Abstract
Endocrine disruption has become a significant human health concern, but is difficult to study outside of the laboratory for several reasons including the multiplicity of exposures, the difficulty in assessing each exposure, and the variety of possible outcomes among human populations. This review summarizes our studies of the relationships of measured persistent organic pollutants (PCBs, p,p′-DDE, HCB and mirex), and heavy metals (lead and mercury), to outcomes directly related to thyroid function and sexual maturation. These studies were conducted in a sample of Native American youth from the Akwesasne Mohawk community. The participants were first studied during puberty (10–16.9 years of age) and then at approximately 18 years of age. Results from these studies show that PCB levels are positively related to TSH and negatively to free T4. Further, these effects are conditioned by breastfeeding history. Anti-thyroid peroxidase antibody levels also are related to PCB levels suggesting elevated risk of autoimmune disease among the exposed. Earlier age at menarche is associated with higher PCB levels while risk of delay is associated with higher lead levels. Some evidence that the timing of exposure produces different effects is presented, and the level of exposure in the participants suggests that effects observed may be relevant to a considerable proportion of the US population. Further investigations are warranted to determine effect thresholds and mechanisms.
Keywords: Polychlorinated biphenyls; PCBs; persistent organic pollutants; POPs; hexachlorobenzene; p, p′- dichlorophenyldichloroethylene; growth; sexual maturation; thyroid; Mohawk; Native American
Introduction
Widespread concern over the potential effects of persistent organic pollutants (POPs) has been growing for decades, largely due to scientific information from studies of wildlife, carefully controlled laboratory experiments, and associations in human populations between POP exposures with some health related outcomes. Persistent organochlorines are lipophilic, slow to metabolize, and bioaccumulate in animal, bird, and human fat tissue over a lifetime. Exposure to these pollutants usually occurs through ingestion of contaminated water, food, or inhalation of air, and can occur prenatally through passage across the placenta, or post-natally by lactation or food consumption [1].
In recent years, scientific publications and the popular press have raised concern that certain POPs, specifically polychlorinated biphenyls (PCBs), hexachlorobenzene (HCB), and p,p′-dichlorophenyldichloroethylene (DDE), a metabolite of DDT, may have adverse effects on mammals, including humans, and other wildlife by disrupting the endocrine system [2–8]. These exogenous agents can mimic or antagonize natural hormones in the body that are instrumental in controlling myriad functions including growth and development, maturation, metabolism, and reproduction [9].
Additionally, compared to adults, the fetus and child have different sensitivities and reactivities to toxicants [10]. The most obvious examples are exposures to methyl mercury, to alcohol and to diethylstilbestrol which produce quite different effects in the fetus than in the adult [11]. Thus, there is concern that exposure to POPs and consequent hormonal disruption in a fetus or young child may be irreversible, and produce physiological programming with a wide range of possible effects that may arise later in life.
Laboratory evidence of endocrine-like effects is extensive, showing alterations in synthesis, metabolism, distribution, and clearance [12–26]. Predicting effects of toxicants in humans is problematical. While animal studies usually involve single exposure models, humans are subject to daily, multichemical exposure. Also, doses used in laboratory studies may not be comparable to humans’ exposures.
Experimentation on humans in this area is understandably unethical. Studies of human populations must consider the multiple exposures to toxicants (in contrast to the single exposure models most common in laboratory work), the developmental stage(s) of the sample, and the myriad effects possible from exposure at different development stages and from different levels of exposure. Furthermore, most studies of human populations are retrospective making exposure assessment problematic: summary measures cannot distinguish exposures by stage of development and measures that capture recent exposure cannot assess exposure at potentially early, critical stages of development.
Despite all the limitations associated with studies of human populations, the results from in- laboratory studies and the reality of potential effects on human health warrant continued research with humans. Over the past 15 years we have conducted two studies in partnership with the St Regis/Akwesasne Mohawk community, Hogansburg, NY. In these projects we were able to measure multiple toxicants and thereby capture some of the exposure complexity of human populations. By focusing on youth and adolescents, we were able also to address questions of sexual development, among others. Here we describe the results of these investigations that address the issue of human health effects. This article summarizes our publications reporting analyses of thyroid hormone levels, sexual maturation, and related outcomes in relation to specific persistent organic pollutants, all based on a study of young members of the Akwesasne Mohawk Nation who have experienced exposure from local industrial sources of pollutants through contamination of local waterways. Three main questions were addressed by this research: 1) Are current PCB levels of youth associated with levels of hormones involved in thyroid hormone activity (TSH, T4, T3, FT4)? 2) Is current PCB level of young adults associated with a marker of autoimmune disease, specifically antithyroid antibody level? and, 3) Is variation in current PCB levels of youth associated with variation in sexual maturation, specifically, age at menarche? For each of these questions we also seek to learn if specific PCB congeners are more closely associated with the outcomes than other PCB congeners, and if so, what is the meaning of these differences with regard to timing of exposure?
Methods
Sample
The Akwesasne Mohawk Nation is situated on the St. Lawrence River with territory abutting New York State, Ontario and Quebec, Canada. Residents of the community live within the boundaries of the St. Regis Mohawk Reservation/Reserve, and in neighboring communities that are part of traditional Mohawk territory, including Bombay, Fort Covington, Hogansburg, Massena, Rooseveltown (NY), and in Cornwall, Ontario. Recent reports indicate a population of approximately 12,000 –13,000 [27–29], however, Akwesasne is not a federally censused population and published estimates of the Akwesasne community’s population size vary.
Several industrial complexes are located near Akwesasne, a result of industrial development along the St. Lawrence River which began in the 1950s. The Akwesasne Mohawk Nation is located downstream of a National Priority Superfund Site (General Motors Central Foundry Division), and two New York State Superfund Sites (Reynolds Metal Company and Aluminum Company of America); all aluminum foundries, that have contaminated the St. Lawrence River and its three tributaries with PCBs, p,p′-DDE, HCB, mirex, and heavy metals (mercury and lead). In the mid-1980s, local species of fish, birds, amphibians and mammals were found to have levels exceeding the US Food and Drug administration’s tolerance limits for human consumption [30, 31], leading to advisories against the consumption of fish and game in the late 1980s and early 1990s [28, 32]. It is believed that postnatal exposure to toxicants is largely from the consumption of locally caught fish [33]. However, there is some evidence that exposure from other sources occurs also [1].
Data Collection
Two cross-sectional studies were conducted by the University at Albany and the Akwesasne Mohawk Nation. The first, the Mohawk Adolescent Well Being study (MAWBs), was conducted between 1995 and 2000, and involved 271 youth between the ages of 10 and 16.99 years (48% males; 52% females). The second study, the Young Adult Mohawk Study (YAWBs), was a follow-up of participants in the earlier project. Participants were between 17 and 20 years of age, and numbered of 153 individuals. Of the MAWBs participants who were eligible, 25% were lost to follow-up and 9% refused to participate. The participation rate was 66%. It differed slightly by gender (males 40% and females 60%); the loss of male participants was greater. Table 1 illustrates the number of participants (by sex) for both studies with regard to the published articles summarized in this review.
Table 1.
Number of participants in two studies of Mohawk well-being.
| Mohawk Adolescent Well-being Study (MAWBs) | Young Adult Well-being Study (YAWBs) | |||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Interviews | 131 | 140 | 60 | 93 |
| Total PCB levels | 131 | 140 | 60 | 93 |
| Thyroid hormone levels | 112 | 120 | 60 | 93 |
| Thyroid peroxidase antibodies | NA | NA | 58 | 57 |
| Breastfed | 65 | 59 | 30 | 35 |
| Non-breastfed | 65 | 80 | 30 | 56 |
Data on breastfeeding is missing on two individual in MAWBs; and one in YAWBs.
NA: Not applicable
Written informed consent was obtained from the children’s parents/guardian and assent was gained by the participating minor. The study protocols were reviewed and approved by the Institutional Review Board at the University at Albany, S.U.N.Y. Details of recruitment and sampling have been reported earlier for MAWBS [11] and YAWBS [34]. In brief, eligibility required not being a twin, not diagnosed with a psychological or physical impairment, and not diagnosed with fetal alcohol syndrome or effects. These last two were disqualifiers for a component study investigating effects of toxicants on cognition [35], that are not described here. Sample sizes for the analyses described below vary slightly depending on the outcome and variables needed as covariates in multivariate analyses.
All data were collected by members of the Akwesasne Nation, without prior knowledge of participants’ exposure status. In MAWBs each adolescent participant and their mother (or guardian) completed socio-demographic interviews and questionnaires, providing information about their family background, as well as their diet, breastfeeding and reproductive history, educational status, occupational status and living environment. In consultation with their mother, a semi-quantitative food frequency developed by the National Cancer Institute and Block Dietary Data Systems [36–38] was administered by Mohawk data collection staff and completed. For each food item or group of foods (i.e., squash, leafy vegetable, dairy) participants are asked to recall how frequently they consumed the food over a given time period (per day, in a week, in a month, less than once a month, never), and asked to identify their typical serving size. Data from the semi-quantitative food frequency was entered into DietSys 4.0 [38]. DietSys estimates nutrient intakes for 33 major nutrients (macro and micro), as well as intakes of specific foods and food groups. The intake frequencies and nutrient levels calculated by DietSys were imported into the Statistical Package for the Social Sciences (SPSS–15) for statistical analysis [39].
For both studies, fasting blood specimens were collected at first rising by trained Mohawk staff and provided material for analysis of six toxicants (mercury, lead, PCBs, p,p′-DDE, HCB, and mirex), and clinical chemistry assays including but not limited to lipid panels and thyroid hormones. In MAWBs blood was collected when the participant entered the study, and was between 10 and 16.99 years of age. In YAWBs, blood collection occurred when the participant was near 17 years of age.
PCB and organochlorine pesticide analyses were conducted at the University at Albany’s Exposure Assessment Laboratory. High resolution, ultratrace, congener-specific analysis was performed by parallel dual-column (splitless injection) gas chromatography with electron capture detection on an Agilent 6890 instrument, quantitating up to 83 individual PCB congeners and 18 PCB congeners as pairs or triplets, with detection limits in the low ppt range for individual congeners. Complete details of the current laboratory protocol for PCB analysis and recent data on laboratory performance have been published [40, 41]. Data were expressed on a whole-weight basis (i.e., not lipid-adjusted). Individual chlorinated biphenyl (CB) congeners are identified according to the IUPAC numbering system [42, 43]. Analyses of mercury and lead were conducted by Le Centre de Toxicologie du Quebec in Sainte-Foy, Quebec Canada. Blood lead was analyzed by Zeeman-corrected graphite furnace atomic absorption spectrometry. Mercury analysis employed cold-vapor atomic absorption spectrometry and levels are reported as the sum of organic and inorganic mercury in micrograms per deciliter. Clinical chemistry assessment for MAWBs was performed at the Clinical Chemistry and Hematology Laboratory, Wadsworth Center for Laboratories and Research, New York State Department of Health (Albany, NY). For YAWBs, assessment of clinical chemistries and lead were performed at the clinical laboratories of the Albany Medical Center in Albany, NY. Both laboratories are New York State and CLIA accredited laboratories, and meet all proficiency requirements.
Statistical analysis
Toxicants
In both studies, many of the congeners were detected in only a portion of the sample. The undetected values were treated in two ways: 1) following common practice, any individual datum of an organochlorine (OC) that was below minimum detection level (mdl) was replaced with the midpoint value between zero and the mdl of each compound or congener (mdl/2); and 2) following the U.S. EPA recommendation for estimating values for distributions of non-detectable amounts of toxicants in tissue or fluid samples [44], employing the formulae described by Gupta (1952) [45]. For a detailed description of this method, please see Schell et al. 2003 [11].
Due to the intercorrelation of certain congeners, we felt it would be inappropriate to test each congener for effects on biological parameters. Thus, we used an approach recommended by and consistent with toxicologists and other researchers whereby congeners were grouped according to theories of congeners’ behavior based on their structure [1, 46, 47]. In addition, the distribution of values for groups of congeners has better statistical properties than individual congeners. For the purpose of our analyses, grouping was done according to either their persistence (half-life in mammalian tissue) reflecting history of exposure, and by estrogenic properties as determined by laboratory testing. The grouping system employed was matched for each biological outcome being tested, and the groupings are defined with the analysis of each outcome below. To correct for skewness and normalize the distributions, lead, mercury, PCBs, p,p′-DDE, and HCB were natural log transformed.
To test for any problems due to potential collinearity between the toxicants, for each analysis involving multiple toxicants, we checked every result from each multivariate analysis by re-running the analysis with only one toxicant and checking for changes in effect size and direction. We found no problems resulting from collinearity.
Menarche
Menarche was measured as present or absent based on self-report at the time of interview and blood sample collection (see [48]). Probit analysis [49], utilizing the status quo method (presence or absence of menses and the age at the time of interview) was used to calculate an estimation of median age at menarche for the sample. One hundred and forty females completed the study, of which 138 had complete data for this analysis.
To test the relationships between lead, mercury, PCBs, DDE, HCB, and mirex, and the attainment of menarche, binary logistic regression analysis was used controlling for age and SES (socioeconomic status). As a proxy for SES status, a weighted index was created in consultation with Akwesasne community members, and was calculated from maternal education, marital status, employment and living environment (house size and condition), and the number and age of household motor vehicles (for details, see [48]).
All continuous independent variables were mean centered, and squared terms were included to test for nonlinear effects. An alpha level of 0.1 was chosen for inclusion of nonlinear terms. An alpha level of 0.05 was used for testing all other effects. Squared terms that were maintained in the model and their corresponding variable main effects were tested at several toxicant levels.
Given the large number of specific PCB congeners detected in our sample, the number of possible independent variables was reduced to include only those congeners shown to have endocrine like properties by toxicological laboratory studies. Sixteen specific congeners detected in 50% or more of the sample was grouped using results from laboratory experimentation [46, 50] resulting in one group of estrogenic congeners: the sum of PCB IUPAC#s 52, 70, 101, 187.
Thyroid hormones and anti-thyroid peroxidase antibody
In MAWBs, we investigated whether past, chronic exposure of different groupings of PCBs, and of p,p′-DDE, HCB, mirex, lead (Pb), and mercury (Hg), were associated with alterations in levels of thyroid-stimulating hormone (TSH), triiodothyronine (T3), total thyroxine (TT4), and free thyroxine (fT4) among 232 older children and adolescents. Multiple regression analysis was used to examine the effect of each toxicant on thyroid hormones while controlling for all other toxicants, sex, age, triglycerides, cholesterol, breastfeeding, the time of day when blood was collected, and the duration of time between interview and blood draw as well as a PCB-by-breastfeeding interaction. Covariates were chosen on the basis of bivariate associations (p < 0.1, t-tests, and correlations) with thyroid hormones and/or PCBs (for more detail, see [51]).
In YAWBs, we examined the relationship of different groupings of PCBs and other organochlorines, to anti-thyroid peroxidase antibody (TPOAb) among 115 young adults of the Akwesasne Mohawk Nation [34]. PCB congeners were grouped according to chlorination and structure. TPOAb is a marker of thyroid dysfunction, and a risk factor for autoimmune disease, with a high predictive value [52–56]. Two protocols for the analysis of TPOAb levels were employed by our laboratory resulting in two different laboratory reference ranges. Consequently, observed TPOAb values were converted to z-scores for each protocol and thereby standardized, and then were combined as one sample for statistical analysis [34]. Multivariate regression analysis was performed to examine the effect of each toxicant on TPOAb while controlling for covariates and stratifying by breastfeeding status. Covariates were chosen on the basis of bivariate associations (p<0.1, t-tests and correlations) with TPOAb and/or PCBs.
Results
Toxicant Levels
Of the 16 congeners detected in 50% or more of the MAWBs sample, eight were considered highly persistent given the substitutions at both para positions, five were mono-ortho substituted, and 11 are di-ortho substituted. Only p,p′-DDE and HCB were detected in 100% and 97.8% of the sample, respectively. None of the participants had a lead level at or above 10 μg/dL, the current level of concern in the U.S (mean = 1.31 μg/dL). Of the 94% of youth with detectable mercury levels, only one participant had a mercury level above 0.5 μg/dL, the blood level associated with health effects in humans [57]. Overall, POP levels (primarily PCBs) were consistent with a pattern of both cumulative and recent exposure [11].
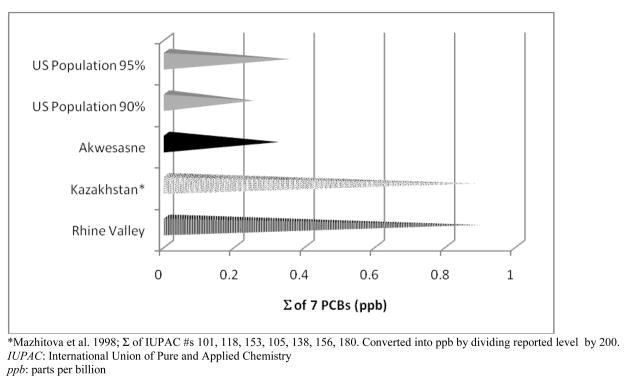
There is a paucity of data for comparison of PCB levels in young Akwesasne youth with other age-comparable and non-occupationally exposed samples. Until recently, the most age-comparable samples were ones that had been studied because of suspected high exposure [58], or that had been hospitalized [59]. These cohorts are far from appropriate comparison groups because they are not representative of the general population. PCBs 118, 138, 153, 170, 180, 183, 187 are seven of the most common and frequently measured congeners [1, 60, 61], and are the most often included in tables of comparison. In comparison to the US population (Figure 1), levels at Akwesasne are high, the average of the sum of seven PCBs being higher than the 90th percentile of US youth (ages 12 to 19 years) [62]. The chronicity of exposure is probably most similar between the US children and the Akwesasne youth.
Figure 1.
Comparison of the sum of seven PCB congeners (IUPAC #118, 138, 153, 170, 180, 187; in ppb) with other studies of similarly aged samples from Rhine Valley [58], Kazakhstan [59] and the United States [62].
An important influence on PCB and p,p′-DDE levels in the sample was breastfeeding even though lactation had ceased many years prior to the blood sampling. Breastfed youth in MAWBS (mean age = 13.2 yrs) had mean levels of total PCBs of 1.84 ppb, and p,p′-DDE levels of 0.54 ppb, whereas non-breastfed youth averaged 1.61 ppb and 0.34 ppb respectively. In breastfed youth, levels of total PCBs (and other PCB congener groupings), and p,p′-DDE were on average 1.3 times those of non-breastfed MAWBs youth. HCB, lead and mercury did not differ by breastfeeding status [11]. Further, breastfed young adults in YAWBs had significantly higher POP levels than non-breastfed young adults.
Sexual Maturation
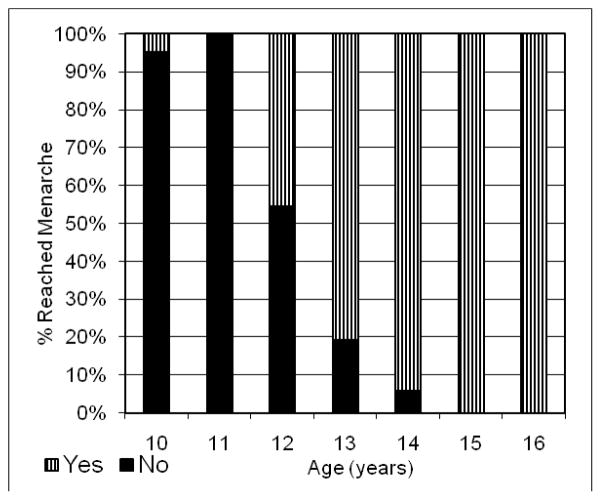
The sample for the analysis of predicted age at menarche consisted of 138 Akwesasne girls between the ages of 10 and 16.99 years, with necessary data available for study. Nearly 60% of the girls had reached menarche (Figure 2). Using probit analysis, the median predicted age at menarche for the total sample was 12.2 years (95% CI: 11.9, 12.5) [63]. This group of Mohawk girls is comparable to a larger sample of 10 to 16 year old American girls (NHANES III) in terms of median age at menarche (12.2 years for both samples), as well as in the distribution of menstrual status by age [64].
Figure 2.
Percent of Akwesasne girls who achieved menarche.
Three different probit analyses tested the relationship of blood lead levels to predicted age at menarche: all girls, girls with lead levels below the median, and girls with levels above the median. Without adjusting for any covariates, girls at or above the median blood lead level of 1.2 μg/dL had a predicted age at menarche of 12.7 years, while those below the median lead level had a predicted age at menarche more than ten months earlier than those girls with higher lead levels (11.8 years), a statistically significant difference [63].
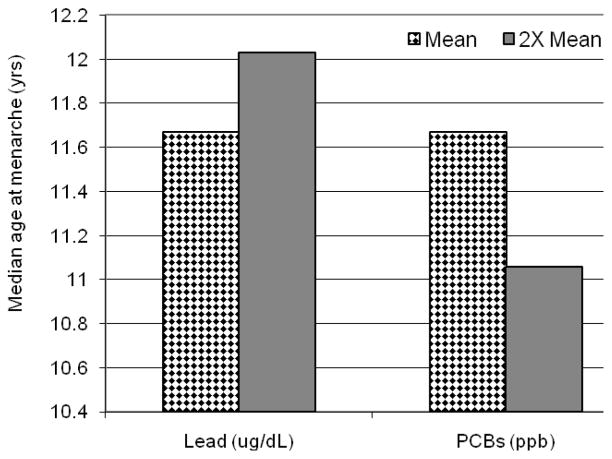
While controlling for other influences on menarche, such as age, SES, and body mass index (BMI), binary logistic regression analysis was used to consider simultaneously the effects of multiple toxicants in predicting menarcheal status (premenarcheal or postmenarcheal). As anticipated, age was found to be a positive, and the strongest predictor of menarche. Binary logistic regression analysis predicting menarche showed a nonlinear effect of lead. At the geometric mean (0.49 μg/dL) lead significantly delayed menarche after adjusting for age, SES, and other toxicants. The odds on reaching menarche decreased by 72% for a one-unit increase in lead level above the mean, and by 98% for a one-unit increase in lead above the 75th percentile (1.66 μg/dL). The grouping of four potentially estrogenic PCBs (Σ PCB4-E) was associated with a significantly earlier menarche after adjusting for age, SES, and other toxicants (Figure 3). Doubling the Σ PCB4-E above the geometric mean (from 0.12 ppb to 0.24 ppb), increases the odds of having reached menarche by 8.4 times. No relationship was observed between menarche, and either Wolff’s antiestrogenic or enzyme-inducing PCB groups. The effect of BMI was tested, but found not to be a significant predictor in the model perhaps due to its association with age (r = 0.322, p<0.001). Adding BMI to the logistic regression model did not change the effects of toxicants as indicated by only small changes in beta coefficients (for example, the coefficient for the PCB variable changed by 7% with no change in significance). Mirex, p,p′-DDE, and HCB were unrelated to menarcheal status.
Figure 3.
Median age at menarche based on logistic regression model controlling for SES, and other toxicants.
Thyroid
We investigated whether levels of persistent organic pollutants reflecting past chronic exposure and two heavy metals (specifically PCBs, p,p′- DDE, HCB, mirex, Pb, and Hg) were associated with alterations in levels of thyroid stimulating hormone (TSH), triiodothyronine (T3), total thyroxine (TT4), and free thyroxine (fT4) among 232 older Akwesasne children and adolescents [51]. While mean levels of T4, fT4, T3, and TSH were within the laboratory reference ranges, the thyroid hormone profile of Akwesasne youth was affected by exposure to a group of eight persistent PCBs (Σ8PerPCBs: IUPAC#S 74, 99, 105, 118, 138[+163+164], 153, 180, 187). This PCB grouping was positively associated with TSH, while inversely related to fT4, while a group of eight non-persistent PCBs were significantly and negatively related to fT4 only. Of the other toxicants, only HCB had a negative association with T4, while the Pb was positively associated with T3.
Additionally, interaction of breastfeeding by Σ8PerPCBs was highly significant (p≤0.001), indicating that the Σ8PerPCBs effect was primarily restricted to youth who had not been breastfed. Among both male and female adolescents who had not been breastfed, estimated TSH levels increased by 1.51 μIU/ml as Σ8PerPCBs levels rose from 0.204 ppb at the 5th percentile to 0.871 ppb at the 95th percentile, whereas among those who had been breastfed no significant relationship was found (Figure 4). Concurrently, predicted fT4 levels among the non-breastfed males and females decreased from 3.1 ng/dL at the 5th percentile of Σ8PerPCBs to 2.9 to 2.7 ng/dL at the 95th percentile, while among those that were breastfed fT4 remained more or less unchanged with increasing Σ8PerPCBs (Figure 4).
Figure 4.
Predicted TSH and fT4 levels in relation to PCB levels among breastfed (BF) and non-breastfed (NBF) Akwesasne youth.
Further thyroid dysfunction was found among 115 Akwesasne young adults in the follow-up study, YAWBs [34]. We examined the relationship of POPs to anti-thyroid peroxidase antibody (TPOAb), a biomarker of autoimmune disease [55, 56]. Autoantibodies to thyroid peroxidase can impair thyroid function [9]. Over 15% of the sample had TPOAb levels above the normal laboratory reference range. Significant, positive relationships between TPOAb levels and all PCB groupings (except the grouping of non-persistent PCBs), and with p,p′-DDE, HCB, and mirex were found among the participants who had been breastfed as infants, yet no association was found among non-breastfed young adults. Levels of POPs (except the group of non-persistent PCBs) were significantly higher among those participants who had been breastfed as infants. (For more details see [34]).
Discussion
Toxicants
The data collected on PCB levels among Akwesasne youth provides a unique opportunity to understand the potential health effects of these contaminants within a young age group. The configuration and levels of serum PCB congeners in Akwesasne youth were consistent with studies of cumulative (prenatal transfer and postnatal uptake via breastfeeding and/or fish consumption), and recent PCB exposure, with the moderately high detection rates of the shorter-lived congeners suggesting a continuing source of exposure [47]. Similar to earlier reported findings [65, 66], and consistent with previous analyses of PCB content in breast milk of Akwesasne Mohawk mothers [28] and other maternal cohorts [67–69], the higher levels of POPs in breastfed participants indicate breastfeeding as a significant source of these pollutants.
Comparisons of published studies of serum PCB levels in children and youth are complicated given the differences in analytic methodologies, the number and choice of specific congeners measured, and the lack of consistency in reporting results by age and breastfeeding status. Past comparisons of PCB levels among the Akwesasne youth to other samples are problematic [11]. Of the two most age-comparable samples, one consists of a younger age group (7–10 years of age) and was originally selected for study because of perceived high exposure [58], and the second, while within the same age range, consisted of a small sample of children hospitalized with locally-defined ecological diseases [59]. Both samples have toxicant levels far higher than in the general population. Moreover, the current POP levels in Akwesasne youth may reflect the decline in local fish consumption following the issuance of advisories against consumption of local fish in the 1980’s [32, 70], subsequent metabolism of stored POPs over many years, and possible dilution effects due to increased body size with growth and development following exposure early in life. In comparison to the US youth of 12 to 19 years of age, the Akwesasne youth have levels of the sum of seven PCB congeners (IUPAC#’s 118, 138, 153, 170, 180, 183, 187) above the US 90th percentile (0.25 ppb) [62]. Further, as the group of seven congeners includes some of the most highly persistent, the level among the Akwesasne youth suggests that exposure was high earlier in life. Measurable levels of nonpersistent congeners also suggests that exposure may be continuing.
Sexual Maturation
The role of toxicants in changing the usual pattern and timing of sexual maturation is of great concern. The scope of adverse reproductive effects caused by POPs is vast, and includes reduced fecundability, menstrual irregularity, decreased sperm motility [13], and effects on steroid hormones and the hypothalamic pituitary axis [71, 72]. However, the relationship between adolescent sexual maturation and POPs is less clear.
The adverse effect of lead on human sexual maturation that we have found is supported by animal studies showing that lead exposure alters steroid hormone levels, delays pubertal development, and reduces reproductive organ growth [73–75]. Lead may also indirectly affect sexual maturation by restricting or delaying growth as has been found in both animals and humans [76–78]. Studies of POPs and sexual maturation differ in the size and direction of effects. Blanck and colleagues [79] found that polybrominated biphenyls in breastfed girls were associated with earlier age at menarche and attainment of pubic hair stages when controlling for socioeconomic factors and PCB exposure. Girls with precocious puberty who immigrated to Belgium from developing countries had significantly higher concentrations of p,p′-DDE [80]. However, delayed attainment of Tanner adult breast stage was related to dioxin-like compounds [81, 82]. The variety of results may be due to exposure to different types and/or combinations of POPs which can have androgenic, estrogenic and antiestrogenic effects, as well as to differences in amount of exposure and its timing relative to developmental stage. Such variation is difficult to analyze in studies of human populations studied retrospectively. Additionally, all of these studies focused on one, or at most two, toxicants in their analyses leaving questions concerning other toxicant exposures unanswered.
In this study we considered multiple common toxicants simultaneously, including both POPs and metals, and tested effects with congener-specific PCB analysis. Despite toxicant levels in our sample that are lower than those in some studies demonstrating effects related to endocrine disruption, both estrogenic PCBs and lead in our sample are associated with differences in age at menarche. Our complementary analyses using statistical models with a single toxicant suggest that the observed lead and estrogenic PCB results are not attributable to intercorrelation among the toxicants.
Thyroid
Non-breastfed adolescents exhibited significant relationships between persistent PCBs and TSH and FT4 while breastfed adolescents did not, even though breastfed adolescents had significantly higher levels of persistent PCBs and p,p′-DDE [51]. It is possible that this is not due to the generally beneficial effects of breastfeeding because the breastfed adolescents had higher levels of those POPs. Another explanation is that breastfeeding introduces a relatively large exposure to POPs postnatally when exposure does not contribute to programming the thyroid hormone levels we observed in adolescents, and is essentially a random exposure with regard to the effects on levels of TSH and T4.
Serum TPOAb titers are a frequently employed biomarker of thyroid dysfunction, and serve as a diagnostic tool for many idiopathic autoimmune diseases [9] such as systemic lupus erythematosus [83], Grave’s disease [84], chronic thyroiditis [55, 56], Hashimoto’s encephalopathy [85], and connective tissue disorders such as rheumatoid arthritis [86] and Sjogren syndrome [87]. Finding an association between TPOAb titers and PCB level is consistent with effects of PCBs on thyroid hormone function as well as with risk of autoimmune diseases. Informal, ancecdoctal reports include autoimmune disease as a health concern by many community residents.
Possible effect modification: the timing of exposure
Some relationships of toxicants to outcomes differ depending on the history of breastfeeding. Breastfeeding is associated with a significant neonatal exposure to lipophilic toxicants such as PCBs, hexachlorobenzene and DDT. This exposure may be important if infancy is a sensitive period for the effects of interest. However, it may be unimportant if the sensitive period for the influence of the exposure precedes lactation, and in actuality it may obscure a relationship established prenatally by the addition of toxicant burden that is random with regard to the effect [88]. Evidence from analyses of TPOAb and thyroid hormones suggest that developmental stage when exposure occurs may be important in mediating effects. (Analysis of age at menarche was not conducted to test this hypothesis.) Effects on thyroid hormone levels appear most evident in relationship to exposures that are likely to have occurred prenatally while relationships with TPOAb are related more to markers of postnatal exposure. The period of exposure is indexed by the history of breastfeeding and the persistence of the PCB congeners. However, a limitation of our set of studies is that exposure in the prenatal period is not known exactly as measures of cord blood, or maternal blood during pregnancy, are not available. Thus, we hypothesize for future testing that the sensitive period for effects of PCBs and other toxicants on thyroid hormone levels may be prenatal, while that for the development of autoimmune responses may be postnatal.
Summary
Our studies have found a reduction in hormones indexing thyroid function among adolescents in relation to their current serum levels of PCBs particularly those related to earlier exposure. Analysis of POPs, lead and age at menarche show that the tempo of sexual maturation is affected by PCBs and lead at levels relevant to many youth in the US and elsewhere. Finally, evidence of a relationship between PCBs and autoimmune disease risk was found. Additional research is necessary to discover the site and mechanism of action of these POPs and of lead, and to determine thresholds for these effects.
Acknowledgments
We thank the Akwesasne Mohawk community for their cooperation and participation in this research. Supported by grants from the National Institute of Environmental Health Sciences (NIEHS-ESO4913–10; ES10904–06), the National Center on Minority Health and Health Disparities (NCMHD - 5RDMD001120), and the National Center on Minority Health and Health Disparities (NCMHD – 1P20MD003373–01)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hansen LG. The ortho side of PCBs: Occurrence and Disposition. Norwell, MA: Kluwer Academic Publishers; 1999. [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for DDT, DDE, DDD. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2002. [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for hexachlorobenzene. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2002. [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for polychlorinated biphenyls (PCBs) Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2000. [PubMed] [Google Scholar]
- 5.Hagmar L. Polychlorinated biphenyls and thyroid status in humans: a review. Thyroid. 2003;13:1021–1028. doi: 10.1089/105072503770867192. [DOI] [PubMed] [Google Scholar]
- 6.Safe SH. PCBs as aryl hydrocarbon receptor agonists. In: Robertson Larry W, Hansen Larry G., editors. PCBs. Recent Advances in Environmental Toxicology and Health Effects. Louisville: University Press of Kentucky; 2001. pp. 171–177. [Google Scholar]
- 7.Safe SH. Endocrine disruptors and human health--is there a problem? An update. Environ Health Perspect. 2000;108:487–493. doi: 10.1289/ehp.00108487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salay E, Garabrant D. Polychlorinated biphenyls and thyroid hormones in adults: asystematic review appraisal of epidemiological studies. Chemosphere. 2009;74:1413–1419. doi: 10.1016/j.chemosphere.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Larsen PR, Kronenberg HM, Shlomo M, Polonsky KS. Williams Textbook of Endocrinology. 10. Philadelphia, PA: Saunders Company; 2003. [Google Scholar]
- 10.Bearer CF. The special and unique vulnerability of children to environmental hazards. Neurotox. 2000;21:925–934. [PubMed] [Google Scholar]
- 11.Schell LM, Hubicki LA, DeCaprio AP, Gallo MV, Ravenscroft J, Tarbell A, Jacobs A, David D, Worswick P. Organochlorines, lead, and mercury in Akwesasne Mohawk youth. Environ Health Perspect. 2003;111:954–961. doi: 10.1289/ehp.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faroon OM, Jones D, de Rosa C. Effects of polychlorinated biphenyls on the nervous system. Toxicol Ind Health. 2001;16:305–333. doi: 10.1177/074823370001600708. [DOI] [PubMed] [Google Scholar]
- 13.Faroon OM, Keith S, Jones D, de Rosa C. Effects of polychlorinated biphenyls on development and reproduction. Toxicol Ind Health. 2001;17:63–93. doi: 10.1191/0748233701th097oa. [DOI] [PubMed] [Google Scholar]
- 14.Fielden MR, Halgren RG, Tashiro CH, Yeo BR, Chittim B, Chou K, Zacharewski TR. Effects of gestational and lactational exposure to Aroclor 1242 on sperm quality and in vitro fertility in early adult and middle-aged mice. Reprod Toxicol. 2001;15:281–292. doi: 10.1016/s0890-6238(01)00129-0. [DOI] [PubMed] [Google Scholar]
- 15.Fischer LJ, Seegal RF, Ganey PE, Pessah IN, Kodavanti PRS. Symposium overview: toxicity of non-coplanar PCBs. Toxicol Sci. 1998;41:49–61. doi: 10.1006/toxs.1997.2386. [DOI] [PubMed] [Google Scholar]
- 16.Gierthy JF, Arcaro KF, Floyd M. Assessment of PCB estrogenicity in a human breast cancer cell line. Chemosphere. 1997;34:1495–1505. doi: 10.1016/s0045-6535(97)00446-3. [DOI] [PubMed] [Google Scholar]
- 17.Goldey ES, Kehn LS, Lau C, Rehnberg GL, Crofton KM. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995;135:77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- 18.Gray LE., Jr . Toxicol Lett. 102–103. 1998. Xenoendocrine disrupters: laboratory studies on male reproductive effects; pp. 331–335. [DOI] [PubMed] [Google Scholar]
- 19.Kim IS. Effects of exposure of lactating female rats to polychlorinated biphenyls (Pcbs) on testis weight, sperm production and sertoli cell numbers in the adult male offspring. J Vet Med Sci. 2001;63:5–9. doi: 10.1292/jvms.63.5. [DOI] [PubMed] [Google Scholar]
- 20.Kim IS, Ariyaratne HB, Chamindrani Mendis-Handagama SM. Effects of continuous and intermittent exposure of lactating mothers to aroclor 1242 on testicular steroidogenic function in the adult male offspring. Tissue Cell. 2001;33:169–177. doi: 10.1054/tice.2000.0168. [DOI] [PubMed] [Google Scholar]
- 21.Kuriyama SN, Chahoud I. In utero exposure to low-dose 2,3′,4,4′,5- pentachlorobiphenyl (PCB 118) impairs male fertility and alters neurobehavior in rat offspring. Toxicology. 2004;202:185–197. doi: 10.1016/j.tox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Loch-Caruso R. Uterine muscle as a potential target of polychlorinated biphenyls during pregnancy. Int J Hyg Environ Health. 2002;205:121–130. doi: 10.1078/1438-4639-00137. [DOI] [PubMed] [Google Scholar]
- 23.Sager DB, Girad D, Nelson D. Early postnatal exposure to PCBs and sperm function in rats. Environ Toxicol Chem. 1991;10:737–746. [Google Scholar]
- 24.Sager DB, Girard DM. Long-term effects on reproductive parameters in female rats after translactational exposure to PCBs. Environ Res. 1994;66:52–76. doi: 10.1006/enrs.1994.1044. [DOI] [PubMed] [Google Scholar]
- 25.Zoeller RT, Dowling ALS, Vas AA. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology. 2000;141:181–189. doi: 10.1210/endo.141.1.7273. [DOI] [PubMed] [Google Scholar]
- 26.Zoeller RT. Polychlorinated biphenyls as disruptors of thyroid hormone action. In: Robertson Larry W, Hansen Larry G., editors. PCBs. Recent Advances in Environmental Toxicology and Health Effects. Louisville: University Press of Kentucky; 2001. pp. 265–271. [Google Scholar]
- 27.Akwesasne Task Force On The Environment. Superfund clean-up at Akwesasne: a case study in environmental justice. Int J Contemp Sociol. 1997;34:267–290. [Google Scholar]
- 28.Fitzgerald EF, Hwang SA, Bush B, Cook K, Worswick P. Fish consumption and breast milk PCB concentrations among Mohawk women at Akwesasne! Lost Data. 1998;148:164–172. doi: 10.1093/oxfordjournals.aje.a009620. [DOI] [PubMed] [Google Scholar]
- 29.George-Kanentiio D. Iroquois population in 1995. Akwesasne Notes. 1995;1:61. [Google Scholar]
- 30.Forti A, Bogdan KG, Horn E. Health Risk Assessment for the Akwesasne Mohawk Population from Exposure to Chemical Contaminants in Fish and Wildlife. Albany, NY: New York State Department of Health; 1995. [Google Scholar]
- 31.Sloan RJ, Jock K. New York State Department of Environmental Conservation. Albany, NY: New State Department of Environmental Conservation; 1990. Chemical contaminants in fish from the St. Lawrence River drainage on lands of the Mohawk nation at Akwesasne and near the General York Motors Corporation/Central Foundry Divisioin, Massena, NY Plant. [Google Scholar]
- 32.Fitzgerald EF, Hwang SA, Brix KA, Bush B, Cook K, Worswick P. Fish PCB concentrations and consumption patterns among Mohawk women at Akwesasne. J Expo Anal Environ Epidemiol. 1995;5:1–19. [PubMed] [Google Scholar]
- 33.Fitzgerald EF, Hwang SA, Langguth K, Cayo MR, Yang BZ, Bush B, Worswick P, Lauzon T. Fish consumption and other environmental exposures and their associations with serum PCB concentrations among Mohawk women at Akwesasne. Environ Res. 2004;94:160–170. doi: 10.1016/s0013-9351(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 34.Schell LM, Gallo MV, Ravenscroft J, DeCaprio AP. Persistent organic pollutants and anti-thyroid peroxidase levels in Akwesasne Mohawk young adults. Environ Res. 2009;109:86–92. doi: 10.1016/j.envres.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman J, Aucompaugh A, Schell LM, Denham M, DeCaprio AP, Gallo MV, Ravenscroft J, Kao CC, Hanover MR, David D, Jacobs A, Tarbell A, Worswick P Akwesasne Task Force On The Environment. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol Teratol. 2006;28:439–445. doi: 10.1016/j.ntt.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire:development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 38.National Cancer Institute. HHHQ-DietSys Analysis Software. 1999. [Google Scholar]
- 39.SPSS I. Statistical Package for the Social Sciences. Chicago, Illinois: SPSS, Inc; 2001. [Google Scholar]
- 40.DeCaprio AP, Tarbell AM, Bott A, Wagemaker DL, Williams RL, O’Hehir CM. Routine analysis of 101 polychlorinated biphenyl congeners in human serum by parallel dual-column gas chromatography with electron capture J. detection. Anal Toxicol. 2000;24:403–420. doi: 10.1093/jat/24.6.403. [DOI] [PubMed] [Google Scholar]
- 41.DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ. Polychlorinated biphenyl. (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Ballschmiter K, Zell M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography. Fresenius Z. Anal Chem. 1980;302:20–31. [Google Scholar]
- 43.Guitart R, Puig P, Gomez-Catalan J. Requirement for a standardized nomenclature criterium for PCBs: Computer-assisted assignment of correct congener denomination and numbering. Chemosphere. 1993;27:1451–1459. [Google Scholar]
- 44.U.S. Environmental Protection Agency. Practical Methods for Data Analysis. QA/G-9. Washington, DC: Environmental Protection Agency; 1998. Guidance for Data Quality Assessment. [Google Scholar]
- 45.Gupta AK. Estimation of the mean and standard Dviation of a normal population from a censored sample. Biometrika. 1952;39:260–273. [Google Scholar]
- 46.Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen LG. Identification of steady state and episodic PCB congeners from multiple pathway exposures. In: Robertson Larry W, Hansen LG., editors. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. pp. 48–56. [Google Scholar]
- 48.Denham M, Deane G, Gallo MV, Ravenscroft J, DeCaprio A. Akwesasne Task Force On The Environment. Age at menarche among Akwesasne Mohawk girls exposed to lead, mercury, mirex, dichlorodiphenyldichloroethane (p,p′-DDE), hexachlorobenzene (HCB) and polychlorinated biphenyls (PCBs) In: Schell LM, editor. Pediatrics. 2. Vol. 115. 2003. pp. e127–e134. 25. [DOI] [PubMed] [Google Scholar]
- 49.Finney DJ. A Statistical Treatment of the Sigmoid Response Curve. 2. Cambridge: Cambridge University Press; 1952. Probit Analysis. [Google Scholar]
- 50.Wolff MS, Toniolo PG. Environmental organochlorine exposure as a potential etiologic factor in breast cancer. Environ Health Perspect. 1995;103:141–145. doi: 10.1289/ehp.95103s7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p′-DDE, and other toxicants in Akwesasne Mohawk youth. Environ Health Perspect. 2008;116:806–813. doi: 10.1289/ehp.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Premawardhana LD, Parkes AB, John R, Harris B, Lazarus JH. Thyroid peroxidase antibodies in early pregnancy: utility for prediction of postpartum thyroid dysfunction and implications for screening. Thyroid. 2004;14:610–615. doi: 10.1089/1050725041692828. [DOI] [PubMed] [Google Scholar]
- 53.Okosieme OE, Evans C, Moss L, Parkes AB, Premawardhana LD, Lazarus JH. Thyroglobulin antibodies in serum of patients with differentiated thyroid cancer: relationship between epitope specificities and thyroglobulin recovery. Clin Chem. 2005;51:729–734. doi: 10.1373/clinchem.2004.044511. [DOI] [PubMed] [Google Scholar]
- 54.Lazarus JH, Premawardhana LD. Screening for thyroid disease in pregnancy. J Clin Pathol. 2005;58:449–452. doi: 10.1136/jcp.2004.021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langer P, Tajtáková M, Fodor G, Kocan A, Bohov P, Michálek J, Kreze A. Increased thyroid volume and prevalence of thyroid disorders in an area heavily polluted by polychlorinated biphenyls. Eur J Endocrinol. 1998;139:402–409. doi: 10.1530/eje.0.1390402. [DOI] [PubMed] [Google Scholar]
- 56.Langer P, Kocan A, Tajtakova M, Radikova Z, Petrik J, Koska J, Ksinantova L, Imrich R, Huckova M, Chovancova J, Drobna B, Jursa S, Bergman A, Athanasiadou M, Hovander L, Gasperikova D, Trnovec T, Sebokova E, Klimes I. Possible effects of persistent organochlorinated pollutants cocktail on thyroid hormone levels and pituitary-thyroid interrelations. Chemosphere. 2007;70:110–118. doi: 10.1016/j.chemosphere.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 57.Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury. Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- 58.Osius N, Karmaus W, Kruse H, Witten J. Exposure to polychlorinated biphenyls and levels of thyroid hormones in children. Environ Health Perspect. 1999;107:843–849. doi: 10.1289/ehp.99107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazhitova Z, Jensen S, Ritzen M, Zetterstrom R. Chlorinated contaminants, growth and thyroid function in shoolchildren from the Aral Sea region in Kazakhstan. Acta Paediatr. 1998;87:991–995. doi: 10.1080/080352598750031671. [DOI] [PubMed] [Google Scholar]
- 60.Safe SH. PCBs and human health. In: Safe Stephen H, Hutzinger O., editors. Polychlorinated Biphenyls (PCBs): Mammalian and Environmental Toxicology. Berlin: Springer-Verlag; 1987. pp. 133–146.pp. 1 [Google Scholar]
- 61.Schecter AJ, Stanley J, Boggess K, Masuda Y, Mes J, Wolff M, Furst P, Furst C, Wilson-Yang K, Chisholm B. Polychlorinated biphenyl levels in the tissues of exposed and nonexposed humans. Environ Health Perspect. 1994;102 (Suppl 1):149–158. doi: 10.1289/ehp.94102s1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control. Third National Report on Human Exposure to Environmental Chemicals. NCEH 05–0570. Atlanta, GA: Deartment of Health and Human Services; 2005. pp. 1–467. [Google Scholar]
- 63.Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio A The Akwesasne Task Force on the Environment. Relationship of Lead Mercury, Mirex, Dichlorodiphenyldichloroethylene, Hexachlorobenzene and Polychlorinated Biphenyls to Timing of Menarche Among Akwesasne Mohawk Girls. Pediatrics. 2005;115:e127–e134. doi: 10.1542/peds.2004-1161. [DOI] [PubMed] [Google Scholar]
- 64.Wu T, Buck GM, Mendola P. Blood lead levels and sexual maturation in U.S. Girls: the third national health and nutrition examination survey, 1988–1994. Environ Health Perspect. 2003;111:737–741. doi: 10.1289/ehp.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karmaus W, DeKoning EP, Kruse H, Witten J, Osius N. Early childhood determinants of organochlorine concentrations in school- aged children. Pediatr Res. 2001;50:331–336. doi: 10.1203/00006450-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Patandin S, Weisglas-Kuperus N, de Ridder MA, Koopman-Esseboom C, van Staveren WA, Van Der Paauw CG, Sauer PJ. Plasma polychlorinated biphenyl levels in Dutch preschool children either breast-fed or formula-fed during infancy. Am J Public Health. 1997;87:1711–1714. doi: 10.2105/ajph.87.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duarte-Davidson R, Jones KC. Polychlorinated biphenyls (PCBs) in the UK population: estimated intake, exposure and body burden. Sci Total Environ. 1994;151:131–152. doi: 10.1016/0048-9697(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 68.Ribas-Fito N, Cardo E, Sala M, Eulalia dM, Mazon C, Verdu A, Kogevinas M, Grimalt JO, Sunyer J. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111:e580–e585. doi: 10.1542/peds.111.5.e580. [DOI] [PubMed] [Google Scholar]
- 69.Ribas-Fito N, Grimalt JO, Marco E, Sala M, Mazon C, Sunyer J. Breastfeeding and concentrations of HCB and p,p′-DDE at the age of 1 year. Environ Res. 2005;98:8–13. doi: 10.1016/j.envres.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Fitzgerald EF, Deres DA, Hwang SA, Bush B, Yang BZ, Tarbell A, Jacobs A. Local fish consumption and serum PCB concentrations among Mohawk men at Akwesasne. Environ Res. 1999;80:S97–S103. doi: 10.1006/enrs.1998.3908. [DOI] [PubMed] [Google Scholar]
- 71.McKinney JD, Waller CL. Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect. 1994;102:290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korach KS, Sarver P, Chae K, Mclachlan JA, Mckinney JD. Estrogen receptor-binding activity of polychlorinated hydroxybiphenyls - conformationally restricted structural probes. Mol Pharmacol. 1988;33:120–126. [PubMed] [Google Scholar]
- 73.Dearth RK, Hiney JK, Srivastava V, Burdick SB, Bratton GR, Dees WL. Effects of lead (Pb) exposure during gestation and lactation on female pubertal development in the rat. Reprod Toxicol. 2002;16:343–352. doi: 10.1016/s0890-6238(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 74.Corpas I, Castillo M, Marquina D, Benito MJ. Lead intoxication in gestational and lactation periods alters the development of male reproductive organs. Ecotoxicol Environ Saf. 2002;53:259–266. doi: 10.1006/eesa.2002.2230. [DOI] [PubMed] [Google Scholar]
- 75.McGivern RF, Sokol RZ, Berman NG. Prenatal lead exposure in the rat during the third week of gestation: long-term behavioral, physiological, and anatomical effects associated with reproduction. Toxicol Appl Pharmacol. 1991;110:206–215. doi: 10.1016/s0041-008x(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 76.Bellinger DC, Leviton A, Rabinowitz M, Allred E, Needleman HL, Schoenbaum S. Weight gain and maturity in fetuses exposed to low levels of lead. Environ Res. 1991;54:151–158. doi: 10.1016/s0013-9351(05)80097-0. [DOI] [PubMed] [Google Scholar]
- 77.Shukla R, Dietrich KN, Bornschein RL, Berger O, Hammond PB. Lead exposure and growth in the early preschool child: a follow-up report from the Cincinnati Lead Study. Pediatrics. 1991;88:886–892. [PubMed] [Google Scholar]
- 78.Schneider D, Freeman N. Children’s Environmental Health: Reducing Risk in a Dangerous World. Washington, DC: American Public Health Association; 2000. The legacy of lead; pp. 75–92. [Google Scholar]
- 79.Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11:641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Krstevska-Konstantinova M, Charlier C, Craen M, Du Caju M, Heinrichs C, de Beaufort C, Plomteux G, Bourguignon JP. Sexual precocity after immigiration from deveoping countries to Belgium:evidence of previous exposure to organochlorine pesticides. Hum Reprod. 2001;16:1020–1026. doi: 10.1093/humrep/16.5.1020. [DOI] [PubMed] [Google Scholar]
- 81.Den Hond E, Roels HA, Hoppenbrouwers K, Nawrot T, Thijs L, Vandermeulen C, Winneke G, Vanderschueren D, Staessen JA. Sexual maturation in relation to polychlorinated aromatic hydrocarbons: Sharpe and Skakkebaek’s hypothesis revisited. Environ Health Perspect. 2002;110:771–776. doi: 10.1289/ehp.02110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staessen JA, Nawrot T, Hond ED, Thijs L, Fagard R, Hoppenbrouwers K, Koppen G, Nelen V, Schoeters G, Vanderschueren D, Van Hecke E, Verschaeve L, Vlietinck R, Roels HA. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: A feasibility study of biomarkers. Lancet. 2001;357:1660–1669. doi: 10.1016/s0140-6736(00)04822-4. [DOI] [PubMed] [Google Scholar]
- 83.Kohno Y, Naito N, Saito K, Hoshioka A, Niimi H, Nakajima H, Hosoya T. Anti-thyroid peroxidase antibody activity in sera of patients with systemic lupus erythematosus. Clin Exp Immunol. 1989;75:217–221. [PMC free article] [PubMed] [Google Scholar]
- 84.Antonelli A, Fazzi P, Fallahi P, Ferrari SM, Ferrannini E. Prevalence of hypothyroidism and Graves disease in sarcoidosis. Chest. 2006;130:526–532. doi: 10.1378/chest.130.2.526. [DOI] [PubMed] [Google Scholar]
- 85.Mocellin R, Walterfang M, Velakoulis D. Hashimoto’s encephalopathy :epidemiology, pathogenesis and management. CNS Drugs. 2007;21:799–811. doi: 10.2165/00023210-200721100-00002. [DOI] [PubMed] [Google Scholar]
- 86.Lee DH, Steffes M, Jacobs DR. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Environ Health Perspect. 2007;115:883–888. doi: 10.1289/ehp.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tunc R, Gonen MS, Acbay O, Hamuryudan V, Yazici H. Autoimmune thyroiditis and anti-thyroid antibodies in primary Sjogren’s syndrome: a case-control study. Ann Rheum Dis. 2004;63:575–577. doi: 10.1136/ard.2003.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p′-DDE, and other toxicants in Akwesasne Mohawk youth. Environ Health Perspect. 2008;116:806–813. doi: 10.1289/ehp.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]