Abstract
An important aspect of cognitive control consists in the ability to stop oneself from making inappropriate responses. In an earlier study we demonstrated that there are different mechanisms for stopping: global and selective. We argued that participants are more likely to use a global mechanism when speed is of the essence, whereas they are more likely to use a selective mechanism when they have foreknowledge of which response tendency they may need to stop. Here we further investigate the relationship between foreknowledge and selective stopping. In Experiment 1 we adapted the earlier design to show that individual differences in recall accuracy for the stopping goal predict the selectivity of the stopping. This confirms that encoding and using a foreknowledge memory cue is a key enabler for a selective stopping mechanism. In Experiment 2, we used transcranial magnetic stimulation (TMS), to test the hypothesis that foreknowledge “sets up” a control set whereby control is applied onto the response representation that may need to be stopped in the future. We applied TMS to the left motor cortex and measured motor evoked potentials (MEPs) from the right hand while participants performed a similar behavioral paradigm as Experiment 1. In the foreknowledge period, MEPs were significantly reduced for trials where the right hand was the one that might need to be stopped relative to when it was not. This shows that having a goal of what response may need to be stopped in the future consists in applying advance control onto a specific motor representation.
Keywords: Stop signal task, Transcranial Magnetic Stimulation, Working Memory, Cognitive Control
INTRODUCTION
Much human cognitive control consists in the ability to stop inappropriate action. In real-world scenarios such control is usually proactive in the sense that people have goals (or foreknowledge) of what they need to stop even if they are not currently trying to stop any impulse or action. Here we explore the neurocognitive basis for how foreknowledge is used to stop particular response tendencies.
A good experimental paradigm for examining the stopping of an incipient response tendency is the stop signal paradigm (reviewed by Verbruggen & Logan, 2009). In the standard paradigm, on each trial, the participant initiates a choice response, and then, on a minority of trials, must try to stop the initiated response when a stop signal occurs. The main dependent measure is the speed of the stopping process, stop signal reaction time. In the standard version, the participant has a general goal to stop when a stop signal occurs, but he or she does not need to deploy this selectively for one motor representation rather than another. In such situations, a fast but ‘global’mechanism is probably used to stop the response tendency. The mechanism is global in the sense that it has effects on muscle representations over and above the particular muscle that needs to be stopped (Badry et al., 2009; Coxon, Stinear, & Byblow, 2006; Coxon, Stinear, & Byblow, 2007; Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000; Sohn, Wiltz, & Hallett, 2002). However, stopping may also be achieved by means of a selective mechanism - i.e. one that has specific effects on particular motor representations rather than many (or all) possible representations. We provided evidence for dissociable global and selective stopping mechanisms by developing a novel version of the stop signal paradigm (Aron & Verbruggen, 2008). On each trial participants initiated a coupled response with fingers of both hands, and then, when a stop signal occurred, the participant tried to stop one response while continuing with the other one (see Figure 1A,B). This design allows a measurement of the selectivity of the stopping in terms of the degree of interference that is produced in the alternative (non-stopped) response - we refer to this as the ‘stopping interference effect’. We compared a condition in which foreknowledge was provided of which response(s) may need to be stopped compared to a condition where no foreknowledge was provided. To do this, we presented the cues “Maybe Stop Left”, “Maybe Stop Right” and “Maybe Stop XXX” in the foreknowledge period. Our key finding was that the stopping interference effect was reduced whereas stop signal reaction time was increased when foreknowledge was provided compared to when it was not. We argued that when foreknowledge is provided stopping is more selective precisely because the participant uses the stopping goal (foreknowledge) to prepare to stop a specific response tendency. We speculate that this type of selective stopping is slower because it involves a fronto-basal-ganglia circuit with more synapses than the one that is putatively used to stop quickly (and globally) (c.f. Aron et al., 2007; Aron & Verbruggen, 2008) - although this remains to be established empirically.
Figure 1.
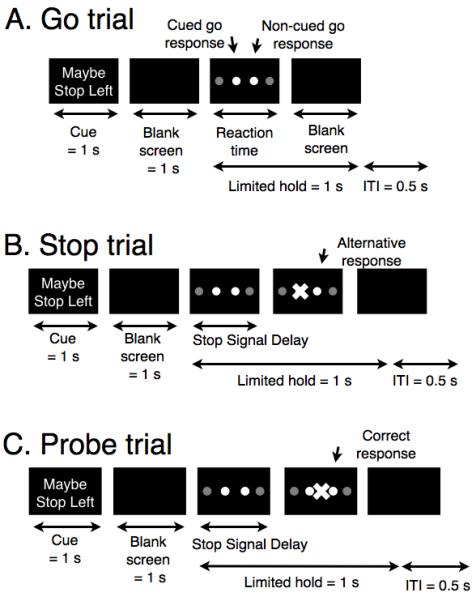
Task design. A. Go trials. The cue “Maybe Stop Left” or “Maybe Stop Right” (or, in the no foreknowledge condition, “Maybe Stop XXX”) is presented for 1 second followed by a blank screen for 1 second, after which time the imperative go stimulus is presented as two blue colored circles (either outer or inner). Participants initiate left and right index finger presses if the blue circles are on the outside, or left and right index finger presses if the blue circles are on the inside. Limited hold refers to the time period during which the circles are displayed if there is no response. ITI is the inter-trial-interval. B. Stop trials. These make up one-third of trials and are identical to go trials, except that a stop signal is presented with a variable stop-signal delay subsequent to the go stimulus. If a stop signal occurs, the subject must try to stop the indicated hand while completing a key press with the other (‘alternative response’) as quickly as possible. C. Probe trials. 25% of stop trials were probe trials. For these, the stop signal appeared in the middle of the screen, probing the participant‘s knowledge of the cue. The participant tries to stop the hand indicated by the cue and to respond with the other hand. The percent of trials for which this was performed correctly provides the cue recall accuracy measure. The stop signal delay on all probe trials was 50 ms.
Here we investigate the neurocognitive mechanisms by which foreknowledge is used to stop particular response tendencies. For Experiment 1 we had two objectives: To replicate our earlier result that stopping is more selective (and slower) for a foreknowledge vs. no foreknowledge condition (Aron & Verbruggen, 2008); and to show that, across participants, those with better recall of the foreknowledge rule are those who are able to stop more selectively. This would help to establish an important link between working memory for stopping goals and the mechanism of inhibitory control that ostensibly underlies the behavioral stopping itself. To examine the relationship between foreknowledge and selective stopping a key addition was made to the design used in Aron and Verbruggen (2008). On a minority of stop signal trials in the foreknowledge condition, the stop signal was uninformative about which response to stop, thus serving as a memory probe. On probe trials, the stop signal was presented at the center of the screen (Figure 1C). When this ‘probe’ occurred the participant was required to stop the response that had been indicated by the foreknowledge cue. This design feature allowed us to compute ‘cue recall accuracy’ - a measure of working memory for the foreknowledge cue. This accuracy measure reflected the proportion of probe trials when the participant correctly stopped the required hand out of all probe trials when they stopped one or both hands. We predicted that those participants with higher cue recall accuracy scores would be those with smaller stopping interference effects. This result would provide further evidence that knowledge of the stopping goal is a key enabler of selective stopping.
Experiment 2 tested a neurocognitive hypothesis about how foreknowledge enables selective stopping. We hypothesized that foreknowledge “sets up” a control set whereby control is applied onto the response representation that may need to be stopped in the future. This predicts that the motor representation of the response that might need to be stopped in the future is affected by the foreknowledge before the response is even initiated. Testing this idea requires a technique that can measure the state of specific motor representations with high temporal resolution. Here we used TMS of the primary motor cortex, using surface electromyography to record evoked potentials from intrinsic muscles of the hand. We delivered TMS stimuli to the left primary motor cortex at specific time-points in the foreknowledge period while participants performed a behavioral paradigm similar to Experiment 1, i.e. making coupled responses with little or index fingers of both hands, and trying to stop one hand when indicated, for both foreknowledge and no foreknowledge conditions. We recorded MEPs from the right first dorsal interosseous muscle (FDI). These MEPs provide a measure of corticomotor excitability for the index finger response representation. We predicted that when the cue was ‘Maybe Stop Right’ the MEP for the right index finger would be significantly reduced compared to when the cue was ‘Maybe Stop Left’. We expected that corticomotor excitability for the ‘Maybe Stop XXX’ condition would either be similar to ‘Maybe Stop Left’ (participants do not prepare to stop the right hand) or else intermediate between ‘Maybe Stop Left’ and ‘Maybe Stop Right’ (participants expect to stop the right hand to some extent). An alternative outcome was that there would be no effect of foreknowledge cue on MEPs in the foreknowledge period. This would indicate that preparing to stop happens at a purely cognitive level, without any effects on the motor system until stopping itself is needed.
METHODS
EXPERIMENT 1: Behavioral study
Participants
16 young adults participated (5 male; all right handed, mean age = 21.2, range 18 – 27 years). All participants provided written consent in accordance with the Internal Review Board guidelines of the University of California at San Diego..
Apparatus
Participants were seated 50 cm in front of an iMac (19“ monitor). The experiment was run using Matlab (Mathworks, Natick, MA) and the PsychToolBox3 (www.psychtoolbox.org). Participants sat with their forearms resting on the table surface in front of four keypads (19 Key Numeric Keypad, Adesso, Walnut, CA). Two of the keypads were mounted vertically with the key surfaces facing laterally (Figure 2A). Participants placed their index finger against a key on each vertical keypad such that they could respond with a key press by moving the index fingers of each hand inward in a lateral abduction. [This movement is optimal for measuring EMG from the index (FDI) muscle, as we did in Experiment 2 below]. The other two keypads lay in a normal position on the table. Participants positioned these keypads so that they could comfortably make a downward key press using their little finger.
Figure 2.
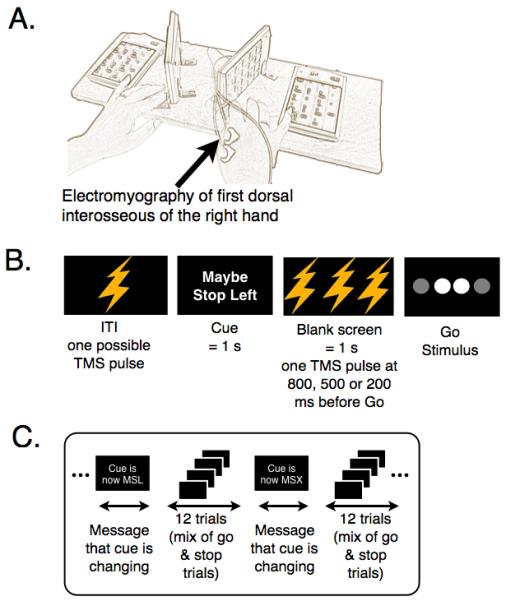
Experimental setup and procedure. A. Response boxes. Keypads were positioned vertically to utilize first dorsal interosseous (FDI) muscle of the index finger for making key press responses. The FDI muscle is well isolated and can be recorded using electromyography (EMG) with little interference from other muscles. Subjects responded by making key presses with either a lateral movement from both index fingers or a downward movement with both little fingers. B. Trial design for Experiment 2. On each trial a TMS stimulus was delivered. It could be delivered at any of four possible time-points; in the intertrial interval (ITI), or in the foreknowledge interval 800, 500 or 200 ms before the go stimulus. C. Trial sequence procedure. The cue was constant for four trials in a row (experiment 1) and twelve trials in a row (experiment 2). A message “Cue is changing to ‘Maybe Stop Left” (MSL) was presented. The order of cues was randomized. Feedback was provided to the participant after each block of 72 trials.
Task and Procedure
Each trial began with a cue (white text on a black background), followed by a blank screen (black), followed by the go signal (Figure 1A). The go signal consisted of two blue circles and two gray circles in a horizontal row, each 2.3ϒ visual angle in diameter. The two inner circles were separated from one another by 4.6ϒ and each inner circle was separated from the outer ones by 1.2ϒ. The four circles corresponded to the four fingers the participant could potentially use to respond. Participants simultaneously pressed both index fingers if the two inner circles were blue and simultaneously pressed both little fingers if the two outer circles were blue. On a third of trials, a stop signal was presented at some delay after the onset of the circles (Figure 1B). The stop signal was a red “X” superimposed either over one of the circles (typical stop signal) or centered between the two middle circles (probe test of cue recall accuracy on a subset of trials).
In the no foreknowledge condition, the cue that preceded the go signal was “Maybe Stop XXX”. On stop trials, a red “X” would appear over one of the two blue circles. If the “X” was over the blue circle on the left side of the screen, participants were to stop the left hand response. If the “X” was over the blue circle on the right side of the screen, participants were to stop the right hand response. In the foreknowledge condition, the cue was “Maybe Stop Left” or “Maybe Stop Right”. On 75% of stop trials, a red “X” would appear over one of the blue circles as in the no foreknowledge condition. On 25% of stop trials (the probe trials), the red “X” was centered between the two middle circles (Figure 1C). On these probe trials, the stop signal did not provide any information about which hand to stop and participants had to recall the cue in order to know which hand response to stop. For example, if the cue was “Maybe Stop Left” and the red “X” appeared in the center of the screen, the participant had to stop the response with the left hand and only respond with the right hand. The cue remained the same for at least 4 trials in a row and a change in cue was signaled by an instruction “Cue changing to ....”.
All participants learned the task using a structured training program that introduced different aspects of the task sequentially. Participants first practiced responding to go trials, then practiced stopping responses for the stop signal, and then practiced observing foreknowledge cues and using recall on probe trials. Verbal instructions from the experimenter emphasized the importance of responding quickly on each trial with fingers of both hands together once the circles were shown. It was also emphasized that participants were to try their best to stop a particular response when indicated by a stop signal, while simultaneously executing the alternative response as fast as possible. It was explained that stopping would not always be possible, but that trying to stop was important.
To prevent participants from delaying the response for the cued hand in the foreknowledge condition (e.g., the left hand when the cue was “Maybe Stop Left”), the text “buttons not pressed together” was presented after responses for which the difference in RT for the two hands was more than 70 ms. This textual prompt was followed by a 3 second punishing time-out before the next trial ensued. Participants were careful not to uncouple their responses (as will be seen below in terms of the correlation of RT for the two hands, and the small number of uncoupled trials). In the no foreknowledge condition, the same instructions applied, except now the cue was uninformative.
Each participant completed 6 blocks of 72 trials (432 trials total). Each block contained 24 stop trials and 48 go trials. Trials were divided evenly across the three cues (Maybe Stop Left, Maybe Stop Right and Maybe Stop XXX). No set of 4 trials had more than 2 stop trials. The trials were divided evenly between the middle two circles being blue (i.e. index finger response) and the outer two circles being blue (i.e. little finger response). After each block of 72 trials, participants were given graphical feedback in terms of mean correct go RT, number of errors on go trials (i.e. with respect to accuracy of making index or little finger responses), and cue recall accuracy on foreknowledge probe trials.
For each hand, stop-signal delays were adjusted dynamically according to the participant’s performance: If a response was stopped then the stop signal delay increased by 50 ms; but if it was not stopped then the stop signal delay decreased by 50 ms (Osman, Kornblum, & Meyer, 1986). A total of 6 dynamic staircases were used, one for each combination of response (index finger, little finger) and cue (Maybe Stop Left, Maybe Stop Right and Maybe Stop XXX). The starting value for the stop-signal delay was either 50 ms or 300 ms. For all probe trials, the stop-signal delay was set to 50 ms to give participants sufficient opportunity to stop their response and thus reveal their cue recall accuracy of the foreknowledge cue.
Analysis
The analysis steps are described in detail in Aron and Verbruggen (2008); here we explain them briefly. In the foreknowledge condition, data for ‘Maybe Stop Left’ and ‘Maybe Stop Right’ were calculated separately. Taking the example of ‘Maybe Stop Left’: We calculated mean correct RT on go trials for the left hand (i.e. the cued-hand RT) and mean correct RT on go trials for the right hand (i.e. the non-cued hand RT). Trials were deemed correct if both hands were pressed together (i.e. difference between cued-hand RT and non-cued hand RT < 70 ms) and if the correct buttons were pressed. When the cue was ‘Maybe Stop Left’, we calculated the cue recall accuracy as the percent of probe trials when the participant correctly stopped the left hand out of probe trials when they stopped one or both hands. We also calculated the stop signal delay that gave an approximately 50% stop rate. There were two staircases for the left hand and each staircase had 48 stop signal delays over the course of 6 blocks. Inspection of data showed that stop signal delay converged after about 24 moves of each staircase. Therefore, for each participant, we averaged the final 24 moves of each staircase, and these two averages were averaged to get the mean stop-signal delay for the left hand. SSRT for the left hand was estimated by subtracting mean stop-signal delay from cued-hand mean correct go RT for the left hand (Logan, Schachar, & Tannock, 1997). We also calculated the stopping-interference effect when the cue was ‘Maybe Stop Left’ by subtracting the mean correct RT for the non-cued hand (i.e. the right hand) on go trials from the mean RT for that same hand (i.e. the right hand) when subject did stop the left response on stop trials.
The above calculations of mean correct go RT for the cued hand and non-cued hand, SSRT and the stopping-interference effect were also made for the ‘Maybe Stop Right’ condition. Following this, data were collapsed across ‘Maybe Stop Left’ and ‘Maybe Stop Right’ conditions in the foreknowledge condition. All these calculations were also repeated for the no foreknowledge condition (see Aron & Verbruggen, 2008) except for cue recall accuracy (there were no probe trials). For key measures of interest, the group mean values for foreknowledge and no foreknowledge were compared statistically with paired-sample t-tests. In the foreknowledge condition alone, the correlation was assessed between cue recall accuracy and the stopping-interference effect across participants.
EXPERIMENT 2: TMS study
Participants
Fifteen young adults participated (4 male; 1 left handed; mean age = 20.7, range 18–28). These were different participants from Experiment 1. All participants provided written consent in accordance with Internal Review Board guidelines of the University of California, San Diego. Participants also completed a TMS safety screen questionnaire and were found to be free of contraindications.
EMG recordings
Participants were seated comfortably in front of an iMac desktop computer (Apple Corporation, Cupertino, CA). Surface EMG recordings were made via 10-mm-diameter Ag-AgCl hydrogel electrodes (Medical Supplies, Inc, Newbury Park, CA) placed over the first dorsal interosseous muscle (FDI: index finger) (Figure 2A). A ground electrode was placed over the styloid process (wrist) of the right ulna. The EMG signal was amplified using a Grass QP511 Quad AC Amplifier System Grass amplifier (Grass Technologies, West Warwick, RI), with a band-pass filter between 30 Hz and 1 kHz and a notch filter at 60 Hz. Data were sampled at 2 kHz using a CED Micro 1401 mk II acquisition system and displayed and recorded to disk using CED Signal v4 (Cambridge Electronic Design, Cambridge, UK). MEP analysis was performed using custom software in Matlab R2007a (The MathWorks, Natick, MA).
TMS
We used a MagStim 200–2 system with a BiStim module (Magstim, Whitland, UK) and a figure-of-eight coil (7 cm diameter) to deliver TMS test stimuli and conditioning stimuli during task performance. To locate the representation of the FDI muscle in left M1, the coil was initially located at a point 5 cm lateral and 2 cm anterior of the vertex. The coil was incrementally repositioned while administering single stimuli to locate the position that produced the largest, reliable MEPs in right FDI. This location was marked on a snug-fitting cap worn by the participant to ensure the consistent placement of the coil through the experiment. Average location of stimulation was 6.2 cm lateral and 2.1 cm anterior of vertex of the skull. Rest motor threshold was determined by finding the lowest stimulus intensity that produced MEPs of at least 0.05 mV amplitude on at least five out of ten trials (Rossini et al., 1994). Next, the participant‘s maximum MEP size was determined by increasing stimulus intensity in 5% increments, starting at rest motor threshold, until MEP amplitude no longer increased with increasing stimulus intensity. Test stimulus intensity was set to produce a MEP amplitude that was approximately half of the participant’s maximum MEP amplitude. This ensured that the test stimulus intensity was on the ascending limb of the individual’s stimulus-response curve, so that both increases and decreases in corticospinal excitability could be detected (Devanne, Lavoie, & Capaday, 1997).
In addition to delivering single magnetic stimuli (half the trials), we also delivered paired-pulse stimuli (the other half of trials) to probe short interval intracortical inhibition. This paired pulse technique can evaluate whether a reduction in MEP amplitude following the test stimulus could be due to increased GABA-ergic inhibition within M1 (Kujirai et al., 1993). The method requires a sub-threshold conditioning stimulus which is delivered 1 to 5 ms before a suprathreshold TMS test stimulus. The effect of the conditioning stimulus on the test stimulus is compared with trials in which there is a test stimulus alone. However, inspection of such short interval intracortical inhibition results for this experiment showed that the method was not technically satisfactory for a majority of participants. This was evidenced by larger conditioned MEPs than unconditioned MEPs. This probably related to a suboptimal procedure for performing thresholding. We did this while the participant was at rest, when a more appropriate method would have been to perform thresholding during a preparation-to-respond period. Consequently this paired-pulse aspect of the experiment is not discussed further.
A magnetic stimulus was delivered on each trial. Half of the trials had a single unconditioned test stimulus. The other half of trials had a conditioning stimulus followed 3 ms later by a test stimulus (data from these trials are not reported). The type of stimulation varied randomly by trial. Stimulation was delivered at four different time-points. One time-point was during the inter-trial interval (ITI) at 250 ms before the cue. The purpose of this time-point was to serve as a baseline for normalizing foreknowledge period MEPs within each subject. The other three time points were within the foreknowledge period, at 800, 500 and 200 before the go signal appeared (Figure 2B).
Task and Procedure
The task for Experiment 2 was identical to Experiment 1 with the following exceptions: a) To maximize utilization of the cue, the cue remained the same for 12 consecutive trials instead of 4 (Figure 2C), b) In the foreknowledge condition, the red “X” appeared in the center of the screen for every stop trial, not just on probe trials. These changes were intended to maximize the attention to foreknowledge and any corresponding effects on corticospinal excitability.
An additional change relative to Experiment 1 was that the stop signal delays were fixed and evenly distributed among values of 100, 150, 200 and 250 ms. This was done to make the comparison of MEPs across participants more consistent. With the dynamic stop signal tracking method used in Experiment 1, participants would be expecting stop signals at different times, potentially affecting MEPs in the foreknowledge period in different ways for different participants.
Analysis
Behavioral data
All relevant metrics were calculated as for Experiment 1 except for stop signal reaction time. As the use of fixed stop signal delays resulted in p(stop) rates far from 50%, stop signal reaction time was estimated using the integration method (Verbruggen & Logan, 2009) rather than the mean method of Experiment 1.
TMS
MEPs were detected from the EMG data using custom software written in Matlab 2007a. Trials were excluded if the Root Mean Square in the 100 ms before stimulus was > 10 μV or if the MEP amplitude of a trial was more than 3 standard deviations from the mean for a given subject. Mean MEP amplitude was calculated for each subject, cue condition, and stimulation time.
A first statistical analysis of FDI MEP amplitude was performed for MEPs in the ITI period. This used a repeated measures ANOVA with three levels of the factor cue (Maybe Stop Left, Maybe Stop Right, Maybe Stop XXX). This established that there were no differences in the ITI period for cue condition. Therefore we used the average MEP across cue condition in the ITI as a baseline to normalize, within each participant, the MEP in each cue condition and for each TMS stimulation time, i.e. 800, 500 and 200 ms before the go stimulus. We then performed a repeated measures ANOVA with the factors of cue (Maybe Stop Left, Maybe Stop Right, Maybe Stop XXX) and test stimulus time (800, 500 and 200 ms before the go stimulus) with ITI-normalized MEP as the dependent measure. A further analysis repeated this same ANOVA on the non-normalized MEPs for the factors cue and test stimulus time.
Additionally, we computed Root Mean Square EMG activity in the 100 ms preceding the magnetic stimuli for each condition in order to establish if the muscle of interest was at rest at the time of stimulation, and in order to establish if there were systematic differences in pre-TMS muscle activity for the conditions of interest.
RESULTS
Experiment 1: Behavioral Study
Table 1 shows the behavioral data. Consistent with our prior report (Aron & Verbruggen, 2008), participants required significantly more time to successfully stop an initiated response when they had been cued as to which response to stop than when they had not, t(15) = 2.15, p < .05 (Figure 3A). Moreover, we found again that on stop trials, stopping one response had less of an effect on executing the alternative response in the foreknowledge condition than in the no foreknowledge condition. This observation was supported by a significantly smaller stopping-interference effect in the foreknowledge condition than in the no foreknowledge condition, t(15)=−4.74, p < .01 (Figure 3B). Unlike our earlier report (Aron & Verbruggen, 2008) there were differences in RT on go trials. The RT for the cued hand on go trials (e.g., the left hand when the cue was ‘Maybe stop left’) in the foreknowledge condition was significantly slower than the RT for the same hand on go trials in the no foreknowledge condition t(15)=3.48, p < .01. Finally, in the foreknowledge condition, across participants, cue recall accuracy significantly predicted the stopping interference effect - those participants with better cue recall stopped more selectively, robust regression, p < .01 (Figure 3C).
Table 1. Behavioral Results for Experiment 1.
Key measures and standard deviations. Values outside parentheses indicate mean across subjects. Values inside parentheses indicate standard deviation. RT cued hand = RT on go trials for the hand that might have had to stop; RT non-cued hand = RT on go trials for the hand that was not cued to stop; % go trials with decoupling = % of go trials with difference in hand RT was greater than 70 ms; % go trials with other errors = % of go trials when incorrect keys were pressed (e.g. index finger presses instead of little finger); RT alternative hand = RT on alternative hand when cued hand successfully stopped; Cue recall accuracy = accuracy of stopping the hand indicated by the cue in probe trials when at least one response was inhibited; Interference effect = the stopping interference effect; i.e. RT alternative hand - RT non-cued hand; p(stop) = probability of stopping across the whole experiment; SSD = the mean SSD value after convergence of staircases; SSRT = stop signal reaction time, i.e. RT cued hand - SSD.
| Foreknowledge | No Foreknowledge | |
|---|---|---|
| RT cued hand | 547 (105) | 531 (102) |
| RT non-cued hand | 546 (106) | 534 (101) |
| % go trials with decoupling | 3.6% (2.3) | 2.0% (2.4) |
| % go trials with other errors | 3.5% (2.7) | 2.5% (3.2) |
| RT alternative hand | 632 (95) | 649 (94) |
| Cue recall accuracy | 74.1% (20.7) | NA |
| Interference effect | 86 (39) | 114 (30) |
| p(stop) | .50 (.09) | .50 (.05) |
| SSD | 279 (114) | 278 (119) |
| SSRT | 268 (42) | 252 (46) |
Figure 3.
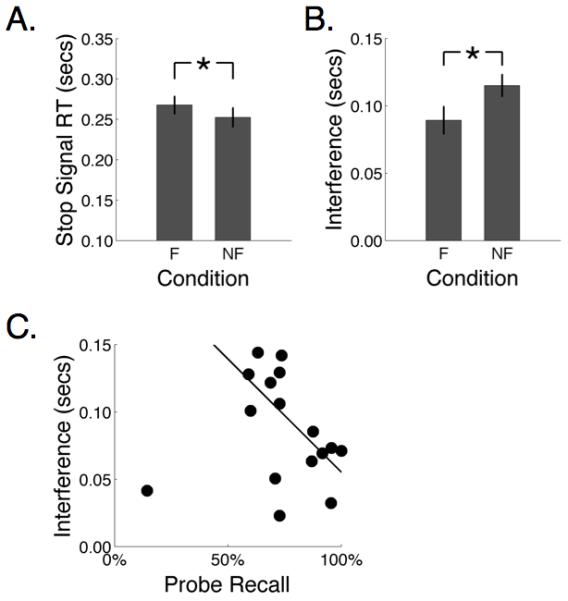
Behavioral results from experiment 1. A. Slower stopping with foreknowledge. Stop signal reaction time (SSRT) is slower in the foreknowledge (F) than no foreknowledge (NF) conditions. B. More selective stopping with foreknowledge. The stopping interference effect (measuring the selectivity of stopping) is smaller in the F than NF condition. C. Degree of foreknowledge predicts the selectivity of stopping. Participants with better cue recall of hand-to-be-stopped have smaller stopping interference effects.
Experiment 2: TMS study
Behavior
Table 2 shows the behavioral data. Importantly, for the interpretation of TMS results, performance was similar for Maybe Stop Left and Maybe Stop Right conditions in terms of the speed of responding on Go trials (Figure 4A), the stopping interference effect and SSRT (all p>0.1). Performance was also similar with respect to the percentage of times participants stopped the relevant hand (i.e. > 85% in both conditions). Note that in this experiment every stop trial in the foreknowledge condition was effectively a ‘memory probe’ trial (i.e. the red X was always presented in the center of the screen, unlike Experiment 1). As the accuracy with which subjects stopped the correct hand was over 85% on average participants certainly encoded the foreknowledge cue and used that information at the time of the stop signal.
Table 2. Behavioral Results for Experiment 2.
The measures are similar to Table 1.
| Foreknowledge | No | ||
|---|---|---|---|
| Maybe Stop Left | Maybe Stop Right | Foreknowledge Maybe Stop XXX |
|
| RT cued hand | 528 (61) | 536 (56) | 519 (67) |
| RT non-cued hand | 530 (57) | 532 (58) | 520 (64) |
| % go trials with decoupling | 2.8% (2.0) | 4.1% (2.9) | 1.9% (1.9) |
| % go trials with other errors | 3.9% (4.1) | 5.2% (7.3) | 3.9% (6.8) |
| % of stop trials on which subject stops correct hand |
86.7% (13.3) | 88.9% (11.8) | NA |
| Stop Signal Reaction Time (SSRT) |
312 (75) | 309 (61) | 303 (84) |
| RT alternative hand | 669 (81) | 666 (84) | 622 (79) |
| Interference effect | 140* (64) | 136* (54) | 103 (41) |
| p(stop)** | 0.69 (.14) | 0.73 (.14) | 0.73 (.14) |
The interference effect is inflated in the Maybe Stop Left and Maybe Stop Right foreknowledge conditions (relative to Experiment 1) because the probe method is used on every stop trial. Even if the participant knows what to stop most of the time (as suggested by mean recall accuracy > 85%) on some trials the participant is likely to forget and this will lead to a longer average stopping interference effect.
In this experiment fixed delays were used for the stop signal delay, rather than the tracking method of Experiment 1. This resulted in a higher p(stop).
Figure 4.
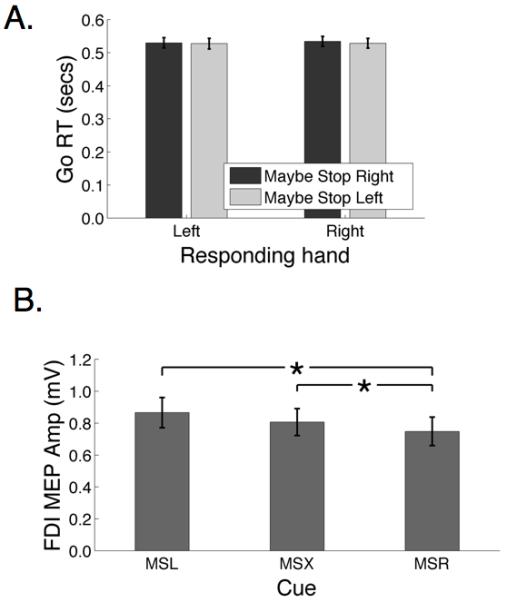
Behavioral and TMS results from Experiment 2. A. On go trials, RT was highly coupled for left and right hands and highly similar regardless of which hand needed to be stopped. B. Corticomotor excitability, measured in terms of motor evoked potential (MEP) for the right FDI muscle is different according to cue condition. When the cue is ‘Maybe Stop Right’ (MSR) the MEP is smaller than when it is ‘Maybe Stop Left’ (MSL) and also compared to when it is ‘Maybe Stop XXX’ (MSX).
An unexpected result arose for the comparison of foreknowledge and no foreknowledge conditions. Here the interference effect for the foreknowledge condition was larger than for the no foreknowledge condition, and this was a significant difference (t(14)=3.6, p<0.01). This ran against the findings of Experiment 1, as well as our earlier report (Aron & Verbruggen, 2008) in which the stopping interference effect was smaller in the foreknowledge condition. This anomalous finding must relate to the change in procedure - in the foreknowledge condition of this experiment, the stopping cue was always presented in the center of the screen (as opposed to just 25% of stop (i.e. probe) trials in Experiment 1). This meant that participants had to recall what to stop on every stop trial. Although participants stopped the correct hand on over 85% of trials, it’s likely that occasional lapses in attention meant that participants used a nonselective (global) stopping some of the time in the foreknowledge condition, followed by a re-initiation of the correct responding hand. It is likely that such a global-stop-plus-re-initiation would produce some trials with very long RT (i.e. an inflated overall observed interference effect). This occasional stop-plus-restart requirement in the foreknowledge condition of this experiment is probably an exaggeration of that in the no foreknowledge case. In the latter, the participant can use the explicit stop signal to know which response to restart - whereas in the foreknowledge condition, the participant has to refer to memory to recall which response needs to be continued. The possibility that participants sometimes forget what to do on foreknowledge trials when the stop signal occurs (in the center of the screen), predicts greater variability of RT for the alternative (i.e. responding hand) on stop trials compared to the no foreknowledge condition. This was in indeed the case: the standard deviation of the alternative response was significantly greater for foreknowledge (M = 0.10, SD=0.02) compared to no foreknowledge (M = 0.07, SD = 0.02), t(14)=6.44, p < .001. Regarding SSRT, participants stopped slightly more quickly in the no foreknowledge compared to foreknowledge condition, as expected, although in this experiment this was not a significant difference (t(14)<1. n.s.). Thus, although the change of procedure altered some of the key measures of selectivity of stopping, it is clear that the procedure was very effective at requiring participants to engage foreknowledge. Moreover, as there were no differences on behavioral measures for the key comparison of Maybe Stop Left and Maybe Stop Right, the comparison between these conditions for the TMS experiment remained very interesting. We note that SSRT was longer for Experiment 2 than Experiment 1. This could be explained by the fact that the average stop signal delay was smaller in Experiment 2 than in Experiment 1. Logan & Cowan (1984) showed that SSRT decreases when stop signal delay increases.
Corticomotor excitability
Average stimulator intensity for the test stimulus was 53.5% maximum stimulator output (SD = 7.15). A first analysis on MEPs in the ITI (baseline) period established that there was no main effect of cue condition F(2,26)<1, n.s. Therefore we used the average MEP across cue condition in the ITI as a baseline to normalize, within each participant, the MEP in each cue condition and for each TMS stimulation time. The mean baseline MEP was 0.715 mV, SEM = 0.027. For the baseline-normalized analysis, there was a significant main effect of cue F(2,26)=5.47, p<0.05, but no main effect of stimulation time F(2,26)=1.91, n.s, nor an interaction F(2,26)=1.80, n.s. This analysis was repeated with non-normalized MEP values, for which there was one additional participant (one was lost from the normalized analysis because that person did not have any valid MEPs in the ITI period). The same cue (3) x stimulation time (3) ANOVA was performed on non-normalized MEPs. There was a significant main effect of cue F(2,28)=8.38, p<0.001, but no main effect of stimulation time F(2,28)=2.0, n.s., nor an interaction F(2,28)=1.20, n.s. Post-hoc pairwise analysis applying Bonferroni correction showed that MEPs were significantly smaller for Maybe Stop Right than Maybe Stop Left (mean difference = 0.14 mV, SE = 0.043, p<0.0167), and that MEPs were significantly smaller for Maybe Stop Right than Maybe Stop XXX (mean difference = 0.09 mV, SE = 0.03, p<0.0167), but there was not a significant difference between Maybe Stop Left and Maybe Stop XXX (mean difference = 0.05 mV, SE = 0.029, p=0.102) (Figure 4B).
Finally, pre-TMS EMG validation was performed to make sure the muscle was equivalently ‘quiet’ across conditions. We analyzed these data with a repeated measures ANOVA including cue (3) and time point (800 ms, 500 ms, and 200 ms before the go signal), with Root Mean Square EMG as the dependent variable. The main effect for cue was not significant, F(2,28)=2.96, p>0.05. The main effect of time point was not significant, nor was the interaction. Overall, therefore, the FDI muscle was similarly ‘at rest’ for the different conditions prior to magnetic stimulation (mean Root Mean Square 3.36 μV, SE = .32 μV).
DISCUSSION
Much human control requires us to keep in mind our goals to stop specific actions or tendencies should they be provoked or initiated by environmental contingencies. Here we studied how such stopping goals (foreknowledge) are set up and how they are proactively deployed to target specific response tendencies. There were two main findings. First, those participants with greater recall accuracy for the foreknowledge cue were able to stop their responses more selectively. This establishes an important link between having a goal in working memory and the recruitment of a selective stopping mechanism. Second, during the foreknowledge period, corticomotor excitability for a specific hand was significantly reduced when that hand was the one that might have needed to be stopped relative to when it was not. This shows that having a goal of what response may need to be stopped in the future consists in applying advance control onto a specific motor representation.
In Experiment 1 we replicated our earlier results (Aron & Verbruggen, 2008) by showing that stopping is more selective but also slower in a foreknowledge compared to a no foreknowledge condition. It is unlikely that stopping is slower in the foreknowledge condition because a more potent motor response is generated for the “non-stopped hand” for two reasons: first, this runs against the observation that there was slower RT in the foreknowledge compared to no foreknowledge conditions and second, the response times for the two hands in the foreknowledge condition were highly coupled. Instead, we suspect that the difference in stopping speed could be explained by differences in the putative fronto-basal-ganglia circuits used to stop with and without foreknowledge. Several lines of evidence suggest that stopping without foreknowledge engages a non-selective (i.e. global) mechanism (Badry et al., 2009; Coxon et al., 2006; Coxon et al., 2007; Leocani et al., 2000; Sohn et al., 2002). This is consistent with a putative role for the STN in stopping (Aron & Poldrack, 2006; Eagle et al., 2007; Kuhn et al., 2004; Li, Yan, Sinha, & Lee, 2008; Ray et al., 2009; van den Wildenberg et al., 2006), as it is positioned to broadly block thalamocortical output (Gillies & Willshaw, 1998). By contrast, we speculate that mechanistically selective stopping is implemented via the so-called ‘indirect pathway’ of the basal ganglia, i.e. frontal-striatal-pallidal connections (rather than fronto-subthalamic). This greater number of synaptic connections could slow down the stopping speed, and we observed such slowing here and previously (Aron & Verbruggen, 2008).
As we noted previously (Aron & Verbruggen, 2008), our identification of a selective stopping mechanism should be contrasted with selective stopping at the behavioral level. Previous studies using “selective” versions of the stop-signal paradigm showed that stopping latency is typically longer for such versions compared to the standard one (Bedard et al., 2003; Bedard et al., 2002; Coxon et al., 2007; De Jong, Coles, & Logan, 1995; van den Wildenberg & van der Molen, 2004). However, longer stopping latencies could be attributed to several factors and these studies cannot apparently distinguish between a truly selective mechanism and a global stopping mechanism. For example, the study by Coxon and colleagues (2007) clearly shows that subjects can stop one response (behaviorally) and continue executing another. However, the execution of the alternative response was substantially delayed, and this probably reflects use of a global suppression mechanism followed by re-initiation of the response in a second step. Thus, prior experiments that purported to measure selective stopping may only have done so at the behavioral level and not at the mechanistic level. By contrast, the present study clearly demonstrates that selective stopping in the foreknowledge condition reduced the stopping-interference effect, showing that the stopping process can be mechanistically more selective.
In relation to these prior studies of behaviorally selective stopping it is also important to note that SSRT was longer in such conditions than standard signal paradigms. This likely reflects the additional choice element that is needed to stop one response and not another (van de Laar, van den Wildenberg, van Boxtel, & van der Molen). This predicts that foreknowledge should remove the choice element and make SSRT quicker. We suspect that such a reduction in the choice element does indeed occur, but that the benefits to SSRT are negated by the additional time added to stop selectively, using a fronto-basal-ganglia pathway with more synapses. Future research is clearly need to validate this model of different basal ganglia pathways for global compared to selective stopping.
Experiment 1 also incorporated a novel design feature that allowed us to test the hypothesis that foreknowledge enables the use of a more selective stopping mechanism precisely because the participant uses the stopping goal (foreknowledge) to prepare to stop a specific response tendency. This predicts that participants with higher levels of foreknowledge, which is to say better memory for the foreknowledge cue, will be able to stop more selectively when they are required to. Consistent with this prediction there was a highly significant relationship between foreknowledge recall and the selectivity of stopping. This provides further support for our model of mechanistically selective stopping - according to which a key requirement is that the participant has advance access to a goal about which response to stop. It also helps to make an important link between the encoding and/or retrieval of stopping goals and the engagement of subsequent inhibitory control itself (Verbruggen, Schneider, & Logan, 2008).
Experiment 2 tested the idea that foreknowledge “sets up” a control set whereby control is applied onto the response representation that may need to be stopped. This predicts that the motor representation of the response that might need to be stopped in the future is affected by foreknowledge even before the response is initiated. To examine this we used TMS to probe corticomotor excitability for the right index finger muscle during the foreknowledge period. Consistent with the prediction, we found that MEPs for the right index finger were significantly smaller during the foreknowledge period for Maybe Stop Right than Maybe Stop Left. We also observed that MEPs were significantly smaller for Maybe Stop Right than Maybe Stop XXX. There are at least two possible explanations for the reduction of corticomotor excitability for the hand that might have to stop in the foreknowledge period: reduced motor facilitation and proactive inhibitory control.
According to the reduced motor facilitation account, when the cue is Maybe Stop Right the participant can be certain that if a stop signal occurs it will be for the right hand (requiring stopping of either the index or little finger movement) and this results in less facilitation of the representation of the right hand. By contrast, when the cue is Maybe Stop Left, the participant can be certain that if a stop signal occurs it will not require the right hand to be stopped, and this may encourage the participant to facilitate the right hand response more. This predicts a reduction of MEP for Maybe Stop Right compared to Maybe Stop Left, as we observed. This account also predicts that for the Maybe Stop XXX cue, the MEP will be intermediate, as we found here for the comparison of Maybe Stop XXX and Maybe Stop Right at least (the comparison of Maybe Stop XXX and Maybe Stop Left was not significantly different). When the cue is Maybe Stop XXX, the probability that a right hand response will need to be stopped, if any stop signal occurs, is 50%, which is more than when the cue is Maybe Stop Left (0%) and less than when it is Maybe Stop Right (100%). This account of reduced facilitation for a hand that might need to be stopped fits with the extant TMS literature on motor preparation. In particular, Bestmann et al (2008) showed that corticomotor excitability, assessed using the MEP measure as we do here, varied according to the amount of information conveyed by sensory cues guiding action. Other TMS studies have shown similar effects in a motor preparation period (Mars, Bestmann, Rothwell, & Haggard, 2007; van Elswijk, Kleine, Overeem, & Stegeman, 2007). Yet the reduced motor facilitation account of our findings is challenged by the fact that reaction times were highly coupled for left and right hands together on go trials, so that when the cue was Maybe Stop Right, the right hand response did not lag the left response; and when the cue was Maybe Stop Left, the left hand response did not lag the right hand response (overall mean cued hand RT: 547 ms; mean non-cued hand RT: 546 ms). Moreover, in the foreknowledge condition the percentage of Go trials with uncoupled responses (i.e., delay between two responses > 70 ms) was only 3.5%. It is difficult to reconcile this observation of highly coupled responses with the theory that a reduction in motor facilitation explains the smaller MEPs for Maybe Stop Right compared to Maybe Stop Left. An additional observation that speaks against the reduced motor facilitation account is the comparison of Maybe Stop Left and Maybe Stop XXX. The reduced motor facilitation account predicts that RT should be faster for the Maybe Stop Left compared to Maybe Stop XXX condition because the MEP is greater (at trend level significance). Yet this is not the case, RT is significantly slower for the Maybe Stop Left condition than Maybe Stop XXX. This speaks against the reduced motor facilitation account.
An alternative account for why MEPs were smaller for Maybe Stop Right than Maybe Stop Left (and for Maybe Stop XXX than Maybe Stop Left) is in terms of proactive inhibitory control. On this account, the participant anticipates that the right hand may need to be stopped, and uses a proactive inhibitory control mechanism to target the right index and little finger representations either at the level of the motor cortex, or perhaps upstream of this in the basal ganglia (e.g. striatum). Proactive inhibitory control that is selective to a particular response tendency may be implemented via input from the prefrontal cortex to the ‘indirect pathway’ of the basal ganglia. Consistent with this many studies have argued for the involvement of frontostriatal pathways in inhibitory control as assessed with Go/No-Go and related paradigms (e.g. Casey et al., 1997; Kelly, Hester, Murphy, Javitt, & Foxe, 2004; Pollux, 2004; Robbins & Rogers, 2000) and at least three studies have implicated prefrontal and/or striatal regions in putative forms of inhibitory control that are proactive, i.e. operating before the response is initiated (Hester, Murphy, Foxe, Foxe, & Javitt, 2004; Jaffard et al., 2008; Jahfari, Stinear, Claffey, Verbruggen, & Aron, 2009). Some recent TMS studies have also argued for inhibitory control in a foreperiod before responses are initiated (e.g. Davranche et al., 2007; Duque & Ivry, 2009), although this is more in the context of preventing impulsive responding (i.e responding too quickly) rather than preparing to stop a particular response tendency. This proactive inhibitory control account is also compatible with our finding that participants executed the go response with the two hands in a highly coupled fashion. This could be the case if it is assumed that proactive inhibitory control can be applied via a separate neural mechanism than that which facilitates the motor response itself (Davranche et al., 2007; Duque & Ivry, 2009; Reynolds & Ashby, 1999).
Although the distinction between these two accounts of reduced motor facilitation and proactive inhibitory control is a substantive one in terms of neural mechanism, the current data cannot distinguish clearly between these. Future research using functional imaging and paired pulse TMS may be able to elucidate the underlying mechanistic basis of the advance control we observe here. However, regardless of the question of neural mechanism, the current results clearly demonstrate that foreknowledge is important for selective stopping. They also show that how well people monitor their stopping goals is a critical factor in how targeted their subsequent stopping can be. And the TMS findings show that having a stopping goal in this paradigm appears to be associated with advance effects on particular motor representations. Thus, the control goal is ‘embodied’ insofar as it is manifest in the motor system and not purely cognitive. This ability to ‘observe’ the control goal within the motor system even before action ensues highlights the power of the TMS technique. It motivates the current behavioral and TMS paradigm as a useful model to address a range of questions related to how we are able to keep inappropriate or unwanted response tendencies at bay.
ACKNOWLEDGEMENTS
Frederick Verbruggen is a PostDoctoral Fellow of the Research Foundation - Flanders (FWO-Vlaanderen). This study was supported by an Alfred P Sloan Fellowship to Adam Aron and NIH Grant 1R01DA026452-01A109.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci. 2007;27(44):11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychological science: a journal of the American Psychological Society / APS. 2008;19(11):1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, Foly N, Nagiub H, Nagamine T, Fukuyama H. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol. 2003;31(3):315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- Bedard AC, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Dev Neuropsychol. 2002;21(1):93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Harrison LM, Blankenburg F, Mars RB, Haggard P, Friston KJ, Rothwell JC. Influence of uncertainty and surprise on human corticospinal excitability during preparation for action. Curr Biol. 2008;18(10):775–780. doi: 10.1016/j.cub.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. Implication of right frontostriatal circuitry in response inhibition and attention- deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36(3):374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear C, Byblow WD. Intracortical Inhibition During Volitional Inhibition of Prepared Action. J Neurophysiol. 2006;95(6):3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow W. Selective inhibition of movement. J Neurophysiol. 2007 doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- Davranche K, Tandonnet C, Burle B, Meynier C, Vidal F, Hasbroucq T. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25(12):3766–3774. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MG, Logan GD. Strategies and Mechanisms in Nonselective and Selective Inhibitory Motor Control. J Exp Psychol. 1995;21(3):498–511. doi: 10.1037//0096-1523.21.3.498. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114(2):329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Duque J, Ivry RB. Role of Corticospinal Suppression during Motor Preparation. Cereb Cortex. 2009 doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-Signal Reaction-Time Task Performance: Role of Prefrontal Cortex and Subthalamic Nucleus. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Gillies AJ, Willshaw DJ. A massively connected subthalamic nucleus leads to the generation of widespread pulses. Proc Biol Sci. 1998;265(1410):2101–2109. doi: 10.1098/rspb.1998.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester, Murphy K, Foxe JJ, Foxe DM, Javitt DC. Predicting Success: Patterns of Cortical Activation and Deactivation prior to Response Inhibition. Journal of Cognitive Neuroscience. 2004 doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42(3):1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with Restraint: What Are the Neurocognitive Mechanisms? J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004 doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, Yarrow K, Brown P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain. 2004;127(Pt 4):735–746. doi: 10.1093/brain/awh106. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, Lee TW. Subcortical processes of motor response inhibition during a stop signal task. Neuroimage. 2008;41(4):1352–1363. doi: 10.1016/j.neuroimage.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan G, Schachar R, Tannock R. Impulsivity and Inhibitory Control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- Logan GD, Cowan WB. On the Ability to Inhibit Thought and Action: A Theory of an Act of Control. Psych Rev. 1984;91:295–327. [Google Scholar]
- Mars RB, Bestmann S, Rothwell JC, Haggard P. Effects of motor preparation and spatial attention on corticospinal excitability in a delayed-response paradigm. Exp Brain Res. 2007;182(1):125–129. doi: 10.1007/s00221-007-1055-4. [DOI] [PubMed] [Google Scholar]
- Osman A, Kornblum S, Meyer DE. The Point of No Return in Choice Reaction Time: Controlled and Ballistic Stages of Response Preparation. J Exp Psychol Hum Percept Perform. 1986;12(3):243–258. doi: 10.1037//0096-1523.12.3.243. [DOI] [PubMed] [Google Scholar]
- Pollux PM. Advance preparation of set-switches in Parkinson’s disease. Neuropsychologia. 2004;42(7):912–919. doi: 10.1016/j.neuropsychologia.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ray NJ, Jenkinson N, Brittain J, Holland P, Joint C, Nandi D, Bain GP, Yousif N, Green A, Stein JS, Aziz TZ. The role of the subthalamic nucleus in response inhibition: Evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53(4):730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Rogers RD. Control of cognitive processes: Attention and performance XVIII. MIT Press; Cambridge, MA: 2000. Functioning of Frontostriatal Anatomical “Loops” in Mechanisms of Cognitive Control. [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88(1):333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- van de Laar M, van den Wildenberg WP, van Boxtel GJM, van der Molen MW. Processing of Global and Selective Stop Signals: Application of Donders’ Subtraction Method to Stop-signal Task Performance. Experimental Psychology. doi: 10.1027/1618-3169/a000019. In press. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18(4):626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, van der Molen MW. Developmental trends in simple and selective inhibition of compatible and incompatible responses. J Exp Child Psychol. 2004;87(3):201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- van Elswijk G, Kleine BU, Overeem S, Stegeman DF. Expectancy induces dynamic modulation of corticospinal excitability. J Cogn Neurosci. 2007;19(1):121–131. doi: 10.1162/jocn.2007.19.1.121. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33(5):647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Schneider D, Logan G. How to stop and change a response: The role of goal activation in multitasking. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(5):1212–1228. doi: 10.1037/0096-1523.34.5.1212. [DOI] [PubMed] [Google Scholar]


