Abstract
Recent evidence has emerged demonstrating that metabolic hormones such as ghrelin and leptin can act on ventral tegmental area (VTA) midbrain dopamine neurons to influence feeding. The VTA is the origin of mesolimbic dopamine neurons that project to the nucleus accumbens (NAc) to influence behavior. While blockade of dopamine via systemic antagonists or targeted gene delete can impair food intake, local NAc dopamine manipulations have little effect on food intake. Notably, non-dopaminergic manipulations in the VTA and NAc produce more consistent effects on feeding and food choice. More recent genetic evidence supports a role for the substantia nigra-striatal dopamine pathways in food intake, while the VTA-NAc circuit is more likely involved in higher-order aspects of food acquisition, such as motivation and cue associations. This rich and complex literature should be considered in models of how peripheral hormones influence feeding behavior via action on the midbrain circuits.
Keywords: leptin, dopamine, midbrain, ventral tegmental area, nucleus accumbens, prefrontal cortex, obesity, striatum
Introduction
Emerging evidence has suggested that peripheral hormones can act on the midbrain dopaminergic systems to control food intake. For instance the adipocyte-derived hormone leptin, in addition to acting through the hypothalamus, also acts on midbrain circuits to influence food intake [1], providing a molecular and cellular mechanism for predicted cross-talk between metabolic hormones and reward systems [2]. These results effectively connect the peripheral hormone system to dopamine circuitry that has been long studied for its role in behavior, including food intake. The literature on dopamine and food intake is vast and here we attempt to distill some of the major findings to set the stage for understanding how metabolic factors might influence feeding via these networks.
The acquisition of food requires motivation, goal-directed behavior, motor control, and the recognition of rewarding stimuli. Dopamine-containing brain systems have been implicated in these behavioral processes. Studies of drug addiction have clearly established that recognition of reward is intimately involved with projections of dopamine-containing neurons in the midbrain to the ventral striatum (or nucleus accumbens, NAc), cortex, and subcortical nuclei [3; 4]. Similarly, it has been proposed that dopamine mediates the motivational and rewarding aspects of food seeking [5; 6; 7; 8; 9] via specific dopaminergic projections from the VTA to the NAc (the ‘mesolimbic’ projection) [5], and that dysfunction of reward processing may contribute to the pathogenesis of obesity [6].
Metabolic signals act on the midbrain
The majority of leptin-responsive neurons in the midbrain are dopaminergic, although non-dopaminergic leptin-responsive cells exist [1; 10]. Leptin administered to animals reduces the firing of dopmanergic neurons[1]. Furthermore, leptin administration to the VTA decreases food intake, as well as decreasing the firing of dopaminergic neurons [1]. In addition, leptin receptor knockdown in the VTA increases food intake without changing body weight [1].
Other metabolic factors from the periphery also influence midbrain function. Ghrelin is a peripheral orexigenic peptide secreted from the stomach [11]. Receptors for ghrelin are expressed in mesolimbic circuits [12], and ghrelin administration to the VTA or to the NAc stimulates feeding in a dose-dependent manner [12; 13]. Ghrelin modulates midbrain dopaminergic electrical and synaptic activity, and stimulated dopamine release in the NAc through the ghrelin receptor[12].
These lines of evidence, along with data suggesting that insulin also modulates dopamine circuits [2; 14], invite inquiry into how these peripheral hormones might act on midbrain circuits to influence feeding. Here, we will review experimental evidence linking mesolimbic systems with feeding behavior. We will explore both dopaminergic as well as non-dopaminergic mechanisms by which these peripheral hormones might alter intake by acting on the midbrain.
Neural substrates of feeding behavior
Feeding is intimately associated with energy homeostasis whereby the perceptions of caloric need leads to subsequent intake of food. One approach to determining the role of brain regions or molecules in feeding is to assess food intake following lesions or genetic mutations. As an example, damaging the lateral hypothalamus causes dramatic losses in body weight [15], while stimulating the lateral hypothalamus causes an increase in food intake [16]. Based on results from this early stimulation and lesion work, most mechanistic and molecular work has focused on the hypothalamus, where peripheral signals can influence the brain [17; 18]. More recently, genetic studies have revealed that ob/ob mice lacking a functional version of leptin, an adipocyte-derived hormone, have increased feeding and dramatic gain weight [19; 20], whereas leptin administration reduces body weight [21; 22]. Work on leptin has traditionally focused on the hypothalamus but is now being recognized as now acting in multiple brain regions [23]. While hypothalamic lesions produce the most dramatic effects on feeding and weight, manipulations of many other regions has been shown to influence food intake. For example, neurochemical disruptions in the NAc can influence food intake [24; 25; 26] and it is clear that food intake involves a diverse neurohumoral ensemble that engages a number of extrahypothalamic brain areas [27; 28].
The simplest way to measure feeding behavior and the one we focus on in this review is free feeding. This consists of recording food intake (i.e., by measuring food intake) of normal chow over time. This measure, albeit crude, is relatively simple and consistently reported in the literature, enabling more direct comparisons of present-day manipulations with previous studies [29; 30]. In addition, it is an innate behavior that does not require training. Free feeding can be measured over several minutes, or several days and the microstructure of feeding (i.e., patterns) can be measured for more detailed assessment [31]. However, several pitfalls exist with using free feeding as the sole behavioral assessment. First, the complex behavioral repertoire underlying food intake cannot be dissociated into its component processes by only recording food intake. Second, in order to acquire food, an animal must be able to detect the presence of food, move towards a food source, and then chew and swallow this food source. Interfering with movement would decrease animals’ food intake during free feeding necessitating motor controls for studies of food intake. Thus, while we emphasize free-feeding in this review, we acknowledge the limitations and also address the role of dopamine in other more complex feeding-related behaviors.
Disrupting dopamine and feeding
Mesolimbic circuits have been implicated in many behaviors, such as reward prediction [32], hedonia [33], reinforcement [5], motivation [4], or incentive salience [34] and it would seem that such processes are involved in ad-libitum feeding, [6]. If dopaminergic mesolimbic circuitry is necessary in feeding [5; 6; 7; 8; 9], then disrupting dopamine in the mesolimbic system should affect the overall food intake of the animal. Some of this work, and the complexities of its interpretation, is reviewed below.
The hypothesis that midbrain dopamine provides a specific motivation to eat [4; 5; 6] is based on dopamine neuron depletion studies using the neurotoxin 6-hydroxydopamine, which irreversibly depletes cathecholamines (including dopamine) and eventually leads to neuronal degeneration of these cathecholaminergic neurons depending on dosage used [35]. Intraventricular administration of 6-hydroxydopamine dramatically impairs free feeding and decreases body weight [36; 37]. Interestingly, dopamine neuron depleted rats gradually recover the ability to eat and drink despite a permanent depletion of dopamine (as well as some other catecholamines) from central dopaminergic nuclei [38]. In addition, peripheral dopamine blockade can block feeding elicited by electrical stimulation of the lateral hypothalamus [39]. Some investigators have described intact movement in these animals [40] although no movement controls were reported in original studies [36]. Peripheral dopamine blockade also can disrupt feeding elicited via hunger [33; 41; 42], and this work was influential in bringing attention to the role of dopamine systems in feeding behavior. However, this line of research used pimozide, an antipsychotic drug with a complex receptor binding profile [43], and the effects were not localized to a central brain structure. As reviewed below, effects on feeding due to specific dopamine receptor antagonists in the NAc have not produced as dramatic results.
Dopamine’s role in feeding was further advanced by unique data from animals in which tyrosine hydroxylase is genetically removed [44; 45]. These animals cannot synthesize dopamine and have dramatically decreased eating and drinking. These dopamine deficient animals only survive a few weeks after birth. However, daily L-DOPA (3,4-dihydroxy-L-phenylalanine; a precursor of dopamine synthesis converted from L-tyrosine) injections enabled survival and subsequent analysis of animals lacking detectable dopamine (hours after the L-DOPA injections). While these animals are hypophagic, they also exhibit deficits in movement. Subsequent experiments have suggested that dopamine deficient animals can exhibit preferences for sucrose [46], raising the possibility that preference and reward-related decisions are possible without dopamine. Taken together, studies in which dopaminergic transmission is disrupted allude to a clear role for dopamine in feeding, though they did not initially identify which dopaminergic pathways are involved (see below) and whether food intake reduction is a primary behavioral effect.
Central dopaminergic pathways
There are a limited number of central dopaminergic pathways that arise from the mediobasal hypothalamus (tuberoinfundibular neurons), or the midbrain (substantia nigra and VTA). Deficits in feeding with dopamine neuron depletion described above likely involve one of these nuclei [36; 37; 38], although it is possible that one of several other minor dopaminergic nuclei might be also involved in feeding.
Of the three major dopaminergic nuclei, the mediobasal hypothalamic dopaminergic neurons are primarily involved in prolactin secretion [47; 48]. To our knowledge, deficits in free feeding have never been reported after dopamine neuron depletion of this area and most experiments have focused on the two other dopaminergic nuclei, the substantia nigra and the VTA, and their projection sites in the brain.
Substantia nigra
There is evidence that the substantia nigra is involved in intake of food. In humans, dopaminergic neurons in the substantia nigra preferentially degenerate in Parkinson’s disease [49], although some ventral tegmental neurons may be involved [50; 51; 52]. Notably, patients with Parkinson’s disease experience mild weight loss [53] relative to controls (statistically significant only in women), although impaired movement or cognition may contribute to this phenomenon [54; 55].
In rats, dopamine neuron depletions of the substantia nigra produce animals with deficits in feeding and drinking [37; 56]. Animals with unilateral lesions of the substantia nigra also demonstrated persistent deficits in body weight [57], though with partial nigral dopamine neuron depletions, there appears to be no effect on free-feeding, but deficits in fine motor movement [30]. Interestingly, in tyrosine hydroxylase deficient mice [44], restoring tyrosine hydroxylase in the dorsal striatum (the major target of the substantia nigra) but not the ventral striatum (i.e., NAc) [58] corrects the feeding. These data point to a clear link between nigrostriatal dopamine and food intake.
Complicating the interpretation of these data are alterations in movement with nigrostriatal dopamine neuron depletion, as well as deficits in sensorimotor performance [59; 60]. Although a deficit in movement does not rule out an effect in feeding, it requires more extensive experiments to establish that animals can acquire food in spite of movement deficits. For example, dopamine deficient animals will consume food placed into their mouth and will show normal preference for sucrose when experiments are designed to limit the movement required for ingestion [46]. This confound notwithstanding, these studies provide compelling data that nigrostriatal dopamine is necessary for intact feeding behavior, likely acting as a permissive signal for goal-directed behaviors [61].
Finally, PET imaging studies, which image dopamine release through the D2 agonist C11-raclopride, have shown that dopamine is released during feeding from dorsal striatal regions (which receive projections from the substantia nigra) in humans. Visual display of food stimulated dopamine release in the dorsal striatum only when dopamine uptake was blocked by methylphenidate [62]. A subsequent study reported that eating a meal increased dopamine release in the dorsal striatum, and that this release correlated with the pleasantness of a meal [63]. These data in humans that striatal dopamine is released during eating support a role for the nigrostriatal pathway in eating and the response to food intake.
Ventral tegmental area
Experiments exploring the role of VTA dopamine in feeding suggest that mesolimbic dopamine can influence, but is not necessary for, feeding behavior. Initial studies that depleted dopamine neurons in the VTA [29] reported no effect on body weight despite increased locomotor activity and deficits in passive-avoidance behavior. To our knowledge, while VTA dopamine neuron depletions can influence a wide range of higher-order behavior, such as drug self-administration [64], motivation [65; 66], perseveration [4; 30], and memory [67], no data suggests that mesolimbic dopamine neuron depletions specifically impact feeding. However, negative data in dopamine neuron depletion experiments are somewhat difficult to interpret, as small amounts of residual dopamine function may be sufficient for critical feeding-related signals. As reviewed below, some studies suggest that lesions (not dopamine specific) in the region do affect food intake. Moreover, specific pharmacological or viral manipulation of neurons in the VTA can produce changes in food intake. This approach, which modulates specific populations of VTA neurons, has demonstrated clear effects on feeding behavior [1; 13; 68], in contrast to the negative data seen with traditional lesion strategies.
Supporting a role for the VTA in feeding is extensive literature establishing dopamine release in the NAc during feeding. These data derive chiefly from microdialysis studies [69], in which changes in extracellular dopamine and breakdown products are seen in the NAc in response to both feeding and hypothalamic stimulation [70]. Food anticipation corresponds with dopamine release in the shell; whereas food stimuli promote dopamine release in the core [71]. Voltammetry studies, which detect changes in dopamine efflux with high temporal resolution, reported rapid dopamine release (~70 ms) in response to food cues and food seeking behavior [72].
In-vivo recording studies also suggest that mesolimbic dopaminergic neurons are modulated in pursuit of food rewards. Such experiments are difficult to interpret outside of a specific behavioral context and free-feeding has not been directly studied in detail. However, the work of Wolfram Schultz and colleagues has shed considerable light on the activity of VTA dopaminergic neurons [32; 73; 74]. These studies recorded from non-human primate dopaminergic VTA neurons and reported phasic modulations in activity following liquid or food rewards [73; 75]. Food rewards also change activity among midbrain dopaminergic neurons in rodents [76]. Increases in activity are seen in response to stimuli that predict rewards, and decreases in activity are observed when rewards are omitted [77]. Dopaminergic neurons increase in firing rate when an animal touches food [78; 79] and when liquid reward is administered to the animal outside of a behavioral context [77]. Such neurons respond primarily to novel rewards, and when outcomes are somewhat unexpected [80; 81]. These persuasive data have led to the hypothesis that dopamine neurons in the midbrain encode reward prediction errors [32; 73; 74]. These experiments suggest that mesolimbic dopamine neuron response is more consistent with a limited role in ad-libitum free feeding and a potentially greater role in signaling unexpected reward, cue associations, or reward valence. These data are also consistent with the finding that novel or unexpected food causes more dopamine release in the NAc shell [71].
Dopamine terminal depletion in mesolimbic target areas
This more restricted, yet important role for the mesolimbic system, is supported from studies that depleted dopamine terminals in major brain structures targeted by VTA projections: the NAc, amygdala and prefrontal cortex. Of these, the NAc (ventromedial striatum) is the primary target of mesolimbic dopaminergic projections [82]. Initial studies indicated that dopamine terminal depletions in the NAc produced mild short-term but not long-term increases in feeding [24] and changes in the rate of feeding. However, this study also depleted dopamine terminals in the olfactory tubercle along with the prefrontal cortex. Subsequent studies have indicated that NAc dopamine terminal depletions alone do not impact feeding [83; 84]. Conversely, dopamine terminal depletions in regions targeted by nigrostriatal projections (i.e., ventrolateral striatum) reliably diminish feeding [83]. NAc dopaminergic terminal depletions have a transient impact on instrumental bar-pressing for food [85], and they can impair bar-pressing when more effort is required [86; 87]. NAc D1/D2 receptor blockade affected motor behavior, had small effects on feeding patterns, but did not reduce the amount of food consumed [88]. These data provide support for the involvement of mesolimbic circuitry in effort-related functions [89] rather than in free feeding. In addition, dopamine in the NAc potentiates responses to cues associated with food intake [28; 90], again suggesting additional roles for dopamine in tasks beyond free feeding.
The amygdala is another structure that influences feeding [91], in combination with prefrontal-hypothalamic networks [92]. Some evidence indicates that dopamine may be released in the amygdala in response to food [93; 94]. However, amygdalar dopamine terminal depletion only transiently reduces feeding while having long term impact on other behaviors, such as conditioned avoidance [95].
Finally, prefrontal cortex, particularly medial regions, have extensive top-down projections to the VTA, amygdala, and NAc [96]. Although feeding can induce dopamine release in the prefrontal cortex [97], no data has described an effect of prefrontal dopamine terminal depletion in feeding. Prefrontal dopamine terminal depletions do not influence more complex behaviors such as cocaine self-administrations [98]. Furthermore, lesions of ventromedial prefrontal cortex do not appear to influence free-feeding in familiar contexts; although lesions do impair cue-driven food consumption [99].
There are extensive minor to moderate targets of midbrain dopaminergic projections, including arcuate and medial hypothalamic nuclei, habenula, the olfactory tubercle, septal nuclei, and medial temporal regions. To our knowledge, there are no data that implicate these projections specifically in feeding.
Non-dopaminergic signaling in midbrain neurons
Midbrain nuclei contain dopaminergic and non-dopaminergic neurons [82]. Trudeau and colleagues report evidence that the glutamate transporter VGLUT2 is expressed in midbrain neurons that do not express tyrosine hydroxylase [100; 101], demonstrating that glutamatergic, non-dopaminergic neurons are intermixed with dopaminergic neurons in the VTA. Some VTA neurons also express presynaptic machinery for the release of GABA [102; 103]. These neurons can project specifically to distant areas, such as prefrontal cortex [104] or receive distant cortical input [102]. Such GABAergic neurons can also interact locally to regulate dopamine neurons and potentiate learning within dopaminergic networks [105].
If non-dopaminergic neurons were important for feeding behavior, then gross lesions or stimulation of mesolimbic networks (which would affect all cell types and all forms of neurotransmission) might produce distinct effects when compared with studies of dopamine neuron-specific depletions. Lesion data lends some support to this notion. In contrast to the dopamine neuron depletion literature reviewed above, electrolytic [106] or radiofrequency lesions of the midbrain [107] produce marked aphagia. The effect of these lesions on aphagia was more pronounced than dopamine neuron depletions [107], although in this work, the amount of dopaminergic cell loss was not quantified. Direct electrical stimulation of the midbrain [108], or specifically the VTA can elicit feeding [109]. Furthermore, stimulation-induced feeding depended on intact mesolimbic projections to the NAc shell [110]. One critique of these techniques is lesions or stimulation may affect fibers of passage in addition to midbrain neurons. Future approaches could address these concerns by using channelrhodopsin-guided stimulation [111; 112; 113] of specific populations of VTA neurons.
Despite these data, there are no experiments to date that specifically investigate the role of non-dopaminergic/glutamatergic/GABAergic subpopulations of midbrain neurons in feeding. However, evidence from opioid manipulation of mesolimbic systems may shed light on this, as the mu-opioid receptor is expressed primarily in non-dopaminergic neurons [114; 115]. It is unknown if these receptors are expressed on GABAergic projection neurons [104]; however, opioid receptors can powerfully modulate dopaminergic projection neurons [116] from the VTA. Microinjections of morphine into both the VTA and NAc triggered food intake in both sated and hungry animals [117]. This effect does not exhibit tolerance, and can be potentiated by amphetamine [118]. Opioid-receptor antagonists such as naloxone into the VTA produce anorexia [119]. Opioid-induced feeding depends on the mu-opioid receptor, and is interdependent with dopaminergic transmission [68]. These data clearly indicate that specific opioid manipulation of the VTA influences feeding.
Taken together, this evidence illustrates how non-dopaminergic circuit elements might interact with dopaminergic projection neurons to control food intake, and provides some insight into how peripheral hormones might influence food acquisition via direct modulation of non-dopamine neurons of the midbrain. Thus, while the limited feeding effects of NAc dopamine blockade indicate that dopamine is not necessary for feeding, the midbrain manipulations above suggest that altered patterns of dopamine release may be sufficient to alter feeding.
Non-dopaminergic signaling in mesolimbic target areas
The work of Kelley and colleagues has linked neuronal excitability of the NAc shell to feeding behavior [26]. For example, blocking glutamate receptor in the NAc stimulates feeding via projections to the lateral hypothalamus [120]. GABAergic agonists in the NAc shell also stimulate feeding behavior [121; 122]. Moreover, opiate administration specifically into the NAc triggers feeding via the mu-opioid receptor [123]. NAc administration of the lateral hypothalamic-derived melanin-concentrating hormone also drives feeding via inhibition of neuronal firing [124; 125]. Combined with data from dopamine receptor blockade [88; 89] and dopamine neuron depletions [83; 84], these experiments establish a relationship between NAc neurochemistry, neuronal exitability, and food intake.
The robust feeding changes with GABA and glutamate manipulations may reflect the important role of non-mesolimbic glutamate projections from the cortex, or other afferent areas. It is also possible that non-dopaminergic signaling in mesolimbic target areas may be sufficient to control feeding beyond pure dopaminergic mechanisms (e.g. via glutamate or GABA release), and the above results invite consideration of how non-dopaminergic mesolimbic projections might influence feeding.
Complexities in dopaminergic signaling and feeding
Interpreting the evidence reviewed thus far can be difficult in light of dopamine’s cellular effects as a synaptically released signal. For instance, dopamine is not a high-fidelity signal of neural activity (like glutamate, GABA, or acetycholine); rather it is neuromodulator acting on a somewhat slower time scale [126]. Typically, dopamine binds to one of 5 known G-protein linked receptors and leads to distributed protein phosphorylation and diverse downstream changes in cellular machinery. These receptors can have opposing effects on intracellular signaling [127]. Furthermore, the relationship between dopaminergic neuron discharge and dopamine release is complex [128]. Dopamine can also have distinct effects at different time scales [32; 129]. Manipulations of the dopaminergic system may change tonic discharge of dopaminergic neurons [130; 131; 132], whereas phasic discharges from dopaminergic neurons that convey feeding signals [32] may be preserved. Moreover, dopaminergic signaling is nonlinear, and may merely ‘optimize’ neural activity on an inverted U-shape curve [132; 133], further obscuring interpretations from dopamine neuron depletion studies. Finally, dopamine alone may not be necessary nor sufficient for signaling an event, but may modulate corticostriatal pathways [134].
These complexities demonstrate the difficulty in understanding the role of dopamine and in developing models that connect feeding with drug addiction [6; 25]. Mesolimbic circuitry is involved in processes thought to be crucial for food intake i.e., reward [73], hedonia, [33], reinforcement, motivation [4], or incentive salience [34], though little data exist to suggest that mesolimbic dopamine is crucial for free feeding. Moreover, without dopamine, animals can still exhibit preferences for rewards [46; 135]. These data raise the possibility that mesolimbic dopamine is not necessary for feeding, but may play an important modulatory role in feeding pathways. Despite this view, it is likely that dysfunction of mesolimbic circuitry is intertwined with obesity. Sated organisms in stimulus-rich environments with complex reward payoffs may be dramatically influenced by small changes in their reward-acquisition circuitry [136; 137] that could contribute to the pathogenesis of obesity [6].
For instance, it is possible that mesolimbic systems are preferentially engaged when animals have access to highly reinforcing foods and food of different reward valences, or when animals must make choices in an environment with complex stimuli. Pharmacological manipulations of opiate receptors in the NAc is consistent with a role in choice of highly reinforcing food. This hypothesis can be extended to dopamine neurons by exploring if animals with VTA manipulations (via lesion or genetic approaches) can become obese in these environments.
Some possibilities for how mesolimbic dopamine might influence feeding
Considering this broad literature, it is difficult to really specify the role of mesolimbic dopamine in free feeding: whereas mesolimbic dopamine neuron depletions do not specifically impact free feeding, neurochemical manipulations of the VTA and NAc [25; 26] as well as infusions of opioid, leptin or ghrelin directly into the VTA prominently modulate free feeding [1; 12; 13; 117]. There are many potential explanations for this and here we present some of the more likely models.
A first possibility, consistent with data reviewed above from the NAc, [120; 121; 122] is that midbrain neurons are involved in feeding chiefly through non-dopaminergic mechanisms of influencing the NAc. As discussed above, non-dopaminergic neurons can express receptors to feeding hormones, such as leptin [1; 10; 14], and may control feeding via these neurons. However, such an account unjustifiably ignores the vast majority of dopaminergic neurons that express receptors for metabolic factors and does not seem likely to explain the complete role of the midbrain in modulating food intake. Moreover, pharmacological manipulations of the VTA that drive food intake depend upon NAc dopamine [68].
A second possibility is that dopaminergic neurons in the midbrain are involved in feeding through glutamate cotransmission. Electrical stimulation of VTA neurons produces fast transients in the NAc [138] and in the prefrontal cortex [139], implying fast rather than slow neurotransmission. As noted above, midbrain dopaminergic neurons express glutamate transporters [101] and glutamate-dopaminergic co-transmission may be behaviorally relevant in addiction [140]. This could account for the finding that VTA dopamine neuron depletions do not impact feeding, whereas lesions of the VTA [107] and modulation of NAc glutamate [26] can profoundly impact feeding. However, the relevance of such cotransmission in feeding behavior has not been established.
A third possibility is that subpopulations of dopaminergic neurons exist in balance with other types of dopaminergic neurons, which effectively masks their role in food intake. There clearly exist pools of neurons expressing receptors for metabolic signals that can control feeding [1; 12; 14], and these neuromodulators may change the balance of dopaminergic transmission that may in turn facilitate feeding. In this scenario, modulating a subpopulation of midbrain neurons would produce distinct results from removing the entire nuclei. Some data indicate that complete dopamine neuron depletion of the VTA produces distinct effects from incomplete dopamine neuron depletions [141], demonstrating the difficulty of interpreting dopamine neuron depletion data. Future experiments might test the hypothesis by studying feeding behavior when mesolimbic systems are partially inactivated by manipulation of genetically identified cell-classes.
Some electrophysiological evidence supports a variant of this possibility, whereby changes in specific firing patterns and dopamine release might encode information. Dopaminergic neurons maintain prominent tonic activity [130]. Grace and colleagues have proposed that behaviorally salient stimuli could trigger phasic deviations in this tonic activity and might constitute a behaviorally relevant signal to the animal [74; 131]. Phasic stimulation of dopaminergic neurons is sufficient for behavioral conditioning [113]. According to this scenario, depleting dopamine would deprive target nuclei of phasic and tonic stimulation, eliminating transmission of information from this nucleus. Both leptin and ghrelin have been shown to influence midbrain dopamine neuron firing rates [1; 12], however, it is unclear if they influence phasic or tonic patterns of dopaminergic discharge, or both. Future studies that specifically stimulate [112] leptin-expressing dopaminergic neurons, or that simultaneously record [142] from ensembles of midbrain neurons and neurons in target areas will address this hypothesis.
A fourth possibility that accounts for discrepancies in the literature is our lack of understanding of the most relevant circuits. For instance, midbrain dopaminergic projections directly target a variety of brain structures, including NAc, prefrontal cortex, and the amygdala. If leptin expressing midbrain neurons projected preferentially to the amygdala or prefrontal cortex, they could directly control feeding (perhaps via projections involving the lateral hypothalamus). Without detailed information on the projections of leptin receptor-expressing and ghrelin receptor-expressing neurons, speculation on how these neurons achieve control over feeding is limited. Once such targets are identified, lesion, receptor knockdown and recording experiments can be designed in order to elucidate this pathway in pursuit of a common pathway regulating feeding. It is notable that recent detailed anatomical and functional analysis suggests that properties of dopamine neurons projecting from the VTA vary according to projection sites [143].
A fifth possibility, is that dopamine is strictly modulatory or state-dependent [126] and not necessary for free-feeding behavior. For instance, GABAergic medium spiny neurons in the NAc may control feeding via direct projections to the lateral hypothalamus [28; 144]. Dopamine release may simply modulate this circuit [134] rather than being solely necessary for feeding behavior. This scenario accounts for inconsistencies in dopamine neuron depletion data; indeed, if dopamine only modulated feeding circuitry, then depletion or removal would not directly influence feeding because critical circuitry would still be in place. Peripheral or whole animal dopamine manipulations could disturb dopaminergic tone [36; 37; 44] and dysregulate this network, whereas area-specific dopamine manipulations may allow dopamine-modulated networks to compensate. Of course, as noted above, free-feeding of laboratory chow in a cage does not mimic the human condition and it is possible that dopamine plays a greater role in food intake under changing environments that humans experience. In particular, NAc dopamine likely plays a role in effort to obtain food[89] as well as responses to cues associated with food intake [28; 90]. While we have emphasized free-feeding in this review, it is critical that future research on dopamine’s role continues to evaluate other aspects of behavior that are relevant to food intake.
These possibilities are not mutually exclusive and even could be seen as overlapping. Distinguishing between these possibilities will require careful experiments that manipulate pools of dopamine neurons that respond to different neuromodulators. For such experiments, using genetic tools to identify and manipulate both genes and firing patterns in populations of subpopulations of neurons within the midbrain [1; 44; 45; 112; 145] will be critical for better defining mesolimbic control of feeding.
Conclusion
Emerging evidence has suggested that peripheral hormones such as ghrelin and leptin can influence feeding via mesolimbic circuits. Here we have reviewed how mesolimbic systems might, in turn, control food intake. Peripheral or whole brain disruptions of dopamine function markedly impair feeding behavior. Studies of dopaminergic neuron depletion and genetic studies have implicated nigrostriatal dopaminergic pathways in feeding, whereas mesolimbic dopaminergic pathways seem to be involved in higher-order aspects of feeding, such as motivation and response to novelty or food-associated cues. Furthermore, while dopamine neuron depletions in the NAc do not directly impair feeding, non-dopaminergic manipulations of glutamate, GABA, and opioids in the NAc or VTA can dramatically influence feeding and food choice. We discussed several possible explanations for the complex data that connects dopamine systems and feeding. Further experiments will elucidate these mechanisms, and isolate specific circuits within the midbrain that are in command of food acquisition and are also responsive to metabolic factors.
Figure 1.
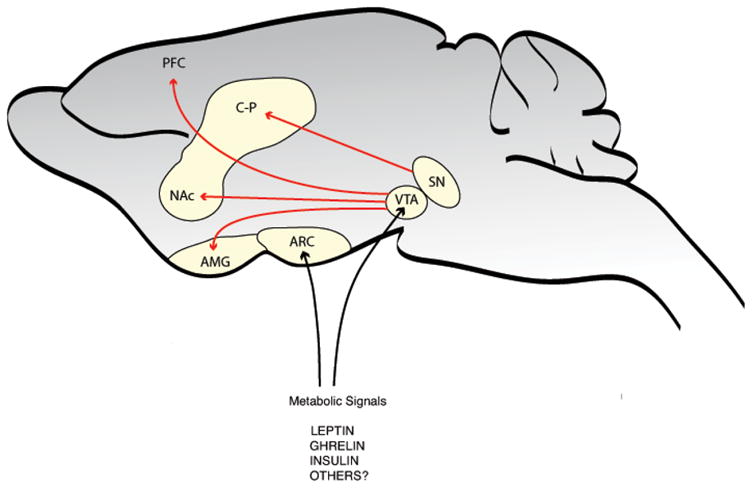
Dopamine Circuits. The midbrain is the primary site of dopaminergic neurons in the rodent brain. A schematic of the major dopamine projections from the midbrain are shown in red. The substantia nigra (SN) neurons project primarily to the caudate putamen (C-P), while the ventral tegmental area (VTA) neurons project primarily to the nucleus accumbens (NAc), the prefrontal cortex (PFC) and the amygdala (AMG). Metabolic signals emanating from the periphery, such as leptin and ghrelin, have recently been shown to not only act in the arcuate nucleus of the hypothalamus (ARC), but also in the VTA.
Figure 2.
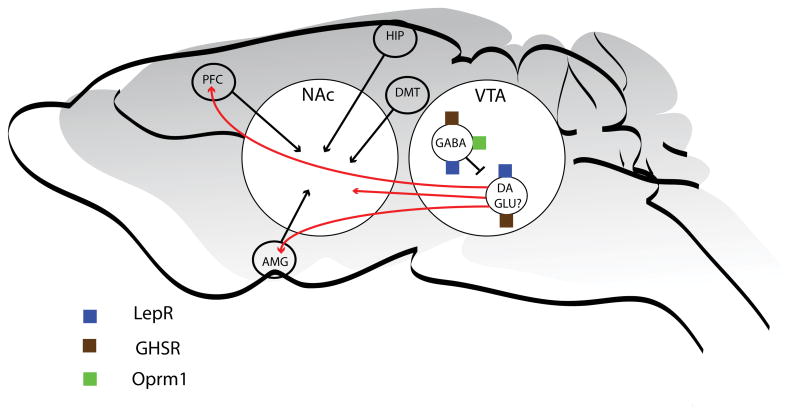
Focus on midbrain circuits and their potential role in influencing free feeding behavior. Midbrain (VTA) and nucleus accumbens are expanded disproportionately to demonstrate some of the mechanisms discussed in the text. We have concluded with five non-mutually exclusive possibilities to explain the discrepancy between dopamine depletion and midbrain lesions in mediating free feeding. The ventral tegmental area (VTA) contains dopamine (DA) neurons and GABAergic (gamma-aminobutyric acid) interneurons. Both cell types express receptors for leptin (LepR) and ghrelin (GSHR). The mu-opioid receptor (Oprm1) is expressed on primarily non-dopaminergic neurons and may account for some aspects of possibility one (GABA projections neurons are not shown). Glutamate (GLU) may be co-released from DA neurons and this is basis of possibility two. There may be subpopulations of DA neurons involved in feeding, perhaps expressing a subset or specific combination of metabolic signal receptors, and these may account for possibility three. The DA neurons also project to the prefrontal cortex (PFC) and amygdala (AMG) and this accounts for possibility four. Finally DA may be strictly a neuromodulator of glutamate transmission coming from the PFC, AMG, the dorsal medial thalamus (DMT) or the hippocampus (HIP) and this is the basis of possibility five.
Acknowledgments
This work is supported by NIH grants DK073677 and DK076964 to R.J.D. as well as by the State of Connecticut, Department of Mental Health and Addiction Services (R.J.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882–92. doi: 10.1152/ajpregu.00602.2002. [DOI] [PubMed] [Google Scholar]
- 3.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–51. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 4.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 5.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–60. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 7.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T. Neural substrates for the processing of cognitive and affective aspects of taste in the brain. Arch Histol Cytol. 2006;69:243–55. doi: 10.1679/aohc.69.243. [DOI] [PubMed] [Google Scholar]
- 9.Stricker EM, Zigmond MJ. Brain catecholamines and the central control of food intake. Int J Obes. 1984;8(Suppl 1):39–50. [PubMed] [Google Scholar]
- 10.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 12.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–15. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 15.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science. 1962;135:374–5. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 16.Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172:162–8. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- 17.Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14(Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 19.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–8. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 20.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 21.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–6. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 22.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–23. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–27. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- 25.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann N Y Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 26.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Balleine BW. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–30. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 28.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 29.Galey D, Simon H, Le Moal M. Behavioral effects of lesions in the A10 dopaminergic area of the rat. Brain Res. 1977;124:83–97. doi: 10.1016/0006-8993(77)90865-4. [DOI] [PubMed] [Google Scholar]
- 30.Pioli EY, Meissner W, Sohr R, Gross CE, Bezard E, Bioulac BH. Differential behavioral effects of partial bilateral lesions of ventral tegmental area or substantia nigra pars compacta in rats. Neuroscience. 2008;153:1213–24. doi: 10.1016/j.neuroscience.2008.01.084. [DOI] [PubMed] [Google Scholar]
- 31.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–67. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–4. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- 34.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 35.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–10. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 36.Zigmond MJ, Stricker EM. Deficits in feeding behavior after intraventricular injection of 6-hydroxydopamine in rats. Science. 1972;177:1211–4. doi: 10.1126/science.177.4055.1211. [DOI] [PubMed] [Google Scholar]
- 37.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:95–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 38.Zigmond MJ, Stricker EM. Recovery of feeding and drinking by rats after intraventricular 6-hydroxydopamine or lateral hypothalamic lesions. Science. 1973;182:717–20. doi: 10.1126/science.182.4113.717. [DOI] [PubMed] [Google Scholar]
- 39.Phillips AG, Nikaido RS. Disruption of brain stimulation-induced feeding by dopamine receptor blockade. Nature. 1975;258:750–1. doi: 10.1038/258750a0. [DOI] [PubMed] [Google Scholar]
- 40.Price MT, Fibiger HC. Discriminated escape learning and response to electric shock after 6-hydroxydopamine lesions of the nigro-neostriatal dopaminergic projection. Pharmacol Biochem Behav. 1975;3:285–90. doi: 10.1016/0091-3057(75)90159-8. [DOI] [PubMed] [Google Scholar]
- 41.Wise RA, Colle LM. Pimozide attenuates free feeding: best scores analysis reveals a motivational deficit. Psychopharmacology (Berl) 1984;84:446–51. doi: 10.1007/BF00431448. [DOI] [PubMed] [Google Scholar]
- 42.Wise RA, Spindler J, Legault L. Major attenuation of food reward with performance-sparing doses of pimozide in the rat. Can J Psychol. 1978;32:77–85. doi: 10.1037/h0081678. [DOI] [PubMed] [Google Scholar]
- 43.Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–10. [PubMed] [Google Scholar]
- 44.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 45.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Cannon CM, Palmiter RD. Reward without dopamine. J Neurosci. 2003;23:10827–31. doi: 10.1523/JNEUROSCI.23-34-10827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gudelsky GA. Tuberoinfundibular dopamine neurons and the regulation of prolactin secretion. Psychoneuroendocrinology. 1981;6:3–16. doi: 10.1016/0306-4530(81)90044-5. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22:12–9. doi: 10.1177/0269216307087148. [DOI] [PubMed] [Google Scholar]
- 49.Hornykiewicz O. Basic research on dopamine in Parkinson’s disease and the discovery of the nigrostriatal dopamine pathway: the view of an eyewitness. Neurodegener Dis. 2008;5:114–7. doi: 10.1159/000113678. [DOI] [PubMed] [Google Scholar]
- 50.Bogerts B, Hantsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry. 1983;18:951–69. [PubMed] [Google Scholar]
- 51.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(Pt 8):1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 52.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain. 1999;122(Pt 8):1421–36. doi: 10.1093/brain/122.8.1421. [DOI] [PubMed] [Google Scholar]
- 53.Durrieu G, MELL, Rascol O, Senard JM, Rascol A, Montastruc JL. Parkinson’s disease and weight loss: a study with anthropometric and nutritional assessment. Clin Auton Res. 1992;2:153–7. doi: 10.1007/BF01818955. [DOI] [PubMed] [Google Scholar]
- 54.Hodge GK, Butcher LL. Pars compacta of the substantia nigra modulates motor activity but is not involved importantly in regulating food and water intake. Naunyn Schmiedebergs Arch Pharmacol. 1980;313:51–67. doi: 10.1007/BF00505805. [DOI] [PubMed] [Google Scholar]
- 55.Bachmann CG, Trenkwalder C. Body weight in patients with Parkinson’s disease. Mov Disord. 2006;21:1824–30. doi: 10.1002/mds.21068. [DOI] [PubMed] [Google Scholar]
- 56.Smith GP, Strohmayer AJ, Reis DJ. Effect of lateral hypothalamic injections of 6-hydroxydopamine on food and water intake in rats. Nature New Biology. 1972;235:27–29. doi: 10.1038/newbio235027a0. [DOI] [PubMed] [Google Scholar]
- 57.Baez LA, Ahlskog JE, Randall PK. Body weight and regulatory deficits following unilateral nigrostriatal lesions. Brain Res. 1977;132:467–76. doi: 10.1016/0006-8993(77)90195-0. [DOI] [PubMed] [Google Scholar]
- 58.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–28. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 59.Marshall JF, Berrios N, Sawyer S. Neostriatal dopamine and sensory inattention. J Comp Physiol Psychol. 1980;94:833–46. doi: 10.1037/h0077825. [DOI] [PubMed] [Google Scholar]
- 60.Smith GP. Accumbens dopamine is a physiological correlate of the rewarding and motivating effects of food. In: Stricker EM, Woods SC, editors. Neurobiology of Food and Fluid Intake New York. New York: 2004. pp. 15–42. [Google Scholar]
- 61.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. “Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–80. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 63.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–15. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 64.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 65.Papp M, Bal A. Motivational versus motor impairment after haloperidol injection or 6-OHDA lesions in the ventral tegmental area or substantia nigra in rats. Physiol Behav. 1986;38:773–9. doi: 10.1016/0031-9384(86)90042-9. [DOI] [PubMed] [Google Scholar]
- 66.Papp M, Bal A. Separation of the motivational and motor consequences of 6-hydroxydopamine lesions of the mesolimbic or nigrostriatal system in rats. Behav Brain Res. 1987;23:221–9. doi: 10.1016/0166-4328(87)90022-2. [DOI] [PubMed] [Google Scholar]
- 67.Wisman LA, Sahin G, Maingay M, Leanza G, Kirik D. Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci. 2008;28:7797–807. doi: 10.1523/JNEUROSCI.1885-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 69.Church WH, Justice JB, Jr, Neill DB. Detecting behaviorally relevant changes in extracellular dopamine with microdialysis. Brain Res. 1987;412:397–9. doi: 10.1016/0006-8993(87)91150-4. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- 71.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–41. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- 72.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–7. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 74.Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- 75.Nishino H, Hattori S, Muramoto K, Ono T. Basal ganglia neural activity during operant feeding behavior in the monkey: relation to sensory integration and motor execution. Brain Res Bull. 1991;27:463–8. doi: 10.1016/0361-9230(91)90143-8. [DOI] [PubMed] [Google Scholar]
- 76.Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–92. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 77.Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]
- 79.Schultz W, Romo R. Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J Neurophysiol. 1990;63:607–24. doi: 10.1152/jn.1990.63.3.607. [DOI] [PubMed] [Google Scholar]
- 80.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–63. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 81.Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–7. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- 82.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 83.Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–10. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- 84.Weissenborn R, Winn P. Regulatory behaviour, exploration and locomotion following NMDA or 6-OHDA lesions in the rat nucleus accumbens. Behav Brain Res. 1992;51:127–37. doi: 10.1016/s0166-4328(05)80206-2. [DOI] [PubMed] [Google Scholar]
- 85.Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–20. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- 86.Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- 88.Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–77. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- 89.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 90.Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology (Berl) 1993;110:355–64. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]
- 91.Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–29. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- 92.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fallon S, Shearman E, Sershen H, Lajtha A. Food reward-induced neurotransmitter changes in cognitive brain regions. Neurochem Res. 2007;32:1772–82. doi: 10.1007/s11064-007-9343-8. [DOI] [PubMed] [Google Scholar]
- 94.Hajnal A, Lenard L. Feeding-related dopamine in the amygdala of freely moving rats. Neuroreport. 1997;8:2817–20. doi: 10.1097/00001756-199708180-00033. [DOI] [PubMed] [Google Scholar]
- 95.Ashford J, Jones BJ. The effects of intra-amygdaloid injections of 6-hydroxy-dopamine on avoidance responding in rats. Br J Pharmacol. 1976;56:255–61. doi: 10.1111/j.1476-5381.1976.tb07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–77. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 97.Hernandez L, Hoebel BG. Feeding can enhance dopamine turnover in the prefrontal cortex. Brain Res Bull. 1990;25:975–9. doi: 10.1016/0361-9230(90)90197-8. [DOI] [PubMed] [Google Scholar]
- 98.Martin-Iverson MT, Szostak C, Fibiger HC. 6-Hydroxydopamine lesions of the medial prefrontal cortex fail to influence intravenous self-administration of cocaine. Psychopharmacology (Berl) 1986;88:310–4. doi: 10.1007/BF00180830. [DOI] [PubMed] [Google Scholar]
- 99.Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–41. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- 101.Mendez JA, Bourque MJ, Dal Bo G, Bourdeau ML, Danik M, Williams S, Lacaille JC, Trudeau LE. Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci. 2008;28:6309–18. doi: 10.1523/JNEUROSCI.1331-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–15. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983;218:220–38. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- 104.Carr DB, Sesack SR. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–23. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 105.Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol. 2008;586:1487–93. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gold RM. Aphagia and adipsia produced by unilateral hypothalamic lesions in rats. Am J Physiol. 1966;211:1274–6. doi: 10.1152/ajplegacy.1966.211.5.1274. [DOI] [PubMed] [Google Scholar]
- 107.Nadaud D, Simon H, Herman JP, Le Moal M. Contributions of the mesencephalic dopaminergic system and the trigeminal sensory pathway to the ventral tegmental aphagia syndrome in rats. Physiol Behav. 1984;33:879–87. doi: 10.1016/0031-9384(84)90222-1. [DOI] [PubMed] [Google Scholar]
- 108.Mogenson GJ, Wu M. Neuropharmacological and electrophysiological evidence implicating the mesolimbic dopamine system in feeding responses elicited by electrical stimulation of the medial forebrain bundle. Brain Res. 1982;253:243–51. doi: 10.1016/0006-8993(82)90691-6. [DOI] [PubMed] [Google Scholar]
- 109.Trojniar W, Staszewska M. Unilateral damage to the ventral tegmental area facilitates feeding induced by stimulation of the contralateral ventral tegmental area. Brain Res. 1994;641:333–40. doi: 10.1016/0006-8993(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 110.Trojniar W, Plucinska K, Ignatowska-Jankowska B, Jankowski M. Damage to the nucleus accumbens shell but not core impairs ventral tegmental area stimulation-induced feeding. J Physiol Pharmacol. 2007;58(Suppl 3):63–71. [PubMed] [Google Scholar]
- 111.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–8. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 112.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–92. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 113.Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science. 2009 doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Svingos AL, Garzon M, Colago EE, Pickel VM. Mu-opioid receptors in the ventral tegmental area are targeted to presynaptically and directly modulate mesocortical projection neurons. Synapse. 2001;41:221–9. doi: 10.1002/syn.1079. [DOI] [PubMed] [Google Scholar]
- 115.Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–28. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- 116.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–8. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397lco:214–24. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- 118.Nencini P, Stewart J. Chronic systemic administration of amphetamine increases food intake to morphine, but not to U50-488H, microinjected into the ventral tegmental area in rats. Brain Res. 1990;527:254–8. doi: 10.1016/0006-8993(90)91144-6. [DOI] [PubMed] [Google Scholar]
- 119.Segall MA, Margules DL. Central mediation of naloxone-induced anorexia in the ventral tegmental area. Behav Neurosci. 1989;103:857–64. doi: 10.1037//0735-7044.103.4.857. [DOI] [PubMed] [Google Scholar]
- 120.Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–88. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behav Neurosci. 1999;113:324–36. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- 122.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–60. [PubMed] [Google Scholar]
- 124.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–40. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sears RM, Liu RJ, Nandakumar NS, Sharf R, Yeckel MF, Maratos-Flier E, Laubach M, Aghajanian GK, DiLeone RJ. Mechanisms underlying a neuropeptide-mediated hypothalamic-limbic feeding circuit. 2009 Under Review. [Google Scholar]
- 126.Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–30. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- 127.Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–4. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- 128.Yavich L, MacDonald E. Dopamine release from pharmacologically distinct storage pools in rat striatum following stimulation at frequency of neuronal bursting. Brain Res. 2000;870:73–9. doi: 10.1016/s0006-8993(00)02403-3. [DOI] [PubMed] [Google Scholar]
- 129.Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007;191:609–25. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 131.Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–7. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–76. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 133.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–84. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 134.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 135.Cannon CM, Bseikri MR. Is dopamine required for natural reward? Physiol Behav. 2004;81:741–8. doi: 10.1016/j.physbeh.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 136.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–8. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 137.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 138.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–81. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lavin A, Nogueira L, Lapish CC, Wightman RM, Phillips PE, Seamans JK. Mesocortical dopamine neurons operate in distinct temporal domains using multimodal signaling. J Neurosci. 2005;25:5013–23. doi: 10.1523/JNEUROSCI.0557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lapish CC, Seamans JK, Chandler LJ. Glutamate-dopamine cotransmission and reward processing in addiction. Alcohol Clin Exp Res. 2006;30:1451–65. doi: 10.1111/j.1530-0277.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 141.Koob GF, Stinus L, Le Moal M. Hyperactivity and hypoactivity produced by lesions to the mesolimbic dopamine system. Behav Brain Res. 1981;3:341–59. doi: 10.1016/0166-4328(81)90004-8. [DOI] [PubMed] [Google Scholar]
- 142.Narayanan NS, Laubach M. Methods for studying functional interactions among neuronal populations. Methods Mol Biol. 2009;489:135–65. doi: 10.1007/978-1-59745-543-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–73. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 144.Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- 145.Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–44. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]