Abstract
The opioid system modulates ethanol intake and reinforcement in adult and preweanling rodents. While adult heterogeneous rats normally do not show ethanol-mediated locomotor stimulation, preweanling rats show it quite clearly. We recently observed that naloxone, a non-specific opioid antagonist, attenuated ethanol-induced locomotor activation in preweanling rats. In the present study we tested the role of specific opioid receptors (mu, delta and kappa) in ethanol-mediated locomotor stimulation and ethanol intake. In Experiment 1 13-day-old rats received naloxonazine (mu antagonist: 0, 7.5 or 15 mg/kg), naltrindole (delta antagonist: 0, 2 or 4 mg/kg) or nor-binaltorphimine (kappa antagonist: 0, 2, 4 or 8 mg/kg) before an intragastric administration of ethanol (0 or 2.5 g/kg), and subsequent locomotor activity assessment. In Experiment 2, the same opioid antagonists were administered on postnatal days 13 and 14 before consumption of ethanol (6%), saccharin (0.05%) or distilled water. In Experiment 1 only naloxonazine reduced ethanol-mediated locomotor stimulation. None of the opioid antagonists affected locomotor activity in water controls. In Experiment 2 naloxonazine and naltrindole suppressed ingestion of all the solutions tested. Similar to what has been reported in adult rodents, mu-opioid receptors seem to modulate ethanol-activating effects during early ontogeny. Hence, there seems to be a partial overlap of neurochemical mechanisms involved in the rewarding and stimulating effects of ethanol in preweanling rats. Mu receptor antagonists reduced both ethanol-induced activity and ethanol intake, but it is unclear whether the latter effect is specific to ethanol or only a reflection of an effect on consummatory behavior generally, since mu and delta receptor antagonists also suppressed ingestion of water and sacccharin.
Clinical and preclinical studies indicate that early prenatal or postnatal ethanol experience predicts later responsiveness to the drug (for reviews, see Spear & Molina, 2005; Chotro, Arias & Laviola, 2007). This association emphasizes the importance of understanding the neurobiological mechanisms that regulate ethanol effects early in development, especially considering the growing number of studies showing a particular sensitivity to ethanol’s motivational and motor effects during the infantile period of the rat (see Chotro et al., 2007; Spear & Molina, 2005). By the end of the gestational period (Chotro & Arias, 2003) or soon after birth (Nizhnikov, Varlinskaya, Petrov & Spear, 2006), rats are highly sensitive to the reinforcing effects of ethanol. This sensitivity seems to extend to the first (Arias & Chotro, 2006) or second postnatal week of life (Molina, Pautassi, Truxell & Spear, 2007), during which even relatively high ethanol doses (1–2.5 g/kg) can promote rapid appetitive learning. This sensitivity to ethanol reinforcement is accompanied by a predisposition to consume relatively high amounts of ethanol, with no initiation procedures required (Sanders & Spear, 2007). High acceptance of ethanol early in ontogeny seems to be associated with the pharmacological effects of ethanol rather than its orosensory properties (Kozlov, Varlinskaya & Spear, 2008; Spear & Molina, 2005).
We have recently observed that preweanling rats are also sensitive to locomotor activating effects of ethanol (Arias, Mlewski, Molina, & Spear, 2009a, 2009b; Arias, Molina, Mlewski, Pautassi, & Spear, 2008; Arias, Mlewski, Miller, Molina & Spear, 2009). The stimulating effect of ethanol is observed with relatively high ethanol doses (1.25 or 2.5 g/kg) during the rising phase of the blood ethanol curve (Arias et al., 2008; Arias et al., 2009). When blood ethanol concentration reaches peak values, ethanol suppresses locomotor activity (Arias et al., 2008). This biphasic effect of ethanol is likely to be associated with the biphasic (appetitive and aversive) reinforcing effects induced by relatively high ethanol doses in preweanling rats (Molina et al., 2007), since both follow a similar time course. During the rising phase of the blood ethanol curve 2 g/kg of ethanol induced appetitive learning, but the same dose induced aversion learning when blood ethanol concentration reached peak values. Hence the understanding of mechanisms regulating ethanol mediated locomotor stimulation in preweanling rats may help to understand the neurobiological basis of ethanol-mediated reinforcement.
Similar to adult rodents (Gianoulakis, 2004), ethanol reinforcement and acceptance in preweanling rats seem to be regulated, at least partly, by the opioid system. For example, non-specific opioid antagonists (such as naloxone or naltrexone) can prevent conditioned preferences induced by ethanol during the last days of gestation (Arias & Chotro, 2005; Chotro & Arias, 2003) or by the end of the first postnatal week (Chotro & Arias, 2007). In newborns mu and kappa receptors modulate ethanol-mediated reinforcement (Nizhnikov et al., 2006). Ethanol intake can also be reduced by non-specific or specific (mu or delta) opioid antagonists during the preweanling period (Chotro & Arias, 2003; Hallmark & Hunt, 2004). In addition, naloxone suppresses locomotor activating effects of ethanol in preweanling rats (Arias et al., 2009b).
The present study focused on two main goals. First, we aimed to test the involvement of specific opioid receptors (mu, kappa or delta) in ethanol-mediated locomotor stimulation in infant rats. Recent findings from our laboratory showed that ethanol-induced conditioned place preference in preweanling rats can be reduced by means of delta or mu antagonists (Nizhnikov, Pautassi, Truxell & Spear, 2009). The present experiments may also help to determine the potential relationship between the neurochemical mechanisms supporting ethanol-induced reinforcement and locomotor stimulation in preweanling rats. In adult mice ethanol-mediated locomotor activation seems to be mediated specifically by mu receptors (Pastor & Aragon, 2006; Pastor, Sanchis-Segura & Aragon, 2005). In infant rats naloxone reduced ethanol-mediated stimulation (Arias et al., 2009b), but the participation of the primary subtypes of opioid receptors in this particular effect has not been tested at this early stage of development. Early in ontogeny mu, delta and kappa receptors follow different patterns of ontogenetic development, but all are functional by the second postnatal week of life (Volterra, Brunello, Restani, Galli & Racagni, 1986).
In the absence of ethanol, kappa opioid receptors seem to regulate particular behaviors in a qualitatively different way during infancy and in adulthood. In adult rats U-50,488 (a specific kappa receptor agonist) attenuates locomotor activity (Ukai & Kameyama, 1985) but this same kappa agonist markedly increases locomotor activity in infant rats (Duke, Meier, Bolanos, Crawford & McDougall, 1997). In adult rats ethanol-induced locomotor activation has rarely been reported, especially with high ethanol doses (Correa, Arizzi, Betz, Mingote & Salamone, 2003; Chuck, McLaughlin, Arizzi-LaFrance, Salamone & Correa, 2006), and yet WIN 44441-3, a kappa antagonist, potentiates ethanol’s activating effects at the adult stage of development (Pohorecky, Patel & Roberts, 1989). Hence, it is plausible that the paradoxical role of kappa receptors in infant rats in locomotor activity may help to explain why preweanling rats, unlike adults, are highly sensitive to ethanol’s activating effects.
The second goal of the present study was to analyze the role of specific opioid receptors in ethanol intake in preweanling rats. In adult rats ethanol intake can be suppressed by means of non-specific (Cichelli & Lewis, 2002; Higley & Kiefer, 2006; Kiefer, Hill, Coonfield & Ferraro, 2005; Sable, Bell, Rodd & McBride, 2006) or delta (Higley & Kiefer, 2006; Nielsen, Simms, Pierson, Li, Saini, Ananthan & Bartlett, 2008) opioid receptor antagonists. However, opioid antagonists also reduce adult ingestion of other solutions such as water, saccharin or sucrose (Cichelli & Lewis, 2002). Non-specific opioid antagonists, as well as delta or mu receptor antagonists, also reduce ethanol consumption in preweanling rats (Chotro & Arias, 2003; Hallmark & Hunt, 2004). The present study tested whether the specific opioid receptors that regulate the reinforcing effects of ethanol in preweanling rats (Nizhnikov et al., 2009), also affect regulation of their ethanol consumption. Ethanol intake is controlled by a variety of factors (Samson & Czachowski, 2003), but the high intake of ethanol early in ontogeny seems to be at least partially controlled by its pharmacological effects (Kozlov et al., 2008; Spear & Molina, 2005). Additionally the present study was intended to determine whether the specific opioid antagonists affect only the preweanling rat’s consumption of ethanol, or if, like adults, they affect consummatory behavior more generally.
Material and Methods
Subjects
Five-hundred and forty two Sprague-Dawley pups, representative of 89 litters, were utilized for in the present study (Experiment 1a, n= 92; Experiment 1b, n= 78; Experiment 1c, n= 127; Experiment 2a, n= 78; Experiment 2b, n= 84; Experiment 2c, n= 84). Two-hundred and seventy eight were males, while 264 were females. Animals were born and reared at the vivarium of the Center for Developmental Psychobiology (Binghamton University, NY) under conditions of constant room temperature (22 ± 1.0 °C), on a 12-hour light 12-hour dark cycle. Births were examined daily and the day of parturition was considered as postnatal day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males, whenever possible) within 48 hours after birth. All procedures were in accordance with the guidelines for animal care and use established by the National Institute of Health (1986) and the Guide for Care and Use of Laboratory Animals (1996) as indicated by the Binghamton University’s institutional animal care and use committee.
Procedures
Experiment 1: locomotor activity test
On PD 12, the day before testing, pups were separated from the mother and placed in pairs in a holding maternity cage (45 × 20 × 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 35° C (±1° C) through the use of a heating pad. One hour later subjects were placed for 8 minutes in the testing chamber to habituate them to the experimental procedure. In a recent study we showed that longer preexposure to the testing environment may interfere with the expression of ethanol-induced locomotor activation in preweanling rats (Arias, Mlewski, et al., 2009).
On PD 13 subjects were separated from their mothers and kept under similar environmental conditions as the day before. One hour later body weights were individually recorded (± 0.01 g) and pups received an intra peritoneal administration of a specific mu (naloxonazine: 0, 7.5 or 15 mg/kg, Experiment 1a), delta (naltrindole: 0, 2 or 4 mg/kg, Experiment 1b) or kappa (nor -binaltorphimine: 0, 2, 4 or 8 mg/kg, Experiment 1c) antagonist. In all cases, volume injected in each pup was 1.0% of their body weight. Vehicle for naltrindole and nor-binaltorphimine was an isotonic saline solution, while Naloxonazine was dissolved in saline containing acetic acid (0.2 %). Dosage of these opioid antagonists was selected on the basis of prior studies in which these drugs were employed with results relevant to the goals of the present study. Similar doses of naloxonazine were effective in reducing ethanol-mediated locomotor activation in mice (Pastor et al., 2005). Naltrindole (between 1 and 5 mg/kg) has been found to reduce ethanol-induced conditioned place preference (CPP) in preweanling rats (Nizhnikov et al., 2009). Finally, nor-binaltorphimine (between 2 and 12 mg/kg) reduced locomotor stimulation induced by kappa agonists in preweanling rats (Collins, Zavala, Nazarian & McDougall, 2000).
After receiving the injection, pups were placed again in pairs in the holding chambe r. Thirty minutes after administration of the opioid antagonists pups received an intragastric (i.g.) administration of 0 or 2.5 g/kg ethanol (volume administered was equivalent to 0.015 ml per gram of body weight of a 21% ethanol solution). Pups assigned to the control condition (0 g/kg) received only vehicle (water). Intragastric administrations were performed using a 10-cm length of polyethylene tubing (PE-10Clay Adams, Parsippany, New Jersey) attached to a 1 ml syringe with a 27 G × 1/2 needle. This tubing was gently introduced through the mouth and slowly pushed into the stomach. The entire procedure took less than 20 seconds per pup. Five minutes after ethanol administration locomotor activity was evaluated for 8 minutes.
The testing environment consisted in a Plexiglas open field (42 × 42 × 30 cms; VersaMax Animal Activity Monitoring System, Accuscan Instruments, Columbus, OH, USA). In this apparatus, locomotion was detected by interruption of eight pairs of intersecting photocell beams evenly spaced along the walls of the testing environment. This equipment was situated in sound-attenuating box chambers (53 × 58 × 43 cms) equipped with a light and fan for ventilation and background noise. Consecutive photocell beam interruptions were translated to distance traveled in cm by the VersaMax. This dependent variable takes into account the path the animal takes, and is an accurate indicator of ambulatory activity. Immediately after the locomotor activity test pups were returned to their homecage.
Experiment 2: intake test
On PD 13 and 14 pups were separated from their mother and placed in holding chambers, the same environmental conditions described in Experiment 1. All pups were intraorally cannulated using a procedure described in previous studies (Chotro & Arias, 2007; Hall & Rosenblatt, 1977). Cannulae were made from 5-cm sections of polyethylene tubing (PE 10, i.d. = 0.28 mm) by heating one end of the section to form a small flange. A thin wire attached to the non-flanged end of the cannula was placed on the internal surface of the pup’s cheek and the wire was then pushed through the oral muccosae until the flanged end of the cannula was positioned over the internal surface of the cheek while the remainder of the cannula exits from the oral cavity. The entire procedure took less than 5 sec per pup. These cannulae were later used to infuse the solutions during the intake test.
Three hours after cannulation, pups were quasi-randomly assigned (with the constraint that no more than one pup of a given sex from the same litter be assigned to the same condition) to the independent conditions defined by the solution infused (water, saccharin or ethanol) and the drug administered (Experiment 2a: 0 or 7.5 mg/kg naloxonazine; Experiment 2b: 0 or 4 mg/kg naltrindole Experiment 2c: 0 or 4 mg/kg nor-binaltorphimine). Naloxonazine dose (7.5 mg/kg) was selected because it was effective in Experiment 1a in reducing ethanol-mediated locomotor activation. In addition, similar naloxonazine doses also have been found to reduce ethanol-mediated locomotor stimulation and sensitization in adult rodents (Pastor et al., 2005). Naltrindole (10 mg/kg) reduced ethanol intake in preweanling rats (Hallmark & Hunt, 2004), but lower doses (between 2 and 4 mg/kg) were enough to reduce ethanol-induced CPP in preweanling rats (Niznikov et al., 2009). Similar nor-binaltorphimine doses modulate behavior activation induced by kappa agonists in preweanling rats (Collins et al., 2000).
Thirty minutes after drug treatment, pups’ bladders were voided by gentle brushing of the anogenital area. Body weights were then registered and subjects placed into heated individual chambers, where they received the intraoral infusion of ethanol (6% v/v; Experiment 2a), saccharin (0.05% w/v; Experiment 2b) or water (Experiment 2c). The intraoral administration was performed using a 10-syringe infusion pump (KD Scientific) connected to the oral cannula of each pup by a polyethylene catheter (Clay Adams, PE 50). The volume administered to each subject was equivalent to 5.5% of their body weight and was infused at a constant rate for 15 min. With these parameters pups are able to either consume or reject the infused solution. At the end of the session post-infusion weights were registered. Body weight gained after each conditioning trial was calculated, and fluid intake was estimated through the percentage of body weight gained during the test. Thirty minutes after the intake test all subjects were returned to their maternity cages. The intake test (including the treatment with the opioid antagonists) was conducted on two consecutive days because under similar experimental circumstances it has been observed that two consecutive days of testing are sensitive to effects on ethanol intake at this age (for example, Pueta, Abate, Spear & Molina, 2005, Arias & Chotro, 2006).
Number of subjects included in each experimental condition in Experiment 1 and 2 are sumarized in Tables 1 and 2 respectively.
Table 1.
Number of subjects included in each experimental condition in Experiment 1a, 1b and 1c as a function of the different opioid antagonist treatments and ethanol administration (0 or 2.5 g/kg).
| Opioid antagonist treatment | Intragastric Water (0 g/kg) | Intragastric Ethanol (2.5 g/kg) | |
|---|---|---|---|
| Exp 1a | Naloxonazine 0 mg/kg | 16 (9 male, 7 female) | 15 (8 male, 7 females) |
| Naloxonazine 7.5 mg/kg | 16 (8 male, 8 female) | 15 (8 male, 7 females) | |
| Naloxonazine 15 mg/kg | 15 (9 male, 6 female) | 15 (7 male, 8 females) | |
| Exp 1b | Naltrindole 0 mg/kg | 13 (9 male, 4 female) | 13 (6 male, 7 female) |
| Naltrindole 2 mg/kg | 12 (5 male, 7 female) | 14 (8 male, 6 female) | |
| Naltrindole 4 mg/kg | 13 (7 male, 6 female) | 13 (6 male, 7 female) | |
| Exp 1c | Nor-binaltorphimine 0 mg/kg | 16 (9 male, 7 female) | 16 (5 male, 11 female) |
| Nor-binaltorphimine 2 mg/kg | 16 (10 male, 6 female) | 16 (8 male, 8 female) | |
| Nor-binaltorphimine 4 mg/kg | 15 (11 male, 4 female) | 16 (4 male, 12 female) | |
| Nor-binaltorphimine 8 mg/kg | 16 (9 male, 7 female) | 16 (7 male, 9 females) | |
Table 2.
Number of subjects included in each experimental condition in Experiment 2a, 2b and 2c as a function of the different opioid antagonist treatments and ethanol administration (0 or 2.5 g/kg).
| Solution | Water | Saccharine | Ethanol | |
|---|---|---|---|---|
| Exp 2a | Naloxonazine 0 mg/kg | 14 (7 male, 7 female) | 12 (6 male, 6 females) | 13 (8 male, 5 female) |
| Naloxonazine 7.5 mg/kg | 12 (5 male, 7 female) | 14 (6 male, 8 females) | 13 (7 male, 6 female) | |
| Exp 2b | Naltrindole 0 mg/kg | 14 (7 male, 7 female) | 14 (7 male, 7 female) | 14 (7 male, 7 female) |
| Naltrindole 4 mg/kg | 14 (7 male, 7 female) | 14 (7 male, 7 female) | 14 (7 male, 7 female) | |
| Exp 2c | Nor- binaltorphimine 0 mg/kg | 14 (7 male, 7 female) | 14 (6 male, 8 female) | 14 (7 male, 7 female) |
| Nor- binaltorphimine 4 mg/kg | 14 (8 male, 6 female) | 14 (7 male, 7 female) | 14 (7 male, 7 female) | |
Data analysis
Data from Experiments 1a, 1b and 1c were analyzed by means of between-factor ANOVAs, in which the factors were sex (male or female), ethanol treatment (0 or 2.5 g/kg) and opioid antagonist dosage (naloxonazine: 0, 7.5 or 15 mg/kg, for Experiment 1a; naltrindole: 0, 2 or 4 mg/kg, for Experiment 1b; and nor-binaltorphimine: 0, 2, 4 or 8 mg/kg, for Experiment 1c). The dependent variable was distance traveled in centimeters.
Intake scores (Experiment 2a, 2b and 2c) were analyzed by means of mixed ANOVAs, including sex (male or female), solution (ethanol, saccharin or water) and opioid antagonist (naloxonazine: 0 or 7.5mg/kg, for Experiment 2a; naltrindole: 0 or 4 mg/kg, for Experiment 2b; and nor-binaltorphimine: 0 or 4 mg/kg, for Experiment 2c). as between-group variables, and day (PD 13 and PD 14) as a within-group variable. The dependent variable analyzed was consumption of each solution (ethanol 6% v/v, saccharin 0.05 % w/v or water).
Significant main effects and/or interactions were further analyzed by means of follow-up ANOVAs and post-hoc analysis (Newman-Keuls post-hoc tests). All inferential analyses conducted in the present study employed an α level of 0.05.
Results
Experiment 1
Experiment 1a
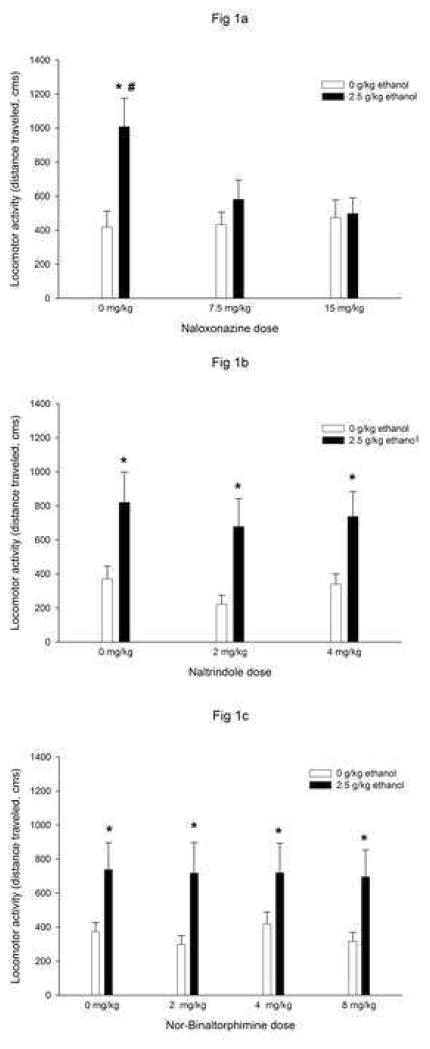
Figure 1a depicts locomotor activity as a function of ethanol and Naloxonazine treatments. The ANOVA indicated a significant main effect of ethanol treatment [F(1,80) = 7.97, p < 0.01]. The interaction between ethanol treatment and naloxonazine also reached statistical significance [F(2,80) = 3.56, p < 0.05]. Post-hoc analyses revealed that pups from groups given ethanol and saline moved significantly more than their respective water-control groups (group water and saline). Naloxonazine significantly attenuated this stimulatory effect of ethanol. Subjects given ethanol and naloxonazine (7.5 or 15 mg/kg) did not differ from their respective water-control groups. In addition, preweanlings administered ethanol and saline also moved more than those given ethanol and naloxonazine (7.5 or 15 mg/kg). Sex did not exert a significant effect or interact with the remaining factors in this experiment.
Figure 1.
Locomotor activity in preweanling rats as a function of ethanol (0 or 2.5 g/kg) and naloxonazine (0, 7.5 or 15 mg/kg, Figure 1a), naltrindole (0, 2 or 4 mg/kg, Figure 1b) or nor-binaltorphimine (0, 2, 4 or 8 mg/kg, Figure 1c) treatments. Vertical lines illustrate standard errors of the means. * Significant difference between ethanol treated group and the corresponding water control. # Significant difference between ethanol- vehicle treated group and ethanol-Naloxonazine treated group.
Experiment 1b
In Experiment 1b, pups given ethanol locomoted over greater distance than those given water. This ethanol-induced activity was not affected by the delta antagonist naltrindole (Figure 1b). The ANOVA revealed only a significant main effect of ethanol treatment [F(1,66) = 17.54, p < 0.005], indicating higher activity scores in pups given ethanol than in those given water. No main effect or interactions involving gender were observed in this experiment.
Experiment 1c
In Experiment 1c, ethanol also induced locomotor activation. This effect seems not to be modulated by kappa receptors since nor-binaltorphimine didn’t modulate locomotor stimulation induced by ethanol (Figure 1c). The ANOVA revealed only a significant main effect of ethanol treatment [F(1,111) = 12.92, p < 0.005], indicating more locomotor activity in subjects treated with ethanol than in water controls. Sex did not exert a significant effect and did not interact with the remaining variables under analysis.
Experiment 2
Experiment 2a
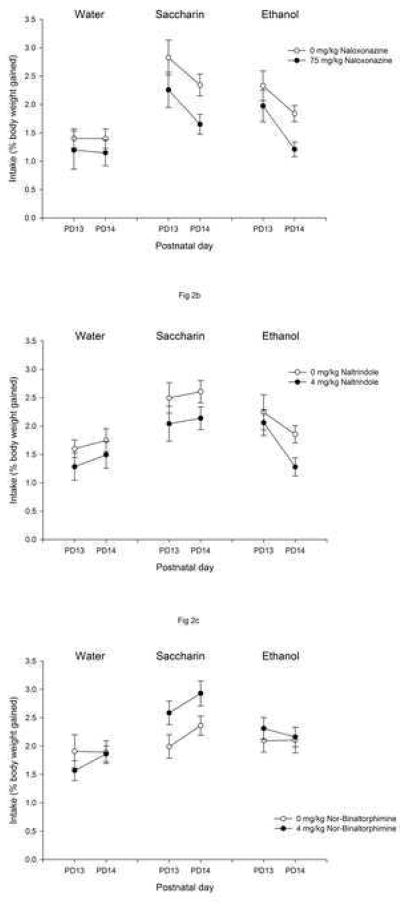
Figure 2a depicts ethanol, saccharin and water consumption scores across testing days as a function of naloxonazine treatment. The ANOVA revealed significant main effects of naloxonazine [F(1,65) = 8.26, p < 0.005], solution [F(2,65) = 14.24, p < 0.001] and day [F(1,65) = 12.65, p < 0.001]. According to the corresponding post-hoc analyses, subjects consumed more saccharin than ethanol or water, and more ethanol than water. Regardless of the solution infused, intake was higher during the first than the second testing day. Finally, naloxonazine significantly reduced consumption overall. Sex did not exert a significant main effect or enter into any interactions.
Figure 2.
Water, saccharin or ethanol intake on postnatal days 13 and 14 as a function of ethanol naloxonazine (0 or 7.5 mg/kg, Figure 2a), naltrindole (0 or 4 mg/kg, Figure 2b) or nor-binaltorphimine (0 or 4 mg/kg, Figure 2c) treatments. Vertical lines illustrate standard errors of the means. In Experiment 2a naloxonazine significantly reduced consumption overall (water, saccharin and ethanol). In Experiment 2b naltrindole suppressed intake regardless the solution. In Experiment 3 no significant effects of nor-binaltorphimine were detected.
Experiment 2b
Figure 2b represents ethanol, saccharin and water intake on PD13 and PD14 as a function of naltrindole administration. The ANOVA revealed significant effects of these main factors: sex [F(1,72) = 6.78, p < 0.05], with males drinking less than females; naltrindole [F(1,111) = 12.92, p < 0.005], indicating that the delta receptor antagonist suppressed intake regardless the solution (no significant interaction between drug and solution); and solution [F(1,72) = 5.94, p < 0.005]. The interaction between solution and day also reached statistical significance [F(1,111) = 12.92, p < 0.005]. Post-hoc analyses indicated that on PD13 pups consumed less water than ethanol or saccharin. On PD 14, saccharin intake was significantly higher than water or ethanol consumption.
Experiment 2c
The overall ANOVA indicated only a significant main effect of solution [F(2,77) = 8.22, p < 0.005]. The corresponding post-hoc analyses revealed that ethanol and saccharin consumption were higher than that of water. In Figure 2c it seems that subjects given nor-binaltorphimine ingested more saccharin than those given saline, an effect that was not observed in the remaining solutions, yet the interaction between nor-binaltorphimine and solution did not quite attain significance [F(2,77) = 2.79, p = 0.06]. No gender effect or interactions involving this factor were observed in the present experiment.
Conclusion
According to the present study, ethanol-mediated locomotor stimulation in infant rats seems to be mediated by mu-opioid receptors: naloxonazine, an antagonist specific to these receptors, reduced the stimulating effects of ethanol (Experiment 1a). Kappa and Delta receptor antagonists (nor-binaltorphimine and naltrindole, respectively) seem not to participate in this ethanol effect (Experiment 1b and 1c). In Experiment 2 we showed that both mu and delta receptors reduced consumption of ethanol. These opioid antagonists also suppressed intake of a sweet solution (saccharin) and water (Experiments 2a and 2b). The Kappa antagonist nor-binaltorphimine did not affect ethanol intake (Experiment 1c).
Ethanol-mediated locomotor activation seems to be partially mediated by a mechanism involving the ventral tegmental area (VTA). According to this hypothesis, ethanol-induced dopamine release is partially modulated by mu opioid and GABA B receptors at the VTA (Boehm, Piercy, Bergstrom & Phillips, 2002; Gianoulakis, 2004). The GABAergic neurons in the VTA are under tonic opioidergic inhibition (Gianoulakis, 2004). When B-endorphin is released by ethanol and stimulates mu receptors, the result is inhibition of these GABA neurons and consequential increase in dopaminergic activity (Gianoulakis, 2004). This hypothesis is supported by pharmacological studies. Non-specific opioid antagonists (Pastor et al., 2005), mu antagonists (Pastor et al., 2005) or GABA B agonists (Chester & Cunningham, 1999) reduce ethanol-mediated locomotor activation in mice. Baclofen (a GABA B receptor agonist) also reduces ethanol-mediated locomotor stimulation in adult rats genetically selected for increased ethanol consumption (Quintanilla, Perez & Tampier, 2008) or in infant heterogeneous rats (Arias et al., 2009b). Naloxone also attenuates the stimulating effects induced by ethanol in preweanling rats (Arias et al., 2009b). The present study provides new evidence for a specific contribution of mu-opioid receptors in ethanol-mediated locomotor stimulation in the preweanling rat.
In Experiment 1b naltrindole did not attenuate ethanol-mediated locomotor activation in infant rats. This suggests that delta opioid receptors may not be involved in the present case of ethanol-induced activation. It is possible that larger naltrindole doses would exert an effect upon ethanol-mediated stimulation. But at least we can conclude that within the range of naltrindole doses that are effective in reducing CPP mediated by ethanol (Nizhnikov et al., 2009) or in reducing ethanol consumption (Experiment 2b) at the present preweanling age, this delta-receptor antagonist did not reduce ethanol-mediated locomotor activation. Results from Experiment 1b are also in accordance with studies conducted with alternative animal models. For example, naltrindole failed to suppress ethanol-mediated locomotor stimulation in mice (Pastor et al., 2005), although it can modulate CPP induced by ethanol (e.g. Matsuzawa, Suzuki, Misawa & Nagase, 1998).
In preweanling rats kappa opioid receptors regulate motor activity in a qualitatively different way than in adults. In preweanling rats kappa agonists stimulate locomotion (Duke et al., 1997), while in adults, kappa agonists induce motor suppression (Ukai & Kameyama, 1985). In adults, administration of kappa agonists reduces ethanol-induced dopamine release and generates aversive states (Gianoulakis, 2004). In contrast, kappa antagonists have been reported to potentiate ethanol’s activating effects in adults (Pohorecky et al., 1989). In the present study we tested whether ethanol-mediated stimulation in preweanling rats was mediated by kappa receptors, but an antagonist for these receptors, nor-binaltorphimine (Experiment 1c), failed to reduce ethanol-mediated stimulation. Hence, it seems that activation of Kappa opioid receptors is not necessary for ethanol’s stimulating effects in rats near athe end of their second postnatal week. This conclusion has to be carefully considered since it has been reported that in adult rats, nor-binaltorphimine is not selective for kappa antagonism over mu opioid antagonism for 24 hours or more after administration (Metcalf & Coop, 2005), although some studies have shown selective effects on kappa receptors within an hour of the antagonist’s administration in newborn (Petrov, Varlinskaya & Smotherman, 2000) or adult animals (Campbell, Taylor & Tizabi, 2007). The lack of effect of the kappa antagonists on locomotion and intake tests in the present study may be due to the fact that nor-binaltorphimine was administered 30 minutes before testing, a postadministration time that might be too soon after administration for an effect upon mu and kappa receptors. We predicted that blocking kappa receptors would increase ethanol intake, while blocking mu receptors would reduce ethanol intake. If the kappa antagonist was indeed nonselective, because the test was given too soon after its administration, it would not be surprising to find no change in intake scores. In Experiment 1a ethanol-induced stimulation was reduced by naloxonazine. If nor-binaltorphimine in Experiment 1c were acting upon mu and kappa receptors, this kappa receptor antagonist could be expected to enhance ethanol-mediated locomotor activation in preweanling rats, similar to what has been observed in adult rodents (Pohorecky et al., 1989). The lack of effect of nor-binaltorphimine may be due to competing antagonism action of kappa receptors and mu receptors. But at least we can conclude that the stimulating effect of relatively high ethanol doses, consistently observed in preweanling rats although rarely seen in adults, is not due to the paradoxical role that kappa receptors play in locomotor activity during infancy (Duke et al., 1997).
In Experiments 2a and 2b the mu antagonist Naloxonazine and the delta antagonist Naltrindole attenuated ethanol intake in preweanling rats. Yet this effect was not specific to ethanol, since these antagonists also reduced saccharin and water consumption. Hence, we can not conclude that these opioid antagonists reduced ethanol intake by blocking ethanol’s rewarding effects. Similar to what has been observed in studies conducted with adult rats, the opioid antagonists seemed to interfere with consummatory behavior in general (Cichelli & Lewis, 2002), likely by reducing motivation to consume or palatability. The opioid system seems to modulate the hedonic value of natural sensory pleasures as well as rewarding drugs (Pecina & Berridge, 2005; Pecina, Smith & Berridge, 2006). Particularly, mu receptors in nucleus accumbens and ventral pallidum participate in the incentive motivation of various rewards, such as food or drugs of abuse (Kelley, Bakshi, Haber, Steininger, Will & Zhang, 2002; Pecina et al., 2006; Smith & Berridge, 2007).
The present results are consistent with those of studies conducted with adult rodents. For example, it has been reported that mu-receptor antagonists reduce ingestion of ethanol, sucrose or laboratory chow in adult rats (Leventhal, Kirkham, Cole & Bodnar, 1995; Mhatre & Holloway, 2003; Stromberg, Casale, Volpicelli, Volpicelli & O’Brien, 1998). Other authors found that Naloxone (1 mg/kg), at doses more specific for mu than other opioid receptors (Takemori & Portoghese, 1984), suppresses water, saccharin and ethanol intake in adult rats (Cichelli & Lewis, 2002). Similarly, delta receptors seem to regulate ingestion of saccharin (Beczkowska, Bowen, & Bodnar, 1992; Beczkowska, Koch, Bostock, Leibowitz, & Bodnar, 1993) and of ethanol (Higley & Kiefer, 2006; Hyytia & Kiianmaa, 2001; Nielsen et al., 2008). Our study extends these observations to the infant rat, showing that opioid mechanisms acting in adult rats to regulate consummatory responses are already present and functional early in ontogeny. Previous findings showing that antagonists of mu or delta receptors reduced ethanol intake in preweanling rats (Hallmark & Hunt, 2004) may be explained by an effect of these opioid antagonists on consummatory behavior generally rather than by blocking specific rewarding effects of ethanol.
The kappa antagonist (nor-binaltorphimine) did not affect intake of ethanol or the other solutions. In adult rats, blocking the kappa opioid system increases ethanol consumption (Mitchell, Liang & Fields, 2005), while kappa receptor agonists suppress ethanol intake (Lindholm, Werme, Brene & Franck, 2001). It can be argued that higher Nor-binaltorphimine doses may result in changes in ethanol acceptance in preweanling rats. However, such higher doses (e.g., 10 mg/kg nor-binaltorphimine) did not affect ethanol intake in 16-day-old rats (Hallmark & Hunt, 2004). Surprisingly we found that nor-binaltorphimine tended to increase saccharin intake, an unexpected result considering that kappa receptor antagonists have been found to reduce intake of sucrose (Beczkowska et al., 1992; Leventhal et al., 1995) or saccharin (Calcagnetti, Calcagnetti & Fanselow, 1990) in adult rats. Because kappa receptors can modulate some behavioral responses in a qualitatively different way in preweanling rats than in adults, further studies are required to understand the role of kappa receptors in the acceptance of palatable sweet solutions during early ontogeny.
In summary, in rats during their second postnatal week locomotor activation induced by ethanol seems to be specifically modulated by mu opioid receptors. Recent evidence from our laboratory has indicated that mu as well as delta opioid receptors are also implicated in ethanol-mediated CPP at about the same age (Nizhnikov et al. 2009). Hence, it seems that there is a partial overlap of neurochemical mechanisms involved in the rewarding and stimulating effects of ethanol in preweanling rats. Finally, delta and mu receptors regulate ethanol intake, but also help regulate consummatory behavior in general during the preweanling period, since mu and delta receptor antagonists also suppressed acceptance of water and saccharin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005;82(3):434–442. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006;120(3):710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Miller S, Molina JC, Spear NE. Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav. 2009;92(3):448–456. doi: 10.1016/j.pbb.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009a;43(1):13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and baclofen attenuate ethanol’s locomotor-activating effects in preweanling Sprague-Dawley rats. Behav Neurosci. 2009b;123(1):172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589(2):291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Koch JE, Bostock ME, Leibowitz SF, Bodnar RJ. Central opioid receptor subtype antagonists differentially reduce intake of saccharin and maltose dextrin solutions in rats. Brain Res. 1993;618(2):261–270. doi: 10.1016/0006-8993(93)91274-v. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Calcagnetti RL, Fanselow MS. Centrally administered opioid antagonists, Nor-binaltorphimineNor-binaltorphimine, 16-methyl cyprenorphine and MR2266, suppress intake of a sweet solution. Pharmacol Biochem Behav. 1990;35(1):69–73. doi: 10.1016/0091-3057(90)90206-w. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Taylor RE, Tizabi Y. Effects of selective opioid receptor antagonists on alcohol-induced and nicotine-induced antinociception. Alcohol Clin Exp Res. 2007;31(8):1435–1440. doi: 10.1111/j.1530-0277.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav. 1999;63(2):325–331. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30(1):19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behav Pharm. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neurosci Biobehav Rev. 2007;31(2):181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Cichelli MJ, Lewis MJ. Naloxone nonselective suppression of drinking of ethanol, sucrose, saccharin, and water by rats. Pharmacol Biochem Behav. 2002;72(3):699–706. doi: 10.1016/s0091-3057(02)00736-0. [DOI] [PubMed] [Google Scholar]
- Collins RL, Zavala AR, Nazarian A, McDougall SA. Kappa-Opioid receptors in the substantia nigra pars reticulata mediate the U-50,488-induced locomotor activity of preweanling rats. Dev Brain Res. 2000;119(1):97–103. doi: 10.1016/s0165-3806(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res Bull. 2003;62(3):197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Duke MA, Meier TL, Bolanos CA, Crawford CA, McDougall SA. Paradoxical effects of kappa-opioid stimulation on the locomotor activity and Fos immunoreactivity of the preweanling rat: role of dopamine receptors. Behav Neurosci. 1997;111(5):1114–1122. doi: 10.1037//0735-7044.111.5.1114. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4(1):39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Hall WG, Rosenblatt G. Suckling behavior and intake control in the developing rat pup. J Comp Physiol Psychol. 1977;91:1232–1247. [Google Scholar]
- Hallmark RA, Hunt PS. Social learning about ethanol in preweanling rats: role of endogenous opioids. Dev Psychobiol. 2004;44(2):132–139. doi: 10.1002/dev.10163. [DOI] [PubMed] [Google Scholar]
- Higley AE, Kiefer SW. Delta receptor antagonism, ethanol taste reactivity, and ethanol consumption in outbred male rats. Alcohol. 2006;40(3):143–150. doi: 10.1016/j.alcohol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and NaltrindoleNaltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25(1):25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academic Press; 1996. National Research Council. [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76(3):365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Hill KG, Coonfield DL, Ferraro FM., 3rd Ethanol familiarity and naltrexone treatment affect ethanol responses in rats. Alcohol. 2005;37(3):167–172. doi: 10.1016/j.alcohol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kozlov AP, Varlinskaya EI, Spear NE. Ethanol, saccharin, and quinine: early ontogeny of taste responsiveness and intake. Alcohol Clin Exp Res. 2008;32(2):294–305. doi: 10.1111/j.1530-0277.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- Leventhal L, Kirkham TC, Cole JL, Bodnar RJ. Selective actions of central mu and kappa opioid antagonists upon sucrose intake in sham-fed rats. Brain Res. 1995;685(1–2):205–210. doi: 10.1016/0006-8993(95)00385-4. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Werme M, Brene S, Franck J. The selective kappa-opioid receptor agonist U50,488H attenuates voluntary ethanol intake in the rat. Behav Brain Res. 2001;120(2):137–146. doi: 10.1016/s0166-4328(00)00368-5. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res. 1998;803(1–2):169–177. doi: 10.1016/s0006-8993(98)00679-9. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa opioid antagonists: past successes and future prospects. AAPS J. 2005;7(3):704–722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhatre M, Holloway F. Micro1-opioid antagonist NaloxonazineNaloxonazine alters ethanol discrimination and consumption. Alcohol. 2003;29(2):109–116. doi: 10.1016/s0741-8329(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist Nor-binaltorphimineNor-binaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182(3):384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No. 86–23) Washington, DC: Government Printing Office; 1986. [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64(11):974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43(5):347–58. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120(2):267–280. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. The role of opioid receptor subtypes in the development of behavioral sensitization to ethanol. Neuropsychopharmacology. 2006;31(7):1489–1499. doi: 10.1038/sj.npp.1300928. [DOI] [PubMed] [Google Scholar]
- Pastor R, Sanchis-Segura C, Aragon CM. Effect of selective antagonism of mu(1)-, mu(1/2)-, mu(3)-, and delta-opioid receptors on the locomotor-stimulating actions of ethanol. Drug Alcohol Depend. 2005;78(3):289–295. doi: 10.1016/j.drugalcdep.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25(50):11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12(6):500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Smotherman WP. The first suckling episode in the rat: the role of endogenous activity at mu and kappa opioid receptors. Dev Psychobio. 2000;37(3):129–143. doi: 10.1002/1098-2302(200011)37:3<129::aid-dev2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Patel V, Roberts P. Effects of ethanol in an open field apparatus: modification by U50488H and WIN 44441-3. Physiol Behav. 1989;45(2):273–287. doi: 10.1016/0031-9384(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Pueta M, Abate P, Spear NE, Molina JC. Interactions between ethanol experiences during late gestation and nursing: Effects upon infantile and maternal responsiveness to ethanol. Int J Comp Psychol. 2005;18:207–224. [Google Scholar]
- Quintanilla ME, Perez E, Tampier L. Baclofen reduces ethanol intake in high-alcohol-drinking University of Chile bibulous rats. Addict Biol. 2008;13(3–4):326–336. doi: 10.1111/j.1369-1600.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- Sable HJ, Bell RL, Rodd ZA, McBride WJ. Effects of naltrexone on the acquisition of alcohol intake in male and female periadolescent and adult alcohol-preferring (P) rats. Int J Adolesc Med Health. 2006;18(1):139–149. doi: 10.1515/ijamh.2006.18.1.139. [DOI] [PubMed] [Google Scholar]
- Samson, Czachowski Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27(7):1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29(6):909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, NaltrindoleNaltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15(4):281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. Comparative antagonism by naltrexone and naloxone of mu, kappa, and delta agonists. Eur J Pharmacol. 1984;104(1–2):101–104. doi: 10.1016/0014-2999(84)90374-1. [DOI] [PubMed] [Google Scholar]
- Ukai M, Kameyama T. Multi-dimensional analyses of behavior in mice treated with U-50,488H, a purported kappa (non-mu) opioid agonist. Brain Res. 1985;337(2):352–356. doi: 10.1016/0006-8993(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Volterra A, Brunello N, Restani P, Galli CL, Racagni G. Ontogenetic studies on mu, delta and kappa opioid receptors in rat brain. Pharmacol Res Commun. 1986;18(10):979–990. doi: 10.1016/0031-6989(86)90100-1. [DOI] [PubMed] [Google Scholar]