Abstract
Background
Metalloproteinase-9 (MMP-9) is a type IV Collagenase found at elevated levels in chronic wounds. As wounds heal, MMP-9 diminishes. In this study, we investigated whether MMP-9 directly contributes to chronic wound pathogenesis.
Methods
Recombinant proMMP-9 was prepared using immortalized keratinocytes transduced by a lentivirus. ProMMP-9 was purified from cell culture media and activated using 4-aminophenylmercuric acetate. Active MMP-9 was then suspended in Xanthan Gum to a concentration paralleling that found in human chronic wounds. Two parallel 6mm punch biopsies were made on the backs of C57BL mice. Wounds were treated daily with MMP-9 or vehicle. Wound areas were measured and tissues examined by densitometry, real-time RT-PCR, histology, and immunohistochemistry at days 7, 10 and 12.
Results
Exogenous MMP-9, at the level found within chronic wounds, delayed wound healing in this animal model. By 7 days, wounds in the MMP-9-injected group were 12% larger than control wounds (p=0.008). By day 12, wounds in the MMP-9-injected group were 25% larger than those of the control group (p=0.03). Histologic examination shows that high levels of active MMP-9 impaired epithelial migrating tongues (p=0.0008). Moreover, consistent with elevated MMP-9, the collagen IV in the leading edge of the epithelial tongue was diminished.
Conclusion
MMP-9 appears to directly delay wound healing. Our data suggests that this may occur through interference with re-epithelialization. We propose that MMP-9 interferes with the basement membrane protein structure, which in turn impedes keratinocyte migration, attachment, and the reestablishment of the epidermis.
Introduction
During the last two decades many investigators have illuminated the complex interaction of cells, proteins, signaling molecules, and the mechanisms whereby organisms heal wounds. Many of these discoveries have improved the ability of clinicians to prevent failed healing of acute wounds and improve the healing of wounds that have become chronic. Nevertheless, billions of dollars are still necessary to treat patients with chronic wounds. Although a considerable effort has been put into the elucidation of the reasons for the chronic wound environment, failed wound healing remains only partially explained.
A prime candidate for a cause for disordered wound healing is a particular proteinase, matrix metalloproteinase type 9 (MMP-9), a type IV collagenase. Increased levels of MMP-9 have been identified by numerous investigators in many chronic wound types. In an acute surgical wound, MMP-9 is transiently expressed, but quickly returns to basal levels (1). MMP-9 is seen in decubitous ulcers and venous stasis ulcers (2-5). As Young and Grinell reported in 1994, and as we have observed in our own lab, MMP-9 is also up regulated in burn wounds (6). In response to injury the cornea also expresses MMP-9 (7). While there is a normal increase in MMP-9 during acute wound healing, MMPs, and in particular MMP-9 are massively elevated in the chronic wound environment (8) an additional fivefold (5). We and others have hypothesized that the prolonged and excessive production of this protease that leads to disordered wound healing. The disappearance of MMP-9 is associated with resolution of the pathologic state. We have observed that healed areas of burn wound lose active MMP-9 expression (unpublished). Trengrove et al described the same disappearance of MMP-9 activity as leg ulcers heal (8). Further, levels of MMP-9 have also been inversely correlated with delayed collagen deposition in alveolar bone healing (9).
We propose that MMP-9 is not simply associated with these wounds, but is in fact one of the causal factors contributing to the development of chronic wounds. Using an animal model we applied clinically-defined, high levels of activated, recombinant MMP-9 to the full-thickness wounds. We found this treatment decreased type-IV collagen in basement membrane, decreased epithelial migration, and ultimately results in delayed wound healing.
Materials and Methods
Animal protocol
Thirty C57-BL mice were used in this study. The study was approved by the Institutional Animal Care and use Committee at the University of Southern California. All animals are used in accordance with the policies of the IACUC of the University of Southern California under protocol 10681. Mice were prepared as described by Gurtner and Colleagues (10). In brief, C57-BL Mice are operated on from ages 8-12 weeks of age. The mouse was initially anesthetized using an intraperitoneal injection of Ketamine and Xylazine. After the anesthetic takes effect, the mouse was shaved, and a depilatory agent is applied. After 4 minutes, the dorsum of the mouse was cleaned using warm moist tap water. Sterile surgical prep was then performed. Two 6 mm punch biopsies were then outlined using a punch biopsy tool on dorsum of the animal. We then excised the skin using a pair of Iris scissors. Krazy Glue™ was applied to the skin around the wound margin and a 5mm thick piece of silicone with a 7mm diameter hole was glued around the wound. This was fixed by the placement of a series of interrupted 4.0 Prolene suture placed circumferentially around the silicone stent. A gauze pledget was then placed inside the stent (that is, in the wound bed). Finally, an occlusive dressing (tegaderm™) was applied with Krazy™ Glue to seal the wound, with the telfa under the occlusive dressing (see fig 1). Injections of bupronorphine were administered for the next 48 hours for post operative pain control. Animals were housed in individual cages.
Fig 1. Animal model of cutaneous wound healing.
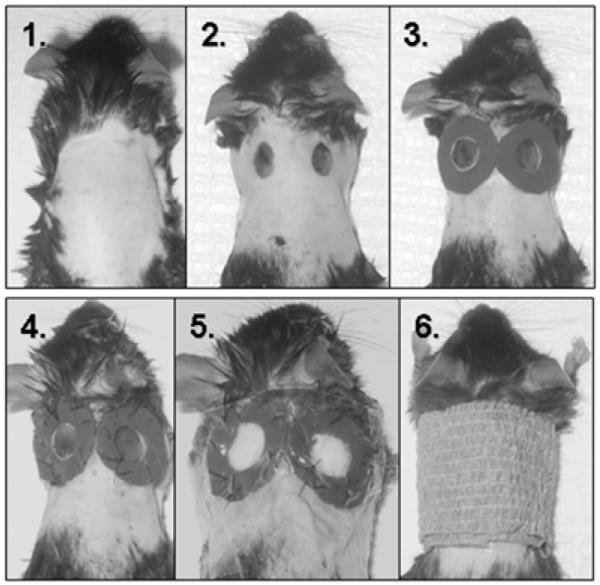
Mice were prepared with a commercial depilatory agent, 6mm punch biopsies made and a silicone stent fixed around the wound with Krazy Glue, and 4.0 prolene suture as described by (Gurtner). A Telfa pledget was then placed in the wound bed and tegaderm is glued to the silicone sheeting and the surrounding skin, creating a pocket into which we injected MMP-9 (right sided wound) or vehicle (left sided wound). Dressing was reinforced with Koban.
At the time of surgery, the first treatment was also applied. 100μl of active MMP-9 was delivered into the telfa pledget in the wound dressing. This was done using a small gage needle (29.5 gauge), to avoid destruction of the dressing. Active drug was always delivered to the wound on the right, control to the wound on the left. Control treatment is explained below under protein purification. Treatment and control were delivered daily to both wounds, until sacrifice.
Protein purification
Conditioned media was harvested from immortalized keratinocytes, transduced by lentivrus expressing human MMP-9 (11). Control media was harvested from similar, but untransduced immortalized keratinocytes. These media was then treated with APMA, a chemical activator for MMP-9. After overnight incubation at 37°C, the media were then purified by passage through a gelatin bead column. Protein was eluted using 6M Urea followed by dialysis against saline. BSA was then added to aid in maintaining the stability of the metalloproteinase. This was then concentrated using ammonium sulfate, and resuspended in a salt buffer. At the same time, control media from untransduced immortalized keratinocytes was treated the same way (addition of BSA, ammonium sulfate concentration).
In order to approximate a clinically-relevant quantity of MMP-9, conditioned media from overnight organ culture of a burn wound skin were compared in a series of titrations to the prepared treatment MMP-9, by zymography (fig 2c). After titration against our human samples, both the control and MMP-9 treatment groups were the mixed in a 1:1 ratio with 0.5% Xanthan Gum as a carrier, yielding a final concentration of 0.25% Xanthan gum, and a quantity of MMP-9 similar to that found in unhealed burn wounds.
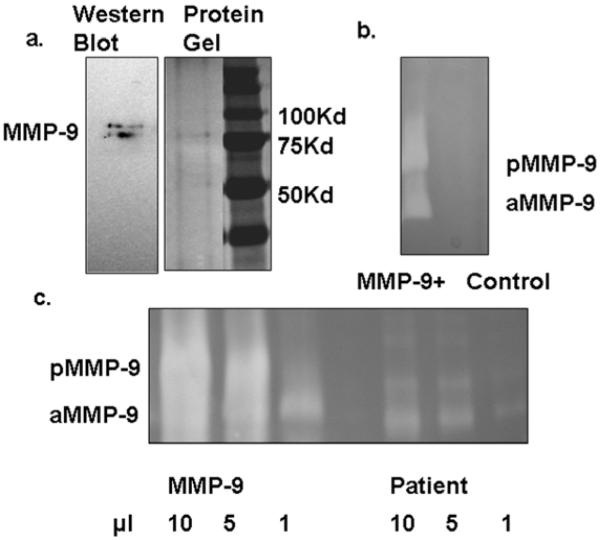
Fig 2. Preparation of MMP-9.
MMP-9 was purified from the conditioned medium of immortalized keratinocyte transuced by lentivrius expressing MMP-9. (2a) The purified MMP- 9 protein was faintly detectable by silver stain and was identified by Western blot analysis. (2b) The gelatinolytic activities of our purified MMP-9 as well as the mock control were examined by zymography, which is much more sensitive. (2c) MMP-9 concentration delivered to mice was determined by titration against condition media from overnight incubation of burned human skin.
Western blot
Protein samples were loaded onto a 10% SDS-PAGE and electrophoresis was performed at room temperature. Samples were then transferred to PVDF membrane, which was then blocked in 5% nonfat milk, 100mM NaCl, and 50mM Tris pH 7.5 for 16 hours. Samples were then incubated with anti- MMP-9 antibody (AB19016, Chemicon international) in 5% milk buffer for two hours. Membranes were then washed and incubated with horseradish peroxidase conjugated second antibody. Antibodies were the visualized using Chemiluminescence (Supersignal West Femto, Maximum sensitivity substrate, Peirce).
Gelatin Zymography
Recombinant protein and secreted protein from wounds were mixed with SDS-PAGE sample buffer without reducing agents. The PAGE gel contains 0.5% gelatin. Electrophoresis was performed at 114 V, 4°C for 16 hours. Gels were then washed in 2% triton X-100. Gels were then incubated at 37°C for 24 hours in a buffer of 50mM Tris pH 7.5, 100mM NaCl, and 5mM CaCl2, to allow the development of gelatinolytic activity. Finally, gels were stained with Coomassie Blue R-250 to visualize protein bands.
Wound Measurements
On the day of sacrifice no treatment is delivered to the wound. The animal was euthanized by intraperitoneal injection of Phenobarbital. After the loss of vital signs (respirations and cardiac function), wounds were processed. The wound and underlying soft tissue was excised as a unit and laid flat next to a ruler. Photographs of the wounds were taken using the Alpha Imager™, and analyzed using the accompanying AlphaEase FC Software V. 4.1.0 (Alpha Innotech Corporation). Using this software wounds were outlined and the circumscribed area was calculated in pixels by the software and then compared to standard areas. Standards were derived from circle of known size drawn on paper which was then analyzed by the method described above. These measurements were not blinded.
Histology
After measurement of the wounds they were embedded in paraffin. After all samples were collected 5μm thick pieces of tissue were used to make a series of slides through the wound bed. The slides were then stained with Hematoxylin and Eosin for analysis of the epithelial tongue size and granulation tissue area. All slides were reviewed by a blinded investigator. 3 to 6 slides were viewed from each wound, and measurements were made of epithelial tongue length and area of granulation. These were then averaged for each wound.
Immunohistochemistry
Frozen sections were sliced at a 5μm thickness. Collagen IV antibody (MD20451, MDBiosciences, St. Paul, MN) or IgG first antibody was used in 5% nonfat milk solution. Attached antibody was visualized using a second antibody conjugated with Cy3. Nuclei were stained with DAPI.
Statistics
Wound size and epithelial tongue length were compared using a paired Students t-test. Control wounds were paired with treatment wounds by animal. Wound sizes were expressed as a ratio of treated vs. control wound. Data was analyzed both at each time point as well as pooled for all animals.
Results
We utilized a full-thickness murine wound model to test our hypothesized pathogenicity for excess activated MMP-9 (aMMP-9) (Fig.1). Paired 6 mm dorsal wounds were created and stented open to prevent healing by contraction (details in methods). We and its originators (10) believe this model more accurately reflects human wound healing than a simple punch wound. To mimic the activated proteinase found in human non-healing wounds recombinant pro-MMP-9 (pMMP-9) was converted to mature active form with apparent molecular weight of 82-kDa. The gelatinase was purified to near homogeneity as shown by silver staining and zymographic activities (Fig.2a and b). The amount of MMP-9 loaded to wounds was evaluated by comparison of its gelatinolytic activities to the MMP-9 from chronic wounds (Fig.2c).
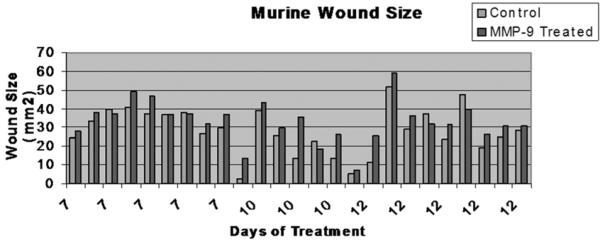
As hypothesized, the exogenous activated MMP-9 delayed wound healing. Figure 3 shows the wound sizes paired by animal. Overall, the wounds exposed to recombinant human MMP-9 healed more slowly than those treated with the control solution. Although there was significant variability in the specific rate of wound closure in the animals as a group, in 19 of 25 animals the MMP-9 exposed wound closed more slowly than did the treatment wound (Fig 3). Because each animal had both a treatment and control wound, this inter-animal variability was normalized by comparing treated to paired control wounds. After 7 days of treatment, healing was significantly affected, with significantly larger open wounds in the MMP-9 treated group than controls. MMP-9 exposed wounds in this group were 12% larger than the untreated wound (p < 0.01). By 12 days this difference was even more dramatic. MMP-9 exposed wounds were 25% larger than their paired normal wounds (p < 0.05). Animals sacrificed at day 10 showed a similar trend towards larger wounds in the treated group, but did not reach statistical significance (fig 4).
Fig. 3. Wounds.
25 Mice were sacrificed at three time points, 7 10 and 12 days. The dorsal skin and underlying muscle were excised, placed on a flat sheet of paper and photographed from a set distance. Here control wounds are picture in blue and MMP-9 exposed wounds in red, paired by animal. Inter-animal variability is notable, but MMP-9 exposed wounds are consistently larger then their paired treatment wound.
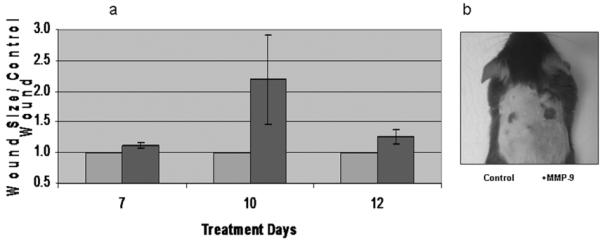
Fig 4. Exogenous MMP-9 delays wound healing.
4a; In order to account for the cross animal variability MMP-9 wounds were normalized to treatment wound. Again, control wounds are pictured in blue, and were set to 1. At day 7 MMP-9 exposed wounds were 12% (p < 0.01) larger, and by day 12 this difference had extended to 25% (p < 0.05) larger. Day 10 wounds had a similar trend but the variability was so great that the difference was not statistically significant (error bars are SEM). 4b; A 12 day mouse demonstrates a dramatically larger mmp-9 exposed wound (right), in contrast to the much smaller control wound (left).
Histological analysis of the wounds suggested a mechanism for this delay in healing after MMP-9 treatment (Fig.5). Wounds were compared by a blinded observer and analyzed in various ways for wound-healing critical activities. We first compared the length of the migrating epithelial tongue. In the MMP-9 treated wounds, epithelial tongues were 40% (p < 0.5) smaller than there counterpart control wounds. Moreover, we observed that the new epithelium had actually detached from the underlying tissue in many of our H&E-stained slides. This finding occurred in 43% (41/96) of the slides from our MMP-9 treated wounds compared to 15 % (13/88) in our control slides (p < 0.001). These findings suggest a defect in attachment of the new epithelium. Specifically this indicates the MMP-9 mediated degradation of the basement membrane zone decreases epithelial migration. This finding may explain, at least in part, the mechanism of delayed wound closure. The analysis of granulation tissue suggests further that there is an increase in the amount of granulation tissue in the wounds exposed to MMP-9. There is slightly greater than twice as much area of granulation tissue (2.1), p <0.005. This finding supports our clinical observation that granulation tissue forms in wounds that are not healing optimally. When analyzed by thickness of granulation tissue, there is no apparent difference between wounds.
Fig 5. Excessive MMP-9 provokes type-IV collagen degradation and delays epithlia migration.
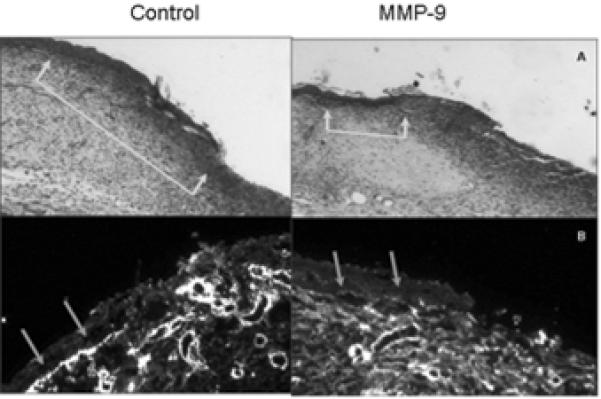
(5a) Epithelial tongue measurements were then made based on Hematoxylin and Eosin staining. In those exposed to MMP-9 epithelial tongues had advanced 62% of the distance measured in the control groups, p < 0.5. (5b) IHC staining for collagen IV at the leading edge of epithelium revealed a clear qualitative difference in the quantity of collagen IV in the basement membrane.
Staining for collagen IV revealed a paucity of staining in the basement membrane at the leading edge of epithelial cell immigration in MMP-9 treated tissues, while the bright staining was seen in the control wounds (Fig 5). While all wounds showed some vascular and lymphatic endothelial cell staining, there were no quantitative or qualitative differences appreciated based on lymphatic markers of LYVE-1 or CD31 staining.
Discussion
MMP-9 is a type IV collagenase that is transiently expressed in the process of normal wound healing but has been found at elevated levels in chronic wounds. This correlation has been observed by a number of investigators in diverse types of slowly or non-healing wounds. MMP-9 synthesis, activation and activity are, in part, regulated by TNF-α (12, 13). We have proposed that the chronic, non-healing wound result from this ongoing inflammatory environment. The ongoing release of TNF-α provides the proximate mechanism for excess and continued MMP-9 production. We propose that normally MMP-9 facilitates reepithelialization by degrading the basement membrane, thereby allowing keratinocyte migration into the wound, and wound closure. In situations where inflammation continues, TNF and subsequently MMP-9 persist, preventing migrating keratinocytes from forming new attachments to a newly synthesized basement membrane. This hypothesis suggests a mechanism whereby the presence of MMP-9 at these high levels delays the process of normal wound healing.
The murine model we used to test our hypotheses is based on the work by Dr. Gurtner and colleagues at NYU (10). The advantage of this model is that it reduces the dramatic wound contraction usually seen in the normal process of murine wound healing. Rather, these mice heal by epithelialization and granulation, better reflecting normal human wound healing. We agree with those authors who have suggested that this model more accurately reflects human wound healing, which depends on reepithelialization and granulation, with lesser importance of wound contraction. The reader might reasonably imagine several ways that a collagenase might affect wound contraction. However, we did not examine this possibility in the current study.
The murine model has of course limitations. Individual members of a species heal at different rates. Mice are of course not human, and we are creating an artificial “human wound.” Mice are however practical, and easy to handle. They also provided us with enough surface area to still have paired treatment and control wounds.
A second point is the choice of control. Media from the same immortalized keratinocyte cell line, but not transduced went through the same extraction process and was then dissolved in the same Xanthan gum carrier that the MMP-9 was delivered in. We acknowledge that there are several other options for controls. Any collagenase at excessively levels might be expected to show similar results. MMP-9 has been a molecule of particular interest and appears to target type IV collagen, and basement membranes. Further, MMP-9 is the only proteinase consistently found in non-healing and chronic wounds. Another potential control would be treatment MMP-9 plus an MMP-9 specific inhibitor. As studies proceed we hope to explore thes possibilities in future studies to determine a specific mechanism for MMP-9 action.
One also must ask what role murine MMP-9 is playing in these wounds. Murine MMP-9 is similar to human and is detected by the same zymography as human MMP-9. It appears not to be significant in our model. Figure 2b shows protein extracted from wound dressings on the day of sacrifice. This fluid lets us assess “wound fluid.” Note both the pro and active MMP-9 is seen only in the treated wound, and not in the control. Thus we conclude that native murine MMP-9 was not and important component of any of the responses found in this study
Between individual mice, there was a fair amount of difference in wound healing. There are many possible causes for this variability, including differences in actual delivered dosage of MMP-9, partial dislodgement of the dressing, loss of MMP-9 into the telfa of the dressing, and most importantly individual differences between mice. In an attempt to mitigate the inter-animal differences we created donor and control wounds on the back of each individual animal. Giving each wound its own “internal control.” This allowed us to normalize wounds between animals. To avoid confusion the left wound was always the control wound, and the right always the treatment wound. We acknowledge that this may introduce some bias. It is possible that all the animals favor one side-for example always sleep on there right, or always scratch on the left as they can better see the door to the lab from that vantage. This might in turn bias our results. Nevertheless, despite this variability, we found that the application of MMP-9 clearly delayed wound healing.
This model has the advantage that it allowed us to have both MMP-9 exposed and treatment wounds on the back of a single animal. In order to provide a stable reservoir for our collagenase, and vehicle, we used a dressing that prevented daily analysis of the wound. We chose three time points 7, 10, and 12 days to sacrifice animals and measure wound size. In the previous description of this model, wounds healed by 12-14 days. Even at the earliest time point, there is already a significant delay in the MMP-9 treated group, with wounds being 12% larger than wounds in the control group. This delay in healing persists so that MMP-9 treated wounds were 25% larger than the control wounds by day 12. While MMP-9 also delayed healing of wounds in the day 10, the small number of animals made it difficult to find statistical significance at this time point, although the trend was the same. The larger average difference seen at day 10 reflects the smaller sample size at this time point, and the inter-animal variability (see figures 3 and 4). Our results provide direct evidence that MMP-9 plays a causal role in delaying wound healing. Clearly, MMP-9 significantly delays wound healing in this model.
What then is the mechanism of the delay in wound healing? Collagen IV, an essential component of basement membranes is one of the major substrates of MMP-9. Hematoxylin and Eosin staining of slides suggested some alteration in the architecture of the basement membrane. In almost half the slides in the MMP-9 treated group we saw the new epithelium lifting away from the underlying tissue bed (43%). In contrast, we seldom saw this in slides from the normal wounds (13%). This finding suggests a likely mechanism for MMP-9-induced delay of wound healing, that the increased fragility of basement ECM impairs keratinocyte attachment. We chose therefore to stain for collagen IV, which fits the criteria of both being important in the epidermal-dermal attachments, as well as being a substrate of MMP-9. This staining yielded striking results. As demonstrated in Fig. 4, MMP-9-treated wounds show almost no staining for collagen IV at the dermal/epidermal junction (fig 4b and c). While it seems likely that a certain level of MMP-9 might be needed in order to release keratinocytes from the underlying matrix and allow migration, persistent excess MMP-9 appeared to interfere with the reattachment of the new epidermis. Keratinocytes are able to migrate into the wound bed, but then fail to reestablish dermal epidermal attachment, and the keratinocytes fall of the wound- leaving a shorter, and likely more fragile epithelial tongue. This is reflected in our epithelial tongue measurements, which were 40% shorter in the MMP-9 exposed wounds.
We also observed an increase of granulation tissue in the MMP-9 treated wounds. The mechanistic reason for this is unknown. Because granulation tissue is primarily newly synthesized blood vessels, it is worthwhile considering how MMP-9 affects this process. There are contradictory reports about the role MMP-9 plays in revascularization of injured tissues, some reports suggest MMP-9 delays angiogenesis, and others that it accelerates it (14-16). When we looked for differences in endothelial cell presence within the wound, there was no difference. Further, the differences observed in granulation were in area not depth i.e. the larger open wounds in MMP-9 exposed mice had a greater area of granulation tissue, but not thicker granulation. It may well be that the greater area of granulation is really a reflection of persistently open wounds rather than the direct impact of MMP-9 on the vascularization that is part of granulation tissue production. MMP-9 plays a role in angiogenesis. Because we applied MMP-9 topically in this model it is difficult to know how deep within the wound our topical MMP-9 penetrated. We did perform staining for both lymphatic and blood vessels, but were unable to appreciate any differences between treated and untreated wounds (data not shown). However because we did not predictably deliver MMP-9 within the deeper layers of the wound bed, we hesitate to draw any conclusions.
Taken together these findings lead us to conclude that when MMP-9 is expressed at excessive levels, it prevents the reestablishment of the dermal/epidermal junction and thereby, limits epithelial migration and wound closure. This is in contrast to normal wound healing, when transient MMP-9 expression may facilitate keratinocyte detachment and migration into the wound bed. During increased MMP-9 expression in chronic wounds, keratinocytes can begin to migrate into the wound, but they are unable to re-anchor themselves to the matrix. This results in the loss of these cells, a shorter epithelial tongue, and a delay or failure of wound closure.
Conclusion
The results presented here demonstrate that a specific proteinase, MMP-9, may have a direct pathogenic role in impaired wound healing. This likely occurs by interfering with the reestablishment of a healthy basement membrane. Although it appears to participate normal healing, MMP-9 is deregulated and expressed at excessive levels in delayed wound healing. The advantages both to the individual patient with a chronic wound, as well as to the healthcare system as a whole in terms of cost are manifold. A number of MMP-9 inhibitors are already available. Further investigations into the use of such drugs will be the focus of future investigations.
Acknowledgement
This work was supported by grants from National Institutes of Health (GM 050967 to W.L.G and AR051558 to Y.P.H) and Robert May Wright Foundation (Y.P.H). We thank Chunli Yan for technical support.
This work was supported by grants from National Institutes of Health (GM 050967 to W.L.G and AR051558 to Y.P.H) and Robert May Wright Foundation (Y.P.H).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaalamo M, Weckroth M, Puolakkainen P, Kere J, Saarinen P, Lauharanta J, et al. Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol. 1996;135(1):52–9. [PubMed] [Google Scholar]
- 2.Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106(5):1119–24. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- 3.Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101(1):64–8. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- 4.Yager DR, Zhang LY, Liang HX, Diegelmann RF, Cohen IK. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107(5):743–8. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 5.Trengove NJ, Langton SR, Stacey MC. Biochemical analysis of wound fluid from nonhealing and healing chronic leg ulcers. Wound Repair Regen. 1996;4(2):234–9. doi: 10.1046/j.1524-475X.1996.40211.x. [DOI] [PubMed] [Google Scholar]
- 6.Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J Invest Dermatol. 1994;103(5):660–4. doi: 10.1111/1523-1747.ep12398424. [DOI] [PubMed] [Google Scholar]
- 7.Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT, Saarialho-Kere U. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003;77(6):653–64. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–52. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 9.Reinhardt RA, Lee HM, Schmid M, Payne JB, Golub L. Relationship between gelatinases and bone turnover in the healing bone defect. J Oral Maxillofac Surg. 2005;63(10):1455–60. doi: 10.1016/j.joms.2005.05.319. [DOI] [PubMed] [Google Scholar]
- 10.Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12(4):485–92. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 11.Han YP, Yan C, Garner WL. Proteolytic Activation of Matrix Metalloproteinase-9 in Skin Wound Healing Is Inhibited by alpha-1-Antichymotrypsin. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem. 2001;276(25):22341–50. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han YP, Nien YD, Garner WL. Tumor necrosis factor-alpha-induced proteolytic activation of pro-matrix metalloproteinase-9 by human skin is controlled by down-regulating tissue inhibitor of metalloproteinase-1 and mediated by tissue-associated chymotrypsin-like proteinase. J Biol Chem. 2002;277(30):27319–27. doi: 10.1074/jbc.M202842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colnot DR, Roos JC, de Bree R, Wilhelm AJ, Kummer JA, Hanft G, et al. Safety, biodistribution, pharmacokinetics, and immunogenicity of 99mTc-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2003;52(9):576–82. doi: 10.1007/s00262-003-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulrich D, Lichtenegger F, Unglaub F, Smeets R, Pallua N. Effect of chronic wound exudates and MMP-2/-9 inhibitor on angiogenesis in vitro. Plast Reconstr Surg. 2005;116(2):539–45. doi: 10.1097/01.prs.0000173447.81513.7a. [DOI] [PubMed] [Google Scholar]
- 16.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, et al. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;290(1):H232–9. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]