Abstract
The tumor necrosis factor (TNF) receptor super family comprises of members that induce two distinct signaling cascades, leading to either cell survival or apoptosis. However, in prostate cancer (PCa), TNF-mediated prosurvival signaling is the predominant pathway that leads to cell survival and resistance to therapy. Although inhibition of TNF signaling by pharmacological agents or monoclonal antibodies has gained importance in the field of cancer therapy, toxicity to normal cells has impaired their extensive use for cancer treatment. We previously identified a natural, nontoxic compound psoralidin that inhibited viability and induced apoptosis in androgen independent prostate cancer (AIPC) cells. Thus, the goal of our study is to investigate whether psoralidin inhibits TNF-mediated prosurvival signaling in AIPC cells. Our results suggest that psoralidin inhibits constitutive and TNF-induced expression of TNF-α and its downstream prosurvival signaling molecules such as NF-κB and Bcl-2 in AIPC cells. On the other hand, psoralidin simultaneously induces the death receptor (DR)-mediated apoptotic signaling eventually causing the activation of caspase cascade and resultant induction of apoptosis. Oral administration of psoralidin inhibits expression of TNF-α and NF-κB/p65 in tumor sections, resulting in tumor regression in PC-3 xenografts. Our results suggest that psoralidin inhibits TNF-mediated survival signaling in AIPC and thus is a potent therapeutic agent for prostate cancer.
Keywords: Psoralidin, TNF signaling, Death receptors, Caspases, Apoptosis
Introduction
Tumor necrosis factor-α (TNF-α), a potent proinflammatory cytokine, exists both in a membrane-bound and soluble form and is released by various immune and non-immune cells (1). Based on the stimuli, TNF-α can function both as a prosurvival and proapoptotic factor thereby leading to cell survival or cell death. Elevated levels of TNF in the serum of cancer patients (2) and increased expression of TNF in pre-cancerous and tumor tissues (3) have been extensively reported, implying that TNF plays a major role in tumor progression. High expression of TNF-α has been correlated with proliferation and survival of malignant cells, stimulation of angiogenesis and metastases and alteration of response to chemotherapeutic agents (4). Activation of several pathways, namely NF-κB (5), PKC-α, AP-1 (6), or ROS production (7, 8), by TNF has been established in many cancer types including PCa.
Cellular response to TNF is mediated by either TNFR1 or TNFR2 (9); the receptors have no homology in their intracellular domains, suggesting that they may activate different intracellular signaling pathways within a cell. Canonical TNF signaling consists of ligand binding and activation of its concomitant receptor that results in the recruitment of TRADD. TRADD in turn recruits TRAF2, RIP, FADD, cIAPs and A20 to the ligand/receptor (10) complex, which results in the activation of proteases, phospholipases, protein kinases and transcription factors (11) that are involved in the regulation of several physiological processes. On the other hand, TNF has the ability to cause apoptosis by clustering of death domain-containing proteins leading to caspase activation and resulting in the disruption of tumor vasculature (12). Considering the diverse role of TNF in tumor initiation, development, progression, angiogenesis and metastasis, targeting TNF signaling may be an important step towards both cancer prevention and therapy.
Several inhibitors of TNF-α like Infliximab, Etanercept, Adalimumab and thalidomide have been approved by the FDA for the treatment of various human illnesses (13), but risk of toxicity, development of secondary cancers and severe allergic reactions (14) have raised important questions regarding TNF-α targeted therapy for cancer. However, several new approaches for targeting TNF are currently being investigated namely radio-inducible TNF-expressing adenoviral vectors for the treatment of inoperable pancreatic cancer (15), NGF-hTNF that targets TNF to CD13 on blood vessels in the tumor for the treatment of colorectal cancer, liver cancer and mesothelioma, and also a modified TNF mutant that is administered intravenously that has been reported to show relatively less systemic toxicity (16). Thus, investigating compounds that inhibit or overcome TNF-mediated prosurvival signaling in various cancer types will be of significant value in treating various cancer types.
In this context, we have identified a natural compound, psoralidin, that is isolated from the seeds of Psoralea corylifolia, which has previously been reported to inhibit the PI3K/Akt/NF-κB signaling pathway in prostate cancer cells (17). Psoralea corylifolia Linn. (Family, Fabaceae), the source of psoralidin, has been extensively used in traditional medicine as an anti-inflammatory agent. It is well established that chronic inflammation plays a major role in the development of cancer, and thus we intended to investigate whether psoralidin overcomes the prosurvival function of TNF-α in AIPC. In this study, we investigated the role of psoralidin in TNF-mediated prosurvival signaling, and our results suggest that psoralidin overcomes TNF-mediated resistance and induces death receptor-mediated apoptosis in AIPC cells.
Materials and methods
Chemicals and Reagents
Psoralidin, the test compound, was obtained as described previously (17). Human recombinant TNF-α (hr TNF-α) and Caspase-9 inhibitor were purchased from Calbiochem (Gibbstown, NJ) and used at a concentration of 10 ng/ml and 10 μM, respectively. Caspase-3 inhibitor was purchased from Promega (Madison, WI), and a 10 μm concentration was used for the assays.
Cell lines and Culture
Androgen independent prostate cancer cell lines PC-3 and DU-145 were purchased from American Type Culture Collection (Manassas, VA). Both cell lines were grown in RPMI 1640 supplemented with 10% fetal bovine serum and antibiotics. Immortalized normal prostate epithelial cells, PzHPV-7, were also obtained from ATCC and were grown in Keratinocyte medium obtained from Invitrogen Corporation (Carlsbad, CA). In all experiments, PC-3 and DU-145 cells were treated with 60 and 45 μM of psoralidin, respectively, unless indicated otherwise.
Cell Viability and Apoptosis assays
PC-3, DU-145 and PzHPV-7 cells were plated in 6-well plates (3×105 cells/well) and treated with either vehicle (DMSO) or psoralidin for 24 h, and percentage of cell viability was determined using the trypan blue exclusion assay as described previously (18). PC-3 and DU-145 cells (70–80% confluent) were treated with psoralidin alone, hrTNF-α alone or a combination of psoralidin and hrTNF-α for 24 h, and percentage of apoptotic cells were quantified using Annexin V-FITC staining and analyzed by flow cytometry as described previously (19). Also, vehicle- and psoralidin-treated tumor sections (17) were subjected to TUNEL assay for which the Dead End Fluorometric TUNEL System was obtained from Promega (Madison, WI) as previously described (20). Apoptotic cells were estimated after counterstaining with DAPI and mounted using VECTASHIELD Mounting medium (Vector laboratories, Burlingame, CA).
ELISA for NF-κB p65 protein levels
PC-3 and DU-145 cells (70–80% confluent) were treated with psoralidin alone, hrTNF-α alone or a combination of psoralidin and hrTNF-α for 24 h, and endogenous levels of total NF-κB/p65 protein were detected using the PathScan® Total NF-κB/p65 Sandwich ELISA Kit (Cell Signaling Technology, Danvers, MA). Briefly, cells (70–80% confluent) were treated as mentioned above and whole cell extracts were obtained. Microwells were coated with NF-κB/p65 mouse monoclonal antibody overnight, which captures both phospho- and nonphospho-NF-κB/p65 proteins in the cell lysate. Microwells were washed extensively, NF-κB/p65 antibody was added, NF-κB/p65 protein was added and then an anti-rabbit IgG, HRP-linked Antibody was used to recognize the bound detection antibody. TMB substrate was used to develop the color for detection by absorbance at 450 nm. The magnitude of optical density was proportional to the quantity of total NF-κB/p65 protein.
RNA extraction and RT-PCR analysis
Total RNA was isolated from control and psoralidin (15 min to 2 h)-treated PC-3 and DU-14 cells using Trizol reagent from Invitrogen Corporation (Carlsbad, CA). The RNA (1 μg) was subjected to reverse transcription using the Two-Step RT-PCR kit from USB (Cleveland, OH) and cDNA was synthesized. PCR primers were designed (PrimerQuest) and purchased from IDT (Coralville, IA), and GAPDH was used as the internal control for PCR. TNF cDNA was synthesized using the forward primer (5′-TGA ACC AGC CTT TAG TGC CTA CCA -3′) and the reverse primer (5′-ACC ATG GTA CCC AGA CAT GCT CAA -3′) using DNA Engine (MJ Research) thermo cycler.
Western Blotting
PC-3 and DU-145 cells (70–80% confluent) were treated with the IC50 dose of psoralidin (60 and 45 μM, respectively) for varying time intervals or human recombinant TNF-α alone or a combination of psoralidin and TNF-α for 24 h, and Western blot analysis was performed using TNFR1, TNF-α, Fas, FasL, DR-4, DR-5, cIAP1/2, XIAP. p-survivin, Bcl-xL, Bid, Bcl-2, Bax and NF-κB/p65 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) and caspase-3, caspase-9 and PARP antibodies from Cell Signaling Technology (Danvers, MA). Additionally, PC-3 and DU-145 cells were treated with psoralidin for varying time intervals, mitochondrial and cytoplasmic fractions were extracted and the cytoplasmic fraction was subjected to Western blot analysis using a cytochrome c antibody from Santa Cruz Biotechnology (Santa Cruz, CA). Actin from Santa Cruz Biotechnology (Santa Cruz, CA) was used as the internal loading control for all the Western blotting experiments.
Fluorometry for Caspase-3 activation
PC-3 and DU-145 cells (70–80% confluent) were treated with psoralidin alone, caspase inhibitor alone or a combination of caspase-9 or caspase-3 inhibitors with psoralidin for 24 h, and caspase-3 activation was measured as described previously (21, 22).
JC-1 staining
PC-3 and DU-145 cells (70–80% confluent) were treated with varying doses of psoralidin for 24 h and JC-1 staining was performed using the JC-1 Mitochondrial Membrane Potential Assay Kit from Cayman Chemical Company (Ann Arbor, MI) as per the manufacturer’s protocol. Briefly, cells were treated with psoralidin and after treatment were harvested using trypsin, washed with PBS twice, resuspended in PBS and finally incubated with JC-1 stain (10 mg/ml) for 20 min at room temperature. Cells were then washed twice with PBS and analyzed by a Flow Cytometer (Becton Dickinson) to detect green and red fluorescence at excitation/emission wavelengths of 485/530 and 485/590 nm, respectively. Mitochondrial membrane depolarization was indicated by a reduction in the red/green fluorescence-intensity ratio. Psoralidin-treated PC-3 and DU-145 cells were subjected to JC-1 staining and shift in fluorescence was observed using confocal microscopy.
Immunohistochemistry
Five micrometer thick sections from vehicle- and psoralidin treated tumors from PC-3 xenografts obtained from our earlier study (17) were subjected to immuno histochemical analysis using TNF-α and NF-κB/p65 antibodies as described previously (23).
Statistical analysis
All experiments were performed three times to ascertain the reproducibility of results. The data shown are representative of three experiments. The Student’s t test was used to calculate statistical significance.
Results
Psoralidin downregulates the expression of TNF-α-mediated NF-κB activation in AIPC cells
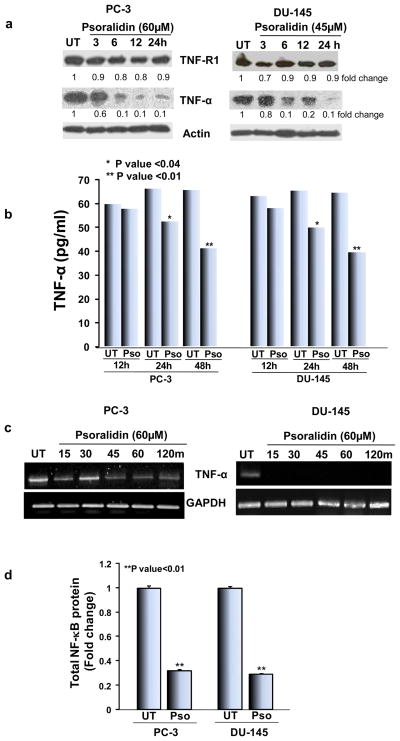
TNF signaling is well known for its survival function especially in PCa cells, and therefore we investigated the effect of psoralidin on the constitutive expression of TNF-α/TNF-R1 in PC-3 and DU-145 cells. In both PC-3 and DU-145 cells, psoralidin downregulated TNF-α expression in a time dependent manner for up to 24 h without altering expression of TNFR1 (Figure 1A). Next, we performed assays to determine the secreted levels of TNF-α expression in response to treatment with psoralidin using an ELISA. Our results show that treatment with psoralidin for 24 and 48 h significantly reduced secreted TNF-α when compared to control cells (Figure 1B). Additionally, RNA analysis in control and psoralidin treated AIPC cells showed a downregulation in TNF-α mRNA in psoralidin treated cells for up to 2 h when compared to control cells (Figure 1C). Previously, we reported that TNF-α induces NF-κB activation in PCa cells (18); thus, we determined the effect of psoralidin on total levels of NF-κB/p65 expression and found that psoralidin significantly inhibits total levels of NF-κB/p65 expression (P value<0.01) in both AIPC cells (17) (Figure 1D).
Figure 1. Effect of psoralidin on TNF-α and NF-κB in AIPC cells.
(A) PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for varying time intervals (3–24 h), and Western blot analysis was performed using TNF-α and TNF-R1 antibodies. Actin was used as the internal loading control. (B) PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for 12, 24 or 48 h, and the supernatant was collected after the treatment periods, protein concentration in the supernatant was quantified and equal amounts of protein were subjected to sandwich ELISA for quantitation of secreted TNF-α levels in control and psoralidin-treated AIPC cells. (C) PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for varying time intervals (3-2 h), and total RNA was isolated using Trizol method, cDNA was synthesized using two step RT-PCR and TNF-α mRNA expression was determined. (D). PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, and cell lysates were subjected to sandwich ELISA for quantitation of total NF-κB protein levels in control and psoralidin-treated AIPC cells. Bars represent mean±SD.
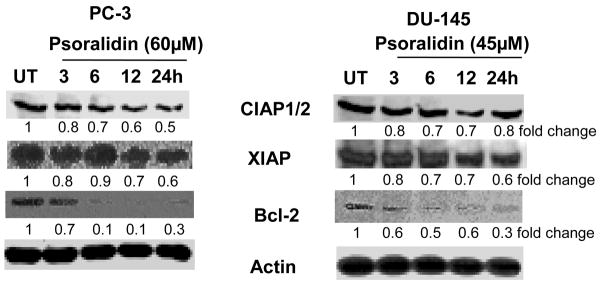
Inhibitor of apoptosis proteins (IAP) (17, 24) and the Bcl-2 family (25) of proteins consist of pro- and anti-apoptotic proteins that determine the fate of a cell. Hence, we examined whether inhibition of TNF-mediated pro-survival signaling resulted in the downregulation of the downstream prosurvival signaling molecules. Treatment with psoralidin significantly decreased expression of cIAP1/2 in PC-3 when compared to DU-145 cells. On the other hand, a moderate downregulation of XIAP was observed in both AIPC cells. Additionally, psoralidin significantly decreased expression of Bcl-2 from 6 h onwards in both AIPC cells (Figure 2). These results suggest that psoralidin significantly decreases expression of prosurvival proteins in AIPC cells.
Figure 2. Effect of psoralidin on pro-survival signaling in AIPC cells.
PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for varying time intervals (3–24 h), and Western blot analysis was performed using cIAP, XIAP and Bcl-2 antibodies. Actin was used as the internal loading control.
Psoralidin induces expression of death receptors and proapoptotic proteins in AIPC cells
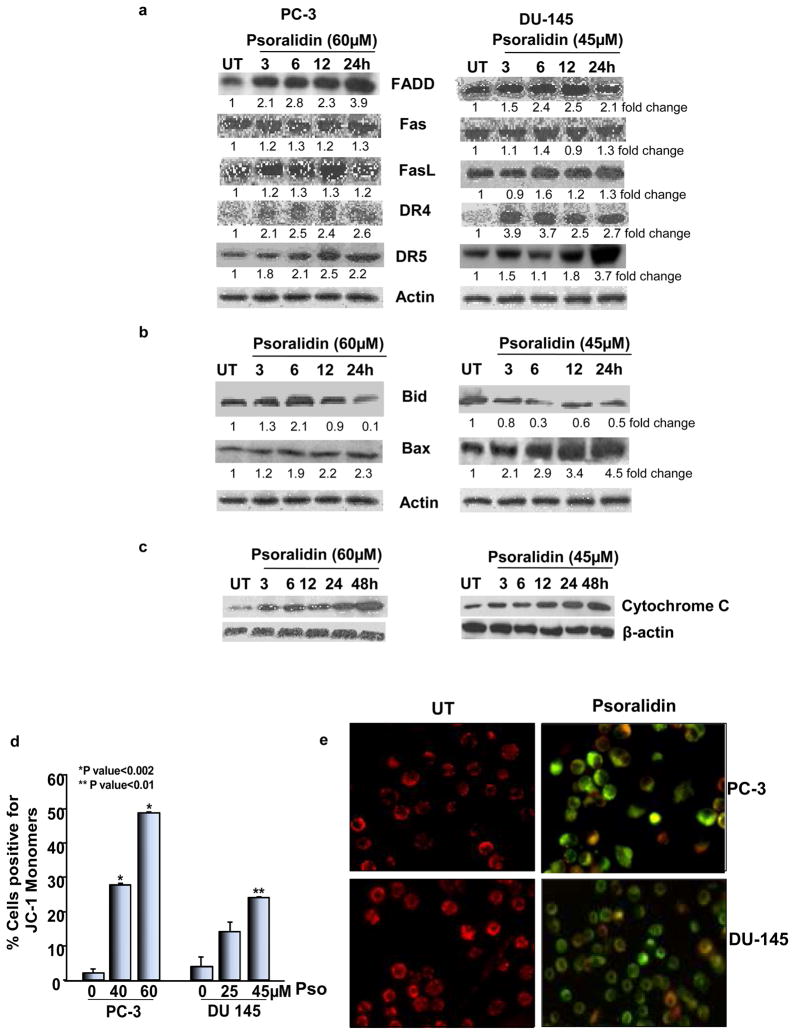
Treatment of PC-3 cells with psoralidin showed a marked increase in the expression of FADD, DR4 and DR5 levels and a moderate increase in the expression of Fas and FasL (Figure 3A). In contrast, in DU-145 cells there was a marked increase in the expression of DR4 and DR5 but no significant changes in the expression of FADD, Fas and FasL were observed (Figure 3A). Additionally, psoralidin treatment caused a decrease in total Bid and a moderate upregulation in Bax in PC-3 cells but a marked increase in Bax expression in DU-145 cells (Figure 3B). Western blot analysis using the cytoplasmic fraction of control and psoralidin treated AIPC cells showed a significant increase in the expression of cytochrome C (Figure 3C) indicating the involvement of mitochondrial membrane depolarization. Additionally, JC-1 staining of control and psoralidin-treated PC-3 and DU-145 cells was performed to confirm the involvement of the mitochondria in psoralidin-mediated apoptosis in AIPC cells. Our flowcytometry (Figure 3D) and confocal microscopy (Figure 3E) data both reveal an increase in JC-1 monomers as seen by a shift towards green fluorescence indicating a disruption in the mitochondrial membrane potential of these cells following treatment with psoralidin.
Figure 3. Effect of psoralidin on death receptor and apoptotic signaling in AIPC cells.
PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for varying time intervals (3–24 h), and Western blot analysis was performed using (A) FADD, Fas, FasL, DR4 and DR5 antibodies and (B) Bid and Bax antibodies. Actin was used as the internal loading control. (C) PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for varying time intervals (3–24 h), and the cytoplasmic fraction of control and psoralidin treated cells were subjected to Western blot analysis using a cytochrome C antibody. (D) PC-3 and DU-145 cells (70–80% confluency) were treated with 40 or 60 μM and 25 or 45 μM psoralidin respectively for 24 h and JC-1 staining was performed using flowcytometry. Bars represent percentage of cells positive for green fluorescence (JC-1 monomers) ±SD. (E) PC-3 and DU-145 cells plated in 8-well chamber slides (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for 24 h, and JC-1 staining was performed and slides were analyzed by confocal microscopy.
Psoralidin induces apoptosis in AIPC cells by activating the caspase cascade
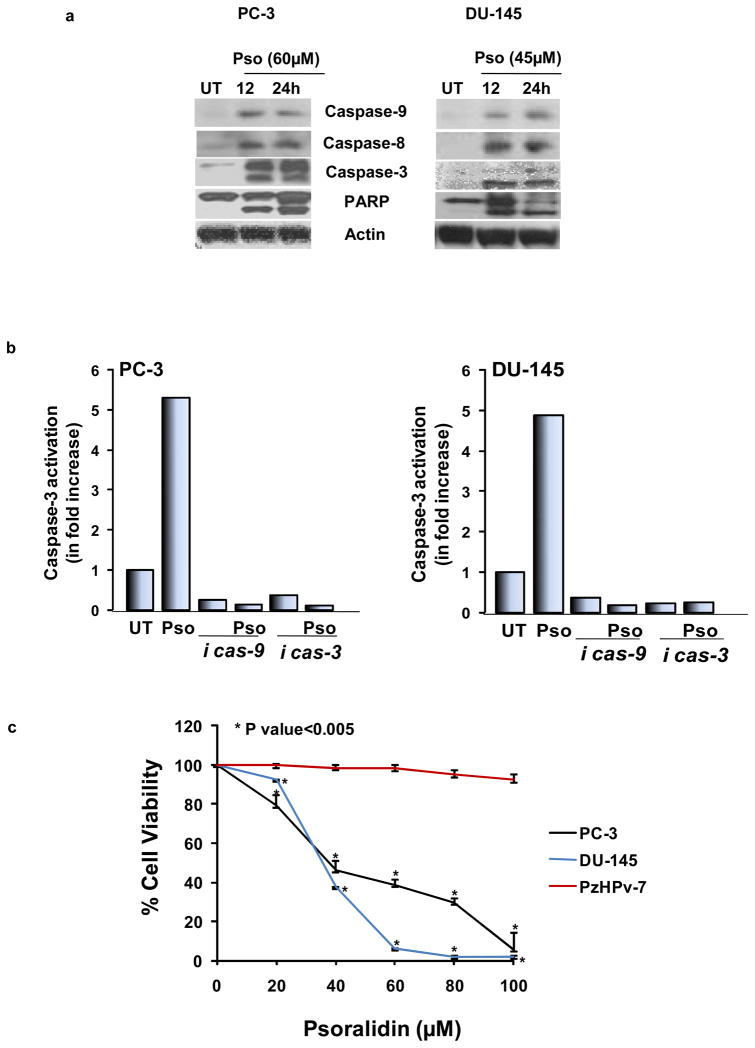
The consequence of activation of both the extrinsic and intrinsic apoptotic pathways is the cleavage and activation of effector caspases namely caspase-3, -6 and -7, which are proteases that result in cell degradation and eventual death (26). Psoralidin treatment led to the activation of caspases-9, -8 and -3 and also a cleavage and inactivation of poly ADP-ribose polymerase (PARP) (Figure 4A) in both AIPC cells. Caspase-3 activation studies using colorimetry indicated that inhibition of caspase-9 nullified psoralidin-mediated activation of caspase-3 in both PC-3 and DU-145 cells (Figure 4B). Collectively, these results indicate that psoralidin causes caspase activation in both PC-3 and DU-145 cells thereby inducing apoptosis in these cells.
Figure 4. Effect of psoralidin on caspase signaling and viability of AIPC cells.
(A). PC-3 and DU-145 cells (70–80% confluency) were treated with 60 and 45 μM psoralidin, respectively, for 12 and 24 h, and Western blot analysis was performed using caspase-9, caspase-8, caspase -3 and PARP antibodies. Actin was used as the internal loading control. (B). PC-3 and DU-145 cells (70–80% confluency) were treated with psoralidin alone, caspase inhibitors alone (3 or 9) and a combination of caspase inhibitors and psoralidin, and a fluorometric assay was performed to determine caspase-3 activation. Bars represent fold increase in caspase activity in each treatment group. (C). PC-3, DU-145 and PzHPv-7 (70–80% confluency) cells were treated with varying concentrations of psoralidin, and cell viability was determined using Trypan blue assay. A dose-response curve was plotted, and each data point represents mean percentage of cell viability±SD.
Control and psoralidin-treated cells were subjected to trypan blue staining to determine cell viability. Our results indicate that there was a significant decrease in the number of viable cells in a dose-dependent manner in both PC-3 and DU-145 when compared to PzHPv-7 (Figure 4C). These results are consistent with our previously published data (17) indicating that there is minimal batch to batch variability in the process of isolation of psoralidin.
Recombinant TNF-α induces NF-κB activation and psoralidin overcomes TNF-mediated resistance in AIPC cells
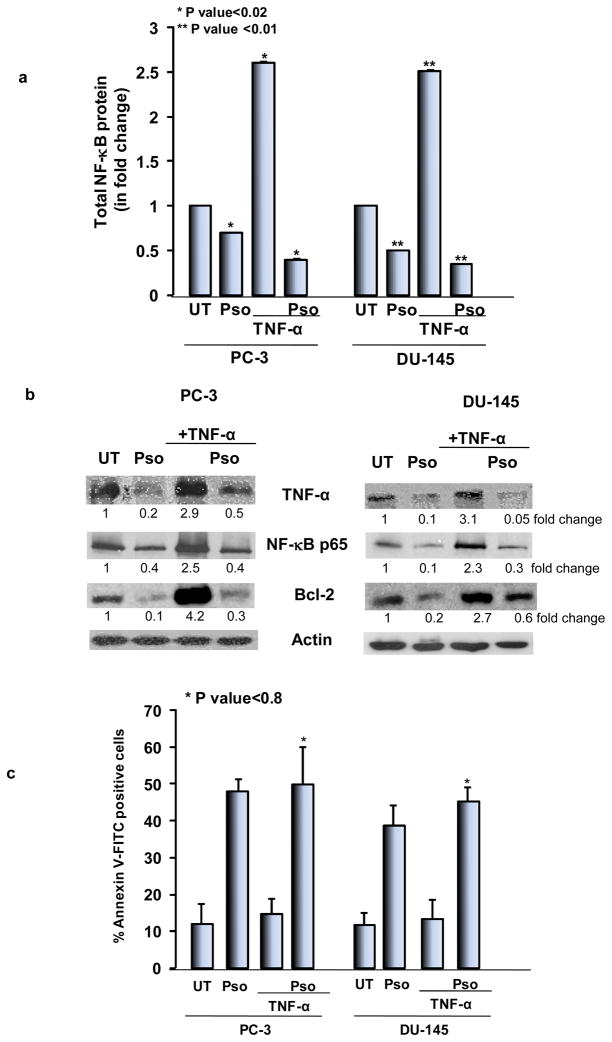
It is well established that treatment with TNF-α activates NF-κB in PCa cells. Therefore, we treated AIPC cells with human recombinant TNF-α followed by treatment with psoralidin. Our studies show that psoralidin inhibited TNF-α-induced NF-κB expression as was seen by the colorimetric assay (Figure 5A); this was further confirmed by Western blot analysis (Figure 5B). We previously published that treatment of AIPC cells with recombinant TNF-α significantly increases Bcl-2 expression, and neutralizing TNF-α expression using antibodies reduced Bcl-2 expression (18). Thus, we intended to determine whether psoralidin downregulates TNF-α-induced Bcl-2 expression in AIPC cells. Western blot analysis showed a significant reduction in Bcl-2 expression upon treatment with psoralidin when compared to the control group; however, treatment with recombinant TNF-α showed a robust increase in Bcl-2 expression, which was again downregulated upon treatment with psoralidin (Figure 5B). Further we intended to determine whether psoralidin overcomes TNF-α-induced NF-κB/Bcl-2-mediated resistance and induces apoptosis in AIPC cells. Our results indicate that psoralidin significantly induced apoptosis in both control and hrTNF-α treated AIPC cells (Figure 5C). These results collectively indicate that psoralidin overcomes TNF-α-mediated expression of pro-survival molecules and thus induces apoptosis in AIPC cells.
Figure 5. Effect of psoralidin on TNF-α signaling in AIPC cells.
(A) PC-3 and DU-145 cells (70–80% confluency) were treated with psoralidin alone, recombinant TNF-α alone or a combination of recombinant TNF-α and psoralidin and cell lysates were subjected to sandwich ELISA for quantitation of total NF-κB protein levels in control and psoralidin-treated AIPC cells. Bars represent mean±SD. (B) PC-3 and DU-145 cells (70–80% confluency) were treated with psoralidin alone, recombinant TNF-α alone or a combination of recombinant TNF-α and psoralidin, and Western Blot analysis was performed using TNF-α, NF-κB p65 and Bcl-2 antibodies. Actin was used as the internal loading control. (C) PC-3 and DU-145 cells (70–80% confluency) were treated with psoralidin alone, recombinant TNF-α alone or a combination of recombinant TNF-α and psoralidin and apoptotic cells were quantified using Annexin V-FITC staining. Bars represent mean±SD.
Psoralidin inhibits TNF-α and NF-κB p65 expression and induces apoptosis in PC-3 xenografts
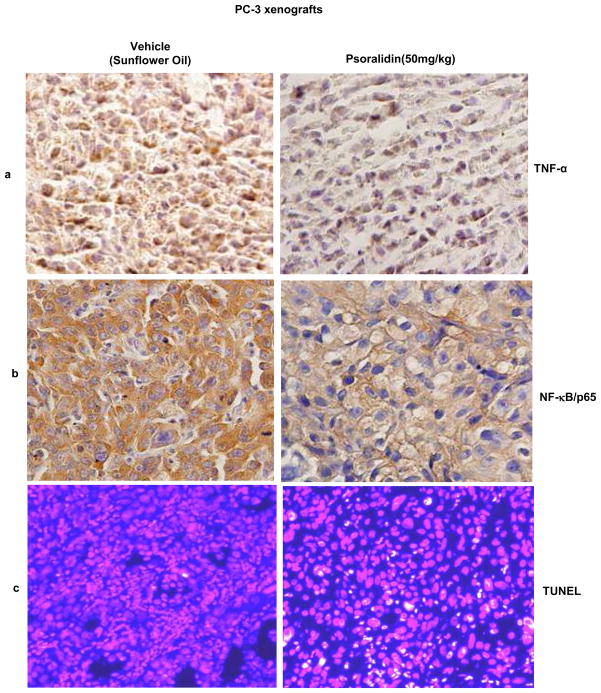
We previously published that psoralidin inhibits prostate tumor growth in a PC-3 xenograft model (17). Our results suggest that oral administration of psoralidin (50 mg/kg) caused significant tumor regression in PC-3 xenografts (data not shown). Immunohistochemical analysis of vehicle- and psoralidin-treated tumor sections showed a significant reduction in the expression of both TNF-α (Figure 6A) and NF-κB/p65 (Figure 6B) in psoralidin -treated sections when compared to control sections. Additionally, psoralidin induced a significant number of TUNEL positive cells when compared to tumors derived from vehicle treated control animals (Figure 6C). These results indicate that psoralidin inhibits tumor growth and induces apoptosis in tumor cells in vivo.
Figure 6. Effect of psoralidin on the expression of TNF-α and NF-κB/p65 in vivo tumor models.
Tumors obtained from PC-3 xenografts treated either with vehicle control or psoralidin (50 mg/kg) were paraffin embedded and sectioned (5 μm). The paraffin sections were subjected to Immunohistochemistry to study expression of (A) TNF-α, (B) NF-κB/p65 and (C) TUNEL staining for detection of apoptotic cell death.
Discussion
Psoralidin belongs to the coumestan family of natural compounds and has several biological effects such as antioxidant, antidepressant, antibacterial and anticancer (17, 27–29). In this study, we found that psoralidin downregulates TNF-mediated pro-survival signaling thereby resulting in the inhibition of cell growth and induction of apoptosis in AIPC cells.
TNF family members play a dual role as survival and apoptosis mediators based on the stimuli. It has been reported that there are very high levels of secreted TNF-α in the plasma of PCa patients and also TNF-α is overexpressed in tumors derived from PCa patients and is therefore one of the major pro-survival pathways in PCa. The TNFR family can be subdivided into two groups: the members that cause cell survival (RANK, TNFR2 and CD40) and those that induce apoptosis (TNFR1 and Fas) (30). In our results, we found that psoralidin downregulated TNF-α at RNA and protein levels in both PC-3 and DU-145 cells suggesting that psoralidin may transcriptionally regulate TNF expression in AIPC cells. Secreted TNF-α was also reduced following psoralidin treatment implying that psoralidin may abrogate the autocrine loop in AIPC cells. Previously, we reported that the natural compound curcumin inhibits the constitutive as well as radiation-induced expression of TNF-α in PCa cells (18), thus these results suggest that psoralidin is yet another agent that modulates TNF-mediated survival of PCa cells.
TNF-α is one of the major activators of NF-κB, and published evidence suggests that NF-κB is activated within minutes upon stimulation by TNF-α in PCa cells (31). Inhibition of TNF-α by psoralidin inhibited NF-κB p65 and other pro-survival signaling molecules including IAP (cIAP and XIAP) and the Bcl-2 (Bcl-xL, data not shown) family of proteins. In addition, stimulation of AIPC with recombinant TNF-α induced NF-κB p65 expression and activation, which was also downregulated by psoralidin. These results underscore the fact that psoralidin overcomes TNF-mediated NF-κB activation and resultant resistance in AIPC cells.
Additionally, our results suggest that psoralidin not only inhibits TNF receptor mediated pro-survival, but also activates TNF family death receptor (DR4 and DR5) mediated pro-apoptotic machinery in AIPC cells. Although there was no significant change in TNFR1 expression in psoralidin treated PC-3 and DU-145 cells, we found a moderate increase in Fas/FasL and a marked increase in FADD, DR4 and DR5 resulting in the induction of apoptosis in AIPC cells. Published evidence strongly suggests that natural compounds trigger Fas- and death receptor-mediated apoptosis in PCa cells (32). DR4 and DR5 activation recruits FADD, which in turn recruits pro-caspase-8 for auto activation. Cleaved and activated caspase-8 in turn triggers the mitochondrial apoptotic pathway through activation of caspases 9, 7 and 3 (33). Our results show that psoralidin activated the caspase cascade in AIPC cells.
Pertaining to the role of caspases in psoralidin-induced apoptosis, specific caspase inhibitors for caspase-3, -8 and -9 were used that either completely or partially blocked caspase-3 cleavage and activation resulting in the inhibition of caspase-mediated PARP cleavage. These results indicate that caspase activation is an essential step in psoralidin-mediated apoptosis. The death receptor/Caspase-8 and the cytochrome C/APAF-1/caspase-9 are the two pathways that eventually activate downstream effector caspases to execute apoptosis (34). Although these two pathways are distinguishable, there have been reports about caspase-8 activation being the primary apoptotic executioner while caspase induced cytochrome C release with subsequent activation of caspase-9 is a secondary pathway for induction of apoptosis (35). Our results clearly suggest that psoralidin activates both intrinsic and extrinsic apoptotic pathways in AIPC cells. In general, cytochrome C release from the mitochondria to the cytosol activates caspase-9, which has been established as a major step in apoptosis (36). We determined whether psoralidin induced caspase-9 activation was dependent on cytochrome C release or on activation of caspase-12. Our results show an increase in the expression of cytochrome C in the cytosolic fraction of psoralidin-treated AIPC cells in a time dependent manner. Additionally, evaluation of the mitochondrial membrane potential by JC-1 staining following psoralidin treatment revealed that psoralidin in fact disrupts mitochondrial membrane potential thereby resulting in cytochrome C release, caspase-9 activation, caspase-3 activation and thus induction of apoptosis. Additionally, our results indicated that psoralidin also induces caspase-8-mediated caspase-3 activation and resultant apoptosis.
Our in vivo results indicate that oral administration of psoralidin inhibited TNF-α and NF-κB p65 expression in PC-3 xenografts, which correlated with our in vitro findings. The number of TUNEL positive cells in psoralidin-treated tumor sections was significantly higher when compared to the tumors from control animals suggesting that the tumor regression was due to the induction of apoptosis by psoralidin.
In conclusion, our findings suggest that psoralidin, a natural compound, overcomes the pro-survival effects of the TNF-α-mediated pro-survival signaling with a simultaneous induction of DR-mediated pro-apoptotic signaling resulting in the induction of apoptosis in AIPC cells.
References
- 1.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ferrajoli A, Keating MJ, Manshouri T, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100:1215–1219. [PubMed] [Google Scholar]
- 3.Szlosarek PW, Grimshaw MJ, Kulbe H, et al. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382–390. doi: 10.1158/1535-7163.MCT-05-0303. [DOI] [PubMed] [Google Scholar]
- 4.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 5.Hu J, Nakano H, Sakurai H, Colburn NH. Insufficient p65 phosphorylation at S536 specifically contributes to the lack of NF-kappaB activation and transformation in resistant JB6 cells. Carcinogenesis. 2004;25:1991–2003. doi: 10.1093/carcin/bgh198. [DOI] [PubMed] [Google Scholar]
- 6.Arnott CH, Scott KA, Moore RJ, et al. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene. 2002;21:4728–4738. doi: 10.1038/sj.onc.1205588. [DOI] [PubMed] [Google Scholar]
- 7.Yan B, Wang H, Rabbani ZN, et al. Tumor necrosis factor-alpha is a potent endogenous mutagen that promotes cellular transformation. Cancer Res. 2006;66:11565–11570. doi: 10.1158/0008-5472.CAN-06-2540. [DOI] [PubMed] [Google Scholar]
- 8.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 10.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu ZG, Han J. Cellular responses to tumor necrosis factor. Curr Issues Mol Biol. 2001;3:79–90. [PubMed] [Google Scholar]
- 12.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–592. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 14.Larmonier N, Cathelin D, Larmonier C, et al. The inhibition of TNF-alpha anti-tumoral properties by blocking antibodies promotes tumor growth in a rat model. Exp Cell Res. 2007;313:2345–2355. doi: 10.1016/j.yexcr.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 16.Krippner-Heidenreich A, Grunwald I, Zimmermann G, et al. Single-chain TNF, a TNF derivative with enhanced stability and antitumoral activity. J Immunol. 2008;180:8176–8183. [Google Scholar]
- 17.Kumar R, Srinivasan S, Koduru S, et al. Psoralidin, an herbal molecule, inhibits phosphatidylinositol 3-kinase-mediated Akt signaling in androgen-independent prostate cancer cells. Cancer Prev Res (Phila Pa) 2009;2:234–243. doi: 10.1158/1940-6207.CAPR-08-0129. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–1607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 19.Sowmyalakshmi S, Nur EAM, Akbarsha MA, Thirugnanam S, Rohr J, Chendil D. Investigation on Semecarpus Lehyam--a Siddha medicine for breast cancer. Planta. 2005;220:910–918. doi: 10.1007/s00425-004-1405-4. [DOI] [PubMed] [Google Scholar]
- 20.Liu T, Wu LY, Choi JK, Berkman CE. In vitro targeted photodynamic therapy with a pyropheophorbide--a conjugated inhibitor of prostate-specific membrane antigen. Prostate. 2009;69:585–594. doi: 10.1002/pros.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan S, Koduru S, Kumar R, Venguswamy G, Kyprianou N, Damodaran C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int J Cancer. 2009;125:961–967. doi: 10.1002/ijc.24419. [DOI] [PubMed] [Google Scholar]
- 23.Rabiau N, Dechelotte P, Guy L, et al. Immunohistochemical staining of mucin 1 in prostate tissues. In Vivo. 2009;23:203–207. [PubMed] [Google Scholar]
- 24.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 25.de Bruin EC, Medema JP. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat Rev. 2008;34:737–749. doi: 10.1016/j.ctrv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Cheung YT, Kong LD, et al. Transcriptional regulation of corticotrophin releasing factor gene by furocoumarins isolated from seeds of Psoralea corylifolia. Life Sci. 2008;82:1117–1121. doi: 10.1016/j.lfs.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Pahari P, Rohr J. Total synthesis of psoralidin, an anticancer natural product. J Org Chem. 2009;74:2750–2754. doi: 10.1021/jo8025884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatune NA, Islam ME, Haque ME, Khondkar P, Rahman MM. Antibacterial compounds from the seeds of Psoralea corylifolia. Fitoterapia. 2004;75:228–230. doi: 10.1016/j.fitote.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Gravallese EM, Galson DL, Goldring SR, Auron PE. The role of TNF-receptor family members and other TRAF-dependent receptors in bone resorption. Arthritis Res. 2001;3:6–12. doi: 10.1186/ar134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparian AV, Yao YJ, Kowalczyk D, et al. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 32.Saleem M, Kweon MH, Yun JM, et al. A novel dietary triterpene Lupeol induces fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 33.Wu XX, Ogawa O, Kakehi Y. TRAIL and chemotherapeutic drugs in cancer therapy. Vitam Horm. 2004;67:365–383. doi: 10.1016/S0083-6729(04)67019-1. [DOI] [PubMed] [Google Scholar]
- 34.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 35.Jiang C, Wang Z, Ganther H, Lu J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–3070. [PubMed] [Google Scholar]
- 36.Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]