Abstract
The present experiment examined the influence of excitotoxic lesions of the basolateral amygdala (BLA) on morphine-induced saccharin avoidance. Neurologically intact subjects rapidly learned to avoid drinking the taste conditioned stimulus (CS), an effect that was sustained throughout the experiment. Although the BLA-lesioned (BLAX) rats showed CS avoidance over the first few trials, the effect was not sustained. That is, by the end of the experiment, the BLAX rats were drinking the same amount of saccharin after seven saccharin-morphine trials as they did on the first trial (i.e., prior to the morphine injections). Potential interpretations of the results are discussed including a disruption of the mechanism that governs drug-induced taste avoidance in normal subjects and the more rapid development of tolerance in BLAX rats.
Keywords: Basolateral amygdala, incentive learning, morphine, rat
1. Introduction
The amygdala is a component of the central gustatory system (for reviews see [6, 21, 47]). In the rat, taste information from the mouth synapses first in the nucleus of the solitary tract and then in the parabrachial nucleus in the dorsolateral pons. From the parabrachial nucleus, taste information ascends to the forebrain along two major pathways, frequently referred to as the dorsal and ventral pathways. Taste information in the dorsal pathway travels along axons from the parabrachial nucleus to the parvicellular division of the ventral posteromedial nucleus of the thalamus, also known as the gustatory thalamus (GT). From the GT, neurons project to the insular cortex (IC [16,27]). Taste information traveling in the ventral pathway is not as straightforward. Axons from the parabrachial nucleus send taste information to a number of nuclei including the lateral hypothalamus, the bed nucleus of stria terminalis and the central nucleus of the amygdala. Additionally, interconnections exist between the central nucleus of the amygdala and the basolateral amygdala (BLA [39]), the central nucleus of the amygdala and IC [30], and the BLA and IC [17].
Central gustatory nuclei have been implicated in a number of taste-guided behaviors including the avoidance that occurs when a taste conditioned stimulus (CS) is followed by administration of a drug of abuse unconditioned stimulus (US) such as morphine, cocaine or amphetamine (e.g., [2–4, 8,44,45,48]. More specifically, lesions of the GT [10,42] and IC ([7,19,43] see also [22,51]) each disrupt drug-induced taste avoidance. The specific aim of the present experiment was to investigate the role of the BLA in morphine-induced saccharin avoidance. Given the anatomical interconnectivity between the GT, IC and amygdala, and the avoidance deficits consequent to lesions of the GT and IC, our expectation was that BLA would also have a role in morphine-induced saccharin avoidance. To examine this issue, neurologically intact (SHAM) subjects and BLA-lesioned (BLAX) rats were given eight conditioning trials, each trial spaced 72 hr apart, in which 15-min access to the saccharin CS solution was followed by administration of the drug US (morphine or saline).
To the best of our knowledge, there are no published studies on the effect of BLA lesions on psychoactive drug-induced taste avoidance. There are, however, a few studies that have examined the effects of BLA lesions on toxin-induced conditioned taste aversions (CTAs; for reviews see [40,41]). Our work indicates that BLA lesions disrupt CTA acquisition only when the taste CS is novel ([46] see also [25,26]), a deficit that we attribute to a misperception of taste novelty [20]. Accordingly, to maximize the likelihood of finding a BLA lesioned-induced deficit in this initial research we intentionally used a novel 0.15% saccharin solution as the CS. Similarly, to afford comparability with our GT and IC lesion studies, a 15 mg/kg dose of morphine was used as the US.
2. Materials and methods
2.1. Subjects
Male, CD-IGS rats purchased from Charles River Laboratories (Portage MI) were used in this experiment. All rats were housed individually in hanging, stainless steel cages in a room maintained on a 12-hour light/dark cycle (lights on at 7:00 am). Rodent chow (Lab Diet® 5012, PMI Nutrition International, Brentwood MO) and water were provided ad libitum unless otherwise noted. The University of Illinois at Chicago’s Animal Care Committee approved all procedures. At all times, rats were treated according to guidelines recommended by the National Institutes of Health [28] and the American Psychological Association [1].
2.2. Surgery
Three treatment groups were utilized: bilateral N-Methyl-D-aspartic acid (NMDA) lesions of the BLA (Group BLAX), BLAX surgery without the NMDA infusion and anesthesia without surgery. The latter two groups were combined and served as the control group (Group SHAM). At the time of surgery/anesthesia, all rats weighed between 300 and 314 g. Rats were anesthetized with sodium pentobarbital, 70 mg/Kg IP. Once anesthetized, each animal undergoing surgery received bupivicaine (Hospira Inc, Lakeforest IL), 1.25 mg/Kg subcutaneously at the incision site, and meloxicam (Metacam®, Boehringer Ingelheim, St. Joseph MO), 1 mg/Kg subcutaneously for analgesia. Meloxicam at the same dosage was repeated once daily for two days post-operatively. The head was shaved and the rat placed in a stereotaxic apparatus with blunt earbars (ASI, Warren MI). Body temperature was maintained at 37°C throughout the procedure using a Homeothermic Blanket Control Unit (Harvard Apparatus, Holliston MA). The scalp was prepared with betadine and alcohol. A midline incision was made, tissue cleared from the skull and the head leveled between bregma and lamda by adjusting the bite bar. Trephine holes were drilled over the BLA, bilaterally. For rats in the BLAX group, sterile glass capillary pipettes (tips 70–80 μm in diameter) were backfilled with 0.15 M NMDA (Sigma, St. Louis MO) and lowered into two infusion sites per hemisphere. Coordinates used for site 1 were: A/P −1.5 mm, M/L +4.6 mm, D/V −6.9 mm and for site 2; A/P −2.3 mm, M/L +4.8 mm, D/V −7.5 mm. The NMDA was iontophoretically infused into the target locations with a Midgard Precision Current Source (Stoelting, Wood Dale IL) using a 0.25 mm diameter silver wire with a current of −10 μA for 3.5 min at site 1 and 4 min at site 2. The infusions were repeated in the other hemisphere utilizing a new, NMDA backfilled pipette. For half of the rats in the SHAM group, a sterile glass capillary pipette was backfilled with sterile 0.9% saline, lowered into the BLA, bilaterally, and removed without being connected to current. All incisions were closed with 4-0 monofilament non-absorbable sutures, which were removed one week postoperatively. For the remaining rats in the SHAM group, anesthetic was administered without further manipulation. All animals received 10 ml of warm (37°C), 0.9% saline subcutaneously after surgery and/or anesthesia, were placed under a heat lamp until recovered from anesthesia and then returned to their home cage.
2.3. Procedure
Rats were allowed to recover from surgery/anesthesia for a minimum of 9 days. Thereafter, all rats were placed on a water deprivation schedule which allowed 15 min access to water each day in the home cage. On this schedule, water intakes stabilized after 10 days at which time animals were divided into four groups based on their surgical treatment (SHAM, BLAX) and whether they were to receive injections of 15 mg/Kg morphine sulfate (Morphine) or a comparable volume of 0.9% sodium chloride (Saline). Morphine was obtained from Henry Schein, Indianapolis IN. The morning after groups were assigned, all rats received 15 min access to 0.15% sodium saccharin solution instead of water. Five min following saccharin exposure, rats received an intraperitoneal injection of either saline or morphine depending on group assignment. This was repeated every third day for a total of 8 trials. Rats were given the standard 15 min access to water on the two days between saccharin trials.
2.4. Histology
At the completion of behavioral testing, rats in the BLAX group were deeply anesthetized with sodium pentobarbital (100 mg/ml) and transcardially perfused with buffered saline followed by 4% buffered formalin. The brains were removed and stored in 4% buffered formalin followed by 20% sucrose for a minimum of two days each. The brains were frozen, sliced on a cryostat at 50 μm, stained with cresyl violet and evaluated under a light microscope (Zeiss Axioskop 40). Drawings of the lesions were made on diagrams obtained from the Paxinos and Watson [38] atlas, and representative digital photomicrographs of BLA lesions were taken using Q-Capture software (Quantitative Imaging Corporation, Burnaby BC).
3. Results
3.1 Anatomical
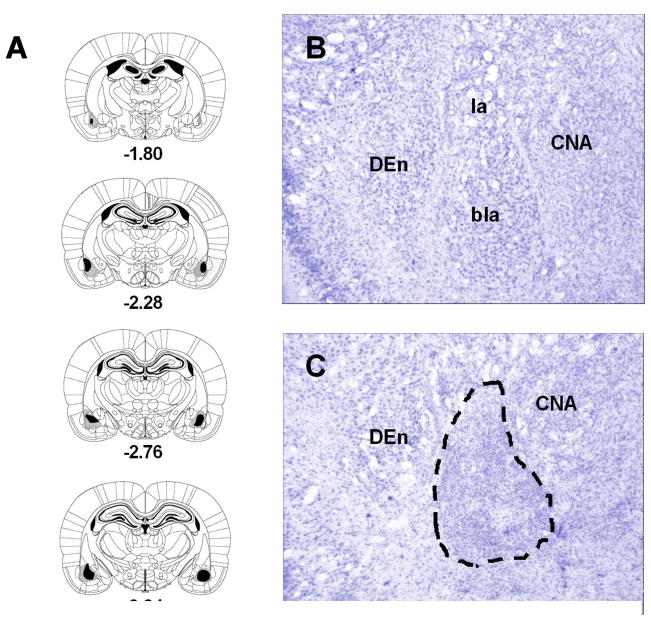
Results of the histological examinations are represented in Fig. 1. The location and extend of the lesions were identified by the loss and shriveling of cell bodies as well as the presence of pyknotic nuclei and/or gliosis. As shown in the figure, the lesions were centered in the BLA. In some cases, damage extended into surrounding areas including the central nucleus of the amygdala, the dorsal endopiriform nucleus as well as layer 3 cortex subjacent to BLA. These encroachments outside the BLA were minimal and unilateral. Rats with unilateral lesions (n = 5) or subtotal lesions (n = 2) were excluded from further analysis. Furthermore, five SHAM subjects were excluded either for failures to drink adequately during water acclimation or for health reasons unrelated to the study. The final numbers of animals in each group were as follows: BLAX-Saline = 8, BLAX-Morphine = 9, SHAM-Saline = 9 and SHAM-Morphine = 5.
Fig. 1.
Series of reconstructions (A) of the largest (gray) and the smallest (black) lesions of the basolateral amygdala (BLA) complex at four coronal levels (−1.80, −2.28, −2.76, −3.24 mm posterior to bregma; the diagrams were adapted with permission from plates in Paxinos and Watson [38] atlas). Digital photomicrographs of coronal brain sections at the level of the amygdala of a neurologically intact subject (B) and a rat with excitotoxic lesions of the BLA (C; the dashed lines shows the extent of cell loss). Abbreviations: bla, basolateral amygdaloid nucleus; CNA, central nucleus of the amygdala; DEn, dorsal endopiriform nucleus; la, lateral amygdaloid nucleus.
3.2. Behavioral
Prior to the first conditioning trial, mean (±SE) water consumption was calculated for SHAM and BLAX groups from the three most recent 15 min water intake trials, with the following results: SHAM-Saline = 18.9 (±1.09) ml, SHAM-Morphine = 18.7 (±1.43) ml, BLAX-Saline = 18.2 (±0.98) ml and BLAX-Morphine = 19.4 (±0.93) ml. An analysis of variance conducted on the data from which the means were derived found no significant effect of Lesion (F < 1) or US (F < 1), a quasi factor at this time, on water consumption prior to the first conditioning trial.
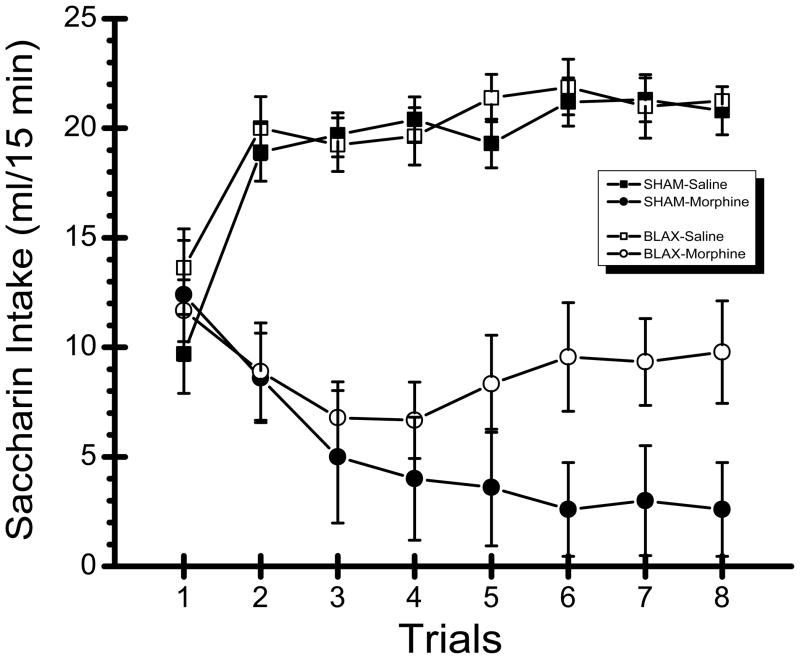
Saccharin consumption data from the eight trials of the experiment are shown in Fig. 2. Inspection of the graph suggests that morphine treated rats drank less saccharin than saline treated rats on Trials 2–8. Furthermore, this intake suppression seems more pronounced in SHAM subjects relative to the BLAX rats. These characterizations of the results were supported by the statistical analysis which revealed significant main effects of US, F(1,27) = 66.42, p < 0.001, and Trial, F(7,189) = 2.25, p < 0.05 as well as significant interactions of US x trial, F(7,189) = 28.31, p < 0.001 and, more importantly, US x trial x lesion, F(7,189) = 4.00, p < 0.001. Post hoc comparisons revealed that the SHAM-Saline group drank significantly less saccharin on Trial 1 than on each of the other seven trials (ps < 0.001); an identical pattern of significance was found in the BLAX-saline rats. In the SHAM-Morphine group, saccharin intake on Trial 1 was significantly higher than on each of the next 7 trials (ps < 0.001). Compared to the steady decline in the saccharin consumption of the SHAM-Morphine subjects, a different pattern of ingestion emerged in the BLAX-Morphine group, which demonstrated some intake suppression over the first few trials and a recovery back to the level of saccharin intake observed on Trial 1. More specifically, post hoc analyses revealed significant differences between saccharin intakes on Trial 1 and each of the next four trials (Trials 2–5; ps < 0.05) for the BLAX-Morphine rats. However, the amount of saccharin consumed on each of the next 3 trials (Trials 6–8) was not significantly different from that of Trial 1 (ps > 0.05). Finally, saccharin intake on Trial 8 was significantly higher for the BLAX-Morphine rats than the SHAM-Morphine subjects (p < 0.001). These results indicate that although contingent injections of morphine initially reduced intake of saccharin in both SHAM and BLAX animals, the BLAX rats, unlike the SHAM subjects, failed to maintain this avoidance over the course of the experiment. Instead, over the final trials of the experiment, the saccharin intake of the BLAX-Morphine rats returned to the pre-morphine injection intake levels of Trial 1.
Fig. 2.
Mean (±SE) 15 min intake (ml) of 0.15% saccharin across 8 trials in non-lesioned control (SHAM) subjects and rats with excitotoxic lesions of the basolateral amygdala (BLAX) that were injected with either 15 mg/kg morphine sulfate (Morphine) or an equivalent volume of physiological saline (Saline).
4. Discussion
On the final trial of the experiment, the SHAM-Morphine rats consumed 2.6 ml of the saccharin CS whereas the SHAM-Saline control subjects drank 20.8 ml. As expected then, neurologically intact animals demonstrated morphine-induced taste avoidance. Although the BLAX-Saline rats performed in a manner identical to that of the SHAM-Saline subjects, BLA lesions were found to disrupt morphine-induced saccharin avoidance. How is this pattern of spared and impaired functions in BLAX rats to be explained?
Before considering interpretations of data from the BLAX-Morphine rats, it is necessary to discuss briefly the performance of the BLAX-Saline subjects. These rats displayed normal taste neophobia. Previous research shows that BLA lesions attenuate the magnitude of the initial neophobic response to a novel saccharin solution without influencing levels of intake at asymptote when the taste is perceived as familiar and safe [20]. It was, therefore, surprising that an attenuation of neophobia was not evident in the performance of the BLAX-Saline rats in the present experiment. Although we have no explanation for this unexpected null effect, clearly the performance of the BLAX-Morphine rats cannot be explained in terms of a lesion-induced impairment in the detection or processing of the saccharin CS.
Relative to their intake on Trial 1, the BLAX-Morphine rats displayed normal levels of saccharin avoidance over the first few trials. But, this avoidance dissipated over the second half of the experiment. Thus, the saccharin intake of the BLAX-Morphine rats on Trial 8 was not significantly different from the amount they consumed on Trial 1 (although, it should be noted, it was substantially lower than the saccharin intake of the BLAX-Saline rats on Trial 8). Explanation of the pattern of results found in the BLAX-Morphine rats is not immediately obvious. We can, however, entertain two potential interpretations.
First, It is by now well established that drugs of abuse suppress CS intake in a manner that is very different from toxin-induced CS suppression. That is, as determined with taste reactivity methodology, whereas the latter involves the acquisition of a conditioned disgust response to the CS, the former does not (e.g., [31–34,36]; for reviews see [35,37]). Unfortunately, the nature of the CS suppression cannot be articulated with any degree of confidence when a drug of abuse serves as the US. Until recently, we would have argued for a lesion-induced disruption of a reward comparison mechanism [9,11] as we did for the effect of GT lesions on the same phenomenon. However, Lin et al. [19] revealed shortcomings that cast doubt on this mechanism as a viable account of CS avoidance. Nonetheless, whatever the nature of the suppression mechanism that underlies drug-induced CS avoidance in neurologically intact rats, one potential interpretation of the present results is that BLA lesions compromise the normal functioning of that mechanism.
An alternative interpretation of the present results is based on the finding reported by Siegel et al. [45] that in neurologically intact rats the magnitude of the morphine-induced taste avoidance attenuates with repeated saccharin-morphine trials. More specifically, Siegel et al. examined saccharin intake in groups of rats given different doses of lithium chloride (6 or 12 mg/kg) or morphine (5, 15 or 40 mg/kg) or control injections of saline over the course of an experiment that involved an unprecedented 40 conditioning trials. The rats in the 15-mg/kg morphine group showed conditioned avoidance of the saccharin CS over the first four trials. Given the large number of conditioning trials, data for the whole experiment were collapsed into four-trial blocks. Siegel et al. reported that the 15-mg/kg group drank less saccharin during the second and third blocks than the first block of trials. Thereafter, the saccharin consumption of these rats began to increase gradually across blocks such that intake during the fourth and later blocks was at least equal to that during block 1. Indeed, by block 10 (i.e., Trials 37–40) saccharin intake was significantly higher than that found during block 1. It should be noted, however, that during block 10 the 15-mg/kg group was drinking less saccharin than the saline-treated control subjects. So, after 40 conditioning trials, these rats were still showing morphine-induced saccharin avoidance, albeit at a significantly weaker level than the maximal avoidance observed during the second and third blocks of conditioning trials. In explanation of this pattern of results, Siegel et al. suggested that, with repeated administrations, tolerance develops to the morphine US thereby leading to a decrease in saccharin avoidance over the course of the later trials relative to the initial trials. An alternative interpretation of the present results, then, suggests that BLA lesions facilitate the development of tolerance to morphine which, presumably, leads to a faster than normal reduction in the effectiveness of the drug US to support conditioned avoidance of the saccharin CS.
In recent years, the BLA has been implicated in a number of psychoactive drug-related tasks. For instance, exposure to a drug-paired cue elicits c-Fos expression in the BLA [5,13,14,29,56]. Furthermore, permanent lesions [24,49] or temporary pharmacological inactivation of the BLA [12,15,23], like the inhibition of protein synthesis in the BLA [18], impair expression of conditioned cue-induced reinstatement of drug-seeking/guided behavior. Thus, irrespective of the nature of the disrupted mechanism, the results of the present experiment add to a growing literature that indicates that the BLA has an important role in some of the processes involved in drug addiction.
There are two clear directions for future research. First, it is imperative to develop new ideas about the nature of the suppression that occurs when orally consumed stimuli are paired with drugs of abuse as well as the role of drug tolerance in this process. Second, more neurobiological research is needed that compares and contrasts the similarities and differences between toxin-induced (i.e., CTA) and drug of abuse-induced CS suppression/avoidance. At present, three forebrain structures have been implicated in drug-induced CS avoidance: GT, IC and BLA. The most immediate goal is to determine whether these nuclei serve interdependent or independent functions. This neurobehavioral approach is expected to contribute to a better understanding of the mechanism(s) involved in drug-induced CS avoidance in neurologically intact subjects.
Acknowledgments
This research was supported by grants DC06456 and DC04341 from the National Institute of Deafness and Other Communication Disorders. The authors would like to thank Kristin Goshorn for her assistance in collecting behavioral data for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychological Association. Guidelines for ethical conduct in the care and use of animals. American Psychological Association; Washington DC: 1996. [Google Scholar]
- 2.Berger B. Conditioning of food aversions by injections of psychoactive drugs. J Comp Physiol Psychol. 1972;81:21–6. doi: 10.1037/h0033316. [DOI] [PubMed] [Google Scholar]
- 3.Cappell H, LeBlanc AE, Endrenyi L. Aversive conditioning by psychoactive drugs: effects of morphine, alcohol and chlordiazepoxide. Psychopharmacology. 1973;29:239–46. doi: 10.1007/BF00414038. [DOI] [PubMed] [Google Scholar]
- 4.Carey RJ. Long-term aversions to a saccharin solution induced by repeated amphetamine injections. Pharmacol Biochem Behav. 1973;1:265–70. doi: 10.1016/0091-3057(73)90115-9. [DOI] [PubMed] [Google Scholar]
- 5.Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci. 2001;98:1976–81. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finger TE. Gustatory nuclei and pathways in the central nervous system. In: Finger TE, Silver WL, editors. Neurobiology of taste and smell. New York: Wiley; 1987. pp. 331–53. [Google Scholar]
- 7.Geddes IR, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122:1038–50. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparison between effects in LEW/N and F344/N rat strains. Psychopharmacology. 1994;114:78–83. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- 9.Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behav Neurosci. 1997;111:129–36. [PubMed] [Google Scholar]
- 10.Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine-, but not LiCl-induced conditioned taste aversions in rats: Evidence for the reward comparison hypothesis. Brain Res. 2000;858:327–37. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- 11.Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-induced suppression of CS intake: Reward, aversion, and addiction. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 74–91. [Google Scholar]
- 12.Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharm. 2000;22:473–9. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 13.Guo N, Garcia MM, Harlan RE. A morphine-paired environment alters c-Fos expression in the forebrain of rats displaying conditioned place preferences or aversions. Behav Neurosci. 2008;122:1078–86. doi: 10.1037/a0012595. [DOI] [PubMed] [Google Scholar]
- 14.Harris GC, Ashton-Jones G. Enhanced morphine preferences following prolonged abstinence: association with increased Fos expression on the extended amygdala. Neuropsychopharm. 2003;28:292–99. doi: 10.1038/sj.npp.1300037. [DOI] [PubMed] [Google Scholar]
- 15.Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–36. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosar E, Grill HJ, Norgren R. Gustatory cortex in the rat. I. Physiological properties and cytoarchitecture. Brain Res. 1996;379:329–41. doi: 10.1016/0006-8993(86)90787-0. [DOI] [PubMed] [Google Scholar]
- 17.Krettek JE, Price JL. A direct input from the amygdala to the thalamus and the cerebral cortex. Brain Res. 1974;67:169–74. doi: 10.1016/0006-8993(74)90309-6. [DOI] [PubMed] [Google Scholar]
- 18.Lee JL, Milton AL, Everitt BJ. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci. 2008;26:5881–87. doi: 10.1523/JNEUROSCI.0323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J-Y, Roman C, Reilly S. Morphine-Induced Suppression of Conditioned Stimulus Intake: Effects of stimulus type and insular cortex lesions. Brain Res. 2009;1292:52–60. doi: 10.1016/j.brainres.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J-Y, Roman C, St Andre J, Reilly S. Taste, olfactory and trigeminal neophobia in rats with forebrain lesions. Brain Res. 2009;1251:195–203. doi: 10.1016/j.brainres.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundy RF, Jr, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. 3. Academic Press; San Diego: 2004. pp. 891–921. [Google Scholar]
- 22.Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24:71–8. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharm (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 24.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–48. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 25.Morris R, Frey S, Kasambira T, Petrides M. Ibotenic acid lesions of the basolateral, but not central, amygdala interfere with conditioned taste aversion: Evidence from a combined behavioral and anatomical tract-tracing investigation. Behav Neurosci. 1999;113:291–302. doi: 10.1037//0735-7044.113.2.291. [DOI] [PubMed] [Google Scholar]
- 26.Nachman M, Ashe JH. Effects of basolateral amygdala lesions on neophobia, learned taste aversions, and sodium appetite in rats. J Comp Physiol Psychol. 1974;87:622–43. doi: 10.1037/h0036973. [DOI] [PubMed] [Google Scholar]
- 27.Nakashima M, Uemura M, Yasui K, Ozaki HS, Tabata S, Taen A. An anterograde and retrograde tract-tracing study on the projections from the thalamic gustatory area in the rat: distribution of neurons projecting to the insular cortex and amygdaloid complex. Neurosci Res. 2000;36:297–309. doi: 10.1016/s0168-0102(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health. Guide for the care and use of laboratory animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- 29.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:12–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- 31.Parker LA. Nonconsummatory and consummatory behavioral CRs elicited by lithium- and amphetamine-paired flavors. Learn Motiv. 1982;13:281–303. [Google Scholar]
- 32.Parker LA. Positively reinforcing drugs may produce a different kind of CTA than drugs which are not positively reinforcing. Learn Motiv. 1988;19:207–20. [Google Scholar]
- 33.Parker LA. Taste reactivity responses elicited by reinforcing drugs: A dose-response analysis. Behav Neurosci. 1991;105:955–64. doi: 10.1037//0735-7044.105.6.955. [DOI] [PubMed] [Google Scholar]
- 34.Parker LA. Taste reactivity responses elicited by cocaine-, phencyclidine-, and methamphetamine-paired sucrose solutions. Behav Neurosci. 1993;107:118–29. doi: 10.1037//0735-7044.107.1.118. [DOI] [PubMed] [Google Scholar]
- 35.Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Reviews. 1995;19:143–51. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- 36.Parker LA. Taste avoidance and taste aversion: Evidence for two different processes. Learn Behav. 2003;31:165–72. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- 37.Parker LA, Limebeer CL, Rana SA. Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 92–113. [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5. Academic Press; San Diego, CA: 2005. [Google Scholar]
- 39.Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosci. 1997;20:517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 40.Reilly S. Central gustatory system lesions and conditioned taste aversion. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 309–27. [Google Scholar]
- 41.Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev. 2005;29:1067–88. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Reilly S, Trifunovic R. Progressive ratio performance in rats with gustatory thalamus lesions. Behav Neurosci. 1999;113:1008–19. doi: 10.1037//0735-7044.113.5.1008. [DOI] [PubMed] [Google Scholar]
- 43.Roman C, Reilly S. Insular cortex lesions and morphine-induced suppression of saccharin intake in the rat. Behav Neurosci. 2009;123:206–11. doi: 10.1037/a0014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherman JE, Pickman C, Rice A, Liebeskind JC, Holman EW. Rewarding and aversive effects of morphine: temporal and pharmacological properties. Pharm Biochem Behav. 1980;13:501–5. doi: 10.1016/0091-3057(80)90271-3. [DOI] [PubMed] [Google Scholar]
- 46.Siegel S, Parker LA, Moroz I. Morphine-induced taste avoidance is attenuated with multiple conditioning trials. Pharm Biochem Behav. 1995;50:299–303. doi: 10.1016/0091-3057(94)00318-d. [DOI] [PubMed] [Google Scholar]
- 47.St Andre J, Reilly S. Effects of central and basolateral amygdala lesions in conditioned taste aversion and latent inhibition. Behav Neurosci. 2007;121:90–9. doi: 10.1037/0735-7044.121.1.90. [DOI] [PubMed] [Google Scholar]
- 48.Travers SP. Orosensory processing in neural systems of the nucleus of the solitary tract. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. Boca Raton: CRC Press; 1993. pp. 339–94. [Google Scholar]
- 49.Vogel JR, Nathan BA. Learned taste aversions induced by hypnotic drugs. Pharmacol Biochem Behav. 1975;3:189–94. doi: 10.1016/0091-3057(75)90147-1. [DOI] [PubMed] [Google Scholar]
- 50.Yun IA, Fields HL. Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience. 2003;121:747–57. doi: 10.1016/s0306-4522(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 51.Zavala AR, Browning JR, Dickey ED, Biswas S, Neisewander JL. Region-specific involvement of AMPA/Kainate receptors in Fos protein expression induced by cocaine-conditioned cues. Eur Neuropsychopharm. 2008;18:600–11. doi: 10.1016/j.euroneuro.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zito KA, Bechera A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30:693–99. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]