 2007;170:1325–36. doi: 10.2353/ajpath.2007.060769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76(2):895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capo-chichi CD, Cai KQ, Testa JR, Godwin AK, Xu XX. Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer. Mol Cell Biol. 2009;29:4766–77. doi: 10.1128/MCB.00087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siemens CH, Auersperg N. Serial propagation of human ovarian surface epithelium in tissue culture. J Cell Physiol. 1988;134:347–56. doi: 10.1002/jcp.1041340305. [DOI] [PubMed] [Google Scholar]
- 35.Kruk PA, Maines-Bandiera SL, Auersperg N. A simplified method to culture human ovarian surface epithelium. Lab Invest. 1990;63:132–6. [PubMed] [Google Scholar]
- 36.Capo-chichi CD, Roland IH, Vanderveer L, Bao R, Yamagata T, Hirai H, Cohen C, Hamilton TC, Godwin AK, Xu XX. Anomalous expression of epithelial differentiation-determining GATA factors in ovarian tumorigenesis. Cancer Res. 2003;63(16):4967–77. [PubMed] [Google Scholar]
- 37.Hermiston T. Adem and for next-generation oncolytic adenoviruses. Curr Opin Mol Ther. 2006;8(4):322–30. [PubMed] [Google Scholar]
- 38.Nettelbeck DM. Cellular genetic tools to control oncolytic adenoviruses for virotherapy of cancer. J Mol Med. 2008;86(4):363–77. doi: 10.1007/s00109-007-0291-1. [DOI] [PubMed] [Google Scholar]