Abstract
The purpose of this study was to determine independent relationships of intra-abdominal adipose tissue (IAAT), leg fat, and aerobic fitness with blood lipids and insulin sensitivity (Si) in European-American (EA) and African-American (AA) premenopausal women. Ninety-three EA and ninety-four AA with BMI between 27 and 30 kg/m2 had IAAT by computed tomography, total fat and leg fat by dual-energy X-ray absorptiometry, aerobic fitness by a graded exercise test, African admixture (AFADM) by ancestry informative markers, blood lipids by the Ektachem DT system, and Si by glucose tolerance test. Independent of age, aerobic fitness, AFADM, and leg fat, IAAT was positively related to low-density lipoprotein–cholesterol (LDL-C), cholesterol-high-density lipoprotein (HDL) ratio, triglycerides (TGs), and fasting insulin (standardized β varying 0.16–0.34) and negatively related to HDL-cholesterol (HDL-C) and Si (standardized β −0.15 and −0.25, respectively). In contrast, independent of age, aerobic fitness, AFADM, and IAAT, leg fat was negatively related to total cholesterol, LDL-C, cholesterol-HDL ratio, TGs, and fasting insulin (standardized β varying −0.15 to −0.21) and positively related to HDL-C and Si (standardized β 0.16 and 0.23). Age was not independently related to worsening of any blood lipid but was related to increased Si (standardized β for Si 0.25, insulin −0.31). With the exception of total cholesterol and LDL-C, aerobic fitness was independently related to worsened blood lipid profile and increased Si (standardized β varying 0.17 to −0.21). Maintenance of favorable fat distribution and aerobic fitness may be important strategies for healthy aging, at least in premenopausal EA and AA women.
INTRODUCTION
Prevention of cardiovascular disease (CVD) can be attained by improvement of cardiovascular and metabolic risk factors such as blood lipids and insulin sensitivity (Si) (1). It is evident that obesity is an important contributor to increased risk of CVD and type 2 diabetes (2,3). It is also evident that not all stored fat contributes equally to risk of disease, with intra- abdominal adipose tissue (IAAT) most strongly correlated with cardiovascular risk factors in both men and women (4–8). In contrast, several studies suggest that leg fat is associated with an improvement in blood lipid profile and blood pressure after adjusting for the potential confounding effects of either overall fat mass or IAAT (9–11).
Both cross-sectional and intervention studies show that high aerobic fitness (maximum oxygen uptake; VO2max) attenuates CVD risk independent of weight status (12,13). Physical activity (especially relatively high-intensity aerobic exercise) is an important determinant of aerobic fitness (14), with genetic factors also accounting for a sizable proportion of the between-subject variability in VO2max (15). The beneficial effects of physical activity and aerobic fitness on blood lipids may be partially related to the lower adiposity, and therefore lower IAAT levels found in more active women and men (15). Because exercise training may affect fat distribution, it has been difficult to establish the independent relative importance of fat distribution and aerobic fitness in influencing blood lipids and Si (16,17). We know of no studies that have attempted to determine the independent relationship between IAAT, leg fat, and aerobic fitness with blood lipids, fasting insulin, and Si. This is the primary goal of this article.
Previous studies examining the relationship between fat distribution and aerobic fitness with blood lipids and Si have been with European-American (EA) subjects (4,18,19). To our knowledge, these factors have not been examined in a mixed population of EA and African-American (AA) subjects. This may potentially be important because there are differences in fat distribution and aerobic fitness, as well as blood lipids and Si between BMI-matched EA and AA women. For example, AA women have lower IAAT, triglycerides (TGs), and cholesterol-high- density lipoprotein (HDL) ratios as well as higher HDL-cholesterol (HDL-C) than EA women (20–22). Paradoxically, AAs have lower Si than EAs (23–25).
Racial categorization is a potential confounder when exploring phenotypic differences among individuals, particularly because of the multiple factors (physiological, genetic, environmental, and behavioral) that are associated with racial categories. Genetic admixture can be used to tease apart the genetic component underlying racial categorization that contributes to a phenotype profile, assigning individuals with a quantitative value that represents their ancestral background and reducing the confounding effects of population stratification. This technique has been used previously to explore physiological and metabolic population differences (26).
The purpose of this study is to determine the independent relationships of IAAT, leg fat, and aerobic fitness with blood lipids, fasting insulin, and Si in a mixed population of EA and AA premenopausal women. Because increasing age is generally associated with increased risk of disease as well as changes in aerobic fitness and fat distribution, it is a potential confounder in the modeling of blood lipids, fasting insulin, and Si. We therefore include age in the modeling. Specifically, we hypothesize that independent of fat distribution, aerobic fitness, age, and African admixture (AFADM), IAAT will be associated with a worsened blood lipid profile, fasting insulin, and Si, whereas leg fat and aerobic fitness will be associated with improved blood lipid profile, fasting insulin, and Si.
METHODS AND PROCEDURES
Subjects
The study group comprised 187 healthy premenopausal women (93 EA and 94 AA self-identified), with BMI between 27 and 30 kg/m2. Age ranged from 21 to 46 years. All subjects were nonsmokers, of overall good health, and had normal menstrual cycles. Normal glucose tolerance was documented by fasting and 2-h postprandial blood glucose levels after an oral glucose load. None used oral contraceptives at the time of enrollment into the study or medications known to affect body composition. The Institutional Review Board for Human Subjects–approved informed consent was obtained prior to participation in the study in compliance with the Department of Health and Human Services Regulations for Protection of Human Research Subjects.
Study design
Subjects participating in the study were maintained in weight-stable state for 4 weeks prior to evaluation in the General Clinical Research Center (GCRC), as part of a larger ongoing study on the role of metabolic factors on obesity. During those 4 weeks, body weight measurements were made at visits to the GCRC 3 days/week for the first 2 weeks, and 5 days/week for the last 2 weeks. During the final 2-week period, all meals were provided through the GCRC Research Kitchen to ensure weight stability of <1% variation from initial weight and to maintain daily macronutrient intake within the range of 20–22% of energy as fat, 17–22% as protein, and 57–62% as carbohydrate. Subjects were then admitted to the GCRC for 4 days, during the follicular phase of the menstrual cycle, and underwent assessment of body composition/fat distribution, aerobic fitness, blood lipids, and Si. After all assessments were completed, subjects were discharged from the GCRC.
Body composition
Lean and fat tissue for the whole body and legs were determined with the use of dual-energy X-ray absorptiometry (Prodigy; Lunar Radiation, Madison, WI). The scans were analyzed with the use of ADULT software, version 1.33 (Lunar Radiation).
IAAT
Cross-sectional area of IAAT was determined by CT with the use of a HiLight/HTD Advantage scanner (General Electric, Milwaukee, WI) set at 120 kVp (peak kilovoltage) and 40 mA. Subjects were examined in the supine position with their arms stretched above their heads, taking a 5-mm scan for 2 s at approximately the level of the fourth and fifth lumbar vertebrae. With the use of procedures established by Kvist et al. (27), the attenuation range for adipose tissue was −30 to −190 Hounsfield units. Cross sections of adipose tissue were determined by using a computerized fat tissue–highlighting technique. IAAT was measured by separating adipose tissue areas by encircling the muscle wall surrounding the abdominal cavity with a cursor. Tissue cross-sections between −30 and −190 Hounsfield units in the respective areas were considered to be IAAT. Both intra- and interobserver test–retest reliability had an r value of 0.99 with a CV of <2% for re-evaluation of 20 scans.
Aerobic fitness (Vo2max)
VO2max was determined during a maximal modified Bruce graded treadmill protocol (28). O2 consumption and CO2 production were measured continuously via open-circuit spirometry and analyzed using a SensorMedics metabolic cart (Model No. 2900; SensorMedics, Yorba Linda, CA). Criteria for achievement of VO2max were heart rate (within 90% of age-predicted maximum), respiratory quotient (>1.1), and plateauing (<1.0 ml/kg/min increase in oxygen uptake when grade was increased 2.5% at a speed of 3 mph) (29). All subjects reached at least two criteria and 82% reached the plateauing criteria.
Collection of sera and frequently sampled, intravenous glucose tolerance test
At ~7 am, after a 12-h fast, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three blood samples were drawn over a 40-min period, and sera subsequently separated and pooled for analysis of lipids. Three additional blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time “0”, glucose (50% dextrose: 11.4 g/m2) was administered intravenously. At 20 min following glucose administration, subjects received an intravenous bolus of insulin (0.02 units/kg). Blood samples were collected at the following times (min) relative to glucose administration at 0, 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 27, 30, 35, 40, 50, 60, 70, 80, 90, 100, 120, 140, 160, and 180 min. Sera were analyzed for glucose and insulin, and values were entered into the MINMOD computer program (version 3.0; Richard N. Bergman) for determination of Si and acute insulin response to glucose (30–32).
Assay of glucose, insulin, and lipids
Analyses were performed in the Core Laboratory of the General Clinical Research Center and the Clinical Nutrition Research Center. Glucose was measured in 10 μl sera using an Ektachem DT system (Johnson and Johnson Clinical Diagnostics, Rochester, NY). In the Core Laboratory, this analysis has a mean intra-assay coefficient of variation of 0.61% and a mean interassay coefficient of variation of 1.45%. Insulin was assayed in duplicate 100 μl aliquots with reagents obtained from Linco (St Charles, MO). In the Core Laboratory, this assay has a sensitivity of 1.0 μIU/ml, a mean intra-assay coefficient of variation of 5%, and a mean interassay coefficient of variation of 5.57%. Commercial quality control sera of low, medium, and high insulin concentration are included in every assay to monitor variation over time. Total cholesterol, HDL-C, and TG were measured with the Ektachem DT II system. With this system, HDL-C is measured after precipitation of low-density lipoprotein (LDL) and VLDL with dextran sulfate and magnesium chloride. Control sera of low and high substrate concentration are analyzed with each group of samples, and values for these controls must fall within accepted ranges before samples are analyzed. The DT II is calibrated every 6 months with reagents and supplied by the manufacturer. LDL-cholesterol (LDL-C) was estimated using the Friedewald formula (33).
Admixture
Genotyping of the ancestry informative markers for the measurement of genetic admixture was performed at PreventionGenetics (http://www.preventiongenetics.com) using the Chemicon Amplifluor SNPs Genotyping System (34) coupled with Array Tape technology (http://www.douglasscientific.com/global-array-array-tape.html). A panel of 85 ancestry informative markers was used to estimate the genetic admixture proportion of each subjects. Molecular techniques for the allelic identification and methodology for genetic admixture application have been described elsewhere (35), and information regarding marker sequences, experimental details, and parental population allele frequencies has been submitted to dbSNP (http://www.ncbi.nlm.nih.gov/SNP) under the handle PSU-ANTH. The information from the ancestry informative markers was translated into estimates of African (AFADM) and European admixture for each subject using ADMIXMAP software (36). Admixture estimate ranged from 1–100, for a total of 100 on each subject.
Statistical analysis
T-tests were used to examine racial differences in descriptive variables. Simple Pearson correlation coefficients were calculated for all the variables of interest: IAAT, leg fat, ratio of leg fat-IAAT, VO2max, age, AFADM with lipid variables, Si, and insulin. To evaluate independent contributions of IAAT, leg fat, and VO2max on blood lipids and Si, multiple regression was used to model each of the seven dependent variables (cholesterol, LDL-C, HDL-C, cholesterol-HDL ratio, TGs, fasting insulin, and Si). Besides inclusion of IAAT, leg fat, and VO2max in the models, AFADM and age were also included to negate any potential confounding effects. In order to further explore the interrelationship between IAAT and leg fat on blood lipids and Si, subjects were characterized into four groups based on IAAT and leg fat. One quarter of a standard deviation above and below the race-specific mean was used to classify the individuals into groups that were appropriate for race. This supplied separation between groups and allowed adequate sample size in each of the four groups (29, 42, 38, and 24 subjects, respectively): (i) Low IAAT (AA <57.6 cm2 and EA <87.4 cm2) and low leg fat (AA <13.2 kg and EA <12.55 kg); (ii) High IAAT (AA >71.0 cm2 and EA >101.6 cm2) and low leg fat (AA <13.2 kg and EA <12.55 kg); (iii) Low IAAT (AA <57.6 cm2 and EA <87.4 cm2) and high leg fat (AA >14.55 kg and EA >13.83 kg); and (iv) High IAAT (AA >71.0 cm2 and EA >101.6 cm2) and high leg fat (AA >14.55 kg and EA >13.83 kg). Subjects were divided into low, medium, and high tertiles for aerobic fitness–based, race-specific frequency distributions. Following the approach recently used by Arsenault et al. (4), three (tertile groups for aerobic fitness) by two (race) analysis of covariance was used to determine whether high aerobic fitness resulted in reduced IAAT (after adjusting for % body fat). This approach allowed adequate sample size in each of the six subgroups: high aerobic fitness (AA VO2max >28.7 ml O2/kg/min with 31 subjects, and EA VO2max >31.0 ml O2/kg/min with 32 subjects), medium aerobic fitness (AA VO2max <28.7 and >25.8 ml O2/kg/min with 33 subjects, and EA VO2max <31.0 and >27.0 ml O2/kg/min with 32 subjects) and low aerobic fitness (AA VO2max <25.8 ml O2/kg/min with 30 subjects, and EA VO2max <27.0 ml O2/kg/min with 29 subjects). Where appropriate, post hoc tests were run with Bonferroni corrections. Fasting insulin, Si, and TGs were log-transformed. All analyses were done using the Statistical Package for the Social Sciences, version 10 (SPSS, Chicago, IL).
RESULTS
Descriptive characteristics of the 94 AA and 93 EA subjects are contained in Table 1. Subjects were healthy, premenopausal, overweight women. No racial differences were observed for age, height, weight, BMI, % fat, leg fat, cholesterol, LDL-C, or fasting Si, although the height difference of 1.7 cm had a probability of 0.07. However, AA had lower IAAT, VO2max, TGs, cholesterol-HDL ratio, and SI but higher HDL-C. Pearson product correlations (Table 2) showed that increased IAAT and age were associated with increased significant positive correlations with total cholesterol, LDL-C, cholesterol-HDL ratio, TGs, and fasting insulin. IAAT was also significantly negatively related to HDL-C. Age tended to be related to improved insulin variables having a significant negative relationship with fasting insulin and a borderline positive relationship with Si (r = 0.12, P = 0.06). Leg fat was significantly associated with improved blood lipids, fasting insulin, and Si. AFADM was negatively related to total cholesterol, cholesterol-HDL ratio, TGs, and Si, and positively related to HDL-C. VO2max was negatively related to cholesterol-HDL ratio and TGs, and positively related to Si. The ratio of leg fat-IAAT was significantly related to all blood lipids and fasting insulin.
Table 1.
Descriptive variables
| African American | European American | P | |
|---|---|---|---|
| Age (years) | 34.0 ± 6.1 | 34.7 ± 6.3 | 0.39 |
| African admixture (%) | 65.8 ± 11.0 | 17.9 ± 5.1 | <0.01 |
| Height (cm) | 164.1 ± 6.4 | 165.8 ± 6.4 | 0.07 |
| Weight (kg) | 76.6 ± 6.4 | 78.1 ± 7.4 | 0.12 |
| BMI (kg/m2) | 28.3 ± 1.3 | 28.3 ± 1.4 | 0.92 |
| % Fat | 43.2 ± 3.7 | 44.0 ± 3.4 | 0.11 |
| Leg fat (kg) | 13.8 ± 2.4 | 13.2 ± 2.6 | 0.11 |
| IAAT (cm2) | 64.4 ± 26.2 | 94.5 ± 28.5 | <0.01 |
| VO2max (ml/kg/min) | 27.5 ± 3.3 | 29.0 ± 3.7 | <0.01 |
| Cholesterol (mg/dl) | 153.6 ± 33.2 | 159.5 ± 31.9 | 0.21 |
| LDL-cholesterol (mg/dl) | 96.9 ± 31.4 | 100.4 ± 28.3 | 0.45 |
| HDL-cholesterol (mg/dl) | 42.9 ± 11.1 | 35.9 ± 9.2 | <0.01 |
| Cholesterol-HDL ratio | 3.8 ± 1.2 | 4.7 ± 1.4 | <0.01 |
| Triglycerides (mg/dl) | 68.7 ± 25.8 | 116.0 ± 52.7 | <0.01 |
| Fasting insulin | 12.2 ± 4.4 | 11.5 ± 3.4 | 0.20 |
| Insulin sensitivity | 2.4 ± 1.3 | 3.6 ± 1.9 | <0.01 |
Values are expressed in mean ± s.d. N = 187 except for fasting insulin and Si for which N = 172.
HDL, high-density lipoprotein; IAAT, intra-abdominal adipose tissue; LDL, low-density lipoprotein; Si, insulin sensitivity; VO2max, maximal oxygen uptake.
Table 2.
Pearson product moment correlations between % fat and fat distribution with blood lipids, fasting insulin, and insulin sensitivity
| Age | % Fat | Leg fat | IAAT | Ratio of leg fat-IAAT | AFADM | VO2max | |
|---|---|---|---|---|---|---|---|
| Total cholesterol | 0.20** | −0.09 | −0.20** | 0.24** | −0.20** | −0.12* | −0.06 |
| LDL-cholesterol | 0.18** | −0.12 | −0.21** | 0.24** | −0.24** | −0.09 | −0.06 |
| HDL-cholesterol | 0.01 | −0.03 | 0.16* | −0.31** | 0.35** | 0.32** | 0.10 |
| Cholesterol/HDL-cholesterol | 0.16* | −0.02 | −0.24** | 0.43** | −0.38** | −0.37** | −0.15* |
| Log triglycerides | 0.12* | 0.01 | −0.22** | 0.44** | −0.34** | −0.50** | −0.13* |
| Log fasting insulin | −0.18** | 0.10 | −0.16* | 0.17* | −0.19** | 0.05 | −0.02 |
| Log insulin sensitivity | 0.12 | −0.01 | 0.17* | −0.03 | 0.09 | −0.35** | 0.20** |
AFADM, African admixture; HDL, high-density lipoprotein; IAAT, intra-abdominal adipose tissue; LDL, low-density lipoprotein.
P < 0.05.
P < 0.01.
Table 3 contains the multiple regression models for each of the seven dependent variables. Based on potential confounding effects, five variables were entered into each of the seven models: leg fat, AFADM, IAAT, VO2max, and age. Independent of all other variables, leg fat was significantly related to improved blood lipids, fasting insulin, and Si, whereas IAAT was significantly related to worsening of blood lipids, fasting insulin, and Si (except total cholesterol for which significance was only approached (standardized β = 0.14, P = 0.07). Independent of the other variables, AFADM was positively related to HDL-C and fasting insulin, and inversely related to cholesterol-HDL ratio, TGs, and Si. VO2max was independently and positively related to HDL-C and Si, and inversely related to cholesterol-HDL ratio and TGs. After adjusting for potential confounders, age was not independently related to any dependent variable, whereas it did have a significant positive correlation with Si and negative correlation with fasting insulin.
Table 3.
Multiple regression models for estimation of blood lipids, fasting insulin, and insulin sensitivity
| Model variables | Intercept | Slope | Model R | Standardized β | P | |
|---|---|---|---|---|---|---|
| Total cholesterol | 168.7 ±35.5 | 0.31 | <0.01 | |||
| Leg fat | −2.07 ±0.98 | 0.16 | 0.04 | |||
| AFADM | −5.30 ±10.6 | −0.05 | 0.60 | |||
| IAAT | 0.141 ±0.09 | 0.14 | 0.07 | |||
| VO2max | −0.541 ±0.71 | −0.06 | 0.44 | |||
| Age | 0.641 ±0.39 | 0.12 | 0.11 | |||
| LDL-cholesterol | 111.0 ±32.5 | 0.30 | <0.01 | |||
| Leg fat | −2.00 ±0.90 | −0.17 | 0.03 | |||
| AFADM | −0.25 ±9.7 | −0.01 | 0.98 | |||
| IAAT | 0.15 ±0.09 | 0.16 | 0.04 | |||
| VO2max | −0.45 ±0.65 | −0.06 | 0.48 | |||
| Age | 0.44 ±0.36 | 0.09 | 0.22 | |||
| HDL-cholesterol | 8.31 ±11.2 | 0.43 | <0.01 | |||
| Leg fat | 0.67 ±0.31 | 0.16 | 0.03 | |||
| AFADM | 10.0 ±3.36 | 0.28 | <0.01 | |||
| IAAT | −0.051 ±0.03 | −0.15 | <0.05 | |||
| VO2max | 0.519 ±0.23 | 0.18 | 0.02 | |||
| Age | 0.212 ±0.12 | 0.12 | 0.09 | |||
| Cholesterol/HDL-cholesterol | 7.76 ±1.37 | 0.53 | <0.01 | |||
| Leg fat | −0.11 ±0.04 | −0.21 | <0.01 | |||
| AFADM | −1.44 ±0.41 | −0.30 | <0.01 | |||
| IAAT | 0.008 ±0.004 | 0.19 | 0.01 | |||
| VO2max | −0.078 ±0.03 | −0.21 | <0.01 | |||
| Age | 0.005 ±0.02 | 0.02 | 0.75 | |||
| Log triglycerides | 2.43 ±0.19 | 0.68 | <0.01 | |||
| Leg fat | −0.017 ±0.005 | −0.20 | <0.01 | |||
| AFADM | −0.321 ±0.055 | −0.44 | <0.01 | |||
| IAAT | 0.001 ±0.001 | 0.22 | <0.01 | |||
| VO2max | −0.001 ±0.004 | −0.17 | <0.01 | |||
| Age | −0.0001 ±0.002 | 0.01 | 0.94 | |||
| Log fasting insulin | 1.25 ±0.16 | 0.38 | <0.01 | |||
| Leg fat | −0.008 ±0.004 | −0.15 | <0.05 | |||
| AFADM | 0.108 ±0.047 | 0.21 | 0.02 | |||
| IAAT | 0.0015 ±0.001 | 0.34 | <0.01 | |||
| VO2max | −0.0003 ±0.003 | −0.01 | 0.91 | |||
| Age | −0.0071 ±0.002 | −0.31 | <0.01 | |||
| Log insulin sensitivity | −0.218 ±0.25 | 0.52 | <0.01 | |||
| Leg fat | 0.022 ±0.007 | 0.23 | <0.01 | |||
| AFADM | −0.376 ±0.073 | −0.44 | <0.01 | |||
| IAAT | −0.0019 ±0.001 | −0.25 | <0.01 | |||
| VO2max | 0.0117 ±0.005 | 0.17 | 0.02 | |||
| Age | 0.0097 ±0.003 | 0.25 | <0.01 |
AFADM, African admixture; HDL, high-density lipoprotein; IAAT, intra-abdominal adipose tissue; LDL, low-density lipoprotein.
Boldface values indicate P < 0.05.
Table 4 shows the results for the analysis of blood lipids and Si for groups characterized into low or high IAAT, and leg fat. The analysis of covariance analysis group effect was significant for all variables at P < 0.01 level except for HDL-C that was significant at P < 0.05. In general, post hoc results show that the low IAAT/high leg fat group had a significantly better blood lipid profile and higher Si than either the low IAAT/low leg fat group or the high IAAT/low leg fat group, whereas the low IAAT/high leg fat group had significantly higher HDL-C and lower cholesterol-HDL ratio than the high IAAT/high leg fat group. The high IAAT/low leg fat group had significantly higher cholesterol-HDL ratio and TGs than the low IAAT/low leg fat group. The high IAAT/high leg fat group had lower cholesterol, LDL-C, TGs, and log insulin but higher log Si than the high IAAT/low leg fat group.
Table 4.
Blood lipids, fasting insulin, and insulin sensitivity according to classification of low and high IAAT and leg fat
| Low IAAT Low leg fat (n = 29) | High IAAT Low leg fat (n = 42) | Low IAAT High leg fat (n = 38) | High IAAT High leg fat (n = 24) | |
|---|---|---|---|---|
| Total cholesterol | 162.0 ± 5.9 | 170.5 ± 5.1 | 146.1 ± 5.2a,b | 150.1 ±6.4b |
| LDL-cholesterol | 104.7 ± 5.4 | 111.1 ± 4.6 | 88.0 ± 4.7a,b | 95.6 ± 5.8b |
| HDL-cholesterol | 39.2 ± 1.8 | 36.8 ± 1.5 | 42.2 ± 1.5b,c | 36.2 ± 1.9 |
| Cholesterol/HDL-cholesterol | 4.3 ± 0.2 | 4.9 ± 0.2a | 3.6 ± 0.2a,b,c | 4.5 ± 0.3 |
| Triglycerides | 90.0 ± 6.9 | 113.1 ± 5.9a | 79.9 ± 6.0b | 91.3 ± 7.4b |
| Log fasting insulin | 11.9 ± 3.8 | 13.3 ± 5.0 | 10.4 ± 2.7a,b | 11.0 ± 3.3b |
| Log insulin sensitivity | 2.5 ± 1.3 | 2.8 ± 1.6 | 3.9 ± 2.3a,b,d | 3.3 ± 1.6b |
ANCOVA analysis adjusted for African admixture and age. ANCOVA analysis group effect significant for all variables significant at <0.01 level except for HDL-cholesterol that was significant at <0.05. ANCOVA analysis was done on log triglyceride, log insulin, and log insulin sensitivity. Back-transformed values are displayed in table. ANCOVA, analysis of covariance; HDL, high-density lipoprotein; IAAT, intra-abdominal adipose tissue; LDL, low-density lipoprotein.
Significantly different from low IAAT and low leg fat group at <0.05.
Significantly different from high IAAT and low leg fat group at <0.05.
Significantly different from high IAAT and high leg fat group at <0.05.
Significantly different from high IAAT and high leg fat group at <0.10.
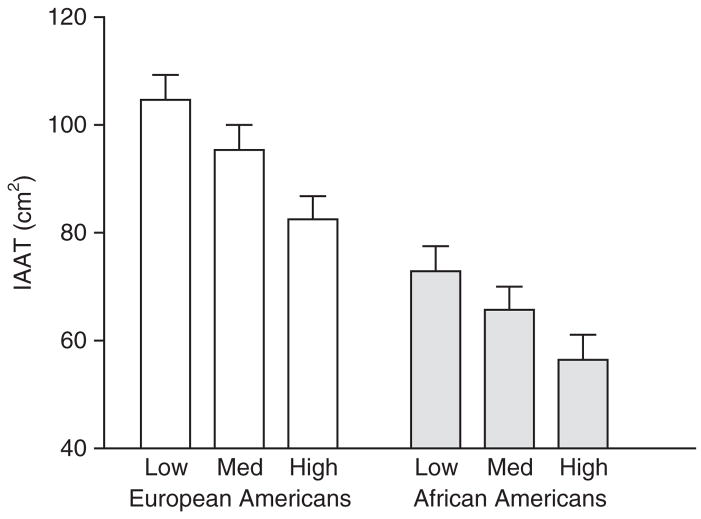
Figure 1 shows the results of the IAAT analysis of covariance by VO2max tertiles. After adjusting for % body fat, there were significant tertile group and race effects (both <0.01), but no significant tertile group by race interaction (P = 0.73). Post hoc analysis revealed that the high tertile group for VO2max had significantly lower IAAT than the low tertile group for VO2max. No other significant differences were observed.
Figure 1.
IAAT by tertiles of aerobic fitness. IAAT, intra-abdominal adipose tissue; Med, medium.
DISCUSSION
Consistent with our hypotheses, IAAT was independently and positively related to worsening of blood lipids and Si, whereas both leg fat and aerobic fitness were independently and positively related to improved blood lipids and Si. Previous studies have shown similar relationships for IAAT and leg fat (9–11,37–39). However, to our knowledge, this is the first study to show these results to be independent of age and aerobic fitness in a mixed group of AA and EA women. Although not the focus of the study, it is interesting to note that age was not related to worsening of the blood lipid profile after adjusting for fat distribution and aerobic fitness. In fact, the significant negative adjusted β for age and fasting insulin, and significant positive adjusted β for age and Si suggested less risk for type 2 diabetes with increasing age if IAAT is kept low and aerobic fitness is kept high. The women in this study were relatively homogeneous for body composition, being neither obese nor lean. In addition, none of the women were diabetic and few had an abnormal blood lipid profile. Therefore, caution must be made in applying these results to a more heterogeneous population. However, within confines of these limitations, the results suggest that both maintenance of a favorable fat distribution and aerobic fitness may be important strategies for healthy aging, at least in premenopausal AA and EA women.
In previous studies, the negative relationship of leg fat with cardiometabolic risk only occurred after adjusting for trunk fat, IAAT, or total fat (9–11). In fact, leg fat has usually been positively related to increased risk for CVD and type 2 diabetes when no adjustments were made (9,10). This was not the case in this study. Leg fat was negatively related to fasting insulin and all blood lipids, except HDL-C for which it was positively related. In addition, it was positively related to Si (Table 2). It continued to be significantly and inversely related when expressed as a ratio (leg fat-IAAT) or after adjustments in a linear regression (Table 3). Our analysis in which subjects were divided into low and high IAAT, and low and high leg fat groups further supports the potential importance of leg fat in improving blood lipid profile and Si (Table 4). The group that had relatively low IAAT and relatively high leg fat consistently had the best blood lipid profile, fasting insulin, and Si. Of particular note is that the high IAAT/high leg fat group actually had a better fasting blood lipids, Si, and blood lipid profile (with the exception of HDL-C and cholesterol-HDL ratio) than the high IAAT/low leg fat group (Table 4). Regardless of IAAT increases, increased leg fat seems to be related to improved blood lipid profile and Si in these women.
IAAT may be associated with increased risk of CVD and type 2 diabetes through its detrimental effect on metabolism in the liver. Hepatic insulin extraction is decreased when exposed to increases in IAAT-derived free fatty acids, which in turn leads to increased fasting insulin. Both risk for CVD and type 2 diabetes is thus increased. On the other hand, leg fat has been proposed to have a protective effect because it has a low rate of lipolysis (40) when compared to IAAT, and may act as a sink for storing fat, thus improving lipid profile and Si (11). A relatively new alternative hypothesis for explaining the positive relationship between IAAT, and blood lipids and Si may involve a larger production of cytokines in IAAT (41). IAAT is associated with the pro-inflammatory cytokine tumor necrosis factor-α and its receptors tumor necrosis factor-RI and tumor necrosis factor-RII, whereas subcutaneous body fat is not associated with this cytokine in premenopausal overweight women (42). In addition, tumor necrosis factor-α has been implicated with a reduction in insulin signaling (43,44), suggesting that inflammation is associated adversely with Si.
The data from the present study do not necessarily show cause and effect between leg fat, and blood lipids and Si, but do lend support for the intriguing possibility that leg fat may convey a protective effect on individuals with genetic propensity to store fat in the gluteofemoral region. Another equally plausible explanation for an apparent protective effect of leg fat involves the endocrine environment. It is possible that women who tend to deposit fat in the legs when they gain weight may have a particular hormone profile that is protective. For example, a more estrogenic environment may direct fat storage toward the legs, whereas a more androgenic environment may direct fat storage toward IAAT (45). Estrogens also have a beneficial effect on lipid profile and Si through action on lipid metabolism and glucose uptake (46–48).
The inverse association between aerobic fitness and blood lipids is highly consistent across studies (49). Low aerobic fitness is considered a predictor of CVD as well as low Si (50,51) and all-cause mortality in both men and women (52–54). We found that aerobic fitness was an independent predictor of HDL-C, cholesterol-HDL ratio, TGs, and Si (Table 3). It is important to point out that this is the first time that these relationships with aerobic fitness have been shown to be independent of fat distribution and African admixture in a mixed population of AA and EA women. Aerobic fitness has a genetic component as well as a training component, but it is impossible to separate their respective contributions to blood lipids and Si in this study.
Consistent with the results of Arsenault et al. (4), it is important to point out that women in the highest tertile for aerobic fitness had lower IAAT than women in the lowest tertile for aerobic fitness even after adjusting for percent body fat, suggesting exercise training may have a positive effect on maintaining a favorable fat distribution (Figure 1). If this is the case, aerobic fitness would possibly have both an independent effect on blood lipids and Si (as shown by the relationships discussed in the previous paragraph), and an indirect one through improvement of fat distribution.
Inclusion of AFADM as a covariate strengthened this study because we accounted for the ancestral genetic variation that underlies racial classification, and we did not limit our design to a dichotomous (AA or EA) race variable. The worsened metabolic profile found with increased IAAT and improved metabolic profile found with increased leg fat were unaffected by adjustments for AFADM. After adjustments for fat distribution, aerobic fitness, and age, AFADM was related to an improved lipid profile (positive relationship with HDL and negative relationship with cholesterol-HDL ratio and TGs) but lower Si and higher fasting insulin. Thus, aside from any genetic contribution to fat distribution and aerobic fitness, genetic differences still account for variability in cardiometabolic profile.
In conclusion, our findings suggest that IAAT is a consistent independent predictor of increased worsened blood lipid profile, fasting insulin, and Si, whereas leg fat and aerobic fitness are consistent independent predictors of improved blood lipid profile, fasting insulin, and Si in a mixed group of EA and AA premenopausal women. Age was not related to any blood lipid after adjustments were made for fat distribution and aerobic fitness. However, age was related to decreased fasting insulin and increased Si after adjusting for aerobic fitness and fat distribution in these premenopausal EA and AA women.
Acknowledgments
We thank Robert Petri, David Bryan, Amy Thomas, Paul Zuckerman, and Betty Darnell who provided invaluable technical assistance in the conduct of the study. Supported by NIH grants R01 DK 49779 and R01 DK51684, General Clinical Research Center grant M01-RR00032, Clinical Nutrition Research Unit grant P30-DK56336, and UAB University-wide Clinical Nutrition Research Center grant. Stouffer’s Lean Cuisine entrées were provided by the Nestlé Food (Solon, OH), and Smart Ones entrées were provided by H.J. Heinz Frozen Foods.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Vartiainen E, Puska P, Pekkanen J, Tuomilehto J, Jousilahti P. Changes in risk factors explain changes in mortality from ischaemic heart disease in Finland. BMJ. 1994;309:23–27. doi: 10.1136/bmj.309.6946.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ. Prevalence of overweight and weight gain in the United States. Am J Clin Nutr. 1992;55:495S–502S. doi: 10.1093/ajcn/55.2.495s. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Arsenault BJ, Lachance D, Lemieux I, et al. Visceral adipose tissue accumulation, cardiorespiratory fitness, and features of the metabolic syndrome. Arch Intern Med. 2007;167:1518–1525. doi: 10.1001/archinte.167.14.1518. [DOI] [PubMed] [Google Scholar]
- 5.Pouliot MC, Després JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 6.Després JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- 7.Peiris AN, Sothmann MS, Hoffmann RG, et al. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 8.DeNino WF, Tchernof A, Dionne IJ, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–932. doi: 10.2337/diacare.24.5.925. [DOI] [PubMed] [Google Scholar]
- 9.Hunter GR, Kekes-Szabo T, Snyder SW, et al. Fat distribution, physical activity, and cardiovascular risk factors. Med Sci Sports Exerc. 1997;29:362–369. doi: 10.1097/00005768-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 11.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab. 2005;90:4573–4578. doi: 10.1210/jc.2004-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young DR, Steinhardt MA. The importance of physical fitness versus physical activity for coronary artery disease risk factors: a cross-sectional analysis. Res Q Exerc Sport. 1993;64:377–384. doi: 10.1080/02701367.1993.10607590. [DOI] [PubMed] [Google Scholar]
- 13.McMurray RG, Ainsworth BE, Harrell JS, Griggs TR, Williams OD. Is physical activity or aerobic power more influential on reducing cardiovascular disease risk factors? Med Sci Sports Exerc. 1998;30:1521–1529. doi: 10.1097/00005768-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Duncan JJ, Gordon NF, Scott CB. Women walking for health and fitness. How much is enough? JAMA. 1991;266:3295–3299. [PubMed] [Google Scholar]
- 15.Bouchard C, Perusse L. Heredity, activity level, fitness, and health. In: Bouchard C, Shephard RJ, Stephens T, editors. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement. Human Kinetics; Champaign, IL: 1994. pp. 106–118. [Google Scholar]
- 16.Wong SL, Katzmarzyk P, Nichaman MZ, et al. Cardiorespiratory fitness is associated with lower abdominal fat independent of body mass index. Med Sci Sports Exerc. 2004;36:286–291. doi: 10.1249/01.MSS.0000113665.40775.35. [DOI] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Gagnon J, Leon AS, et al. Fitness, fatness, and estimated coronary heart disease risk: the HERITAGE Family Study. Med Sci Sports Exerc. 2001;33:585–590. doi: 10.1097/00005768-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60:695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. Effects of diet- and exercise-induced weight loss on visceral adipose tissue in men and women. Sports Med. 1997;24:55–64. doi: 10.2165/00007256-199724010-00005. [DOI] [PubMed] [Google Scholar]
- 20.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes. 1997;46:456–462. doi: 10.2337/diab.46.3.456. [DOI] [PubMed] [Google Scholar]
- 21.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan SR, Wattigney W, Webber LS, Berenson GS. Race and gender differences in serum lipoproteins of children, adolescents, and young adults—emergence of an adverse lipoprotein pattern in white males: the Bogalusa Heart Study. Prev Med. 1991;20:671–684. doi: 10.1016/0091-7435(91)90063-a. [DOI] [PubMed] [Google Scholar]
- 23.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 24.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 25.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 26.López-Alarcón M, Hunter GR, Gower BA, Fernández JR. IGF-I polymorphism is associated with lean mass, exercise economy, and exercise performance among premenopausal women. Arch Med Res. 2007;38:56–63. doi: 10.1016/j.arcmed.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–1361. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 28.Hellerstein HK, Franklin BA. Exercise testing and prescription. In: Wenger NK, Hellerstein HK, editors. Rehabilitation of the Coronary Patient. Wiley; New York: 1984. pp. 197–284. [Google Scholar]
- 29.Holly RG. Measurement of maximal rate of oxygen uptake. In: Blair SN, Painter P, Pate RR, Smith LK, Taylor CB, editors. Resource Manual for Guidelines for Exercise Testing and Prescription. Lea & Febiger; Philadelphia, PA: 1988. pp. 171–177. [Google Scholar]
- 30.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 32.Yang YJ, Youn JH, Bergman RN. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol. 1987;253:E595–E602. doi: 10.1152/ajpendo.1987.253.6.E595. [DOI] [PubMed] [Google Scholar]
- 33.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 34.Myakishev MV, Khripin Y, Hu S, Hamer DH. High-throughput SNP genotyping by allele-specific PCR with universal energy-transfer-labeled primers. Genome Res. 2001;11:163–169. doi: 10.1101/gr.157901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeigue PM. Multipoint admixture mapping. Genet Epidemiol. 2000;19:464–467. doi: 10.1002/1098-2272(200012)19:4<464::AID-GEPI17>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 37.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40:733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 38.Sjöström CD, Håkangård AC, Lissner L, Sjöström L. Body compartment and subcutaneous adipose tissue distribution—risk factor patterns in obese subjects. Obes Res. 1995;3:9–22. doi: 10.1002/j.1550-8528.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 39.Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24:923–929. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
- 40.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 42.Hyatt T. Ethnic Differences in Markers of Inflammation with Weight Loss. University of Alabama at Birmingham; 2007. Thesis/Dissertation. [Google Scholar]
- 43.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 44.Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 45.Elbers JM, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol. 1999;276(2 Pt 1):E317–E325. doi: 10.1152/ajpendo.1999.276.2.E317. [DOI] [PubMed] [Google Scholar]
- 46.Walsh BW, Schiff I, Rosner B, et al. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Engl J Med. 1991;325:1196–1204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer EJ, Foster DM, Zech LA, et al. The effects of estrogen administration on plasma lipoprotein metabolism in premenopausal females. J Clin Endocrinol Metab. 1983;57:262–267. doi: 10.1210/jcem-57-2-262. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai S, Holmäng A, Björntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–97. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 49.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6 Suppl):S379–S399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 50.Nyholm B, Mengel A, Nielsen S, et al. Insulin resistance in relatives of NIDDM patients: the role of physical fitness and muscle metabolism. Diabetologia. 1996;39:813–822. doi: 10.1007/s001250050515. [DOI] [PubMed] [Google Scholar]
- 51.Rosenthal M, Haskell WL, Solomon R, Widstrom A, Reaven GM. Demonstration of a relationship between level of physical training and insulin-stimulated glucose utilization in normal humans. Diabetes. 1983;32:408–411. doi: 10.2337/diab.32.5.408. [DOI] [PubMed] [Google Scholar]
- 52.Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 53.Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10:417–423. doi: 10.1038/oby.2002.58. [DOI] [PubMed] [Google Scholar]
- 54.Blair SN, Kampert JB, Kohl HW, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]