Abstract
Although albumin (Alb) is the most abundant plasma protein, it is considered to be non-adhesive to platelets, as it lacks any known amino acid sequences for binding platelet receptors. Recent studies have suggested that platelets adhere to adsorbed Alb by mechanisms linked to its conformational state. To definitively address this issue we used circular dichroism (CD) spectropolarimetry to characterize the conformation of Alb adsorbed on a broad range of surface chemistries from a wide range of Alb solution concentrations, with platelet adhesion examined using a lactate dehydrogenase (LDH) assay and scanning electron microscopy (SEM). Our results prove that platelets bind to adsorbed Alb through receptor-mediated processes, with binding sites in Alb exposed and/or formed by adsorption-induced protein unfolding. Most importantly, beyond a critical degree of unfolding, the platelet adhesion levels correlated strongly with the adsorption-induced unfolding in Alb. The blockage of Arg-Gly-Asp (RGD) specific platelet receptors using an Arg-Gly-Asp-Ser (RGDS) peptide led to significant inhibition of platelet adhesion to adsorbed Alb, with the extent of inhibition and morphology of adherent platelets being similar for both Alb and Fg. Chemical neutralization of arginine (Arg) residues in the adsorbed Alb layer inhibited platelet–Alb interactions significantly, indicating that Arg residues play a prominent role in mediating platelet adhesion to Alb. These results provide deeper insight into the molecular mechanisms that mediate the interactions of platelets with adsorbed proteins, and how to control these interactions to improve the blood compatibility of biomaterials for cardiovascular applications.
Keywords: Albumin, Protein adsorption, Platelet adhesion, Haemocompatibility
1. Introduction
The adsorption of blood plasma proteins to the surfaces of synthetic materials used in cardiovascular applications is widely recognized in the biomaterials field as being an important mediator of platelet adhesion, thrombus formation, and blood compatibility. Human albumin (Alb), with a plasma concentration of 40 mg/mL [1], is the most abundant protein in blood. Its high concentration, combined with a moderately low molecular weight (68.3 kDa) [2] causes it to be one of the first proteins to adsorb on the surface of implanted biomaterials [3]. Since Alb lacks any known amino acid sequences for binding platelet receptors, this protein has been considered to be unable to support platelet adhesion and hence is widely used for blocking non-specific platelet–surface interactions in platelet adhesion studies [4] as well as for a hemocompatible coating for biomaterial surfaces [5,6]. In cases where platelet adhesion has been observed to occur on Alb-coated surfaces, it has generally been attributed to some non-specific process [4], such as the interaction between the platelets and exposed surface area of the substrate, as opposed to actually representing an interaction with Alb itself.
Two recent studies, however, have suggested that platelets may actually have specific mechanisms to adhere to adsorbed Alb [7,8], and that these mechanisms may be linked to the conformational state of the protein [7]. Unfortunately, these studies did not rule out the possibility that these responses may have been due to platelet interactions with some other residual protein in the system, thus leaving this question still open to debate. To clarify this issue, we conducted studies to definitively determine whether non-activated platelets can adhere to adsorbed Alb; and if so, whether the adhesion response is due to receptor-mediated processes that can be directly correlated with the degree of adsorption-induced unfolding of the protein, which we measured by adsorbed-state circular dichroism (CD) spectropolarimetry.
CD spectropolarimetry was first used by McMillin et al. [9] to examine the adsorbed conformation of fibrinogen and factor XII on quartz substrates. Subsequent CD studies by Norde and Favier [10] showed that proteins tend to undergo a loss in α-helical content upon adsorption, with this effect being diminished with increasing protein solution concentration, which translated to higher protein surface coverage. A more recent study done by our group [11] investigated the role of surface chemistry and protein solution concentration on the adsorbed conformation of fibrinogen (Fg) and Alb, and showed that Fg and Alb undergo a greater degree of unfolding with increasing surface hydrophobicity and decreasing solution concentration, which is in agreement with the results obtained by Norde and Favier, as well other groups via grazing-angle infrared spectroscopy [12] and total internal reflectance fluorescence (TIRF) [13]. These studies have thus demonstrated that CD spectropolarimetry is a powerful technique for examining post-adsorptive conformational changes in proteins on biomaterial surfaces.
The role of the adsorbed conformation of the protein in mediating platelet interactions has come into prominence relatively recently [7,14,15], whereas previously the amount of adsorbed protein has generally been considered to be the primary determinant of the hemocompatibility of biomaterials [16,17]. Tanaka et al. [15] indicated that although poly(2-methoxyethylacrylate) (PMEA) and poly(2-hydroxyethylmethacrylate) (PHEMA) adsorbed similar amounts of Fg, the PHEMA surface exhibited higher platelet adhesion compared to PMEA, which was believed to be due to a higher degree of conformational change in the adsorbed Fg (as measured by the loss in α-helix using CD) on PHEMA. In support of the findings by Tanaka et al., a recent study by Hylton et al. [7] also showed a strong correlation between platelet adhesion and the adsorption-induced loss in percentage α-helix in adsorbed proteins, providing further evidence that conformation is a critical determinant of the ability of an adsorbed protein layer to mediate platelet adhesion. The fact that non-activated platelets do not become activated when they interact with proteins in their native state in the bloodstream, but readily bind and become activated when they interact with adsorbed proteins such as fibrinogen, vitronectin, and fibronectin [18], also strongly suggests that the adsorption process causes structural changes in the protein leading to the exposure of platelet binding sites which would otherwise have remained in a nonactive form in their native state.
In order to further investigate the role of adsorption-induced conformational changes in mediating platelet adhesion and whether Alb itself can support platelet adhesion by specific mechanisms, we established test conditions to provide a broad range of conformational states of adsorbed Alb following which platelet adhesion studies were carried out, including the use of several different blocking agents to verify whether or not the platelet responses were indeed being mediated by specific interactions with the adsorbed Alb. A broad range of conformational states of the adsorbed protein was obtained by using (i) a wide range of surface chemistries and (ii) a wide range of Alb solution concentrations to influence the degree of protein unfolding that was induced during the protein adsorption process. For our surfaces, we used alkanethiol self-assembled monolayers (SAMs) on gold-coated substrates functionalized with OH, COOH, NH2, OCH2CF3, and CH3 head-groups to provide a broad range of surface hydrophobicity. These types of surfaces have been widely used in previous studies dealing with both plasma protein adsorption [11] and platelet adhesion [8,19]. Alb was adsorbed to each of these surfaces from three different solution concentrations (0.1, 1.0, and 10 mg/mL) to vary the rate of protein adsorption, following which the conformation of the adsorbed Alb was assessed by adsorbed-state CD spectropolarimetry [11]. Platelet adhesion studies were then conducted and correlated with the degree of adsorption induce Alb unfolding, with the platelet adhesion response assessed by a lactate dehydrogenase (LDH) assay and scanning electron spectroscopy (SEM). Platelet blocking studies were performed using a set of antibody, soluble peptide, and protein side-chain modification studies.
2. Materials and methods
2.1. Gold substrates
Quartz slides (0.375″ × 1.625″ × 0.0625″, Chemglass) were used for CD experiments, while 18 mm square cover glasses (VWR Scientific, Catalog No. 48368-040) were used as substrates for the platelet adhesion experiments. These substrates were cleaned as previously described [11] prior to the deposition of chromium and gold films via thermal vapor deposition. The cover glasses were coated with a 50 Å chromium adhesion layer and 1000 Å of gold, while the quartz slides for CD were coated with 30 Å of chromium and 100 Å of gold. The thicknesses of the gold and chromium layers were verified using a DekTak profilometer and a GES5 ellipsometry (Sopra, Inc., Palo Alto, CA).
2.2. Formation of self-assembled monolayers (SAMs) of alkanethiols
The following alkanethiols were prepared in 100% ethanol (Pharmco-Aaper; Catalog No. 111000200), and used for creating the SAM surfaces:
1-Dodecanethiol (SH–(CH2)11CH3; Aldrich; CH3),
11-(2,2,2-Trifuoroethoxy) undecane-1-thiol (SH–(CH2)11OCH2CF3; Aldrich; CF3)
11-Amino-1-undecanethiol, hydrochloride (SH–(CH2)11NH2HCl; Prochimia; NH2)
-
11-Mercaptoundecanoic acid (SH–(CH2)11COOH; Aldrich; COOH), and
11-Mercapto-1-undecanol (SH–(CH2)11OH; Aldrich; OH).
The gold-coated substrates were cleaned by dipping them in a modified piranha wash (4:1 v/v H2SO4/H2O2), followed by a Radio Corporation of America (RCA) basic wash (1:1:5 v/v NH4OH/H2O2/H2O), for 1 min each and then rinsed copiously with 100% ethanol, prior to incubating them in a 1.0 mM alkanethiol solutions for 24 h, as per the established protocols [20,21], described previously [11].
After SAM formation on the gold substrates, the SAM surfaces were cleaned to remove any traces of hydrophobic contaminants on their surface prior to surface characterization and protein adsorption [22]. The CH3 and OCH2CF3 SAMs were cleaned by sonication in ethanol, hexane and ethanol, and then rinsed with nanopure water. The NH2, COOH, and OH SAMs were sonicated in ethanol, and then incubated in a 25 mM potassium phosphate buffer containing 0.005 volume % Triton-X-100 (Sigma; Catalog No. T-9284), in order to block off any hydrophobic defect sites (e.g. grain boundaries), and then rinsed thoroughly with acetone, ethanol and nanopure water to remove loosely-bound Triton.
2.3. Contact angle goniometry
Advancing contact angle measurements on the SAM surfaces were carried out using a CAM 200 Optical Contact Angle/Surface Tension Meter (KSV Instruments Ltd.) and the CAM 200 software, as previously described [11], with the results presented in Table S1 of the Supporting Information section.
2.4. Buffers
Protein adsorption experiments were carried out using a 25 mM potassium phosphate buffer (pH 7.4), which was prepared by combining appropriate amounts of the mono- and dibasic salts (Sigma–Aldrich) to maintain the pH at 7.4. This buffer is recommended for protein structural determination by CD experiments [3,11,23,24] as it permits measurement of CD spectra with minimal noise below 200 nm, especially the positive CD peaks at 193 and 195 nm, which are critical in determining the α-helix and β-sheet content of the proteins, respectively.
The platelet suspension buffer (PSB, pH 7.4) contained 137 mM NaCl, 2.7 mM KCl, 5.5 mM Dextrose, 0.4 mM sodium phosphate monobasic, 10 mM HEPES and 0.1U/mL apyrase [25]. 2.5 mM CaCl2 and 1.0 mM MgCl2 was added to the PSB to give a platelet suspension buffer with metal ions (PSB + MI). It should be noted that bovine serum Alb (BSA) was not added to the platelet suspension buffer as a blocking agent as is commonly used in platelet adhesion studies. Preliminary studies were also conducted, however, with 4 mg/mL BSA added to this buffer to assess the influence of this blocking agent on the platelet adhesion response, with the use of BSA not resulting in any significantly differences (see Supporting Information, Section S.4).
2.5. Protein adsorption
Human Alb (Sigma, Catalog No. A9511) was dissolved in 25 mM phosphate buffer solution (pH 7.4), to prepare the protein stock solutions, and protein adsorption was carried out as described previously [11], at three different protein concentrations of 0.1 mg/mL,1.0 mg/mL, and 10.0 mg/mL. At the end of the protein incubation step, the surfaces with preadsorbed protein were either used for CD studies to analyze the structure of the adsorbed layer, or the surfaces were incubated with the platelet suspension.
2.6. CD studies to quantify the adsorption-induced conformational changes and total surface coverage of Alb on SAM surfaces
The native and adsorbed secondary structures of Alb, as well as the surface coverage of adsorbed Alb, was determined using a Jasco J-810 spectropolarimeter (Jasco, Inc., Easton, MD), and the secondary structural content was determined using the SELCON software package [26], as described earlier [11].
Since proteins exhibit an absorbance peak at 195 nm [27], we used the height of this absorbance peak (A195) for determining the surface coverage of Alb (Qads) on the SAMs, as described in our previous work [11]. This method for measuring the surface coverage of adsorbed protein has been validated by independent measurement of Qads from the thickness of the adsorbed protein film obtained by ellipsometry [11] using de Feijter’s formula [28].
2.7. Platelet adhesion
Platelet adhesion was carried out, using a suspension of washed platelets from human blood. Briefly, 25.0 mL of blood was collected from volunteers as per protocols approved by the Institutional Review Board (IRB) and Institutional Biosafety Committee (IBC) at Clemson University, in BD Vacutainer tubes (Becton–Dickinson, Catalog No. 364606) containing an acid-citrated dextrose (ACD) anticoagulant. The first few mL of blood was discarded, as it is rich in clotting factors and then the 25 mL of blood was collected.
The ACD-anticoagulated blood was centrifuged (225g, 15 min, 25 °C) to generate platelet rich plasma (PRP), and platelets were separated from PRP via a gel separation method [25], using a liquid chromatography column (Sigma–Aldrich, Catalog No. C4169) containing Sepharose 2B (Sigma–Aldrich, Catalog No. 2B-300). The Sepharose column was allowed to equilibrate with PSB, after which the PRP was carefully pipetted on to the column and allowed to pass into the column. PSB was then added to the column from a reservoir, while keeping the column running. Fractions were collected from the bottom of the column, with the platelets being identified by their increased turbidity. The platelet rich fractions were pooled, and platelet concentration was measured using a Beckman Coulter Z2 Coulter Particle Count and Size Analyzer (Beckman Coulter, Fullerton, CA). The platelet count was adjusted to 108 platelets/mL with PSB, and CaCl2 and MgCl2 were added to give 2.5 mM and 1.0 mM concentrations of these salts, respectively. Platelet suspension was allowed to rest for 30 min, and the platelet adhesion step was carried out on the protein-coated SAMs for 1 h at 37 °C.
The platelet suspension was then aspirated from each well at the end of the platelet adhesion step, and the non-adherent platelets were rinsed away by filling and aspirating the wells five times with PBS. Platelet adhesion levels were quantified using a lactate dehydrogenase (LDH) assay, and the morphology of the adherent platelets was visualized using scanning electron microscopy (SEM).
2.8. Measurement of platelet adhesion using lactate dehydrogenase (LDH) assay
The platelet adhesion levels on the Alb-coated SAMs was quantified by measuring the lactate dehydrogenase (LDH) released when the adherent platelets were lysed with a Triton-PSB buffer, using a CytoTox96® Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI), as indicated in our previous study [29].
2.9. Scanning electron microscopy (SEM)
The samples for SEM were fixed, as described in our previous study [29]. Briefly, the samples were immersed in a special fixing buffer (3% glutaraldehyde, 0.1 mM sodium cacodylate, pH 7.4), for 30 min at room temperature, after which they were rinsed with PBS thrice. The fixed samples were then incubated in ascending ethanol:water mixtures (50%, 60%, 70%, 80%, 90% and 99% ethanol) for 10 min to dehydrate them. These samples were finally treated with 0.02 mL of hexamethyldisilazane (Sigma) and allowed to dry overnight. The morphology of the adherent platelets was examined using a FESEM-Hitachi S4800 scanning electron microscope (Electron Microscopy Facility, Advanced Materials Research Laboratory, Clemson University, Pendleton, SC).
2.10. SDS-PAGE analysis of platelet suspension
The platelet suspensions obtained from two separate gel separation runs were centrifuged at 1200 rpm, 1500 rpm, and 1800 rpm, and the supernatant was aspirated and analyzed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for any traces of residual plasma proteins. Silver staining was done using a Silver SNAP Stain Kit II (Pierce, Rockford, IL), which provides a highly sensitive detection system for sub-nanogram levels of proteins. This is essential in ensuring that the platelet suspension was free of any residual plasma proteins, which may potentially complicate our analysis by displacing the preadsorbed protein and then binding platelets.
2.11. Study using polyclonal antibodies against Alb
Polyclonal antibodies against human Alb (Anti-Alb Rabbit pAb; Calbiochem, La Jolla, CA) at a concentration of 4 μg/mL were incubated for 30 min at room temperature, on the Alb surfaces in order to inhibit Alb recognition by the platelet receptors. As a control, SAM surfaces coated with Fg (FIB 3; plasminogen, von Willebrand factor, and fibronectin depleted; Enzyme Research Laboratories, South Bend, IN) were incubated with 4 μg/mL of the anti-Alb polyclonal antibodies, and platelet adhesion was quantified using an LDH assay.
2.12. Study using polyclonal antibodies against Fg
Polyclonal antibodies against human Fg (Anti-fibrinogen Rabbit pAb; Calbiochem, La Jolla, CA) at a concentration of 4 μg/mL were incubated for 30 min at room temperature, on the Alb-coated surfaces in order to verify whether the inhibition of platelet–Alb interactions was due to the effects of the anti-Alb polyclonal antibodies or the adsorption of these antibodies on bare surface exposed between the adsorbed Alb molecules. Platelet adhesion was characterized as described above.
2.13. Inhibition of platelet–protein interactions using Arg-Gly-Asp (RGD)-containing peptides
The platelet suspension was incubated with 300 μM of an Arg-Gly-Asp-Ser (RGDS) peptide (Calbiochem, La Jolla, CA) for 30 min at room temperature, in order to inhibit interactions between RGD-binding platelet receptors and Alb. These RGDS-treated platelets were then allowed to adhere on the Alb-coated SAM surfaces, after which the platelet adhesion was quantified using an LDH assay and the morphology of the adherent platelets was examined using SEM.
Platelets incubated with 300 μM of a control Arg-Gly-Glu-Ser (RGES) peptide (American Peptide Company, Sunnyvale, CA) were allowed to adhere on the SAM surfaces preadsorbed with Alb.
2.14. Modification of Arg residues in Alb using 2,3-butanedione
Site-specific modification of R residues in Alb was carried out using 2,3-butanedione (Fluka), as described previously [30]. Briefly, an Alb solution was modified by addition of suitable amount of 2, 3-butanedione to borate buffer to give a final concentration of 50 mM of the modifying agent. The modified protein samples were dialyzed for 12 h against 25 mM phosphate buffer, and then analyzed for Arg modification via a protocol described previously [22], using electrospray ionization mass spectrometry (ESI-MS) performed at the Clemson University Genomics Institute. Additionally, we used CD spectropolarimetry to analyze the secondary structure of the modified Alb in solution and compared it with that of unmodified Alb.
2.15. Platelet adhesion to SAM surfaces coated with Alb with modified arginine residues
Alb adsorption was carried out, after which the arginine residues exposed in the adsorbed Alb layer were neutralized by 2, 3-butanedione, using the protocol described above. After washing away the excess butanedione, the surfaces were incubated with platelets, and the platelet adhesion levels were quantified using an LDH assay, with the morphology of the adherent platelets being observed using SEM.
2.16. Statistical analysis
The results we present are the mean values with 95% confidence intervals (CI). Statistical significance of differences between mean values for different samples and conditions was evaluated using a Student’s t-test, with p ≤0.05 considered as statistically significant.
3. Results and discussion
3.1. Circular dichroism studies on native and adsorbed Alb
The secondary structural content of native and adsorbed Alb on the SAM surfaces as a function of surface chemistry and solution concentration, determined via CD spectropolarimetry, are presented in Fig. 1. These results clearly illustrate the higher degree of adsorption-induced conformational changes (i.e., loss of α-helix accompanied by increased β-sheet) as the surfaces became more hydrophobic and when the protein was absorbed from a lower solution concentration, with the combined variation of both surface chemistry and solution concentration effectively providing a wide range of conformational states of the adsorbed Alb.
Fig. 1.
Secondary structure of adsorbed Alb on SAM surfaces at (A) 0.1 mg/mL, (B) 1.0 mg/mL and (C) 10.0 mg/mL bulk solution concentrations (n = 6, mean ± 95% CI). * denotes difference not statistically significant, p > 0.05.
The concentration dependence of adsorption-induced unfolding in Alb is clearly illustrated in Fig. 1, with the degree of adsorption-induced conformational change decreasing with increasing Alb solution concentrations. This can be attributed to the progressively higher transport rate of Alb molecules to the surface from solution with increasing protein solution concentration, as a result of which the adsorbed proteins have less time to unfold and spread out on the surface before it becomes saturated, preventing further protein spreading [12,31]. For Alb adsorbed on the SAM surfaces from 10.0 mg/mL Alb solutions (Fig. 1C), the molecules adsorbed on the surfaces with minimal spreading due to their rapid rate of transport to the surface, resulting in their secondary structure being much closer to their native state.
The surface coverage of albumin adsorbed on the SAMs from the three different bulk concentrations was calculated using the height of the absorbance peak at 195 nm (A195), as described previously [11], and is shown in Table 1. The surface coverage at 0.1 and 1.0 mg/mL bulk Alb solution concentration lie between the theoretical monolayer surface coverage values of 0.72 μg/cm2 for end-on adsorption and 0.21 μg/cm2 for side-on adsorption [13], assuming that the Alb molecule has dimensions of 4.0 × 4.0 × 14 nm3 [32]. The surface coverage at 10.0 mg/mL Alb solution concentration was well beyond the theoretical values for monolayer surface coverage, indicating multilayer adsorption. These results clearly indicate that the surfaces are saturated with Alb, and the amount of Alb adsorbed increases with increasing hydrophobicity of the SAM surfaces. Significantly greater Alb adsorption also occurred on a given SAM surface with increasing Alb solution concentration. This can be explained by the fact that the rate of transport of the protein molecules to the surface increases as solution concentration increases, as a result of which the molecules that adsorb from higher concentration have less time to unfold and spread before the surface becomes saturated with protein [13,31,33].
Table 1.
Amounts of Alb adsorbed (Qads) on SAM surfaces from 0.1, 1.0 and 10.0 mg/mL bulk solution concentrations (n = 6, mean ± 95% CI).
| Albumin concentration [mg/mL] | OH [μg/cm2] | COOH [μg/cm2] | NH2 [μg/cm2] | CF3 [μg/cm2] | CH3 [μg/cm2] |
|---|---|---|---|---|---|
| 0.1 mg/mL | 0.25 ± 0.01 | 0.28 ± 0.01 | 0.46 ± 0.03 | 0.40 ± 0.02 | 0.54 ± 0.03 |
| 1.0 mg/mL | 0.26 ± 0.01 | 0.29 ± 0.01 | 0.49 ± 0.02 | 0.44 ± 0.01 | 0.58 ± 0.01 |
| 10.0 mg/mL | 1.77 ± 0.05 | 2.23 ± 0.09 | 2.26 ± 0.19 | 2.55 ± 0.24 | 2.71 ± 0.18 |
3.2. Platelet adhesion to adsorbed Alb
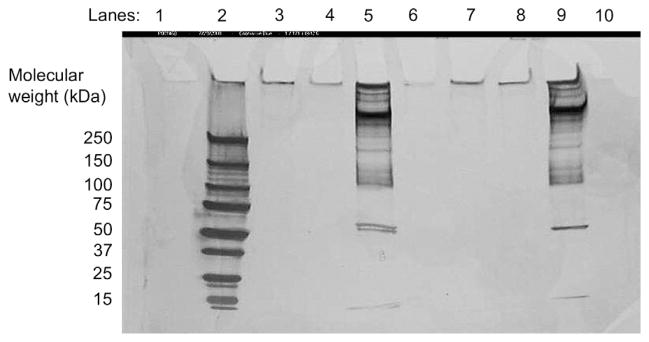
A suspension of washed platelets was used for the platelet adhesion runs. Prior to carrying out the platelet adhesion experiments, it was essential to check for traces of residual proteins that could potentially adsorb onto and/or displace the preadsorbed Alb from the SAM surfaces through the Vroman effect [34]. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis performed on supernatant fluid from centrifugation of the platelet suspensions showed no detectable levels of proteins (Fig. 2) in the suspensions centrifuged at 1200 rpm and 1500 rpm. Since activated platelets are likely to release proteins from their granules into the suspension, this also indicates that the platelets in suspension were not activated during the gel separation process through the Sepharose column, or during any handling prior to the platelet adhesion step. The bands observed for the samples centrifuged at 1800 rpm (Lanes 5 and 9 in Fig. 2) can be attributed to the release of platelet granular contents due to platelet lysis at high centrifugation speeds.
Fig. 2.
SDS-PAGE analysis for residual protein content in supernatant of two separate platelet suspensions, separated from platelet rich plasma by gel separation. (Lane 1: Blank, Lane 2: Protein ladder, Lanes 3 & 7: platelet suspension centrifuged at 1200 rpm, Lanes 4 & 8: Platelet suspension centrifuged at 1500 rpm, Lanes 5 & 9: Platelet suspension centrifuged at 1800 rpm, Lanes 6 & 10: Blank). Staining was done using a SilverSNAP® Stain Kit II.
Platelet adhesion levels on the SAM surfaces preadsorbed with Alb are shown in Fig. 3. It is clearly evident that platelet adhesion did occur on these surfaces, with platelet adhesion being strongly influenced by both surface chemistry and the solution concentration from which the Alb was adsorbed. Platelet adhesion levels followed the same general trends observed for the adsorption-induced conformational changes in Alb (Fig. 1); i.e., increasing with increasing hydrophobicity of the surfaces and decreasing Alb solution concentration.
Fig. 3.
Platelet adhesion on SAM surfaces preadsorbed with Alb from bulk solutions of 0.1 mg/mL, 1.0 mg/mL and 10.0 mg/mL (n = 6, mean ± 95% CI). * denotes no statistically significant difference, p > 0.05.
To investigate the relationship between platelet adhesion and the degree of adsorption-induced protein unfolding, the platelet adhesion levels (Fig. 3) were plotted as a function of the extent of loss of α-helix in Alb measured on these surfaces, as presented in Fig. 4A. As clearly shown in this figure, the platelet adhesion response correlated strongly (r2 = 0.92) with the degree of conformational change in adsorbed Alb when the percentage of α-helix loss was greater than about 34%. For levels of unfolding less than this threshold value, platelet adhesion was negligible, with the slope of the regression line for this region of the plot not being significantly different than zero (p = 0.60). Thus, below this critical level of unfolding, the layer of adsorbed Alb can be considered to be ‘passive’ in terms of its ability to adhere platelets, which supports the conventional understanding that Alb functions as a blocking agent for non-specific interactions [4]. However, once this critical level of unfolding was exceeded, platelets readily adhered to the adsorbed Alb, with the adhesion response increasing linearly as the degree of unfolding increased. Hence, this suggests that platelet binding domains in Alb are being exposed and/or formed beyond this critical level of unfolding, thereby inducing the platelet adhesion response.
Fig. 4.

Platelet adhesion to (A) Alb and (B) Fg on the SAM surfaces as a function of the degree of loss in α-helix (Each point represents the mean of six values for each SAM surface). Fg data is provided for comparison to the Alb results and is reproduced from [29].
A particularly intriguing result from this study is that beyond this critical level of unfolding, the increase in platelet adhesion as a function of continued unfolding of the Alb occurred in a manner very similar to fibrinogen (Fg), which we measured in a related study [29] (Fig. 4B). This finding suggests that, beyond this critical level, platelet adhesion to both of these proteins as a function of the degree of adsorption-induced unfolding may be due to similar mechanisms. Given the fact that there are no known platelet binding sites in albumin, this similarity actually suggests that the platelet binding response to adsorbed Fg as it unfolds is being mediated by processes that are not associated with currently known platelet binding motifs in Fg, but rather are being driven by the exposure and/or formation of new platelet receptor recognition sites similar to the process that is occurring in Alb.
3.3. Delineating the role of RGD-specific receptors in platelet–Alb interactions
To probe the molecular mechanisms underlying the observed platelet adhesion response, four independent blocking studies were conducted for Alb adsorbed from the most dilute Alb solution (0.1 mg/mL) on the two SAM surfaces representing the extremes in hydrophobicity (OH and CH3 SAMs).
First, in order to confirm that platelets were actually adhering to the adsorbed Alb and not to residual proteins that may have adsorbed from the platelet suspension (e.g., proteins released from the platelets granules), the adsorbed Alb layer was treated with polyclonal antibodies against human Alb (pAb-Alb) prior to performing the platelet adhesion studies. These pAb-Alb were then also used to treat adsorbed Fg as a negative control. Secondly, to ensure that the effect of the pAb-Alb was due to their specificity towards Alb, and not due to minimization of interactions between platelets and bare SAMs as a result of the adhesion of these antibodies on the surface of the SAMs (i.e., in the gaps possibly present between adjacent adsorbed Alb molecules), the Alb-coated SAMs were treated with anti-Fg polyclonal antibodies (pAb-Fg) before exposure to the platelet suspension. Thirdly, the platelet suspensions were treated with RGDS peptides prior to exposure to the Alb-coated SAM surfaces, with RGES used as a control, to determine whether platelet adhesion was being mediated by RGD-specific receptors. Finally, the solvent-accessible arginine (R) residues in preadsorbed Alb were chemically modified using 2,3-butanedione [35,36] prior to conducting the platelet adhesion studies, to determine if they were involved in the platelet binding motifs that were being exposed or formed as a function of the adsorption-induced Alb unfolding. Control studies were conducted by using 2,3-butanedione to modify the R residues in Alb in solution, followed by analysis via electrospray ionization mass spectrometry (ESI-MS) to confirm that the modification procedures actually caused the intended site-specific modification of the R residues. In addition, CD studies were conducted to determine the effect of this chemical modification process on the conformation of Alb in both its soluble and adsorbed-states. The results of these four studies are shown in Fig. 5 and in Table S3.
Fig. 5.
Platelet adhesion on SAM surfaces preadsorbed with Alb and Fg from 0.1 mg/mL solutions under various blocking conditions. The blocking treatments included RGDS and RGES peptides, as well as polyclonal antibodies against Alb (pAb vs. Alb) and Fg (pAb vs. Fg) (n = 6, mean ± 95% CI). * denotes no statistically significant difference, p > 0.05. Fg data is provided for comparison to the Alb results and is reproduced from [29].
As clearly shown in Fig. 5, the pAb-Alb nearly completely inhibited platelet adhesion to preadsorbed Alb on both the CH3 and OH SAMs, while not significantly affecting platelet adhesion to either the CH3 or OH SAMs preadsorbed with Fg. Also, the pAb-Fg did not inhibit platelet adhesion to adsorbed Alb, providing further proof that the pAb-Alb were effective in blocking platelet adhesion to adsorbed Alb due to their specificity towards Alb and not due to their adsorption on bare surface exposed between the adsorbed Alb molecules on the SAMs.
Treatment of the platelets with the RGDS peptides resulted in a substantial decrease in platelet binding, with 60.2% and 48.0% reductions in platelet adhesion to the adsorbed Alb on the CH3 and OH SAM surfaces, respectively; while the RGES peptides had no significant effect. These results indicate that non-RGD-specific platelet receptors, which account for nearly 50% of the platelet response on both SAM surfaces, mediate the adhesion of RGDS-treated platelets to adsorbed Alb. When these results were compared with those obtained from our previous related study [29] for RGDS-based inhibition of platelet adhesion to Fg, it was surprising to find that for a given SAM surface, the levels of inhibition observed were nearly identical for both Alb and Fg (58.3% for Fg adsorbed on a CH3 SAM, and 45.7% for Fg adsorbed on the OH SAM). These results further support the possibility of platelet adhesion to Fg and Alb as a function of their unfolded state is mediated by similar specific mechanisms.
Chemical modification of R residues of the preadsorbed Alb also significantly reduced platelet adhesion, with the reduction on the OH SAMs not being significantly different (p = 0.18) than the response from the RGDS peptides, while the reduction on the CH3 SAMs was significantly greater (p < 0.05) than the reduction from treatment with RGDS. This may be due to the fact that R modification is irreversible, while binding of the RGDS peptide by the platelet receptors represents a reversible, competitive binding situation, with some level of RGD-mediated platelet adhesion thus still being expected to occur in the presence of the RGDS peptide. The ESI-MS results confirmed that the chemical modification was specific to only the R residues, with all R residues in the Alb in its solution state being modified under the applied conditions. Table 2 presents the results from the CD analyses of Alb before and after R modification, which confirms that this chemical treatment had minimal influence on the secondary structure of the Alb in either its solution or its adsorbed condition. CD spectra comparing the unmodified and R-modified Alb in solution are presented in Figure S1 (see Supporting Information).
Table 2.
Comparison of secondary structural composition of unmodified Alb and Alb with arginine residues modified using 2, 3-butanedione, in solution and after adsorption on the CH3 and OH SAMs (n = 6, mean ± 95% CI).
| Sample | α-helix (%) | β-sheet (%) |
|---|---|---|
| In solution | ||
| Human Alb-unmodified | 66.5 ± 1.5 | 1.5 ± 0.7 |
| Human Alb with R-modification | 65.8 ± 0.9 | 0.9 ± 0.3 |
| After adsorption | ||
| CH3, 0.1 mg/mL Alb-unmodified | 14.4 ± 0.7 | 28.3 ± 0.7 |
| CH3, 0.1 mg/mL Alb-modified | 15.7 ± 1.3 | 28.6 ± 1.5 |
| OH, 0.1 mg/mL Alb-unmodified | 43.1 ± 2.1 | 13.4 ± 1.1 |
| OH, 0.1 mg/mL Alb-modified | 42.6 ± 1.4 | 12.3 ± 0.5 |
Overall, these results indicate that platelets are able to adhere to adsorbed Alb and that adhesion is strongly correlated with the adsorption-induced loss of α-helix in Alb (Fig. 4A). The adhesion is substantially due to a specific binding mechanism involving RGD-specific receptors on non-activated platelets that are able to recognize motifs involving R residues that are either exposed and/or formed in Alb after it undergoes a critical degree of adsorption-induced unfolding.
The prominent role played by RGD-binding platelet receptors and the R residues of Alb in mediating platelet adhesion to adsorbed Alb is intriguing, considering the conventional view that Alb does not support platelet adhesion through specific binding mechanisms. As shown in Fig. 6, Alb contains a large number of charged amino acids (24 arginine, 59 lysine, 35 aspartic acid, and 62 glutamic acid residues). We hypothesize that in its native state, these residues are not structured in a manner to be recognized by platelet receptors, but after undergoing a critical level of adsorption-induced unfolding, oppositely charged pairs of residues within this protein become spatially positioned such that they become recognizable to RGD-specific receptors in the platelets similar to an RGD motif. We further hypothesize that the incidence of this increases with increased degree of conformational rearrangement, thus resulting in the linear relationship between platelet adhesion and the percent loss in α-helix, observed in Fig. 4A.
Fig. 6.

Molecular structure of human serum Alb (PDB ID: 1E7H) [2] with charged residues displayed in spacefill mode and all other residues displayed in wireframe. Arginine (blue), lysine (green), aspartic acid (red), and glutamic acid (yellow). Figure was generated using RASMOL.
Scanning electron microscopy (SEM) analyses were conducted to assess the effect of RGDS peptides and the R modification of adsorbed Alb on the morphology of adherent platelets on the OH and CH3 SAMs with preadsorbed Alb. Fig. 7A and D, show that the platelets adherent on the CH3 and OH SAMs preadsorbed with Alb underwent a similar moderate degree of spreading and extension of filopodia on both surfaces, even though platelet adhesion on the CH3 SAM was more than six times higher than on the OH SAM. These results indicate that the adhesion mechanism was similar and induced a moderate level of platelet activation on both surfaces. However, when the platelets were treated with an RGDS peptide prior to the platelet adhesion (Fig. 7B and E), which resulted in about 50–60% reduction in adhesion, they exhibited a distinctly different morphology, with much less spreading and minimal extension of filopodia. These results suggest that the mechanism of adhesion to RGDS-treated platelets was different from that of untreated platelets. As shown in Fig. 7C and F, platelets adherent on the adsorbed Alb with R modification exhibited morphologies similar to the RGDS-treated platelets. This supports a hypothesis that R modification of adsorbed Alb results in blocking the same platelet receptors as those blocked by the RGDS peptides.
Fig. 7.
Platelet adhesion to the CH3 (top row) and OH SAMs (bottom row) preadsorbed with 0.1 mg/mL Alb, under different blocking conditions: (A,D) unblocked Alb, (B,E) platelets treated with RGDS, and (C,F) adsorbed Alb layer treated with 2,3-butanedione to neutralize Arg.
The morphology of RGDS-treated platelets adherent to adsorbed Alb on the CH3 and OH SAMs was strikingly similar to those observed to be adherent to Fg in the presence of RGDS on the same SAMs in our previous related study [29], as seen in Fig. 8. This provides further evidence that similar adhesion mechanisms are involved in platelet adhesion to both Alb and Fg as a function of their degree of adsorption-induced unfolding on a surface.
Fig. 8.
Morphology of RGDS-treated platelets adherent to CH3 (top row) and OH SAMs (bottom row), preadsorbed with Fg (A,C) and Alb (B,D). Fg data is provided for comparison to the Alb results and is reproduced from [29].
Taken altogether, the SEM results strongly suggest that there are at least two distinct sets of receptors associated with platelet adhesion to sites in adsorbed Alb that are exposed and/or formed following adsorption-induced unfolding beyond a critical degree; one RGD-specific receptor that recognizes a protein motif involving R that mediates both adhesion along with a moderate degree of activation, and another that induces platelet adhesion with minimal activation. We plan to attempt to identify these receptors and to further examine the mechanisms underlying their interactions with adsorbed Alb in future investigations.
4. Conclusions
The results from these studies conclusively demonstrate that platelets adhesion to adsorbed Alb is substantially mediated by interactions between RGD-specific platelet receptors and R residues in the adsorbed Alb, beyond a critical level of adsorption-induced protein unfolding. Additionally, we provide evidence that the mechanisms underlying platelet adhesion to Fg and Alb as a function of the degree of adsorption-induced unfolding are similar in nature, with platelet adhesion increasing as a function of the degree in adsorption-induced unfolding for both proteins (beyond a critical degree of unfolding of the Alb) and RGDS-treatment induced similar levels of inhibition of platelet adhesion to both proteins, with similar morphology of the adherent RGDS-treated platelets. Overall, these results suggest that platelet adhesion to adsorbed fibrinogen, and possibly many other adsorbed proteins, involves mechanisms that may be quite different than currently understood. Thus, there is still much to learn about the molecular mechanisms that mediate the interactions of platelets with adsorbed proteins and how to control these interactions to improve the blood compatibility of biomaterials used in cardiovascular applications.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge numerous volunteers for donating blood for these studies, as well as the staff at the Redfern Health Center at Clemson University for their assistance with the blood-draw process. We thank Dr. Kenan Fears for technical discussions and Aby Abraham Thyparambil, Dr. Dan Simionescu and Dr. Agneta Simionescu for experimental assistance. We are also grateful to Dr. Yonnie Wu at the Clemson University Genomics Institute (CUGI) for mass-spectrometric analysis of the modified protein samples, and to Dr. James Harriss at Clemson University, for fabrication of the gold-coated surfaces used in our studies. This project was partially supported by NIH Grant Number P20 RR-016461 from the National Center for Research Resources. We also would like to thank Dr. Thomas Horbett (University of Washington), Dr. Andres Garcia (Georgia Institute of Technology), and Dr. Naren Vyavahare (Clemson University) for their insightful comments related to these studies.
Appendix. Supplementary data
Supplementary data associated with this article can be found in the online version, at doi:10.1016/j.biomaterials.2009.10.017.
Appendix
Figures with essential color discrimination. Figs. 1, 3, 5, and 6 in this article may be difficult to interpret in black and white. The full color images can be found in the online version, at doi:10.1016/j.biomaterials.2009.10.017.
References
- 1.Curry S, Mandelkow H, Brick P, Franks N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat Struct Biol. 1998;5(9):827–35. doi: 10.1038/1869. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya AA, Grune T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol. 2000;303(5):721–32. doi: 10.1006/jmbi.2000.4158. [DOI] [PubMed] [Google Scholar]
- 3.Coelho MAN, Vieira EP, Motschmann H, Mohwald H, Thunemann AF. Human serum albumin on fluorinated surfaces. Langmuir. 2003;19(18):7544–50. [Google Scholar]
- 4.Tsai WB, Grunkemeier JM, Horbett TA. Variations in the ability of adsorbed fibrinogen to mediate platelet adhesion to polystyrene-based materials: a multivariate statistical analysis of antibody binding to the platelet binding sites of fibrinogen. J Biomed Mater Res A. 2003;67A(4):1255–68. doi: 10.1002/jbm.a.20024. [DOI] [PubMed] [Google Scholar]
- 5.Kottke-Marchant K, Anderson JM, Umemura Y, Marchant RE. Effect of albumin coating on the in vitro blood compatibility of Dacron arterial prostheses. Biomaterials. 1989;10(3):147–55. doi: 10.1016/0142-9612(89)90017-3. [DOI] [PubMed] [Google Scholar]
- 6.Marois Y, Chakfe N, Guidoin R, Duhamel RC, Roy R, Marois M, et al. An albumin-coated polyester arterial graft: in vivo assessment of biocompatibility and healing characteristics. Biomaterials. 1996;17(1):3–14. doi: 10.1016/0142-9612(96)80749-6. [DOI] [PubMed] [Google Scholar]
- 7.Hylton DM, Shalaby SW, Latour RA. Direct correlation between adsorption-induced changes in protein structure and platelet adhesion. J Biomed Mater Res A. 2005;73A(3):349–58. doi: 10.1002/jbm.a.30295. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues SN, Goncalves IC, Martins MCL, Barbosa MA, Ratner BD. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials. 2006;27(31):5357–67. doi: 10.1016/j.biomaterials.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 9.McMillin CR, Walton AG. Circular-Dichroism technique for study of adsorbed protein structure. J Colloid Interface Sci. 1974;48(2):345–9. [Google Scholar]
- 10.Norde W, Favier JP. Structure of adsorbed and desorbed proteins. Colloids Surf. 1992;64(1):87–93. [Google Scholar]
- 11.Sivaraman B, Fears KP, Latour RA. Investigation of the effects of surface chemistry and solution concentration on the conformation of adsorbed proteins using an improved circular dichroism method. Langmuir. 2009;25(5):3050–6. doi: 10.1021/la8036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roach P, Farrar D, Perry CC. Interpretation of protein adsorption: surface-induced conformational changes. J Am Chem Soc. 2005;127(22):8168–73. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 13.Wertz CF, Santore MM. Effect of surface hydrophobicity on adsorption and relaxation kinetics of albumin and fibrinogen: single-species and competitive behavior. Langmuir. 2001;17(10):3006–16. [Google Scholar]
- 14.Moskowitz KA, Kudryk B, Coller BS. Fibrinogen coating density affects the conformation of immobilized fibrinogen: implications for platelet adhesion and spreading. Thromb Haemost. 1998;79(4):824–31. [PubMed] [Google Scholar]
- 15.Tanaka M, Motomura T, Kawada M, Anzai T, Kasori Y, Shiroya T, et al. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA) – relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21(14):1471–81. doi: 10.1016/s0142-9612(00)00031-4. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara K, Fukumoto K, Iwasaki Y, Nakabayashi N. Modification of polysulfone with phospholipid polymer for improvement of the blood compatibility. Part 2. Protein adsorption and platelet adhesion. Biomaterials. 1999;20(17):1553–9. doi: 10.1016/s0142-9612(98)00206-3. [DOI] [PubMed] [Google Scholar]
- 17.Suhara H, Sawa Y, Nishimura M, Oshiyama H, Yokoyama K, Saito N, et al. Efficacy of a new coating material, PMEA, for cardiopulmonary bypass circuits in a porcine model. Ann Thorac Surg. 2001;71(5):1603–8. doi: 10.1016/s0003-4975(01)02466-3. [DOI] [PubMed] [Google Scholar]
- 18.Grunkemeier JM, Tsai WB, McFarland CD, Horbett TA. The effect of adsorbed fibrinogen, fibronectin, von Willebrand factor and vitronectin on the procoagulant state of adherent platelets. Biomaterials. 2000;21(22):2243–52. doi: 10.1016/s0142-9612(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 19.Sperling C, Schweiss RB, Streller U, Werner C. In vitro hemocompatibility of self-assembled monolayers displaying various functional groups. Biomaterials. 2005;26(33):6547–57. doi: 10.1016/j.biomaterials.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989;111(1):321–35. [Google Scholar]
- 21.Gooding JJ, Mearns F, Yang WR, Liu JQ. Self-assembled monolayers into the 21(st) century: recent advances and applications. Electroanalysis. 2003;15(2):81–96. [Google Scholar]
- 22.Fears KP, Sivaraman B, Powell GL, Wu Y, Latour RA. Probing the conformation and orientation of adsorbed enzymes using side-chain modification. Langmuir. 2009;25(16):9319–27. doi: 10.1021/la901885d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Damodaran S. In situ measurement of conformational changes in proteins at liquid interfaces by circular dichroism spectroscopy. Anal Bioanal Chem. 2003;376(2):182–8. doi: 10.1007/s00216-003-1873-6. [DOI] [PubMed] [Google Scholar]
- 24.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1(6):2876–90. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunkemeier JM, Tsai WB, Horbett TA. Co-adsorbed fibrinogen and von Willebrand factor augment platelet procoagulant activity and spreading. J Biomater Sci Polym Ed. 2001;12(1):1–20. doi: 10.1163/156856201744416. [DOI] [PubMed] [Google Scholar]
- 26.Sreerama N, Woody RW. Estimation of protein secondary structure from circular dichroism spectra: Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem. 2000;287(2):252–60. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 27.Qiu J, Lee H, Zhou C. Analysis of guanidine in high salt and protein matrices by cation-exchange chromatography and UV detection. J Chromatogr A. 2005;1073(1–2):263–7. doi: 10.1016/j.chroma.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 28.de Feijter JA, Benjamins J, Veer FA. Ellipsometry as a tool to study adsorption behavior of synthetic and biopolymers at air–water-interface. Biopolymers. 1978;17(7):1759–72. [Google Scholar]
- 29.Sivaraman B, Latour RA. The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials. doi: 10.1016/j.biomaterials.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McTigue JJ, Vanetten RL. Essential arginine residue in human prostatic acid-phosphatase. Biochim Biophys Acta. 1978;523(2):422–9. doi: 10.1016/0005-2744(78)90044-x. [DOI] [PubMed] [Google Scholar]
- 31.Latour RA. Biomaterials: protein-surface interactions. In: Wnek GE, Bowlin GL, editors. The encyclopedia of biomaterials and bioengineering: informa healthcare. 2008. pp. 270–84. [Google Scholar]
- 32.Sigal GB, Mrksich M, Whitesides GM. Effect of surface wettability on the adsorption of proteins and detergents. J Am Chem Soc. 1998;120(14):3464–73. [Google Scholar]
- 33.Dee KC, Puleo DA, Bizios R. Protein-surface interactions. In: Dee KC, Puleo DA, Bizios R, editors. An introduction to tissue-biomaterial interactions. John Wiley; 2002. pp. 37–52. [Google Scholar]
- 34.Vroman L, Adams AL. Adsorption of proteins out of plasma and solutions in narrow spaces. J Colloid Interface Sci. 1986;111(2):391–402. [Google Scholar]
- 35.Mahley RW, Innerarity TL, Pitas RE, Weisgraber KH, Brown JH, Gross E. Inhibition of lipoprotein binding to cell-surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B-apoproteins. J Biol Chem. 1977;252(20):7279–87. [PubMed] [Google Scholar]
- 36.Riordan JF. Functional arginyl residues in carboxypeptidase A. Modification with butanedione Biochemistry. 1973;12(20):3915–23. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.