Abstract
Invasive fungal infections can be devastating, particularly in immunocompromised patients, and difficult to treat with systemic drugs. Furthermore, systemic administration of those medications can have severe side effects. We have developed an injectable local antifungal treatment for direct administration into existing or potential sites of fungal infection. Amphotericin B (AmB), a hydrophobic, potent, and broad-spectrum antifungal agent, was rendered water-soluble by conjugation to a dextran-aldehyde polymer. The dextran-aldehyde-AmB conjugate retained antifungal efficacy against C. albicans. Mixing carboxymethylcellulose-hydrazide with dextran-aldehyde formed a gel that cross-linked in situ by formation of hydrazone bonds. The gel provided in vitro release of antifungal activity for 11 days, and contact with the gel killed Candida for three weeks. There was no apparent tissue toxicity in the murine peritoneum and the gel caused no adhesions. Gels produced by entrapment of a suspension of AmB in CMC-dextran without conjugation of drug to polymers did not release fungicidal activity, but did kill on contact. Injectable systems of these types, containing soluble or insoluble drug formulations, could be useful for treatment of local antifungal infections, with or without concurrent systemic therapy.
Introduction
Fungal infections are a serious threat to many patient populations, particularly the immuno-compromised [1]. Treatment of localized fungal infections, such as osteoarticular infections, can be particularily refractory, perhaps because they take on an abcess-like or granulomatous form where they are relatively sequestered from circulating drugs. Infection of medical devices can necessitate their removal. Furthermore, systemically delivered antifungal drugs can have severe and potentially lethal side effects.
Controlled release systems can address both issues. Consequently, there has been increasing interest, in both research and clinical practice, in providing localized treatments of infectious processes, e.g. delivery of gentamicin using various materials as delivery systems [2, 3]. Specifically antifungal devices have included antifungal hydrogels for prophylaxis [4] and a range of topical creams for epidermal [5], buccal [6], or vaginal application [7], and medical cements containing antifungal drugs [8]. By providing a drug depot at the site of infection, such devices have the potential to achieve very high local drug concentrations without significant systemic distribution [9].
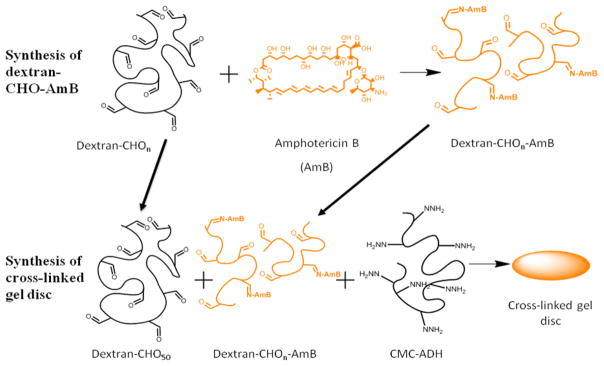
Treatment of localized infections within the body without having to resort to surgery requires that the drug delivery system be injectable. However, since control of drug release is closely related to drug viscosity, formulations that are easy to inject often control drug release poorly [9]. In order to allow the creation of a high-viscosity matrix while maintaining ease of injection, we have used a hydrogel composed of dextran and cellulose that cross-links in situ, forming hydrazone bonds [10]. This approach maintains the hydrogel at the site of injection. Tissue reaction to cellulose-dextran gels has been previously shown to be benign and to prevent adhesions when injected into the peritoneum of rabbits, and the crosslinking chemistry of hydrazone bond formation in vivo does not have any adverse tissue effects [11,12]. Here, to control the release of the antifungal from the hydrogel, we conjugated a potent antifungal agent, amphotericin B (AmB) to that in situ cross-linking hydrogel (Scheme 1), and determined its antifungal activity and intraperitoneal biocompatibility
Scheme 1.
Conjugation of AmB to oxidized dextran and the incorporation of the dextran-CHO-AmB into a CMC-Dextran gel.
Materials and Methods
Materials
Dextran (100–200 kDa), carboxymethylcellulose (250 kDa, medium viscosity), sodium periodate, polyethylene glycol, sodium hydroxide, hydroxylamine, adipic dihydrazide, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC), 1-hydroxybenzotriazole (HOBt), amphotericin B (AmB), glucose, histidine and leucine were obtained from Sigma Aldrich (St. Louis, MO, USA). Phosphate buffered saline (pH 7.4) was obtained from Invitrogen. Yeast nitrogen base (YNB) and yeast extract peptone (YEP) media from Difco (Beckton, Dickinson and Company, Franklin Lakes, NJ, USA) were used.
Oxidation of dextran (to dextran-aldehyde; dextran-CHO)
The oxidation was conducted as reported [13]. Dextran (3 g) was dissolved in 300 ml of deionized water and sodium periodate (1.604 g) suspended in 10 ml of deionized water was added dropwise with vigourous stirring – the reaction mixture was stirred for a further 2 hours. Polyethylene glycol (800 μl) was added to stop the reaction and the reaction mixture was stirred for an additional hour. The solution was dialysed against deionized water in dialysis membranes of 3,500 MWCO, followed by lyophilization. The extent of oxidation was calculated by stirring 0.1 g of oxidized polymer in a 0.25 M solution of hydroxylamine hydrochloride (25 ml) for 3 hours and then titrating against 0.1 M sodium hydroxide [13]. The extent of oxidation was controlled by the amount of sodium periodate added to the reaction mixture, Table 1. The oxidized dextran is called dextran-CHOn where n refers to the number of aldehyde groups per 100 units of glucose (dextran subunits) units.
Table 1.
Characterisation of polymer components by weight average and number average molecular weights (Mw and Mn), the degree of functionalisation by hydrazine (CMC) and the degree of oxidation of the dextrans before and after conjugation with amphotericin B.
| Polymer | Mwa (kDa) | Mna (kDa) | NaIO4 (mol %)c | Measured degree of functionalisation |
|---|---|---|---|---|
| CMC | 250b | - | - | - |
| CMC-ADH | 810.3 | 77.7 | - | 51 % |
| Dextran | 100–200b | - | - | - |
| Dextran-CHO33 | 27.6 | 6.8 | 45 | 35 %d |
| Dextran-CHO50 | 21.4 | 5.7 | 64 | 48 %d |
| Dextran-CHO66 | 19.4 | 5.0 | 90 | 66 %d |
| Dextran-CHO75 | 18.0 | 5.0 | 96 | 75 %d |
| Dextran-CHO33-AmB12.5 | 8.0e | 3.1e | - | 15 %d |
| Dextran-CHO33-AmB62.5 | 8.1e | 3.1e | - | 5 %d |
| Dextran-CHO66-AmB62.5 | 7.9e | 2.6e | - | 13 %d |
| Dextran-CHO75-AmB62.5 | 8.3e | 2.5e | - | 18 %d |
Relative to Pullulan standards,
According to provider,
Mol% defined as [IO4-]/[glucose] × 100
Number of aldehyde groups per 100 glucose units,
Contained multiple components.
Functionalization of carboxymethylcellulose (to CMC-hydrazide; CMC-ADH)
The functionalization of carboxymethylcellulose with hydrazine groups was carried out using the method published by Bulpitt et al [10]. Carboxymethylcellulose (1 g, medium viscosity) was dissolved in 200 ml of deionized water. Adipic dihydrazide (3 g) was added and the pH was measured to be at 7.4. HOBt (258 mg suspended in 2 ml of a dimethylsulfoxide:water (1:1) mixture) was then added followed by EDC (262 mg in 2 ml of a dimethylsulfoxide:water (1:1) mixture). The pH was adjusted to 6.8 and kept there by adding small amounts of 0.1 M sodium hydroxide when necessary while stirring for 6–7 hours. The solution was dialysed against deionized water using 10,000 MWCO membranes, followed by lyophilization. The degree of functionalization was then deduced by the nitrogen content using CHN elemental analysis of the polymer and 1H NMR analysis. Elemental analysis is described below. For 1H-NMR analysis, the polymer was dissolved in D2O and the analysis was conducted on a Varian Unity 300 spectrophotometer.
Conjugation of AmB to dextran-CHO (dextran-aldehyde)
The conjugation was conducted by a modification of the method of Falk et al [14]: Dextran-CHO (750 mg) and AmB were dissolved in 0.2 M sodium tetraborate buffer (10 ml) adjusted to pH 11 with 2 M NaOH and stirred at 37°C for 2 hours in the dark. The solution was then dialysed using membranes (3500 MWCO) at 4°C in 5 l of deionized water in the dark with a minimum of 8 water changes with 4 hours between each water change. The solution was then centrifuged at 2,000 rpm for 10 minutes to ensure removal of any precipitated AmB and the solution was freeze dried and stored at 4°C in the dark before use. The nomenclature dextran-CHOn-AmBm was used where n refers to the number of aldehyde groups per 100 glucose units (%) of the oxidized dextran used and m referes to the loading of AmB (in mg/g) on the dextran-CHO. The n values used are the experimental oxidation values while the m values are the theoretical values for the amount of amphotericin B conjugated to the polymer. The UV absorption spectra, minimum inhibitory concentrations against C. albicans and extinction coefficients of dextran-CHOn-AmBm with varying m and n were examined and compared.
Molecular Weight Measurements
The molecular weight of CMC-ADH, dextran-CHO33, dextran-CHO66 and dextran-CHO75 were deduced by size exclusion chromatography (SEC) using the Waters Alliance 2695 Analytical HPLC coupled with a Waters 2410 Refractive Index Detector (LIMS 899) and a PL Aquagel-OH Mixed 8μm column (Intertek ASG, Manchester, UK). These samples were run with 0.1 M sodium nitrate eluent at a rate of 1 ml/min for a run time of 30 minutes. The molecular weights of dextran-CHO33-AmB62.5, Dextran-CHO66-AmB 62.5 and Dextran-CHO75-AmB62.5 were measured on the same system using a TSK Gel G2000 SWxl column and were run with with 0.1 M sodium nitrate eluent at a rate of 0.5 ml/min for a run time of 30 minutes. The molecular weights were calculated based on pullulan standards. SEC was carried out by Intertek ASG, Manchester, UK.
Elemental Analysis
Elemental analysis (CHN) of the CMC-ADH, dextran-CHOn and dextran-CHOn-AmBm polymer-drug conjugates was conducted by completely burning the polymer/polymer-drug conjugate at 1800 °C, and measuring the concentrations of CO2, N2, and H2O with a CHN analyzer (PE-2400II; Perkin-Elmer, Waltham, MA, USA). The weight percentage of C, H, and N was determined based on the measured gas composition.
Formation of blank and AmB loaded CMC-Dextran gels
Dextran-CHO50 (60 mg) was dissolved in 1 ml of PBS over a few hours. CMC-ADH (25 mg) was dissolved in 1 ml of PBS overnight. Each polymer was loaded separately into a 1 ml syringe and placed in a double barrel injector with one needle (21 guage) at the tip. The gels were injected into a 6 mm diameter and 3 mm high rubber mold between two glass slides and allowed to set for 30 minutes at 37 °C. Gelation occurs in 2–3 seconds. When adding AmB, the AmB formulation was suspended (insoluble form) or dissolved (souble dextran-CHOn-AmBm formulation) in the dextran-CHO50 solution before loading into the syringe. For the insoluble AmB, 3 mg of drug was suspended in 1 ml of dextran-CHO50 solution. For the soluble dextran-CHOn-AmBm, an equivalent amount of AmB was added, e.g. 48 mg of dextran-CHO33-AmB62.5 was dissolved in the dextran-CHO50 solution.
Characterization of CMC-Dextran gels
Scanning electron microscopy was conducted using a FEI/Philips XL30 FEG ESEM (Oregon, USA). Samples were lyophilized before SEM analysis. SEM images were analyzed using Gatan Digital Micrograph Version 3.6.5 (Warrendale, PA, USA). Mechanical strength compression testing was conducted using an Instron (Model 1011, High Wycombe, UK) with a 100 N load cell. The swelling rate and rate of degradation of the CMC-Dextran gels was assessed by incubating each gel (6 mm × 3 mm cylindrical disc) in PBS (1 ml) and shaking at 37°C in the dark. The gel disc was weighed and placed into fresh PBS every 24 hours. Gel discs were also lyophilized and their swelling ratio from dry mass to wet mass in PBS with shaking at 37°C in 24 hours was determined.
In vitro release kinetics from CMC-Dextran gels
Gel discs containing AmB (1.5 mg/ml), dextran-CHO33-AmB62.5 (24 mg/ml), dextran-CHO66-AmB62.5 (24 mg/ml) and dextran-CHO75-AmB62.5 (24 mg/ml) were prepared and placed in 1 ml of PBS in a 24 well plate. The gels were shaken at 150 rpm in an incubator at 37°C in the dark and were placed in a new well containing fresh PBS every 24 hours. The PBS was then analysed by UV-visible spectroscopy for dextran-CHOn-AmBm content.
Antifungal activity assay [4]
Growth of C. albicans strain SC5314 was regrown from frozen stocks from the Whitehead Institute. In all cases, C. albicans was maintained on YEP–agar plates. For experiments, YEP (2x, 50 ml) was inoculated with one colony of Candida overnight. After that time, the suspension was centrifuged (5 min at 8000 g), the supernatant was discarded, and the cells were washed twice with PBS 7.2 (50 ml). The cells were counted on a hemocytometer and diluted to 0.5–1 × 107 or 2–4 × 107 cells/ml in YNB medium with 50 mM glucose. The actual measure of fungal survival was a colony growth assay. The details of these experiments follow.
Drug Potency
To quantify the minimum inhibitory concentration (MIC) of each AmB drug formulation, 225 μl of drug solution was added to 75 μl of fresh YNB containing 2–4 × 107 C. albicans SC5314 cells/ml. After 2 h the suspension was diluted to a concentration of 1:1,000, 200 μl were plated onto YEP agar plates, and incubated at 37°C for 24 h, after which viable colonies were counted. Control samples were prepared by adding 225 μl of YNB to 75 μl of YNB containing 2–4 × 107 cells/ml C. albicans SC5314. The drug solutions/suspensions were tested at different concentrations and at different time points after shaking (150 rpm) at 37°C in the dark. Before testing each solution/suspension was centrifuged ((5 min at 8000 g).
Halo Test
200 μl of fresh YNB containing 0.5–1 × 107 cells/ml C. albicans SC5314 were plated onto a YEP-agar plate. The gel disc (6 mm × 3 mm, 150 mg) being tested was placed onto the centre of the plate and incubated for 24 hours at 37°C in the dark before being examined for a “halo” or “zone of inhibition” surrounding the gel disc.
Fungicidal effect of direct exposure to the gels
The gels (6 mm × 3 mm, 150 mg) were placed in the wells of a 24-well tissue culture plate with 1ml of the Candida suspension (0.5–1 × 107 cells in YNB). The disks were incubated for 2 h, at 37°C while shaking at 100 rpm. Then the disks were removed, and the remaining medium was vigorously stirred and then diluted to a concentration of 1:1,000. Next, 200 μl of the diluted medium were plated on YEP agar plates. The YEP plates were incubated at 37°C for 24 h, and colonies were counted.
Fungicidal effect of drug release
To assess the fungicidal effect of the AmB formulation released from the gel, hydrogels were immersed in 1 ml of YNB medium with 50 mM glucose and incubated at 37°C at 100 rpm. The incubation medium was removed completely, and the gel was immersed in fresh medium daily. To 225 μl of YNB medium collected at each time point, we added 75 μl of fresh YNB containing 2–4 × 107 C. albicans SC5314 cells/ml. After 2 h the suspension was diluted to a concentration of 1:1,000, 200 μl were plated onto YEP agar plates, and incubated at 37°C for 24 h, after which viable colonies were counted. Control samples were prepared by adding 75 μl of YNB containing 2–4 × 107 cells/ml C. albicans SC5314 to 225 μl of YNB.
The lower detectable limit of candidal survival due to the dilution steps in the assays described above was 0.2 %.
Hemolysis assay
0.2 ml of AmB and dextran-CHOn-AmBm suspensions or solutions at concentrations ranging from 5 μg to 1 mg/ml were incubated in human whole blood with shaking for 1 hr. The samples were then centrifuged at 3000 rpm for 20 minutes and the concentration of hemoglobin (absorbance at 545 nm) in the supernatant was determined by UV absorption spectroscopy. A negative control of PBS and positive control of deionized water were used.
In vivo studies
All the animals were cared for in compliance with protocols approved by the Animal Care and Use Committee at the Massachusetts Institiute of Technology, and the Principles of Laboratory Animal Care (NIH publication #85-23, revised 1985). SV129 mice weighing 25 g were purchased from Charles River Labs (Wilmington, MA) and housed in groups in a 6 AM – 6 PM light dark cycle. CMC-ADH and dextran-CHO were sterilized by UV irradiation for 2 h and then dissolved in sterile phosphate buffered saline (CMC-ADH 2.5 % w/v, dextran-CHO50 6% w/v). Anaesthesia was induced briefly, prior to injection, with isofluroane in 100% oxygen. Each mouse received one intraperitoneal injection, from a double barrel syringe, of 1 ml of hydrogel. The three experimental groups were (a) CMC-ADH (12.5 mg), dextran-CHO50 (30 mg), (b) CMC-ADH (12.5 mg), dextran-CHO50 (30 mg), dextran-CHO33-AmB62.5 (0.96 mg) and (c) CMC-ADH (12.5 mg), dextran-CHO50 (30 mg), dextran-CHO66-AmB62.5 (0.96 mg). There were three mice in each group. One mouse from each group was sacrificed 7, 11 and 33 days after injection. Tissues recovered from the necropsy were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for histological examination using standard techniques.
Results
A formulation of amphotericin B (AmB) for treating localized infections in an injectable gel form was achieved by combining two techniques: the solubilization of AmB by the conjugation of the drug to dextran [15] and incorporation into an injectable crosslinking gel. Modifications of the dextran are described as dextran-CHOn-AmBm, where n refers to the number of aldehyde groups per 100 units of glucose (dextran subunits) units and m is the milligrams of AmB per gram of dextran-CHO. CMC-dextran refers to crosslinked gels formed by mixing carboxymethylcellulose-hydrazide (CMC-ADH) and dextran-aldehyde (dextran-CHO50).
Characterization of uncrosslinked AmB formulations
Amphotericin B
In methanolic solution, spectroscopy of AmB showed well resolved peaks at 365, 387 and 406 nm (Fig. 1). An aqueous suspension of AmB exhibited a poorly resolved spectrum (Fig. 1), due to its insolubility. The particle size of the aqueous AmB suspension was ~ 1000 ± 100 nm, in PBS and dextran-CHO50 (6% in PBS).
Figure 1.
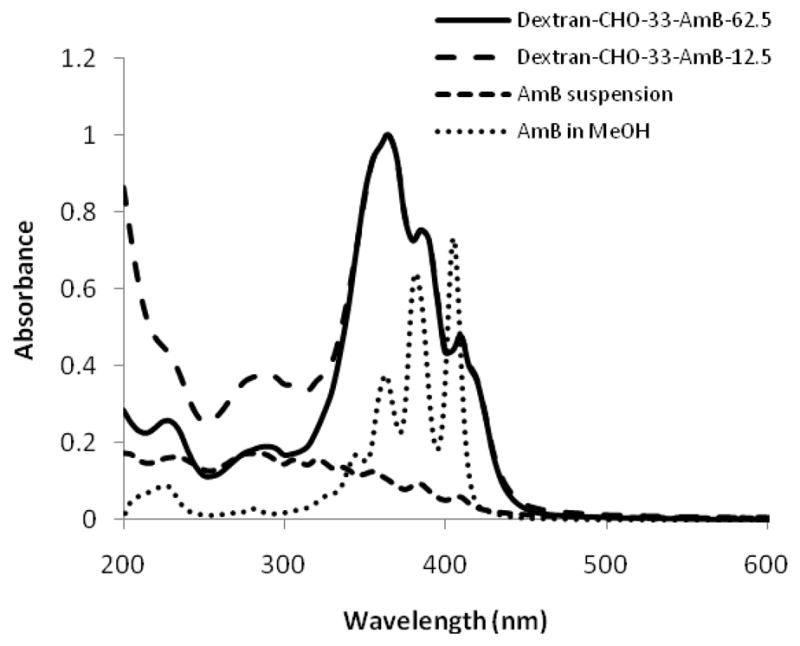
UV-visible absorption spectra of dextran-CHO33-AmB62.5, dextran-CHO33-AmB12.5 (normalised), AmB suspension (after centrifugation) in PBS and AmB in methanol.
Oxidized dextrans
Dextran was modified such that there were 33–75 % of aldehyde groups present on the polymer (Table 1). Dextran-CHOn did not show any absorption over the wavelength range of 300–500 nm, the range at which peaks were seen in the methanolic solution of AmB and the aqueous solutions of dextran-CHOn-AmBm. The molecular weight of the dextran was reduced significantly by oxidation with sodium periodate and the more highly oxidized polymers had the lowest molecular weights, e.g. dextran-CHO33 and dextran-CHO75 had molecular weights of 28 and 18 kDa respectively (Table 1). The degree of oxidation of dextran, measured by reaction with hydroxylamine hydrochloride, was much lower than that predicted from the mole percentage of periodate added, Table 1. This may be due to the due to the formation of a stable six-membered hemiacetal which protects the aldehydes from further reaction [13].
Conjugates of AmB to dextran
The free amine group of AmB reacted with the aldehyde groups of the oxidized dextrans, forming an imine group [14]. When 50 mg of AmB was conjugated to 750 mg of dextran-CHO33, no AmB precipitated during dialysis or centrifugation before lyophilization, suggesting complete conjugation to the dextran-CHO33 (i.e. 62.5 mg AmB per gram of dextran-CHO33). Elemental analysis showed that nitrogen was 0.11 ± 0.03 % of the mass, which was close to what would be expected stoichiometrically (0.09%) if AmB were incorporated in proportion to the quantities of reagents added. Up to 100 mg of AmB could be loaded on to 750 mg of dextran-CHO33 without precipitation; higher loadings were not attempted. The amount of free aldehyde groups remaining on the oxidized dextrans after reaction to AmB (Table 1), measured by reaction with hydroxylamine, was lower than expected from the number of moles of AmB added. Possibly AmB, a relatively large molecule, masked free aldehyde groups from hydroxylamine, giving a falsely low measurement. After the conjugation reaction, the weight average molecular weight of the the dextran-CHO polymers decreased from 18–28 kDa to 8.0 kDa (Table 1). One possible explanation for this is that the physicochemical properties (e.g. intrinsic viscosity and hydrophobicity) of the dextran-CHO polymer may have been altered by the conjugated amphotericin B moieties, which may have lengthened the elution time of the dextran-CHOn-AmBm polymer drug conjugates from the column, resulting in lower calculated molecular weights.
After conjugation of AmB to dextran-CHO, peaks were seen at wavelengths very similar to those seen with AmB dissolved in methanol (362, 385 and 410 nm), but the intensity ratio of the peaks was inverted (Fig. 1). Nishi et al reported that the UV-visible spectra of polyenes are very sensitive to conformational changes induced by different molecular interactions, including aggregation [16]. Thus the dextran polymer can influence the UV-visible spectra of the dextran-CHOn-AmBm formulations (Fig. 1) and the UV-visible spectral changes observed after conjugation of AmB to dextran-CHO may not be solely attributable to aggregation effects.
When the degree of oxidization of the dextran polymer was increased to 66% and the AmB loading kept the same, the UV-visible absorption spectra and MICs remained the same as for dextran-CHO33-AmB62.5, (Fig. S1–S2), although the number of free aldehyde groups present on the dextran-CHO-AmB62.5, measured by hydroxylamine, increased from 6% to 15% (Table 1). Higher amounts of free aldehyde groups will allow for greater crosslinking of the dextran-CHO-AmB to CMC-ADH when incorporated into the CMC-dextran gels. Similarly the extinction coefficient of dextran-CHOn-AmB62.5 at 362 nm (10.5 ± 1.1 mg of dextran-CHOn-AmBm/ml cm−1) was independent of the degree of oxidation (Fig. S3). This extinction coefficient was used to determine the amount of dextran-CHOn-AmBm released over time in vitro from the CMC-dextran gels. Thus, varying the degree of oxidation of the dextran polymer used to solubilise the AmB did not alter the structure (as observed by UV-visible spectroscopy) or antifungal activity of the final drug-polymer conjugate.
AmB could bind nonspecifically to unmodified dextran, in that the polymer turned yellow when mixed with AmB solution, and the polymer then acquired antifungal activity. However, AmB precipitated during dialysis when as little as 10 mg were combined with 750 mg of dextran, and the antifungal activity was much lower per unit of total mass than when conjugated to dextran-CHO33-AmB12.5.
Antifungal activity
The antifungal potencies of a suspension of AmB, dextran-CHO33-AmB12.5 and dextran-CHO33-AmB62.5 were compared (Fig. 2). Dextran-CHO33-AmB62.5 was more potent than the suspension of AmB, with a minimum inhibitory concentration (MIC) of 3 μg/ml of AmB (0.048 mg/ml of dextran-CHO33-AmB62.5 conjugate) while the AmB suspension did not kill below 10 μg/ml (Fig. 2). Dextran-CHO33-AmB12.5 and dextran-CHO33-AmB62.5 were roughly equipotent in antifungal activity for a given concentration of AmB present. Consequently, dextran-CHO33-AmB62.5, was five times more potent per unit mass than dextran-CHO33-AmB12.5.
Figure 2.

Antifungal activity of AmB in suspension, dextran-CHO33-AmB62.5 and dextran-CHO33-AmB12.5. The lower detectable limit of candidal survival was 0.2 %. (The x-axis shows the concentration of AmB present on polymer conjugate or in suspension)
To assess the stability of antifungal activity, a solution of dextran-CHO33-AmB62.5 and a suspension of AmB were incubated in darkness at 37°C with continuous mechanical agitation, and aliquots of these compounds were added to a fungal culture at predetermined time points. At a concentration of 0.1 mg/ml, AmB retained its fungicidal properties for 40 days in YNB media, while 1.6 mg of dextran-CHO33-AmB62.5 per ml of YNB began to lose its antifungal activity by day 28, (Fig. 3).
Figure 3.

Antifungal activity over time of 0.1 mg/ml AmB and 1.6 mg/ml dextran-CHO33-AmB62.5. The lower detectable limit of candidal survival was 0.2 %.
Hemocompatibility
It has been reported that hemolysis occurs with monomeric but not aggregated forms of AmB [17]. None of our formulations - the AmB suspension or the dextran-CHOm-AmBn conjugates - were hemolytic at concentrations of 5 – 1000 μg/ml of AmB. The size of the aggregates in the AmB suspension was ~1 μm and resembled the poly-aggregate form described by Espada et al in that hemolysis did not occur [17].
CMC-Dextran in situ crosslinking gels
CMC-ADH was synthesized as in Methods. Elemental analysis of the carbon, hydrogen and nitrogen content of CMC-ADH indicated a degree of substitution of 51 % and NMR analysis showed the presence of the methylene groups of adipic dihydrazide (1H NMR 1.6 ppm (s) and 2.3 ppm (d)). Gel discs (Fig. 4) were made by mixing CMC-ADH (2.5% w/v) with dextran-CHO50 (6% w/v) leading to hydrazone bond formation [10] and gelation within seconds (see Methods). Dextran-CHO50 was selected for formation of all cross-linked gels below – dextran-CHO with 33 %, 66 % and 75 % aldehyde groups also formed good gel constructs, and could have been used as well. The viscosity of higher concentrations of CMC-ADH prevented good mixing with dextran-CHO50. Higher concentrations of dextran-CHO50 did not add to the strength of the gel formed as assessed by mechanical strength compression tests (data not shown) and thus were not deemed necessary. Gel discs (6 mm × 3 mm) retained their shape (Fig. 4a) for 3 weeks when suspended in PBS or media, and could withstand pressures of up to 4 N before breaking under a compressive strain. Hydrated gels swelled slowly to about 150 % of their original mass over 3 weeks. Lyophilized discs (Fig. 4b) swelled to 20 times their dry mass in PBS at 37°C, to 150 mg in 24 hours.
Figure 4.
(a) Photograph of cross-linked CMC-dextran hydrogel, (6 mm scale bar shown). (b) SEM of same, lyophilised, (20μm scale bar shown).
CMC-dextran-AmB gels; Formation and in vitro release studies
AmB dextran conjugate-containing gels were formed by combining CMC-ADH (25 mg/ml) with a mixture of dextran-CHO50 (60 mg) and dextran-CHOn-AmB62.5 (48 mg) per ml of PBS, by injection from a double barrel syringe. This mixture with dextran-CHO50 unconjugated to AmB was used to compensate for the relatively low number of aldehyde sites available for cross-linking in the AmB-conjugated dextrans (Table 1). (In other words, dextran-CHOn-AmBm was party entrapped in the hydrogel via covalent linkage to CMC-ADH, and partly by entrapment within the CMC-dextran hydrogel.) Gels of poor consistency were formed when CMC-ADH and dextran-CHO66-AmB62.5 were used without additional dextran-CHO50.
Upon release from the gel matrix after 6 days, the UV-visible spectrum of the release media was very similar to that of freshly dissolved dextran-CHOn-AmBm, (compare Fig. S1 and Fig. 5a). This implies that it is dextran-CHOn-AmBm and not free AmB that is released from the gels. The total amount of dextran-CHOn-AmB62.5 released over time was slightly higher with increasing degree of oxidation of the dextran (Fig. 5b). Lower degrees of oxidation may have resulted in less of the dextran-CHOn-AmB62.5 being conjugated to CMC-ADH.
Figure 5.

(a) UV-visible absorption spectra of dextran-CHO75-AmB62.5, dextran-CHO66-AmB62.5 and dextran-CHO33-AmB62.5 released after 144 hours from CMC-Dextran gel. (b) Release kinetics of dextran-CHOn-AmB62.5 from CMC-Dex gel discs into PBS at 37°C; data are means with standad devations (n = 4).
Gels containing the AmB suspension were formed by combining CMC-ADH (25 mg/ml) with a mixture of dextran-CHO50 (60 mg) and AmB (3 mg) per ml of PBS, by injection from a double barrel syringe. The release medium was changed and tested each day. UV-visible spectral analysis of the release media showed that very little AmB was released (data not shown).
Antifungal activity of AmB in CMC-dextran gels
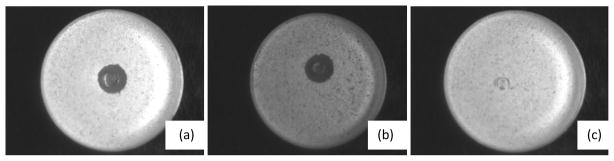
Gel discs were produced by cross-linking CMC-ADH with dextran-CHO50 containing a suspension of AmB or dextran-CHOn-AmBm as described above. The former was yellow and cloudy while the latter was translucent even though very yellow. Gel discs were placed on agar plates that had been inoculated with C. albicans and incubated at 37 °C in darkness. After 1 day, discs containing dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5 were surrounded by a clear halo, a zone free of fungal growth, which remained for four days until the plates were disposed of. There was no such halo around gels made with a suspension of AmB (Fig. 6).
Figure 6.
Petri plate assay for killing of Candida albicans. Each of the plates has a confluent lawn of cells except where the drug diffused out of the central gel disc and killed them leaving a clear zone. The gel discs consisted of CMC-dextran gels containing (a) dextran-CHO33-AmB62.5 (24 mg/ml), (b) dextran-CHO66-AmB62.5 (24 mg/ml) and (c) a suspension of AmB (1.5 mg/ml).
Separate gel discs were incubated in YNB media at 37 °C in darkness. At predetermined intervals, the media (with released fungicide; Fig. 7) were tested for antifungal activity. The release media were added to YNB media containing 107 colonies of C. albicans. Release media from gels containing a suspension of AmB exhibited very little fungal killing after day 1 while the release media from the gels containing dextran-CHO33-AmB62.5 killed for 3–4 days and those containing dextran-CHO66-AmB62.5 killed for as long as 11 days. The antifungal agent (dextran-CHOn-AmBm) is released as the CMC-dextran gels slowly swell and the hydrazone bond between the antifungal agent and the CMC-ADH is hydrolysed. The release media from blank gels had no antifungal activity.
Figure 7.
Antifungal activity of the release media from CMC-Dex gels containing AmB suspension, dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5, each formulation containing 1.5 mg AmB/mL upon gelation of CMC-Dextran gels. The release media (YNB) was changed and tested each day and stored shaking in darkness at 150 rpm at 37°C between tests. The lower detectable limit of candidal survival was 0.2 %.
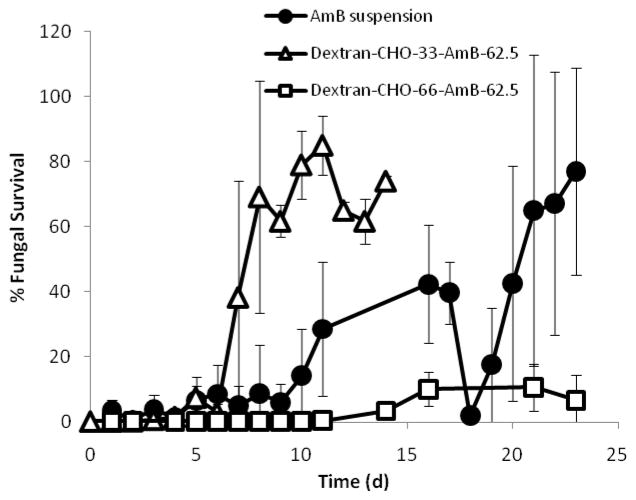
Similarly, gel discs incubated at 37 °C in darkness were placed in YNB media containing 107 colonies of C. albicans at predetermined intervals (Fig. 8). Gels containing dextran-CHO33-AmB62.5 stopped killing after 6 or 7 days, while those containing the more highly oxidized dextran-CHO66-AmB62.5 retained antifungal activity for longer than 3 weeks. Interestingly, discs containing a suspension of AmB exhibited strong antifungal activity for 3 weeks (Fig. 8), even though their release media had only shown antifungal activity for 1 day. Blank gels did not exhibit any antifungal activity and became infected with Candida after the first incubation period.
Figure 8.
Anti-fungal activity of CMC-dextran gels containing AmB suspension, dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5, each formulation containing 1.5 mg AmB/mL upon gelation of CMC-Dextran gels. The release media (YNB) was changed and tested each day and stored shaking in darkness at 150 rpm at 37°C between tests. The lower detectable limit of candidal survival was 0.2 %.
Intraperitoneal biocompatibility
Cross-linked CMC-dextran gels, made with dextran-CHO66-AmB62.5, with dextran-CHO33-AmB62.5 and blank gels were injected into the peritoneum with a double-barrelled syringe (see Methods section for details of groups and sample sizes). We selected the peritoneum for the assessment of biocompatibility as it is relatively prone to adverse tissue reactions [12] compared to subcutaneous tissue [18]. Furthermore, it resembles some of the spaces in which such formulations might be used clinically (peritoneum, pleura, synovium). We have previously shown that tissue reaction to CMC-dextran gels is benign, and in fact the gel prevents adhesions in the injured peritoneum [19]. However, the gels used here contained a high concentration of a hydrophobic drug, which could alter the biocompatibility.
Injected animals did not demonstrate abnormalities in feeding, grooming, or behavior during the time frame of the experiment nor did they exhibit signs of distress. On dissection 1 week, 11 days and 33 days after injection, all gels appeared to have crosslinked rapidly but not immediately, as the gel had spread throughout the peritoneum, coating the viscera (Fig. 9). The gels had a soft consistency, but also formed discrete collections. The gels were difficult to recover completely, but gross observation suggested that a significant fraction of gel mass was retained on day 33. No adhesions were observed at any time point in any group. Light microscopy of hematoxylin-eosin stained sections showed an absence of injury to mesothelium, smooth muscle or any other cell type (Fig. 10). The gels themselves showed relatively little inflammation; the inflammation in Fig. 10(a) is on the high end of what was seen. Inflammatory cells were largely macrophages with occasional neutrophils and plasma cells. Lymphocytes were seen at later time points, as were giant foreign body cells around small collections of gel. The looseness of the association between the gel and the tissues was reflected in the fact that very few slides showed gel remaining in contact with tissue.
Figure 9.
CMC-Dex gels 11 days after intraperitoneal injection. (a) blank, (b) dextran-CHO66-AmB62.5 and (c) dextran-CHO33-AmB62.5. Arrows indicate remaining gel mass.
Figure 10.
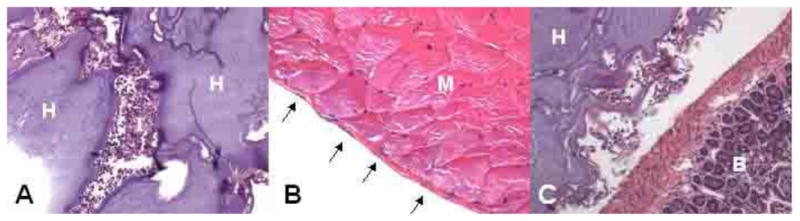
Photomicrographs of hematoxylin-eosin stained sections of injected CMC- dextran-CHO66-AmB62.5 11 days after injection A. Inflammatory reaction to a collection of hydrogel in the peritoneal cavity (magnification 200X); most inflammation seen was less than this. B. Intact peritoneal mesothelium (the thin layer of cells overlying the abdominal wall musculature). C. Interface between gel and the outside of the bowel. H: hydrogel, I: inflammation, M: abdominal wall muscle, B: bowel, arrows: nuclei of mesothelial cells.
Discussion
We produced and characterized a variety of novel fungicidal formulations incorporating AmB. Our principal aim was the development of in situ cross-linking gels for the extended therapy of localized infections, especially of anatomic spaces such as the synovia. The formulations we created, cross-linked gels containing AmB conjugated to the hydrogel or suspended in the matrix, were both effective in killing fungi on contact, suggesting that they both could be useful in preventing infections, in a manner analoguous to our observations in a different non-injectable hydrogel-based system containing AmB [4]. The somewhat unusual property of contact killing of fungi in the absence of detectable release of antifungal activity was also noted in that report. However, one would anticipate that the gel with conjugated AmB would be more likely to be useful in the context of established infections, because it would release antifungal activity, which would then be able to penetrate infected tissues. These in situ cross-linking gels are appealing in that they easy to apply, would conform to the shape of the space in which they are injected, and would provide sustained antifungal therapy. As we show here, they would remain in situ at the site of injection for the duration of therapy. Furthermore, their biocompatibility in the peritoneum was excellent, and their consistency is such that they would not be likely to be deleterious in weight-bearing joints, etc.
Current therapy of invasive candidiasis – and of other fungal infections – requires prolonged administration of antifungal agents [20]. Treatment generally commences with an intravenous course of two weeks or more, followed by protracted therapy with an oral agent (e.g. up to a year for candidal osteomyelitis). During the period of intravenous therapy, patients are exposed to the difficulties and risks of maintaining intravascular access over weeks; then and subsequently, they are at risk for the systemic side effects of the antifungal agents used (e.g. nephrotoxicity for AmB). Formulations like ours – with AmB and/or other compounds - would be able to provide local therapy at the site of infection from a single injection for durations that are comparable to those that are currently recommended, and at concentrations that would be difficult or impossible to achieve safely via systemic therapy. Local therapy could be particularly advantagous in pregnancy, where there may be concerns over birth defects with some antifungal agents [21]. We note that current recommendations for many local infections (e.g. endophthalmitis, osteomyleitis, pericarditis) suggest some form of surgical intervention for diagnostic or therapeutic (debridement) purposes, making it possible to deliver such local treatments to those locations safely. It remains to be seen whether such treatments would result in improved cure rates, and/or mitigate or obviate the need for concurrent systemic therapy. We also note that formulations of this type could in theory act as depots to provide systemic therapy in patient or communities where sustained intravenous access is either impractical or impossible.
There are numerous existant sustained release systems, including hydrogels and particulates. Some have been used to deliver antifungal agents topically or systemically, and it is possible that such could be adapted for use in local infections. The specific advantages of our system include prolonged duration of therapy, high local drug concentrations, ease of application, ability of the material to conform to the shape of the surface to which applied, tunability of a number of physical parameters, and biocompatibility.
AmB conjugated to oxidized dextran was effective against C. albicans. The fact that it was water soluble makes it potentially useful for intravasacular injection. AmB has been conjugated to other hydrophilic polymers and natural polysaccharides including arabinogalactan [14], dextran [15, 22], gum arabic [16] and PEG [23]. These conjugates have been shown to reduce the toxicity of AmB in mice [14, 16, 22, 23].
The polysaccharides used here were selected because of their biocompatility and their slow degradation due to the relatively low concentration (dextranases) or absence (cellulases) of human enzymes that degrade them. Some degradation would, however, be expected to occur by hydrolytic cleavage of the hydrazone bond, and eventually perhaps through the action of inflammatory cells. Other polysaccharides, such as hyaluronic acid, would allow more rapid degradation [12]. The tissue dwell time of the hydrogel matrix could be tuned to match the release kinetics of whatever drug is delivered – this could be desirable if repeated injection was anticipated.
Conclusions
We have produced injectable in situ cross-linking gels with extended antifungal activity. Depending on the formulation, they have the potential to kill fungi by drug release and/or on contact. There was no evidence of tissue injury after intraperitoneal injection, or of hemolysis from these hydrogels. Cross-linked hydrogels containing AmB have the potential to provide sustained local antifungal therapy.
Supplementary Material
UV/visible absorption spectra of dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5.
Antifungal activity of dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5. (The x-axis shows the concentration of amphotericin present on polymer conjugate or in suspension).
Extinction coefficient of dextran-CHOm-AmB62.5 at 360 nm
Acknowledgments
This work was supported by MCOIF-CT2007-040150 (to SH), NIH EB000244 (to RL) and NIH GM073626 (to DSK) and NIH GM040266 and GMO35010 (to GRF). GRF is American Cancer Society Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Naeger-Murphy N, Pile JC. Clinical Indications for Newer Antifungal Agents. J Hosp Med. 2009;4(2):102–111. doi: 10.1002/jhm.412. [DOI] [PubMed] [Google Scholar]
- 2.Ruszczak Z, Friess W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv Drug Deliver Rev. 2003;55(12):1679–1698. doi: 10.1016/j.addr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Zilberman M, Elsner JJ. Antibiotic-eluting medical devices for various applications. J Control Release. 2008;130(3):202–215. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Zumbuehl A, Ferreira L, Kuhn D, Astashkina A, Long L, Yeo Y, et al. Antifungal hydrogels. P Natl Acad Sci USA. 2007;104(32):12994–12998. doi: 10.1073/pnas.0705250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart R, Bell-Syer SEM, Crawford F, Torgerson DJ, Young P, Russell I. Systematic review of topical treatments for fungal infections of the skin and nails of the feet. Brit Med J. 1999;319(7202):79–82F. doi: 10.1136/bmj.319.7202.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vazquez JA. Diagnosing and managing oropharyngeal candidiasis. Infect Med. 2007;24(10):427. [Google Scholar]
- 7.Sobel JD, Chaim W, Nagappan V, Leaman D. Treatment of vaginitis caused by Candida glabrata: use of topical boric acid and flucytosine. Am J Obstet Gynecol. 2003;189(5):1297–1300. doi: 10.1067/s0002-9378(03)00726-9. [DOI] [PubMed] [Google Scholar]
- 8.Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, et al. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can J Surg. 2001;44(5):383–386. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007. [Google Scholar]
- 10.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47(2):152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Yeo Y, Highley CB, Bellas E, Kohane DS. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials. 2007;28(23):3418–3426. doi: 10.1016/j.biomaterials.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Yeo Y, Highley CB, Bellas E, Ito T, Marini R, Langer R, et al. In situ cross-linkable hyaluronic acid hydrogels prevent post-operative abdominal adhesions in a rabbit model. Biomaterials. 2006;27(27):4698–4705. doi: 10.1016/j.biomaterials.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Bruneel D, Schacht E. Chemical Modification of pululan.1. Periodate-oxidation. Polymer. 1993;34:2628–2632. [Google Scholar]
- 14.Falk R, Domb AJ, Polacheck I. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob Agents Ch. 1999;43(8):1975–1981. doi: 10.1128/aac.43.8.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokolsky-Papkov M, Domb AJ, Golenser J. Impact of aldehyde content on amphotericin B-dextran imine conjugate toxicity. Biomacromolecules. 2006;7(5):1529–1535. doi: 10.1021/bm050747n. [DOI] [PubMed] [Google Scholar]
- 16.Nishi KK, Antony M, Mohanan PV, Anilkumar TV, Loiseau PM, Jayakrishnan A. Amphotericin B-gum arabic conjugates: synthesis, toxicity, bioavailability, and activities against Leishmania and fungi. Pharm Res. 2007;24(5):971–980. doi: 10.1007/s11095-006-9222-z. [DOI] [PubMed] [Google Scholar]
- 17.Espada R, Valdespina S, Alfonso C, Rivas G, Ballesteros MP, Torrado JJ. Effect of aggregation state on the toxicity of different amphotericin B preparations. Int J Pharm. 2008;361(1–2):64–69. doi: 10.1016/j.ijpharm.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, et al. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J Biomed Mater Res. 2002;59(3):450–459. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 19.Barwicz J, Christian S, Gruda I. Effects of the Aggregation State of Amphotericin-B on Its Toxicity to Mice. Antimicrob Agents Ch. 1992;36(10):2310–2315. doi: 10.1128/aac.36.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moudgal VV, Sobel JD. Antifungal drugs in pregnancy: a review. Expert Opin Drug Saf. 2003;2(5):475–483. doi: 10.1517/14740338.2.5.475. [DOI] [PubMed] [Google Scholar]
- 22.Shkurupy VA, Selyatitskaya VG, Tsyrendorzhiev DD, Pal’chikova NA, Kurilin VV, Travin MA, et al. Bull Exp Biol Med. 2007;143(4):392–394. doi: 10.1007/s10517-007-0138-3. [DOI] [PubMed] [Google Scholar]
- 23.Sedlak M, Pravda M, Staud F, Kubicova L, Tycova K, Ventura K. Synthesis of pH-sensitive amphotericin B-poly(ethylene glycol) conjugates and study of their controlled release in vitro. Bioorg Med Chem. 2007;15(12):4069–4076. doi: 10.1016/j.bmc.2007.03.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UV/visible absorption spectra of dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5.
Antifungal activity of dextran-CHO33-AmB62.5 and dextran-CHO66-AmB62.5. (The x-axis shows the concentration of amphotericin present on polymer conjugate or in suspension).
Extinction coefficient of dextran-CHOm-AmB62.5 at 360 nm