Abstract
Therapeutic angiogenesis relies on the delivery of angiogenic factors capable of reversing tissue ischemia. Polymeric materials that can provide spatial and temporal over vascular endothelial growth factor (VEGF) presentation provide clear benefit, but the influence of VEGF dose, temporal, and spatial presentation on the resultant angiogenic process are largely unknown. The influence of the temporal profile of VEGF concentration, dose, and the impact of VEGF spatial distribution on angiogenesis in in vitro models of angiogenesis and ischemic murine limbs was analyzed in this study. Importantly, a profile consisting of a high VEGF concentration initially, followed by a decreasing concentration over time was found to yield optimal angiogenic sprouting. A total VEGF dose 0.1 μg/g, when delivered with kinetics found to be optimal in vitro, provided a favorable therapeutic dose in murine hindlimb ischemia model, and distributing this VEGF dose in two spatial locations induces a higher level of vascularization and perfusion than a single location. These findings suggest that material systems capable of controlling and regulating the temporal and spatial presentation of VEGF maybe useful to achieve a robust and potent therapeutic angiogenic effect in vivo.
Introduction
There is a significant need for new approaches to promote blood vessel formation in ischemic diseases, as these diseases remain the dominant cause of mortality worldwide [1, 2]. Further, the engineering or regeneration of many tissues and organs is currently being explored [3], and the success of virtually all strategies will rely on the creation of a functional vascular network capable of providing for the metabolic needs of the cells and facilitating integration with native tissue. The molecular and genetic mechanisms involved in blood vessel formation have been extensively studied in the past decades [4, 5], and several factors have been identified as critical regulators of neovascularization, including vascular endothelial growth factor (VEGF). VEGF has been extensively tested in pre-clinical and clinical studies with a therapeutic objective [6]. However, the predominant delivery strategies used in these studies involved simple infusions or injections of VEGF in solution, and these approaches do not allow one to address many key issues, including tissue-specific targeting, low systemic exposure and extended time of exposure [7]. Further, the effects of angiogenic growth factors are highly dependent on the timing of their expression and their concentration gradients [8].
Polymeric systems had been utilized to bypass limitations of bolus delivery by providing a desirable spatial distribution of angiogenic factors in a localized and sustainable manner to specific cell populations [9-12]. In particular, injectable alginate hydrogels have been utilized to investigate how sustained, localized VEGF delivery can directly stimulate neovascularization in vivo, and return limb perfusion to normal levels and prevent limb necrosis [10] in a mouse model of peripheral vascular disease (PVD) [13]. However, there is currently limited information regarding the influence of the temporal changes in VEGF concentration, the role of VEGF dose, and the impact of VEGF spatial distribution on angiogenesis resulting from this delivery approach.
A series of in vitro and in vivo experiments were performed in this study to examine the role of VEGF presentation on the angiogenic process. In vitro models are useful tools for the study of neovasculatization events, since they allow one to screen the effects of specific conditions on EC behavior in a tightly controlled environment. Here a versatile three-dimensional (3-D) in vitro sprouting assay [14] was utilized that creates a depot of cells able to form capillary structures in a 3-D environment, in order to investigate EC behavior under different VEGF stimuli. The hypercholesterolemic ApoE -/- mice is a widely used model to study angiogenesis in vivo in preclinical models of PAD [13], due to very limited ability of this mouse to promote angiogenesis after hindlimb ischemia. This preclinical model was used here to screen how specific aspects of VEGF delivery influence neovascularization. An injectable alginate hydrogel system was utilized for the in vivo studies, as previous work has demonstrated the ability of this system to control the kinetics of VEGF release and spatial distribution in both ischemic hindlimb and coronary infarct rodent models [10, 15].
Materials & Methods
In vitro studies
For proliferation studies, human microvascular dermal endothelial cells (HMVEC) (Cambrex Corporation, NJ, USA) (passage 6) were seeded into 6 well plates (5,000 cells/cm2) and cultured overnight with EGM-2MV (Cambrex Corporation, NJ, USA). Endothelial cells were then washed twice with PBS and cultured with either EGM-2MV without growth factors, or EGM-2MV without growth factors but supplemented with VEGF at different concentrations (3, 5, 10, 20, 30, 50, 100 ng/ml). Media was changed every day for 3 days. After 72 hours the endothelial cells were detached via trypsinization and counted in a Coulter Counter (Beckman Corp.) and compared and normalized to the cultures with the EGM-2MV without growth factors. For in vitro sprouting analysis, HMVEC (passage 4) in EGM-2MV were combined with 50 mg of hydrated Cytodex 3 microcarriers (Amersham Biosciences, Piscataway, NJ, USA) in a 7:1 (cell:microcarrier) ratio in a spinner vessel (Bellco Glass Inc., Vineland, NJ, USA). The microcarriers with cells were subsequently transferred to tissue culture flasks, and cultured for 1-2 days, until cells reached confluence. Beads in suspension (57 μl) were combined with 170.5 μl of fibrinogen (Sigma) solution (4 mg/ml) and 22.7 μl of aprotinin (Sigma) (500 μg/ml). This solution was then added to 200 μl of thrombin (Sigma) (22.72 units/ml), and incubated at 37°C for 20 min to allow gel formation. Cultures were fed every day with 0.8 ml of EGM-2MV without growth factors, or EGM-2MV with control VEGF (ranging from 25 - 50 - 100 ng/ml at day 1). After 5 days, gels were washed twice with PBS and incubated with 4% formaldehyde overnight at 4°C. The formaldehyde solution was then aspirated, and gels were washed twice with PBS, and sprouts per bead were quantified from microscopic images (average of 100 beads analyzed per condition). A sprout was defined as an elongated structure extending from the bead with the participation of two or more endothelial cells [10, 16].
Binary Molecular Weight Alginate Gel Formulation and Modifications
Ultrapure alginates were purchase from ProNova Biomedical (Norway). MVG alginate, a high G containing alginate was used as the high molecular weight component to prepare gels. Low molecular weight alginate was obtained by gamma (γ)-irradiating high molecular weight alginate with a cobalt-60 source for 4 h at a γ-dose of 5.0 Mrad (Phoenix Lab, University of Michigan, Ann Arbor, USA), as specified [10]. The alginate gels were a combination of the high molecular weight and low molecular weight polymers at a ratio of 75:25. Both alginate (low and high molecular weight) polymer chains were oxidized - 1% of the sugar residues - with sodium periodate (Aldrich, USA) by maintaining solutions in the dark for 17 h at room temperature, as previously described [17]. The solution was sterile filtered, frozen (-20°C overnight), lyophilized and stored at −20°C. To prepare gels, modified alginates were reconstituted in EBM-2 (Cambrex) to obtain 2% w/v solution (75% LMW; 25% HMW used in all experiments) prior to gelation. The 2% w/v alginate solutions were cross-linked with aqueous slurries of a calcium sulfate solution (0.21 g CaSO4/ml d H2O) in a ratio of 25:1 (40 μl of CaSO4 per 1 ml of 2 % w/v alginate solution) using a 1 ml syringe. Reconstituted alginate was stored at 4°C.
Growth Factor Incorporation and Release Kinetics
Alginates were first mixed with recombinant human VEGF165 protein (R and D Systems, USA) by using two syringes coupled by a syringe connector, and the calcium slurry (Sigma, USA) was then mixed with the resulting alginate/VEGF solution using two syringes coupled by a syringe connector to facilitate the mixing process and prevent entrapment of air bubbles during mixing. The mixture was allowed to gel for 30 minutes and then was maintained at 4°C prior to animal injections. For quantification of in vitro VEGF release, alginates were mixed with iodinated growth factor, as described above for VEGF. 125I-VEGF165 was purchased from PerkinElmer Life Sciences (USA). Gels were placed in PBS buffer solution (PBS - Invitrogen with 0.1g/l of MgCl2*6H2O and 0.132g/l of CaCl2*2H2O Sigma) at 37°C, the solution was changed daily, and the radiolabeled growth factor released into the buffer solution at each time point was measured using a gamma counter (1470 WIZARD (PerkinElmer, USA)) and compared to the initial total 125I VEGF165 incorporated into the sample, as described [10].
Animals and Surgical Procedures
All animal work was performed in compliance with NIH and institutional guidelines. Female ApoE-/- mice aged 6 weeks were purchased (Jackson Laboratories, Bar Harbour, ME) and used for these studies. Mice were fed a high fat diet (21% fat, 0.15% cholesterol, Harlan Teklad) for at least 6 weeks prior to enrollment in the study. Mice were anesthetized with an intraperitoneal injection of a mixture of ketamine 80 mg/kg and xylazine 5 mg/kg prior to all surgical procedures. Hindlimb ischemia was induced by unilateral external iliac and femoral artery and vein ligation as previously described [13].
After the vessel ligation, mice were injected with a total volume of 50 μl of alginate gel containing 3 μg, 5 μg and 10 μg VEGF165, or gel with no VEGF165. Intramuscular injections were performed using a 25G needle (Becton Dickinson, USA) directly into the area where the vessels were ligated. The injected gel volume represents approximately 6% of the total ischemic skeletal muscle tissue injection site. The ischemic tissue volume was calculated from direct measurement of the total mice hindlimb volume, assuming that 70% of the tissue is ischemic; this assumption is based on the 70% drop in regional blood flow to the limb following ligation. In certain experiments, two injection sites were used: the first injection of 25 μl alginate gel (1.5 μg VEGF165) into the area where vessels were ligated and other 25 μl alginate gel (1.5 μg VEGF165) at the location where the aorta branches to the iliac artery. Incisions were subsequently surgically closed and animals monitored over time.
Perfusion, Immunohistochemistry, and Blood Vessel Quantification
Before surgery and 0, 1, 3 and 7 days, and 2, 4, and 6 weeks post-surgery measurements of the ischemic/normal limb blood flow ratio were performed on isoflurane (2% v/O2) anesthetized animals (n=6/timepoint/experimental condition) using a Periscan system blood perfusion monitor laser Doppler equipment (Perimed, Sweden). Perfusion measurements were obtained from the right (ischemic) and left (non-ischemic) limb. Laser Doppler Perfusion Imaging (LDPI) non-invasively measures blood flow by analyzing the flow velocity of the red blood cells circulating in the blood vessels, by means of changes in the wavelength of the reflected light [18]. To minimize variability due to ambient light and temperature, the index was expressed as a ratio of ischemic to non-ischemic limb blood flow.
Hindlimb muscle tissues (n=6/timepoint/experimental condition) were retrieved, fixed, paraffin embedded, and stained for mouse CD31 (BD Biosciences Pharmingen, San Diego CA). For measurement of capillary densities, 30 randomly chosen high-power fields of the tissue were analyzed. The number of positively stained blood vessels were manually counted and normalized to the tissue area. Sections from each sample were visualized at 200× and 400× with a Nikon Eclipse E800 light microscope (Japan) connected to an Olympus DP70 digital image capture system (Japan) and analyzed using IPLab 3.7 software (Scanalytics, Rockville, MD). Vessel size was determined using IPLab 3.7 software.
Statistical Analysis
All statistical comparisons were performed using Students t-test (two-tail comparisons) and one-way analysis of variance (ANOVA), and analyzed using InStat 3.0b (Graphpad, USA) software. Differences between conditions were considered significant if p<0.05.
Results
Effect of VEGF dose and dose distribution on HMVEC-d proliferation and sprouting
To confirm the mitogenic potential of VEGF165 on endothelial cells, HMVEC-d were cultured with discrete VEGF doses for 3 days, and the subsequent cell counts were normalized to no growth factor controls. Increasing the VEGF165 dose induced a gradual increase in EC proliferation (Figure 1A). Doses ranging from 5 – 20 ng/ml of VEGF165 resulted in the same level of cell proliferation, approximately 25% more proliferation than the control. The cell proliferation rate reached a plateau at 50 ng/ml, as no further increase was observed with a dose of 100 ng/ml, and for both conditions cells proliferated approximately 60% more than compared to no VEGF treated cells. The effects of VEGF dose distribution were next analyzed in a 3-D sprouting assay, a standard in vitro model that mimics some early events of angiogenesis [14, 19]. In particular, the effect of VEGF dose distribution on HMVEC-d sprouting was examined (Figure 1B). HMVEC-d were exposed to the same total VEGF165 dose (200 ng of VEGF) over 5 days. However four different dose distributions were used, including a constant dose distribution (50 ng/ml every day), a decreasing dose distribution (100 ng/ml, 50 ng/ml, 50 ng/ml, 25 ng/ml, 25 ng/ml; from 1-5 days respectively), a delayed dose distribution (100 ng/ml, 100 ng/ml, 20 ng/ml, 20 ng/ml, 10 ng/ml; from 1-5 days respectively) and an increasing dose distribution (25 ng/ml, 25 ng/ml, 50 ng/ml, 50 ng/ml, 100 ng/ml; 1-5 days respectively). After 5 days the number and length of sprouts formed under these conditions was quantified. Media containing no VEGF was used as the negative control. Cells exposed to higher doses of VEGF in the early days exhibited higher numbers of sprouts, as compared to a constant dose distribution or to an increasing dose with time. Interestingly, no differences in the numbers of sprouts were observed between a constant dose distribution and an increasing dose distribution. The average length of the sprouts that formed under all conditions was constant (data not shown).
Figure 1.
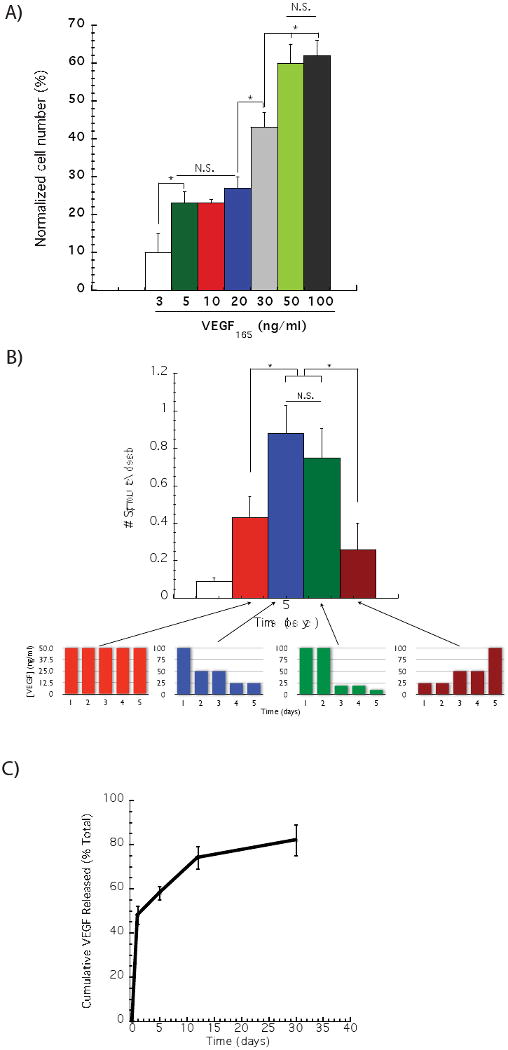
Effect of VEGF dose on in vitro endothelial cell proliferation and sprouting. The proliferation of human microvascular endothelial cells (HMVEC-d) was analyzed after 73 hours of culture with distinct VEGF165 concentrations (A). A statistically significant higher endothelial cell proliferation was observed when cells were cultured with 50 ng/ml of VEGF165, as compared to lower VEGF concentrations, but no statistically difference was noted between 50 and 100 ng/ml. Gradually decreasing the VEGF dose induced a greater number of endothelial cells sprouts, as compared to a constant VEGF doses (50 ng/ml day), or a gradual VEGF dose decrease over time (B). The release kinetics of 125I – VEGF165 from gels formed from binary molecular weight alginate partially oxidized was monitored over time (C). In (A) (B) and (C), values represent mean and standard deviation (A, n=6 and B and C, n=4).
VEGF dose response in ischemic ApoE-/- hindlimb model
Alginate hydrogels with well controlled degradation rates can be formulated by combining control over molecular weight distribution and partial oxidation to make the polymer chains susceptible to hydrolysis [20]. Previous studies with this system have indicated a rapid release of a substantial quantity of encapsulated VEGF, followed by a sustained release at a lower rate – this profile mimics the optimal VEGF exposure for sprout formation identified in the sprouting study and so was utilized for the in vivo studies. Quantification of the VEGF release from the gels used in this study confirmed an initial burst, followed by a sustained VEGF release, and within 7 days approximately 60% of the total VEGF was released (Fig. 1C).
The effects of the dose of VEGF165 released from the gels on vascularization and perfusion of ischemic tissues was next analyzed in ApoE-/- mice that were subjected to femoral artery and vein ligation, a standard model of peripheral ischemia that mimics some aspects of human atherosclerosis [13]. The angiogenic response to delivery of three different doses (3, 5 and 10 μg) of VEGF from alginate gels was analyzed via immunohistochemical evaluation of blood vessel densities and determination of blood perfusion in the ischemic regions (Figure 2). Immunohistochemical analysis revealed that VEGF delivering gels increased blood vessel densities, compared with blank gels (no VEGF), but no statistically significant differences between the various doses of VEGF was noted (Figure 2A). Quantification of this staining confirmed that delivery of VEGF had a significant effect on vascularization (∼3 fold increase in blood vessel densities), as expected, compared with delivery of blank gels (Figure 2B). Animals treated with blank gels were incapable of recovering normal levels of hindlimb blood perfusion after surgery. In contrast, animals treated with alginate releasing VEGF165 displayed a significant recovery of regional blood flow over time. However, no significant differences were observed between the three different doses delivered (Figure 2C), suggesting that 3 μg was a saturating dose of VEGF in this model.
Figure 2.
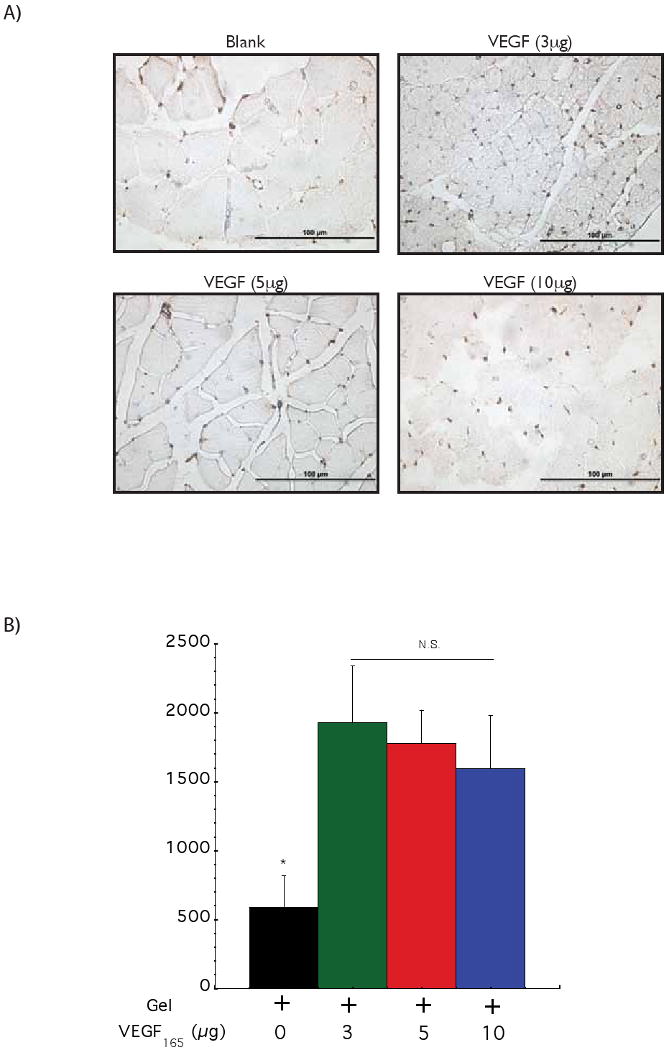

Monitoring the effect of VEGF dose in driving vascularization in ApoE-/- ischemic hindlimbs. Representative photomicrographs from CD31 immunostained sections of hindlimb muscle tissues (A). Quantification of blood vessel densities in hindlimb muscle tissues 6 weeks after treatment with a control blank gel (+ 0); a gel with 3 μg of VEGF (+ 3); a gel with 5 μg of VEGF (+ 5) and a gel with 10 μg of VEGF (+ 10) (B). No statistically significant differences were observed between the different VEGF doses. Tissue perfusion of ApoE-/- mice hindlimbs at various time points following treatment with a control blank gel (◇); 3 μg of VEGF delivered from alginate hydrogels (●); 5 μg of VEGF delivered from alginate hydrogels (○) and 10 μg of VEGF delivered from alginate hydrogels (□) (C). Mean values are presented with standard deviations and * indicates statistically significant differences (p<0.05), as compared to control gels. N.S. displays no statistically significant difference between conditions.
Effects of VEGF spatial distribution in ischemic ApoE-/- hindlimb model
The effects of delivering VEGF from hydrogels placed at two distinct spatial locations (proximal and distal to the ischemic region), as contrasted to the standard single gel injection, on vascularization and perfusion of ischemic tissues was next analyzed in the same animal model described above. Immediately after surgery animals were injected with 50 μl hydrogels gels containing VEGF165 (3 μg) or blank gels. The total gel volume was either injected into the region A – the area proximal to the site of femoral artery ligation, or the gel volume was split into two equal volume injections – one injection in region A and the other in region B, which was an area more distal to the site of femoral artery ligation. After 42 days postoperative, tissue sections were immunostained to identify blood vessels (Figure 3A). Quantification of the blood vessel densities demonstrated no statistically significant difference between the animals treated with one versus two gel injections (Figure 3B). As expected, animals treated with gels loaded with VEGF demonstrated a significant recovery of regional blood flow over time, in contrast to the animals treated with blank gels. Interesting, animals treated with two gel injections demonstrated complete recovery of regional blood perfusion to normal levels, as compared with the ∼80% recovery observed for the animals treated with a single gel injection (Figure 3C).
Figure 3.
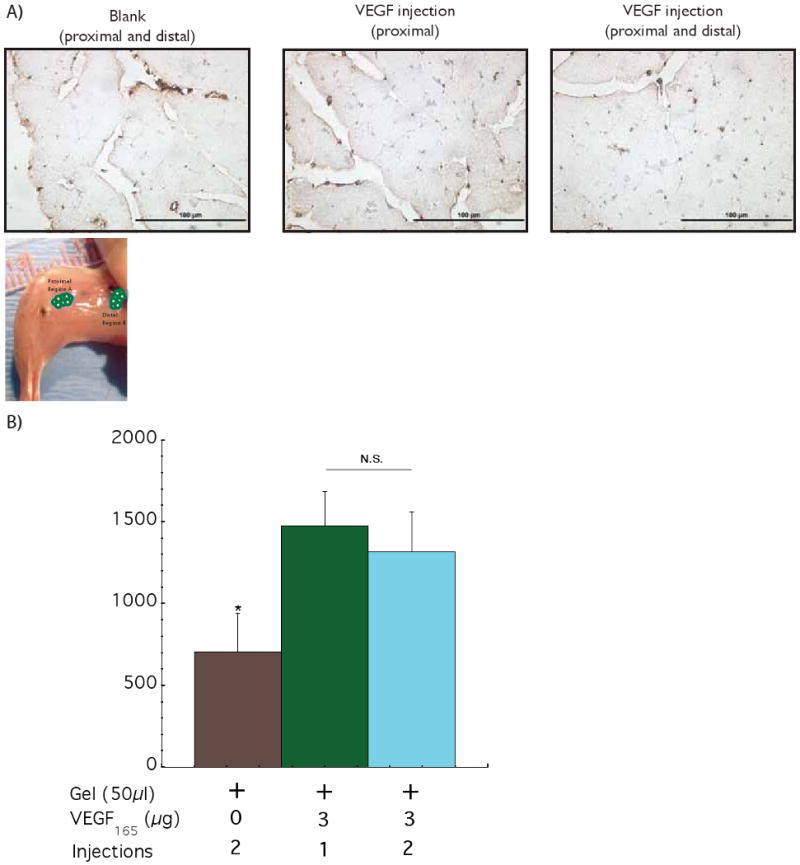
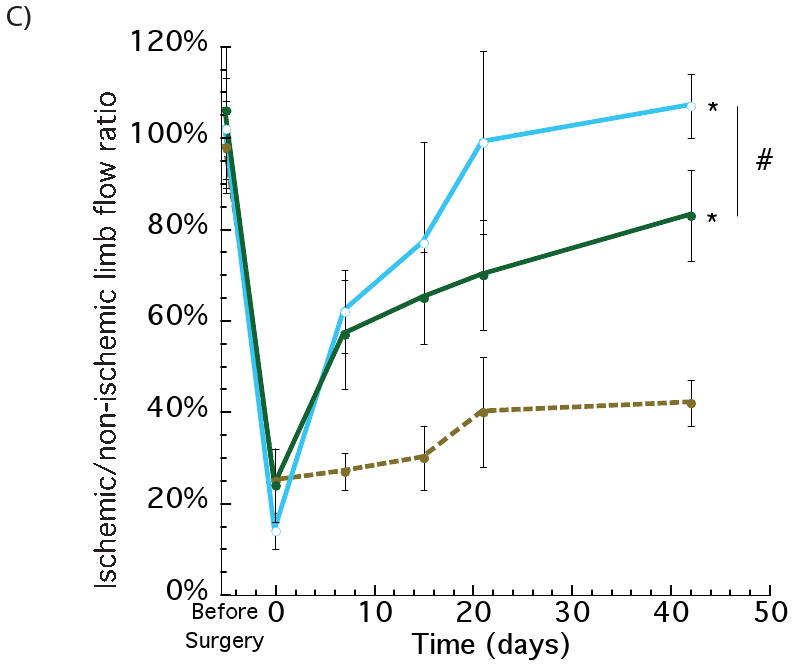
Assessing the effect of spatial distribution of VEGF delivery on the ischemic ApoE-/- hindlimbs. Photomicrographs of representative tissue sections from hindlimbs of ApoE-/- mice at postoperative 42 days, immunostained for the endothelial marker CD31 (A). Quantification of blood vessels densities after 6 weeks with 2 injections (proximal and distal) of control blank alginate hydrogel (+ 0 2); 1 injection of alginate loaded with VEGF (proximal) (+ 3 1) and 2 injections of alginate loaded with VEGF (proximal and distal) (+ 3 2) (B). Similar values of blood vessel densities were obtained for the 1 and 2 injections of VEGF. Perfusion profiles of hindlimbs at various experimental time points with 2 injections of control blank gel (●); VEGF delivered from alginate gels (1 injection - proximal) (●) and VEGF delivered from alginate gels (2 injections – proximal and distal) (○) (C). The two injections of VEGF elicited an increase in the regional perfusion, as compared with a single injection (VEGF loaded), even though the total VEGF dose was the same between the two conditions. Mean values are presented with standard deviations, * indicates statistically significant differences (p<0.05), as compared to control gels, and # represents statistically significant differences (p<0.05) between conditions.
Discussion
The results of this study confirmed that VEGF is capable of governing in vitro EC phenotype in a manner dependent on both its dose and temporal presentation. The proliferation studies revealed that the mitogenic potential of VEGF saturated at 50 ng/ml, suggesting that this concentration is likely to be the optimal dose of VEGF in vitro, in agreement with previous studies [21, 22]. The effect of VEGF temporal presentation on EC sprouting formation was tested by using different dose distributions. High levels of VEGF at early time points resulted in a marked increase in EC sprouting. In contrast, a constant VEGF dose over time or a gradually increasing VEGF dose resulted in noticeably less sprouting. The level of EC sprouting resulting from a constant VEGF presentation over time has been studied [23] as has the importance of VEGF gradients [24], but the importance of VEGF temporal gradients has been neglected. No differences were observed regarding sprouts length with the various treatments, suggesting that either sprouting proceeds autonomously once initiated, or that other factors are needed to promote further maturation and elongation of the structures initiated by VEGF exposure.
Determining the optimum dose of VEGF clearly remains a critical factor in controlling angiogenesis in vivo. In this study we demonstrated that a total dose 3 μg per animal (0.1 μg/g) of VEGF provides a favorable therapeutic dose for this particular atherosclerosis murine hindlimb model. Several past studies have suggested that there is an optimal therapeutic window for VEGF delivery [8, 24] Also, other previous studies have demonstrated that higher doses of VEGF (0.14 - 0.33 μg/g) resulted in an increased level of neovascularization in vivo [25, 26]. However VEGF was delivered in a bolus formulation in those studies. More recently, a relatively low dose of VEGF (0.04 μg/g) was reported to be sufficient to induce significant angiogenesis in vivo by using an alginate gel loaded with PLGA microspheres, however no information on higher doses was provided [27]. Similar to our findings, previous studies have demonstrated that polymer systems that provide a sustained and controlled released of angiogenic factors result in significant benefit in terms of eliciting neovascularization, as compared with simple protein aqueous formulations [7, 9, 16, 27, 28].
Interestingly, binary gel injections led to greater perfusion, even though the increase in blood vessel density was similar to a single injection. This likely resulted from either the initial angiogenic response occurring in a larger tissue volume, resulting in significant increases in blood vessel formation throughout a greater percentage of the ischemic limb, and/or enhanced remodeling of the initially formed vasculature into a more functional network [11] with spatially distrubuted VEGF delivery. Finally, this work showed that the appropriate therapeutic dose distributed in a binary spatial fashion resulted in superior regional blood perfusion and neovascularization. These results stress the need to gain spatiotemporal control over the VEGF presentation in vivo and are in agreement with previous studies that suggest control over local distribution can improve the efficacy of VEGF delivery without a need to change the total dose [8, 29, 30].
Conclusions
The results of this study indicate that temporal gradients of VEGF, and in particular a high early concentration followed by lower concentrations, is capable of inducing more endothelial cell sprouting in vitro than a constant VEGF concentration. It is also demonstrated that a total VEGF dose 0.1 μg/g, when delivered with kinetics found to be optimal in vitro, provides a favorable therapeutic dose in the common pre-clinical model of atherosclerotic murine hindlimb ischemia, and that distributing this VEGF dose in two spatial locations induces a higher level of vascularization and perfusion than a single injection.
Acknowledgments
The authors thank the National Institutes of Health for financial support of this research (RO1 HL069957), and the Biological Resources Branch of the National Cancer Institute (NCI) for generously providing VEGF used in our studies. E.A.S. was supported by a predoctoral fellowship from Fundacao para Ciencia e Tecnologia - FCT (SFRH/BD/9613/2002) and by the Gulbenkian PhD Program in Biomedicine, Portugal for part of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 3.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 8.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 10.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 12.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ Res. 2004;94:1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 13.Couffinhal T, Silver M, Kearney M, Sullivan A, Witzenbichler B, Magner M, et al. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation. 1999;99:3188–3198. doi: 10.1161/01.cir.99.24.3188. [DOI] [PubMed] [Google Scholar]
- 14.Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 15.Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, Lin AS, et al. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21:3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 17.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 18.Hoefer IE, van Royen N, Jost MM. Experimental models of arteriogenesis: differences and implications. Lab Anim. 2006;35:36–44. doi: 10.1038/laban0206-36. [DOI] [PubMed] [Google Scholar]
- 19.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 20.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Christenson LK, Stouffer RL. Isolation and culture of microvascular endothelial cells from the primate corpus luteum. Biol Reprod. 1996;55:1397–1404. doi: 10.1095/biolreprod55.6.1397. [DOI] [PubMed] [Google Scholar]
- 22.Conn G, Soderman DD, Schaeffer MT, Wile M, Hatcher VB, Thomas KA. Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci U S A. 1990;87:1323–1327. doi: 10.1073/pnas.87.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatsu MN, Sainson RC, Perez-del-Pulgar S, Aoto JN, Aitkenhead M, Taylor KL, et al. VEGF(121) and VEGF(165) regulate blood vessel diameter through vascular endothelial growth factor receptor 2 in an in vitro angiogenesis model. Lab Invest. 2003;83:1873–1885. doi: 10.1097/01.lab.0000107160.81875.33. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, et al. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation. 1995;92:II365–371. doi: 10.1161/01.cir.92.9.365. [DOI] [PubMed] [Google Scholar]
- 26.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 28.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 29.Banfi A, von Degenfeld G, Blau HM. Critical role of microenvironmental factors in angiogenesis. Curr Atheroscler Rep. 2005;7:227–234. doi: 10.1007/s11883-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 30.von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, et al. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20:2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]


