Abstract
Objective
The effect of urine leakage on quality of life (QOL) is related to severity of leakage. This study investigates gender and race/ethnic differences in this relationship.
Methods
An epidemiologic survey was conducted with a population-based random sample of 3,202 women and 2,301 men (1,767 Black, 1,877 Hispanic, 1,859 White) aged 30–79 years in Boston MA. Severity of leakage was based on frequency and amount by the Sandvik Severity Scale. QOL was measured with physical (PCS) and mental (MCS) component summaries of Medical Outcomes Study Short-Form-12 (SF-12). Covariates included race/ethnicity, age, socioeconomic status, urinary incontinence risk factors, and comorbidities. Analysis included multivariate linear regression modeling by gender.
Results
30% of women and 18% of men reported urine leakage, most was mild or moderate. Women (5.1%) were more likely than men (0.9%) to report severe leakage. In multivariate analysis, as severity of leakage increased, both components of QOL declined, with decreases in scores of 7–8 points for men (p≤0.001 for each) and 4–6 points (p<0.05 and p=0.001 respectively) for women. Severe leakage was associated with a greater decline in QOL than was observed for most other co-morbidities considered. The impact of urine leakage on QOL was similar among racial and ethnic groups.
Conclusions
Urine leakage impairs QOL for both men and women, with no evidence of different effects by racial/ethnic group. Leakage has clinically significant impacts on physical health related QOL for men and on mental health QOL for both men and women.
Keywords: Urinary incontinence, quality of life
Introduction
Urinary incontinence is a common problem with life-disrupting consequences. With prevalence (ranging from 10%–30% depending on study population and definition) similar to chronic diseases such as asthma and coronary artery disease 1, incontinence causes personal distress, affects daily activities and personal relationships, is costly and negatively impacts on quality of life 2. Although established as a health problem that differs for men and women 1, most studies of the impact of urine leakage have included only women 3–6. Studies including both men and women 7,8 have not investigated gender differences in the impact of leakage on quality of life (QOL) or have reported inconsistent results. Other than one study 8, the impact of urine leakage on QOL in one or more racial/ethnic groups has not been investigated. Reported race-ethnic differences in prevalence of urine leakage 9–12 warrant further investigation of differences in the impact of urine leakage on QOL in these groups.
The Boston Area Community Health (BACH) Survey is a prospective epidemiologic survey of urologic symptoms in a population-based random sample of Black, Hispanic, and White men and women age 30–79 years. Previously we have shown that prevalence of weekly urine leakage was 8% overall, 10.4% in women and 5.3% in men. White women (11.7%) were more likely than Black (9.4%) and Hispanic (7.3%) women to report weekly leakage and to report stress-type (35.4% vs 9.4% and 14.5% respectively) and urge-type leakage (13.4% vs 3.3% and 10.8% respectively). Rates and type of leakage for men did not vary by race/ethnicity. Risk factors for urine leakage varied by gender and by race/ethnic group17.
The objectives of this study were to investigate the relationship between severity of urine leakage and health related quality of life (QOL) in men and women enrolled in the BACH Survey and to determine if the observed relationships varied by race and ethnicity.
Materials and Methods
Description of the BACH Survey
This prospective epidemiologic survey used a multi-stage, stratified cluster sample design to enroll a total of 5,503 study participants (3,202 women and 2,301 men) from April 2002 through June 2005, including 1,767 Black, 1,877 Hispanic, and 1,859 White respondents. The sampling design specified equal numbers of subjects in each of 24 design cells, defined by age (30–39, 40–49, 50–59, 60–79 years), gender, and race/ethnicity (Black, Hispanic, and White). The city of Boston was stratified into 12 strata: four geographic areas formed by grouping the city’s planning districts by three levels of minority density (i.e., primarily White, at least 25 percent Black, and at least 30 percent Hispanic). Census blocks were randomly sampled from 4,266 city blocks by stratum such that approximately 10% of low density minority blocks, 15% of high density Black blocks, and 75% of high density Hispanic blocks were selected.
In-person screening was conducted at the household and individual level with a goal of approximately equal numbers of Black, White, and Hispanic respondents in the four age categories by gender. Individuals were eligible for BACH if a member of the randomly selected household was race/ethnicity and gender compatible with the household’s sampling code, 30–79 years of age, competent to consent, and could speak English or Spanish well enough to complete the interview. Interviews were completed with 63.3 percent of eligible individuals from selected households, with little variation across minority sampling blocks. Data were obtained by in-home interviews by trained survey interviewers. Physiological measures included height, weight, hip and waist circumference, heart rate, blood pressure. The protocol and informed consent procedure were approved by the New England Research Institutes’ Institutional Review Board. Details of the design of the BACH Survey have been reported previously 13.
Measures
Urine leakage was determined with the following question:
“Many people complain that they leak urine (wet themselves) or have accidents. In the last 12 months, have you leaked even a small amount of urine?”
Severity of urine leakage was measured by the Sandvik Severity Scale 14 based on frequency and amount, factors previously shown to be clinically relevant, most likely to result in help-seeking, and associated with increased bother and reduced QOL 3,15. The frequency of urine loss was reported with the following categories: weekly (“one or more times per week” or “everyday”), monthly (“one or more times per month”), or occasionally (“less than once per month”). The amount of urine lost was quantified by the participant as “drops”, “small splashes”, or “more”. Severity was defined as mild (scores of 1–2), moderate (scores of 3–4), and severe (scores ≥ 5).
Health related quality of life (QOL) was measured with the Medical Outcomes Study Short Form-12 (SF-12) and summarized by the physical health (PCS) and mental health (MCS) component scores. 16 This generic measure of health-related QOL is self-administered, taking approximately 3–4 minutes to complete. The measure is considered comparable to the longer SF-36, with established validity and reliability (Ware et al 1996). In adults, scored from 1–100, both scales have a population mean of approximately 50 and a standard deviation of 10 points with higher scores indicating better QOL. A Spanish version of the measure was available from developers of this measure.
Covariates consisted of medical and sociodemographic correlates of leakage by gender as identified previously.17 Medical covariates consisted of the self-reported comorbidities (y/n) of heart disease, hyperlipidemia, asthma, and arthritis previously associated with urine leakage17 and other factors considered to affect QOL, including several comorbid conditions (chronic lung disease, diabetes, history of stroke, vascular disease, hypertension, Parkinson’s disease), smoking status, alcohol intake, and physical activity level. Obstetric history (any vaginal birth) and menopause/hormone status were included in models for women. Sociodemographic covariates included race/ethnicity (Black, White, Hispanic), age group (in 10 year increments), waist circumference (continuous) as a measure of obesity, depressive symptoms (>five symptoms on the abbreviated Center for Epidemiologic Studies-Depression [CES-D] scale 18 and/or taking anti-depression medication [y/n]). Finally, socioeconomic status (SES) was measured by an index that combines education and income 19 and categorized such that one-fourth of the BACH population was lower, one-half was middle, and one-fourth was upper socioeconomic class.
Analysis
Gender differences in characteristics were assessed by chi-square analysis and analysis of variance (ANOVA), as appropriate. The PCS and MCS scores (means ± standard deviations) are reported for all respondents and then by gender and by race/ethnicity within gender. Scores were compared across race/ethnic groups and gender groups with t-tests.
To investigate the relationship between leakage severity and QOL, separate linear regression models were fit for men and for women for the PCS and MCS scores, adjusting for race/ethnicity and covariates. Regression coefficients (β) and standard errors (se(β)) denote the mean increase (or decrease) in QOL score for a level of leakage severity compared to no leakage (reference group) adjusting for covariates associated with urine leakage and co-morbidities that affect QOL. To ascertain the best model, a manual model selection procedure was used, starting with all covariates and removing variables one at a time if they were insignificant (p>0.05) in models for both men and women. In order to better compare the models between men and women, covariates that were significant in models for men were not removed from the models for women and vice versa. Age and race/ethnicity were retained in each model regardless of level of significance. Depressive symptoms and anti-depressant medication were not included in models predicting MCS due to their high correlation with MCS. Two methods were used to determine if the relationship between leakage severity and QOL varied by race/ethnicity: 1) inclusion of an interaction term for severity and race/ethnicity, and 2) stratified models. Stratified models and an interaction term between leakage severity and race/ethnicity allow us to see if there were different associations between leakage severity and QOL in the three race/ethnic groups. The same methods were used to determine if the relationship between leakage severity and QOL varied by SES. P-values < 0.05 are deemed statistically significant, not accounting for multiple comparisons.
Because of the two-stage cluster sampling design, it was necessary to weight observations inversely proportional to probability of selection into the study 20,21 so that results are generalizable to the Boston population. Weights were further post-stratified to the Boston population according to the 2000 Census. SAS version 9.1 (SAS Inst., Cary, NC) was used to impute missing values by multiple imputation within a gender, race/ethnic group22. With the exception of income (6%), missing data was less than 0.5%. Twenty-five multiple imputations were performed by gender and race/ethnicity, using all relevant variables. SUDAAN version 9.0.1 (Research Triangle Institute, Research Triangle Park, NC) was used to conduct weighted analyses.
RESULTS
Selected demographic and clinical characteristics of BACH Survey participants are presented in Table 1. The frequency of urine leakage was substantially greater in women (30.5%) than men (17.9%). Twelve percent of men and 18% of women reported mild leakage. The frequency of urine leakage was greater in women than in men for each level of severity. Notably, women were about five times more likely than men to report severe urine leakage (5.1% versus 0.9%).
Table 1.
Weighted Study Sample Characteristics, Boston Area Community Health (BACH) Survey
| Percent or Mean ± Standard Deviation | |||
|---|---|---|---|
| Variable | Men | Women | p† |
| Age categories | 0.03 | ||
| 30–39 | 26.7% | 24.8% | |
| 40–49 | 28.6% | 26.2% | |
| 50–59 | 22.2% | 24.3% | |
| 60–69 | 14.3% | 16.1% | |
| 70–79 | 8.2% | 8.7% | |
| Race/Ethnicity | 0.02 | ||
| Black | 25.1% | 29.9% | |
| Hispanic | 13.0% | 13.3% | |
| White | 61.9% | 56.8% | |
| Urine leakage | <.001 | ||
| None | 82.1% | 69.5% | |
| Mild | 11.8% | 17.9% | |
| Moderate | 5.2% | 7.5% | |
| Severe | 0.9% | 5.1% | |
| Waist circumference (cm) | 97.9 ± 14.3 | 90.3 ± 16.9 | <.001 |
| Socioeconomic status | <.001 | ||
| Low | 24.3% | 30.8% | |
| Medium | 49.1% | 45.3% | |
| High | 26.6% | 23.9% | |
| Heart disease | 9.6% | 7.5% | 0.08 |
| Asthma | 14.3% | 18.9% | 0.004 |
| Arthritis | 17.3% | 29.0% | <0.001 |
| Hyperlipidemia | 28.4% | 28.4% | 0.99 |
| Depressive symptoms | 14.0% | 20.1% | <0.001 |
| Taking antidepressants | 11.9% | 17.1% | 0.006 |
| Stroke | 1.8% | 1.2% | 0.26 |
| Vascular diseases | 6.4% | 7.3% | 0.35 |
| Chronic lung disease | 5.8% | 7.6% | 0.11 |
| Diabetes | 9.3% | 9.6% | 0.82 |
| High blood pressure | 26.2% | 28.3% | 0.22 |
| Parkinson’s disease | 0.12% | 0.23% | 0.37 |
| Any vaginal birth(s) | --- | 32.4% | --- |
| Menopause/hormone status | --- | ||
| Pre-menopausal | --- | 24.0% | |
| Peri-menopausal | --- | 21.6% | |
| Post-menopausal | --- | 21.9% | |
| Undetermined | --- | 2.9% | |
| Surgical, no hormones | --- | 12.9% | |
| Surgical, with hormones | --- | 2.9% | |
| Hormone replacement therapy | --- | 2.7% | |
| Oral contraceptives | --- | 10.2% | |
| Raloxifene HCL | --- | 0.5% | |
| Progesterone only | --- | 0.4% | |
| Alcohol intake (drinks/day) | <0.001 | ||
| None | 27.5% | 41.7% | |
| <1 | 38.9% | 43.2% | |
| 1–2.9 | 24.1% | 12.9% | |
| 3+ | 9.6% | 2.2% | |
| Smoking status | <0.001 | ||
| Never | 28.9% | 27.1% | |
| Former | 28.9% | 27.1% | |
| Current | 32.2% | 23.1% | |
| Physical activity | <0.001 | ||
| Low | 26.8% | 27.8% | |
| Medium | 47.4% | 53.6% | |
| High | 25.8% | 18.5% | |
p-values for gender difference are from chi-square tests for all variables except waist circumference which was tested using a F test from analysis of variance.
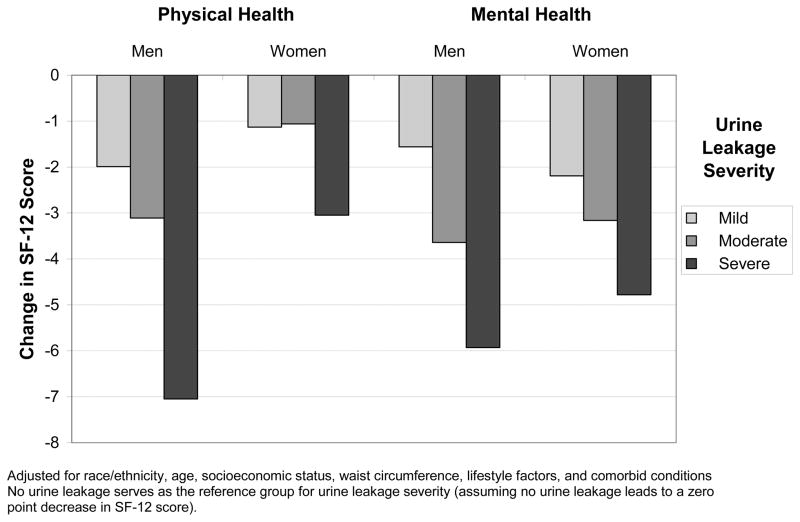
In both men and women, mean PCS and MCS decreased as severity of urine leakage increased, with a greater decrement observed for physical health (Table 2). Table 3 presents the results of the four multivariate regression models describing the relationship of leakage severity with physical and mental health QOL by gender, adjusting for race/ethnicity and other covariates. Among men, leakage severity was associated with lower PCS scores when controlling for race/ethnicity and factors associated with QOL. As shown in Figure 1, mean PCS scores (physical QOL) were 7 points lower in men with severe leakage and 3 points lower in men with moderate leakage compared to men with no leakage. In the multivariate model, PCS was related to SES, alcohol intake, smoking status, waist circumference, physical activity level, and several health conditions in addition to urine leakage. However, the relationship between severe urine leakage and PCS (−7.1) was larger than that for any other factor considered. In comparison to other health conditions, the next largest coefficients were for the relationship between vascular diseases and arthritis and QOL, with coefficients close to −5. Race/ethnicity and age were not significant confounders in the relationship between severity of leakage and PCS score when controlling for the other factors.
Table 2.
Weighted Mean SF-12 Component Scores by Urine Leakage by Gender, Boston Area Community Health (BACH) Survey
| Mean SF-12 Component Scores | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mental Health | Physical Health | |||||||
| Variable | Men | p* | Women | p* | Men | p* | Women | p* |
| Urine leakage | <.001 | <.001 | <.001 | <.001 | ||||
| None | 50.8 | 50.2 | 51.1 | 49.4 | ||||
| Mild | 49.5 | 48.3 | 48.0 | 47.5 | ||||
| Moderate | 47.1 | 46.6 | 45.1 | 45.1 | ||||
| Severe | 43.1 | 44.0 | 35.3 | 39.4 | ||||
p-values for difference in QOL by urine leakage are from analysis of variance tests
Table 3.
Relationship of Severity of Urine Leakage with Quality of Life by Gender Adjusting for Race/Ethnicity and Other Covariates
| Mental Health | Physical Health | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality of Life | Men | Women | Men | Women | ||||||||
| β | 95% CI | p* | β | 95% CI | p* | β | 95% CI | p* | β | 95% CI | p* | |
| Urine leakage severity | <.001 | <.001 | <.001 | 0.05 | ||||||||
| No urine leakage | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| Mild | −1.56 | −3.52, 0.40 | −2.19 | −3.65, −0.73 | −1.99 | −3.52, −0.46 | −1.13 | −2.37, 0.12 | ||||
| Moderate | −3.64 | −6.27, −1.02 | −3.16 | −5.33, −1.00 | −3.11 | −5.85, −0.38 | −1.06 | −2.98, 0.86 | ||||
| Severe | −5.93 | −9.96, −1.90 | −4.78 | −9.24, −0.32 | −7.05 | −10.87, −3.23 | −3.05 | −5.66, −0.44 | ||||
| Race/ethnicity | 0.52 | 0.74 | 0.19 | 0.73 | ||||||||
| Black | 0.02 | −1.50, 1.55 | 0.59 | −0.94, 2.11 | −1.03 | −2.14, 0.08 | 0.29 | −0.80, 1.37 | ||||
| Hispanic | 0.79 | −0.84, 2.41 | 0.13 | −1.88, 2.14 | −0.54 | −1.65, 0.57 | −0.26 | −1.79, 1.27 | ||||
| White | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| Age group | <.001 | <.001 | 0.44 | <.001 | ||||||||
| 30–39y | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| 40–49y | 0.25 | −1.42, 1.92 | 0.79 | −0.92, 2.50 | −0.75 | −1.94, 0.44 | −2.56 | −3.77, −1.36 | ||||
| 50–59y | 1.24 | −0.75, 3.23 | 2.37 | 0.81, 3.93 | 0.09 | −1.24, 1.41 | −1.29 | −2.34, −0.25 | ||||
| 60–69y | 5.81 | 3.58, 8.05 | 4.00 | 1.98, 6.01 | 0.14 | −1.52, 1.81 | −1.38 | −3.10, 0.33 | ||||
| 70–79y | 6.70 | 3.98, 9.43 | 5.78 | 3.65, 7.91 | −1.36 | −3.56, 0.84 | −0.47 | −2.17, 1.23 | ||||
| Socioeconomic status | 0.46 | 0.002 | <.001 | 0.07 | ||||||||
| Low | −0.91 | −2.81, 0.99 | −3.49 | −5.40, −1.58 | −3.30 | −4.63, −1.98 | −0.84 | −2.43, 0.75 | ||||
| Medium | −0.02 | −1.78, 1.73 | −1.63 | −2.95, −0.31 | −1.11 | −2.07, −0.15 | 0.44 | −0.82, 1.69 | ||||
| High | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| Heart disease | −3.22 | −5.27, −1.18 | 0.002 | −3.47 | −5.68, −1.27 | 0.002 | −4.20 | −6.19, −2.20 | <.001 | −1.19 | −3.17, 0.79 | 0.24 |
| Asthma | −0.49 | −2.09, 1.11 | 0.55 | −1.99 | −3.68, −0.31 | 0.02 | −0.55 | −1.62, 0.52 | 0.31 | −2.92 | −4.26, −1.58 | <.001 |
| Arthritis | −1.88 | −3.22, −0.54 | 0.006 | −0.45 | −1.85, 0.96 | 0.53 | −4.75 | −6.05, −3.45 | <.001 | −6.09 | −7.36, −4.83 | <.001 |
| Depressive symptoms | −2.49 | −4.56, −0.42 | 0.02 | −1.60 | −2.94, −0.26 | 0.02 | ||||||
| Vascular diseases | −4.94 | −7.19, −2.68 | <.001 | −3.31 | −5.15, −1.48 | <.001 | ||||||
| Chronic lung disease | −2.07 | −4.02, −0.12 | 0.04 | −1.83 | −3.68, 0.01 | 0.05 | ||||||
| Diabetes | −2.81 | −5.99, 0.38 | 0.08 | −2.22 | −4.29, −0.15 | 0.04 | −1.01 | −2.99, 0.97 | 0.32 | −2.03 | −3.88, −0.17 | 0.03 |
| High blood pressure | −1.33 | −2.44, −0.21 | 0.02 | −1.10 | −2.34, 0.14 | 0.08 | ||||||
| Alcohol intake (drinks/day) | 0.01 | 0.57 | 0.03 | 0.006 | ||||||||
| None | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| <1 | −1.62 | −3.12, −0.11 | 0.82 | −0.64, 2.29 | 1.44 | 0.22, 2.66 | 1.42 | 0.39, 2.44 | ||||
| 1–2.9 | −0.08 | −1.54, 1.37 | 0.85 | −1.23, 2.93 | 1.67 | 0.43, 2.90 | 2.11 | 0.54, 3.68 | ||||
| 3+ | −2.79 | −4.38, −0.74 | 2.19 | −1.59, 5.97 | 2.19 | 0.60, 3.77 | 2.89 | 0.76, 5.03 | ||||
| Smoking status | 0.01 | <.001 | 0.02 | 0.09 | ||||||||
| Never | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| Former | −1.08 | −2.78, 0.62 | −0.28 | −1.57, 1.02 | −0.85 | −1.92, 0.23 | −0.54 | −1.78, 0.69 | ||||
| Current | −2.17 | −3.60, −0.74 | −3.08 | −4.73, −1.43 | −1.84 | −3.10, −0.59 | −1.38 | −2.62, −0.15 | ||||
| Physical activity | <.001 | 0.19 | <.001 | <.001 | ||||||||
| Low | Ref | . | Ref | . | Ref | . | Ref | . | ||||
| Medium | 3.03 | 1.44, 4.62 | 1.34 | −0.11, 2.79 | 3.10 | 1.69, 4.51 | 4.69 | 3.53, 5.86 | ||||
| High | 4.85 | 3.11, 6.58 | 1.31 | −0.61, 3.23 | 4.74 | 3.26, 6.22 | 6.99 | 5.38, 8.60 | ||||
| Waist circumference, cm | −0.07 | −0.13, −0.01 | 0.01 | −0.08 | −0.12, −0.05 | <.001 | ||||||
p-values are from F tests for the relationship between the covariate and quality of life CI: Confidence Interval Ref: Referent
Figure 1.
Change in SF-12 Score by Severity of Leakage in men and Women from Mulitvariate Models
In women reporting severe leakage mean PCS scores were 3 points lower compared to those who reported no leakage. Women who reported mild or moderate leakage were found to have small decrements in PCS scores after adjusting for covariates (Figure 1). In the multivariate model, PCS was related to age, alcohol intake, physical activity, waist circumference, and several health conditions (Table 3). In comparison to these health conditions, the impact of severe leakage on PCS score (−3.1) was similar in magnitude to that observed for vascular disease (−3.3) and asthma (−2.9). Only the relationship between arthritis and PCS score (−6.1) was greater. The relationships between PCS and all other factors were less than that of severe leakage.
Among both men and women, leakage severity was substantially related to mental health QOL (MCS) even after adjusting for other factors (Table 3). Those with severe leakage had mean MCS scores 5 to 6 points lower than those without urine leakage (Figure 1). Moreover, the negative effect of severe leakage was greater than the effects of any other factor considered. Age was associated with a positive effect on MCS for both genders, particularly at the higher ages. For example, the 70–79 year age group was associated with a 6 to 7 point increase in mean MCS score, compared to the 5 to 6 point decrease in score associated with severe leakage. Race/ethnicity was not a significant confounder in the relationship between leakage severity and MCS score for either gender. Other factors related to the MCS score (after adjusting for the other covariates) were heart disease, arthritis, alcohol intake, smoking status, and physical activity in men and SES, heart disease, asthma, diabetes, and smoking status in women.
We found limited evidence of differential effects of UI severity by gender, race/ethnicity, and SES. The two significant interactions occurred in models of women predicting mental health QOL (MCS). There was a weak interaction between race/ethnicity and leakage severity but a strong interaction between leakage severity and SES. In stratified models (results not shown), it is clear that the race/ethnicity interaction is an artifact of the high correlation between SES and race/ethnicity. In multivariate models, leakage severity had a substantially stronger effect on MCS in women with low SES than in women with middle or high SES.
COMMENT
The substantial negative impact of urinary incontinence on quality of life has been well documented.2–5,7,8. However, few studies have included subjects recruited from the community, fewer have included a sufficient number of men, and only rarely have examined the impact on quality of life among different racial and ethnic groups.
The BACH Survey was designed to assess the prevalence, incidence and impact of a large number of urologic symptoms, including urinary incontinence in a community-based sample of white, black and Hispanic men and women. We found that severe urine leakage had greater negative effects than almost all other factors considered, including most other comorbid conditions, on physical and mental health QOL for both genders. After controlling for an interaction with SES, urine leakage appears to have a similar negative effect on physical and mental health QOL in White, Black and Hispanic participants.
Both moderate and severe leakage had a greater negative effect on physical and mental health QOL in men than it did in women. For men, severe urine leakage had a greater effect on physical health QOL than did any other factor considered, including older age, SES, lifestyle factors and a range of comorbid conditions. However, it is quite possible that the urine leakage in men is associated with other co-existing conditions not included in this study that contribute to the impact of these urinary symptoms on physical health QOL. Although women were more likely than men to report severe urine leakage, its impact on physical QOL appeared to be less as the mean reduction in PCS was about one-half of that observed in men with the same severity of urine leakage. One reason for less of an effect observed in women may be that many women consider urine leakage a normal part of aging and more easily accommodate physically to it 7,8 than do men. For men, on the other hand, the leaked urine, often post-void dribbling17, might be visible and interfere more with work and other public daily activities. We also observed a greater negative impact of leakage on mental health QOL in women of low SES compared to women of middle to high SES. For women faced with daily challenges associated with lower income levels, the additional experience of urine leakage has an emotional impact not seen in men.
Severity is an important aspect of urine leakage as it has been related to treatment seeking for urinary incontinence 23–25. We previously reported that 45% of women and 22% of men in the BACH Survey who reported weekly incontinence had sought treatment 26, forty percent of whom reported not receiving treatment, and half of those treated (30%) continued to have daily leakage. Considered with results reported here, findings from the BACH Survey suggest that untreated or under-treated urine leakage has a major impact on global mental health in men and women.
These findings confirm that the effect of urine leakage on health-related QOL is substantial for both genders. For the SF-36, a 5-point difference in the total score suggests a mild but clinically significant condition and a 10-point difference indicates a clinically moderate symptom or condition 16. Extrapolating from these criteria, our findings indicate that severe urine leakage has clinically significant impacts on physical health related QOL for men and on mental health QOL for both men and women. For women, the impact of severe leakage on health related QOL was less than that for arthritis but was similar to or greater than other comorbid conditions considered.
Type of leakage, usually urge and mixed types of leakage, has been associated with the magnitude of impact on QOL 27,28. While prior analysis 17 found effects of race/ethnicity on types of leakage in women, sample size limitations precluded inclusion of type of leakage in this analysis.
This study had a number of strengths. First, the study uses a population-based random sample; thus we are able to generalize these results to the population. Second, our sample included a large number of men, a group which is understudied for the effects of urinary incontinence. Third, our population was racially and ethnically diverse and included whites, blacks and Hispanic participants. We also utilized standardized, validated symptom measures to assess the frequency and severity of urine leakage. Use of a generic measure of quality of life, the SF-12, permitted us to compare the impact of various co-morbid illnesses and conditions on quality of life with the effect of urine leakage.
However, there were limitations to our study. First, an area sample raises questions about generalizability of findings. However, a comparison of overall findings from this study with data from large-scale national studies (NHANES, NHIS and BRFSS) shows that with appropriate adjustments, most of our findings are generalizable, with the possible exception of some data about the Hispanic group. Hispanics in this study are not representative of Hispanics in the US; only 1% is of Mexican origin as compared to 58% of US Hispanics. Second, this is a study of self-reported symptoms. Although the questions about urine leakage are valid and reliable 14, self-report introduces the possibility of reporting or assessment error. Finally, the study is cross-sectional in nature, and a prospective study is needed to assess the effect of urine leakage on quality of life over time. The BACH Survey is ongoing and is currently conducting follow-up assessments.
CONCLUSIONS
Both men and women aged 30–79 with symptoms of urine leakage suffer from marked impairments in QOL, and this effect was similar for White, Black and Hispanic subjects. The effect of leakage on physical health related QOL was greater for men than for women despite their reports of less severe leakage than women. The negative impact of leakage on mental health QOL was generally similar for both genders but more pronounced in women of lower social class.
Acknowledgments
This research was funded by grant DK56842 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Payne CK. Epidemiology, pathophysiology, and evaluation of urinary incontinence and overactive bladder. Urology. 1998;51:3–10. doi: 10.1016/s0090-4295(98)90001-2. [DOI] [PubMed] [Google Scholar]
- 2.Wagner TH, Hu TW. Economic costs of urinary incontinence in 1995. Urology. 1998;51:355–61. doi: 10.1016/s0090-4295(97)00623-7. [DOI] [PubMed] [Google Scholar]
- 3.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–7. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 4.Hagglund D, Walker-Engstrom ML, Larsson G, Leppert J. Quality of life and seeking help in women with urinary incontinence. Acta Obstet Gynecol Scand. 2001:1051–5. [PubMed] [Google Scholar]
- 5.Margalith I, Gillon G, Gordon D. Urinary incontinence in women under 65: quality of life, stress related to incontinence and patterns of seeking health care. Qual Life Res. 2004;13:1381–90. doi: 10.1023/B:QURE.0000040794.77438.cf. [DOI] [PubMed] [Google Scholar]
- 6.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537–42. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 7.Dugan E, Cohen SJ, Robinson D, Anderson R, Preisser J, Suggs P, Pearce K, Poehling U, McGann P. The quality of life of older adults with urinary incontinence: determining generic and condition-specific predictors. Qual Life Res. 1998;7:337–44. doi: 10.1023/a:1024938014606. [DOI] [PubMed] [Google Scholar]
- 8.Fultz NH, Herzog AR. Self-reported social and emotional impact of urinary incontinence. J Am Geriatr Soc. 2001;49:892–9. doi: 10.1046/j.1532-5415.2001.49179.x. [DOI] [PubMed] [Google Scholar]
- 9.Thom DH, van den Eeden SK, Ragins AI, Wassel-Fyr C, Vittinghof E, Subak LL, Brown JS. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259–64. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sze EH, Jones WP, Ferguson JL, Barker CD, Dolezal JM. Prevalence of urinary incontinence symptoms among black, white, and Hispanic women. Obstet Gynecol. 2002;99:572–5. doi: 10.1016/s0029-7844(01)01781-1. [DOI] [PubMed] [Google Scholar]
- 11.Fultz NH, Herzog AR, Raghunathan TE, Wallace RB, Diokno AC. Prevalence and severity of urinary incontinence in older African American and Caucasian women. J Gerontol A Biol Sci Med Sci. 1999;54:M299–303. doi: 10.1093/gerona/54.6.m299. [DOI] [PubMed] [Google Scholar]
- 12.Burgio KL, Matthews KA, Engel BT. Prevalence, incidence and correlates of urinary incontinence in healthy, middle-aged women. J Urol. 1991;146:1255–9. doi: 10.1016/s0022-5347(17)38063-1. [DOI] [PubMed] [Google Scholar]
- 13.McKinlay JB, Link CL. Measuring the Urologic Iceberg: Design and Implementation of The Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389–396. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19:137–45. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: urinary incontinence in women-national trends in hospitalizations, office visits, treatment and economic impact. J Urol. 2005;173:1295–301. doi: 10.1097/01.ju.0000155679.77895.cb. [DOI] [PubMed] [Google Scholar]
- 16.Ware J., Jr . Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute, New England Medical Centre; 1994. [Google Scholar]
- 17.Tennstedt S, Link C, Steers W, McKinlay J. Prevalence and risk factors of urine leakage in a racially and ethnically diverse population of adults: The Boston Area Community Health (BACH) Survey. Am J Epidemiol. 2008;167:390–399. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
- 18.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 19.Green LW. Manual for scoring socioeconomic status for research on health behavior. Public Health Rep. 1970;85:815–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran W. Sampling Techniques. New York: John Wiley & Sons; 1977. [Google Scholar]
- 21.Kish L. Survey Sampling. New York: John Wiley & Sons; 1965. [Google Scholar]
- 22.Rubin D. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 23.Burgio KL, Ives DG, Locher JL, Arena VC, Kuller LH. Treatment seeking for urinary incontinence in older adults. J Am Geriatr Soc. 1994;42:208–12. doi: 10.1111/j.1532-5415.1994.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 24.Seim A, Sandvik H, Hermstad R, Hunskaar S. Female urinary incontinence--consultation behaviour and patient experiences: an epidemiological survey in a Norwegian community. Fam Prac. 1995;12:18–21. doi: 10.1093/fampra/12.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Kinchen KS, Burgio K, Diokno AC, Fultz NH, Bump R, Obenchain R. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health. 2003;12:687–98. doi: 10.1089/154099903322404339. [DOI] [PubMed] [Google Scholar]
- 26.Harris S, Link C, Tennstedt S, Kusek J, McKinlay J. Care Seeking and Treatment for Urinary Incontinence in a Diverse Population. J Urol. 2007;177:680–684. doi: 10.1016/j.juro.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 27.Coyne KS, Zhou Z, Thompson C, Versi E. The impact on health-related quality of life of stress, urge and mixed urinary incontinence. BJU Int. 2003;92:731–5. doi: 10.1046/j.1464-410x.2003.04463.x. [DOI] [PubMed] [Google Scholar]
- 28.Simeonova Z, Milsom I, Kullendorff AM, Molander U, Bengtsson C. The prevalence of urinary incontinence and its influence on the quality of life in women from an urban Swedish population. Acta Obstet Gynecol Scand. 1999;78:546–51. [PubMed] [Google Scholar]