Abstract
Aflatoxin B1 (AFB1) is a major risk factor for hepatocellular carcinoma (HCC) in humans. However, mice, the major animal model for the study of AFB1 carcinogenesis, are resistant, due to high constitutive expression, in the mouse liver, of glutathione S-transferase A3 subunit (mGSTA3) that is lacking in humans. Our objective was to establish a mouse model for AFB1 toxicity could be used to study mechanisms of toxicity that are relevant for human disease, i.e., an mGSTA3 knockout (KO) mouse that responds to toxicants such as AFB1 in a manner similar to humans. Exons 3–6 of the mGSTA3 were replaced with a neomycin cassette by homologous recombination. Southern blotting, RT-PCR, Western blotting, and measurement of AFB1-N7-DNA adduct formation were used to evaluate the mGSTA3 KO mice. The KO mice have deletion of exons 3–6 of the mGSTA3 gene, as expected, as well as a lack of mGSTA3 expression at the mRNA and protein levels. Three hours after injection of 5 mg/kg AFB1, mGSTA3 KO mice have more than 100-fold more AFB1-N7-DNA adducts in their livers than do similarly treated wild-type (WT) mice. In addition, the mGSTA3 KO mice die of massive hepatic necrosis, at AFB1 doses that have minimal toxic effects in WT mice. We conclude that mGSTA3 KO mice are sensitive to the acute cytotoxic and genotoxic effects of AFB1, confirming the crucial role of GSTA3 subunit in protection of normal mice against AFB1 toxicity. We propose the mGSTA3 KO mouse as a useful model with which to study the interplay of risk factors leading to HCC development in humans, as well as for testing of additional possible functions of mGSTA3.
Keywords: GSTA3, knockout mouse, aflatoxin B1, liver, DNA adducts, hepatocellular carcinoma
INTRODUCTION
We report the development of a mouse model for testing of relationship of the sensitivity of aflatoxin (AFB1) carcinogenesis to the level of a glutathionine S-transferase (GST) subunit mGSTA3. AFB1 is metabolized by the P450 enzyme system to active epoxide intermediates, which bind to DNA and induce carcinogenic mutations. These epoxides are detoxified by GST to inactive forms, and are then degraded. There are significant differences in sensitivity to acute (adduct formation, cytotoxic damage) and chronic (carcinogenic) effects of AFB1 among species (Croy and Wogan, 1981; Degen and Neumann, 1981; Gorelick, 1990; Hayes et al., 1992; Hengstler et al., 1999; O’Brien et al., 1983; Wong and Hsieh, 1980). Thus, humans, rats, and trout are very sensitive to low doses of AFB1, whereas adult mice are resistant (Akao et al., 1971). Adult mice can tolerate high doses of AFB1 (up to 60 mg/kg) without manifesting toxic or carcinogenic effects (Wogan, 1969). However, mice are prone to AFB1 toxicity if the toxin is administered during the first week after birth, with males being significantly more sensitive than females (Ueno et al., 1991; Vesselinovitch at., 1972). In addition, the susceptibility to AFB1 increases in adult mice if hepatocyte proliferation is stimulated, such as following partial hepatectomy or CCl4-induced injury (Arora, 1981; Shupe and Sell, 2004), or during chronic regenerative hyperplasia (Ghebranious and Sell, 1998; Sell et al., 1991). The enhanced sensitivity to AFB1 hepatocarcinogenicity in newborn mice and in adult mice after liver injury is closely related to a lower level of GST activity in the liver, as compared to the hepatic activity in normal adult mice (Sell, 2003).
The resistance of adult mice to AFB1 has been suggested to be due to constitutive expression in mouse liver of the A3 subunit of GST (mGSTA3; also known as Yc or Ya3), which exhibits high catalytic activity toward AFB1 (Buetler and Eaton, 1992; Hayes et al., 1992; McDonagh et al. 1999). In support of this suggestion are the findings that mGSTA3 confers protection against 8,9-epoxide when transfected into hamster cells (Fields et al., 1999) and that substitution of the five critical mGSTA3 amino acid residues into the rat GSTA3 sequence increases the conjugation activity over 200-fold (van Ness et al., 1998). A 25,700 Da protein, mGSTA3 accounts for about 35% in male mice, and 47% in female mice, of all GST subunits (Mitchell et al., 1997). Besides AFB1, mGSTA3 appears to have narrow substrate specificity, and is largely unresponsive to drugs (Hayes and Pulford, 1995). However, it may have a function in an antioxidant defense mechanism (Hayes et al., 2000; McWalter et al., 2004; Yang et al., 2002), an idea which is further supported by its possession of a functional antioxidant response element (ARE) within its promoter. Nrf2 transcription factor binds to this promoter and, at least in part, controls its expression (Jowsey et al., 2003).
In order to test the hypothesis that mGSTA3 is critical for protecting mice from AFB1 toxicity, we have generated an mGSTA3 knockout (KO) mouse. Following a single AFB1 injection, the KO mice show more than a 100-fold increase in AFB1-N7-DNA adduct levels in their livers, relative to the levels in wild-type (WT) control mice. Thus, the KO mice show levels of adducts similar to those of AFB1-sensitive newborn mice. We propose the mGSTA3 KO mouse as a pre-clinical model with which we can more accurately study the interplay of risk factors contributing to development of HCC in humans.
METHODS
Targeting vector
For preparation of the targeting vector used to generate the mGSTA3−/− mice, GSTA3 BAC clone RP23-80N7 (Invitrogen, Carlsbad, CA) was grown and DNA extracted and purified using “NucleoBond BAC Maxi Kit”, according to the manufacturer (BD Biosciences Clontech, Palo Alto, CA). Primers specific to mGSTA3 exon 4 and exon 5 (forward primer: 5’ - GGA GTT CAA CCA GGG CAA TA - 3’; reverse primer: 5’ – GGC GGA TCT GGA GAT AAT GA – 3’) were used to PCR amplify this region, and the resulting amplicon sequenced to confirm that the purified BAC DNA was mGSTA3. The purified BAC DNA was then digested with BglII and SpeI in separate reactions to generate 2-kb and 6.7-kb fragments, respectively; these DNA fragments were excised and gel purified. The 6.7-kb fragment was ligated into the EmbryoMax ESTV-Neo targeting vector (Specialty Media, Phillipsburg, NJ). This vector contains a floxed neomycin resistance selection marker. Subsequent screening and sequencing identified a clone with the proper GST fragment in the correct orientation. The 2-kb fragment was then inserted upstream of this and correct insertion and orientation again confirmed by sequencing. We then proceeded to produce a null mGSTA3 by homologous recombination, as described in the results.
Breeding of mGSTA3 KO mice
The mGSTA3 KO mice, generated as described in the results section, have been bred with C57Bl/6J WT mice (Jackson Laboratory, Bar Harbor, ME) and maintained on a C57 background. Routinely, homozygous mGSTA3KO males are bred with mGSTA3 homozygous KO females so that all offspring are expected to be KO mice. For some purposes, KO mice are bred to WT mice, or heterozygous (+/−) mice are mated together. For all offspring, we use PCR screening of the genomic DNA that involves three separate reactions: exon 4 and exon 5 (present only in the WT mice) and Neo (present only in the KO mice). The accuracy of the screens is 100%, since the results of the reactions are always consistent with the expected distribution of the WT and KO mGSTA3 gene.
Measurements
The activity of total hepatic GSTs was measured on liver homogenates, with DCNB as a substrate, by the method of Habig et al. (1974). The AFB1-DNA adducts were measured by liquid chromatography – electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS). This triple-quadrupole mass spectrometry (Thermo-FinniganTSQ mass spectrometer, coupled to a Thermo-Finnigan Surveyor Plus HPLC and autoinjector (ThermoElectron, San Jose, CA) was coupled with the use of a stable isotope-labeled internal standard (AFB1-N7-15N5-Gua) as described by Egner et al. (2006).
Animal care
All animals were treated in accordance with the protocol approved by the Wadsworth Center Institutional Animal Care and Use Committee. Mice were housed in the Animal Facility at the Wadsworth Center and maintained on a 12/12 h light/dark cycle. They were fed “Prolab RMH 3500” lab-chow (PMI Nutrition, Brentwood, MO) and had unlimited access to water.
RESULTS
Generation of mGSTA3 knockout mice
Mice lacking mGSTA3 were generated by replacement of exons 3–6 of the mGSTA3 gene with a neomycin resistance cassette (Neo) (Fig. 1a). The Neo fragment of approximately 4 kb was flanked in the targeting vector on the left arm with a 2-kb genomic fragment cut from intron 2 with BglII enzyme, and on the right arm with a 6.7-kb Spe1 genomic fragment that includes exon 7 and surrounding intronic regions. Homologous recombination with the targeting vector was then used to generate the KO mice (produced by Dr Kerri Kluetzman, Transgenic and Gene Knockout Core facility, Wadsworth Center, NY State Department of Health, Albany, NY). These were then analyzed to determine the success of the knockout procedure.
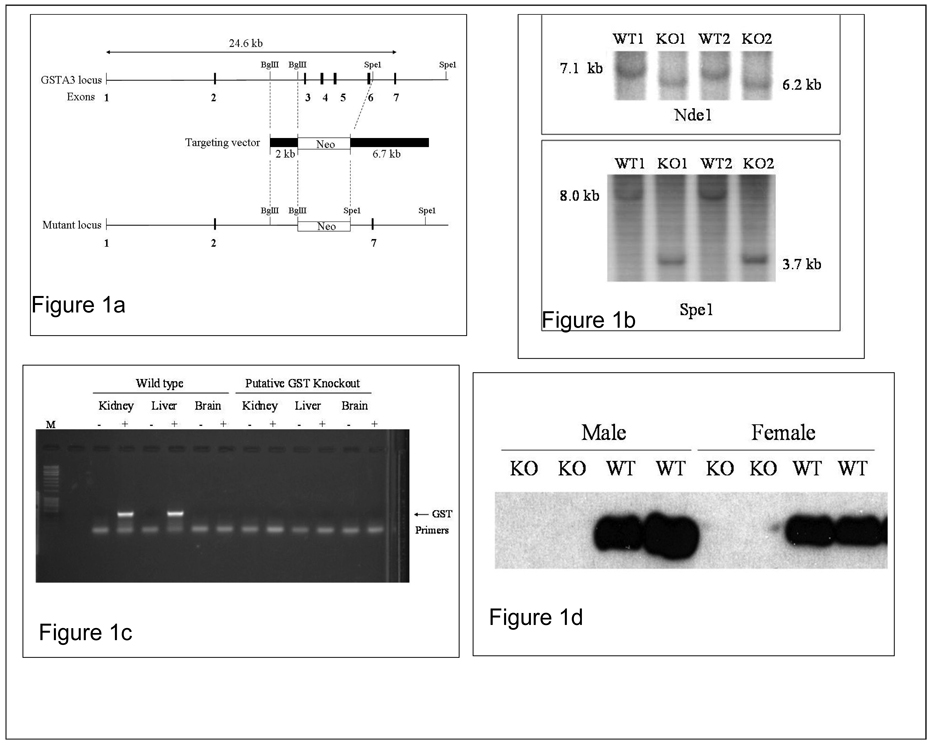
Figure 1. Generation and characterization of mGSTA3 knock-out (KO) mouse.
A. Scheme of mGSTA3 gene deletion. The targeting vector containing a neomycin resistance cassette is shown, along with restriction enzymes on the left and right arms used to generate it from genomic DNA. B. Detection of mGSTA3 DNA by Southern blotting on two mGSTA3 KO and two WT DNA samples isolated from liver. The DNA was digested with either Nde1 or Spe1 and probed with the 2-kb Bgl2 fragment. C. Determination of mGSTA3 MRNA by RT-PCR in liver, kidney, and brain of four mGSTA3 KO and four WT mice. D. Determination of mGSTA3 protein by Western blotting using anti-mGSTA3 specific antibodies, in livers of four mGSTA3 KO and four WT mice. B, C, and D show the absence of targeted DNA and lack of mGSTA3 mRNA and protein expression for the KO mice.
Genomic DNA from two KO and two WT (control) mice was digested with Nde1 and Spe1 in separate reactions, Southern blotted, and then probed with the 2-kb Bgl2 (left arm) fragment depicted in Figure 1a. As predicted, the Nde1 digestion produced a 7.1-kb fragment in the mGSTA3 KO mice and a 6.2-kb fragment in the WT mice, whereas the Spe1 digestion produced a 3.7-kb band in the KO mice and a 8.0-kb band in WT (Fig. 1b). GSTA3 mRNA and protein expression in tissues was also compared between KO and WT. For mRNA, total RNA was extracted from liver, kidney and brain, and RT-PCR carried out using primers specific for mGSTA3 (Jowsey et al., 2003). As shown in Figure 1c, expression of GSTA3 mRNA was observed in liver and kidney, but not brain, in the WT mice. However, no expression was observed for any tissue in any of the KO mice (4 WT and 4 KO). We also carried out GSTA3 protein analysis on liver tissue homogenates (from 4 mGSTA3 KO and 4 WT control mice, with 2 males and 2 females per group). Western blotting, using anti-mouse specific mGSTA3 antibodies (obtained from Dr. John Hayes, University of Dundee, Dundee, Scotland), revealed, as expected, that livers from KO mice do not express mGSTA3 protein (Fig. 1d) whereas expression in WT livers is strong. Thus, our KO mice exhibit appropriate gene alterations and do not express mGSTA3.
General characterization of mGSTA3 KO mice
Both the KO and hemizygous (+/−) mGSTA3 mice, males and females, appear normal, without any visible health problems and, thus so far, they show a normal life span. Both males and females are fertile and produce normal-sized litters (6–8 pups, on average). They have now been bred back onto the C57Bl/6J strain for five generations. By microscopic examination no obvious differences between WT and KO organs (liver, lungs, kidney, heart, GI tract, brain, and spleen) were seen.
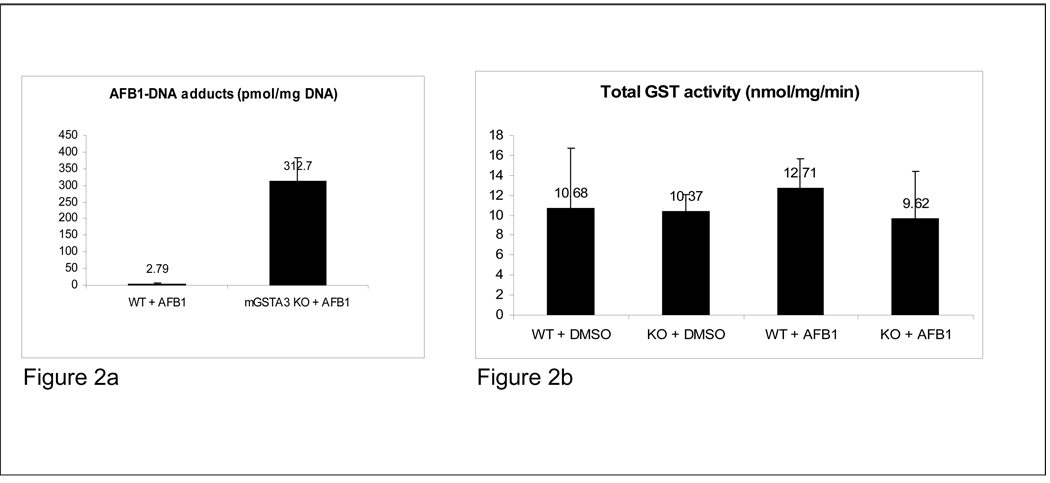
Determination of AFB1-N7-DNA adducts and total GST activity. Given that mGSTA3 mediated glutathione conjugation is the primary detoxification pathway for activated AFB1 metabolites, and that the high levels of mGSTA3 in the mouse are the major - if not the only - reason why adult mice are resistant to the toxic and carcinogenic effects of AFB1, our primary goal was to evaluate whether deletion of the gene (and protein) would render the mice sensitive to AFB1 treatment. Mice (5 mGSTA3 KO and 5 WT, 6 months of age, all males) were injected with a single dose of 5 mg/kg AFB1, dissolved in DMSO, in a volume of 100 µl/30 g of mouse weight, and euthanized 3 h later. Results revealed a striking difference in the levels of AFB1-N7-guanine adducts, with AFB1-treated mGSTA3 KO mice displaying more than a 100-fold more adducts than did their WT counterparts (312.7 vs. 2.79 pmol/mg DNA) (Fig. 2a). One of the WT mice, for undetermined reasons, had aberrant levels of adducts above 1 pmol/mg; if that mouse is excluded, the difference in adduct levels between mGSTA KO and WT increases to more than 300-fold. The individual values were: WT mice: 0.92, 0.44, 0.92, 0.21 and 11.4 pmol/mg DNA; mGSTA3 KO mice: 289.66, 436.40, 254.62, 298.92 and 283.94 pmol/mg DNA. This finding, that an adult mouse can be sensitive to the acute effects of AFB1, and respond similarly to rats when exposed to it, has never been described before. We anticipate that the mGSTA3 KO mice will also become sensitive to the long-term, carcinogenic effects of AFB1, and will develop liver lesions and tumors over time. Thus, we are confident that we have created a true GSTA3 knockout mouse that shows a dramatic phenotype when it is exposed to AFB1 as an adult. This knockout provides a mouse model in which to study the interplay of AFB1 and other risk factors in the development of liver tumors.
Figure 2. Body weights, AFB1-DNA adducts and total GST activity.
A. Determination of AFB1-N7-DNA adducts in livers of mGSTA3 KO and WT mice, by liquid chromatography-mass spectrometry. Male mice (five/group) were injected with 5 mg/kg AFB1 in DMSO and their livers collected 3 h later. KO mice have more than 100-fold more DNA adducts than WT mice. B. No differences were found in the levels of total GST activity between mGSTA3 KO and WT mice (five/group).
Total GST activity in both KO and WT liver extracts (5 mice in each group) was also assessed. No differences were observed, even though the mGSTA3 subunit accounts for about a third of all GST subunits in male mouse liver (Fig. 2b). These results suggest the operation of a redundant compensatory response by other GST forms. Such compensatory increases have been seen in existing GST knockout mouse models (Engle et al., 2004; Fernandez- Canon et al., 2002).
Acute toxicity of AFB1 in mGSTA3 KO mice
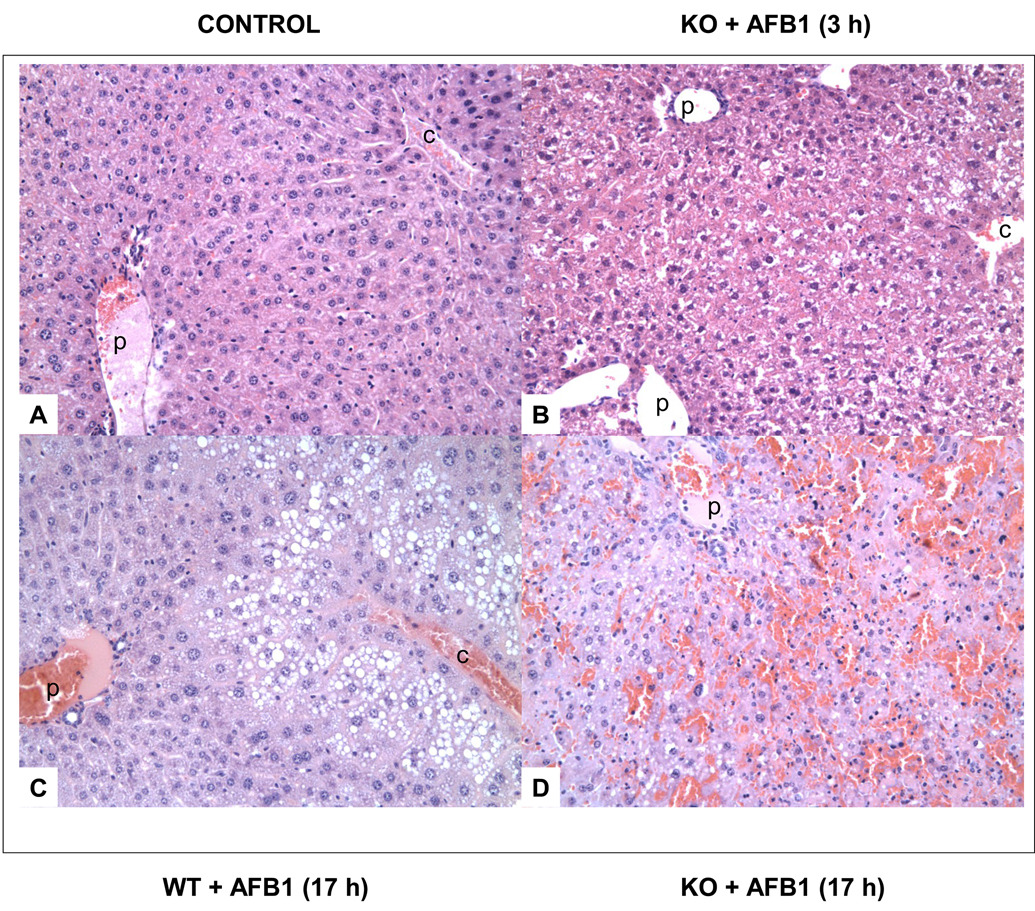
Following 5 mg/kg AFB1 injection, all KO mice tested, 3 males and 3 females, died within 17 h due to massive liver necrosis, whereas WT mice survived. At 3 h after AFB1 administration, some areas of the livers of the KO mice showed loss of hepatocyte cell borders, and pyknotic nuclei (Fig. 3b) in contrast to normal liver(Fig. 3a). At this time, the mid-zonal hepatocytes of the WT mice had slightly vacuolated cytoplasm, but cell membranes and nuclei are intact. At 17 h after AFB1 injection in the mGSTA3 KO mouse, extensive liver necrosis and hemorrhaging are evident in the mid- and central zones, with involvement of 50–80% of the liver, and loss of the central vein (Fig. 3d). All WT mice appeared healthy and unaffected at this time; histologically, they showed fat accumulation in the distal liver cord, but not in the pericentral- or mid- zones (Fig. 3c).
Figure 3. Effect of AFB1 on liver histology for wild-type (WT) and mGSTA3 knock-out (KO) mice.
A. untreated male WT mouse; B. mGSTA3 male KO mouse, 3 h after AFB1 administration; some loss of hepatocyte cell borders is evident, as is some nuclear pyknosis. C. WT mouse, 17 h after AFB1 administration; fat accumulation in hepatocytes is seen in the distal cord, with the exception of immediate layer of cells around the central vein. D. KO mouse, 17 h after AFB1 administration; extensive hemorrhaging and necrosis are evident, with involvement of up to 80% of the liver. All figures are shown at 200× final magnification. p, portal vein; c, central vein.
DISCUSSION
Hepatocellular carcinoma (HCC) is listed by the American Cancer Society (ACS, 2009) as the 6th highest in causing death in men and 9th highest in women in the United States, and its incidence has shown a slow but steady increase since 1970. HCC is the is the 5th or 6th most common cancer worldwide, with number of cases (626,000) closely resembling the number of deaths (598,000), making it the 3rd (after lung and stomach) leading cause of cancer-related death (Bartlett et al., 2008; Gomaa et al., 2008). Most cases occur in Asia and sub-Saharan Africa. In these regions, high incidence has been associated with contamination of foodstuffs, stored grains, water, and soil with aflatoxin mycotoxins, as well as with chronic hepatitis B infection. Aflatoxin B1 (AFB1) is the most potent member of the aflatoxin group. It has been suspected as a major risk factor for HCC since the 1960s, when its carcinogen potency was described in several animal species (Busby, 1984; Eaton and Groopman, 1994; Wogan, 1969; Wogan, 1992). The increase in HCC in the US over the last 40 years is thought to be related to the two main risk factors, chronic hepatitis and AFB1 exposure.
AFB1 requires metabolic activation by cytochrome P450 enzymes, to produce its toxic and carcinogenic effects. Among several oxidative metabolites that are produced, the most important is AFB1-8,9-exo-epoxide (AFBO), which is AFB1’s ultimate carcinogenic product (Busby, 1984). This electrophilic epoxide reacts with nucleophilic centers in the DNA and proteins, forming a covalently bound aflatoxin-N7-guanine (AFB1-N7-Gua) DNA adduct (Essigman et al., 1977). AFB1-DNA adduction is believed to be the source of point mutations that initiate AFB1-induced hepatocarcinogenesis (Bailey et al., 1996). However, cells have an important protective mechanism that can detoxify the AFB1-8,9-epoxide; this mechanism involves conjugation of the epoxide functional group to glutathione (GSH), catalyzed by glutathione S-transferase (GST), with the subsequent excretion of the water-soluble AFB1-GSH conjugate in the bile and urine (Moss et al., 1985). The balance between the rate of AFB1-8,9-epoxide production and the rate of inactivation by GST-conjugation determine the susceptibility of the individual and species to AFB1 carcinogenesis (Eaton and Gallagher, 1994). Although not examined yet in the mGSTA3 mice, in our previous studies of AFB1 metabolism we found no evidence for variances in metabolic activation or glutathione levels to explain differences in susceptibility of adult, neonatal or adult partially hepactectomized mice to AFB1 hepatocarcinogenicity (Shupe and Sell, 2004). In addition, although there may be differences in CYP levels between WT and mGSTA3KO mice, the finding of high adduct formation in the KO mice after AFB1 administration (Fig 2a) indicates that bioactivation is intact in the KO mice.
Due to their high levels of mGSTA3 expression, adult WT mice are resistant to the AFB1-induced DNA lesions and liver tumor development. Thus, for its effects to be studied, AFB1 must be injected into mice shortly after birth (Vesselinovitch et al., 1972). In contrast, we find that, following the disruption of the functional mGSTA3 gene, adult mice are sensitive to the acute genotoxic effects of AFB1 treatment. The high levels of AFB1-DNA adducts that we observed in mGSTA3 KO mice confer a dramatic phenotype. We expect that these mice will also become sensitive to the long-term, carcinogenic effects of AFB1, and will develop liver lesions and tumors over time more like AFB1-sensitive species such as rat. We are confident that the mGSTA3 KO mouse can serve as an animal model of AFB1 toxicity, one that is more relevant for the study of this ubiquitous carcinogen than are isolated liver cells in vitro. Long-term experiments are currently under way in our laboratory, to explore the interplay of AFB1 with other risk factors (chronic hepatic injury and p53 mutation) in the development of HCC. The mGSTA3 KO mice will be crossbred with other genetically modified mice, in an extension of our already published findings (Ghebranious and Sell, 1994; Sell, 2003).
The mGSTA3 KO mice can also provide confirmation of and valuable insights into other proposed metabolic functions of the mGSTA3 enzyme, other than the known detoxification of AFB1. GSTA3, along with several other GST subunits, may play a significant role in cellular defense against reactive oxidative stress and damage (Hayes and McLellan, 1999). For example, another catalytic activity that is highly specific for mGSTA3 is the reduction of cumene hydroperoxide (CuOOH) (Hayes and Pulford, 1995); the enzyme also reduces endogenously produced phospholipid hydoxyperoxides (Yang et al., 2002). Mouse GSTA3 can additionally be induced following treatment of either isolated cells or entire mice with a cytoprotecive agents, such as a synthetic phenolic antioxidant (butylated hydroxytoluene [BHT], butylated hydroxyanisole [BHA] or ethoxyquin) or a natural antioxidant, extracted from broccoli seeds (the isothyocyanates) (Hayes et al., 2000; McWalter et al., 2004).
Further strengthening mGSTA3’s role in antioxidant defense is the finding that promoter regions of the gene that encodes it (as well as the genes encoding mGSTA4, mGSTP1, mGSTZ1, mGSTM1 and mGSTM2) contain functional antioxidant response element (ARE) to which transcription factor Nrf2 binds, following induction with any of various antioxidants (Hayes et al., 2000; Hayes et al., 2005; Jowsey et al., 2003a; McWalter et al., 2004). A reduction in the level of mGSTA3 (about 40%) was observed in male mice that lack Nrf2 (Nrf2 KO mice), under basal conditions, or when administered an antioxidant rich (BHA) diet or a dithiolethione chemopreventive agent (Chanas et al., 2002; Kwak et al., 2001). Furthermore, when treated with AFB1, Nrf2 KO mice accumulate several-fold more AFB1-DNA adducts than do WT mice (Kwak et al., 2001). Clearly, these data necessitate further examination of the antioxidant role of the mGSTA3 and the enzyme’s activation and control by the Keap1/Nrf2/ARE transcriptional pathway. Similarly, the generation and use of the mGSTA4 KO mouse have provided significant information on the protective actions of that subunit against lipid peroxidation products (Dwivedi et al., 2006; Engle et al., 2004).
A finding of undetermined significance is that the mGSTA3 subunit is markedly induced during adipocyte differentiation, specifically by the transcriptional action of pro-adipogenic prostaglandin, PGJ2 (Jowsey et al., 2003b). Study of the mGSTA3 KO mouse will allow us to analyze this effect in more detail, and evaluate its physiological importance.
To date, six KO mouse models entailing the knockout of different GST subunits have been reported: mGSTA4, mGSTM1, mHSTO1, mGSTP1/2, mGSTS1 and mGSTZ1 (reviewed by Board, 2007). The addition of the mGSTA3 KO mouse model can provide a significant insight of this subunit’s functions, principally the one related to AFB1 detoxification and liver cancer development, and also a possible role in cellular antioxidant protection.
ACKNOWLEDGEMENTS
This work was supported by NIH R01 CA112481 and R01 ES09495 grants. Patricia A. Egner’s work is supported by grant NIH PO1 ES006052.
ABBREVIATIONS
- AFB1
aflatoxin B1
- DMSO
dimethyl sulfoxide
- HCC
hepatocellular carcinoma
- KO
knockout
- LC-ESI/MS/MS
liquid chromatography/electrospray ionization tandem mass spectrometry
- mGSTA3
mouse glutathione S-transferase A3 subunit
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
Contributor Information
Zoran Ilic, Email: zxi01@health.state.ny.us.
Dana Crawford, Email: crawfod@mail.amc.edu.
Patricia A. Egner, Email: pegner@jhsph.edu.
Stewart Sell, Email: ssell@wadsworth.org.
REFERENCES
- ACS. American Cancer Society. Cancer Facts and Figures. 2009 ( www.cancer.org/docroot/STT/STT_0.asp)
- Arora RG. Enhanced susceptibility to aflatoxin B1 toxicity in weaniling mice pretreated with carbon tetrachloride. Acta. Pathol. Microbiol. Scand. [A] 1981;89:303–308. doi: 10.1111/j.1699-0463.1981.tb00225.x. [DOI] [PubMed] [Google Scholar]
- Bailey EA, Iyer RS, Stone MP, Harris TM, Essigmann JM. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1535–1539. doi: 10.1073/pnas.93.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DL, Di Bisceglie AM, Dawson LA. Cancer of the liver. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer. Principles & practice of oncology. eighth ed. New York: Lippincott Williams & Wilkins; 2008. pp. 1129–1156. [Google Scholar]
- Board PG. The use of glutathione transferase-knockout mice as pharmacological and toxicological models. Expert. Opin. Drug. Metab. Toxicol. 2007;3:421–433. doi: 10.1517/17425255.3.3.421. [DOI] [PubMed] [Google Scholar]
- Buetler TM, Eaton DL. cDNA cloning, mRNA expression and induction of alpha-class glutathione S-transferases in mouse tissues. Cancer Res. 1992;52:314–318. [PubMed] [Google Scholar]
- Busby WF. Aflatoxins. In: Searle C, editor. Chemical carcinogens. Washington, DC: American Chemical Society; 1984. pp. 945–1136. [Google Scholar]
- Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the GST gsta1, gsta2, gstm1, gstm3 and gstm4 genes in the livers of male and female mice. Biochem J. 2002;365:405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy RG, Wogan GN. Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse liver and kidneys. J. Natl. Acad. Sci. 1981;66:761–768. [PubMed] [Google Scholar]
- Degen GH, Neumann H-G. Differences in aflatoxin B1 susceptibility of rat and mouse are correlated with the capability in vitro to inactivate aflatoxin B1 epoxide. Carcinogenesis. 1981;2:229–306. doi: 10.1093/carcin/2.4.299. [DOI] [PubMed] [Google Scholar]
- Dwivedi S, Sharma R, Sharma A, Zimniak P, Ceci JD, Awasthi YC, Boor PJ. The course of CCl4 induced hepatotoxicity is altered in mGSTA4-4 null mice. Toxicology. 2006;218:58–66. doi: 10.1016/j.tox.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Eaton DA, Groopman JD. The toxicology of aflatoxins: human health, veterinary and agricultural significance. San Diego: Academic Press; 1994. [Google Scholar]
- Eaton DL, Gallagher EP. Mechanism of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- Egner PA, Groopman JD, Wang J-S, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: a generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Essigmann JM, Croy RG, Nadzan AM, Busby WF, Reinhold VN, Buchi G, Wogan GN. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc. Natl. Acad. Sci. U.S.A. 1977;74:1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Canon JM, Baetcher MW, Finegold M, Burlingame T, Gibson KM, Grompe M. Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism. Mol. Cell. Biol. 2002;22:4943–4951. doi: 10.1128/MCB.22.13.4943-4951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields WR, Morrow CS, Doehmer J, Townsend AJ. Expression of stably transfected murine glutathione s transerase A3-3 protects against nucleic acid alkylation and cytotoxicity by aflatoxin B1 in hamster V79 cells expressing rat cytochrome P450-2B1. Carcinogenesis. 1999;20:1121–1125. doi: 10.1093/carcin/20.6.1121. [DOI] [PubMed] [Google Scholar]
- Ghebranious N, Sell S. Hepatitis B injury, male gender, aflatoxin and p53 each contribute to hepatocarcinogenesis in transgenic mice. Hepatology. 1998;27:383–391. doi: 10.1002/hep.510270211. [DOI] [PubMed] [Google Scholar]
- Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J. Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick NJ. Risk assessment for aflatoxin B1. I. Metabolism of aflatoxin B1 by different species. Risk Analysis. 1990;10:539–559. doi: 10.1111/j.1539-6924.1990.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Wolf CR, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of GSTs in mouse liver and to their induction by the chemopreventive synthestic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Judah DJ, McClellan LI, Neal GE. Contribution of the glutathione S-transferases to the mechanisms of resistance to aflatoxin B1. Pharmac. Ther. 1991;50:443–472. doi: 10.1016/0163-7258(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Judah DJ, Neal GE, Nguyen T. Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem. J. 1992;285:173–180. doi: 10.1042/bj2850173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Van der Burg B, Steinberg P, Oesch F. Interspecies differences in cancer susceptibility and toxicity. Drug. Metab. Rev. 1999;31:917–970. doi: 10.1081/dmr-100101946. [DOI] [PubMed] [Google Scholar]
- Jowsey IR, Jiang Q, Itoh K, Yamamoto M, Hayes JD. Expression of the aflatoxin B1-8,9-epoxide-metabolizing murine glutathione S-transferase A3 subunit is regulated by the Nrf2 transcription factor through an antioxidant response element. Mol. Pharmacol. 2003a;64:1018–1028. doi: 10.1124/mol.64.5.1018. [DOI] [PubMed] [Google Scholar]
- Jowsey IR, Smith SA, Hayes JD. Expression of the murine glutathione S-transferase alpha3 (GSTA3) subunit is markedly induced during adipocyte differentiation: activation of the GSTA3 gene promoter by the pro-adipogenic eicosanoid 15d-PGJ2. Biochem. Biohys. Res. Comm. 2003b;312:1226–1235. doi: 10.1016/j.bbrc.2003.11.068. [DOI] [PubMed] [Google Scholar]
- Kwak M-K, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW. Role of phase 2 enzyme induction in chemoprevention by dithiolethiones. Mut. Res. 2001:480–481. 305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- McDonagh PD, Judah DJ, Hayes JD, Lian LY, Neal GE, Wolf CR, Roberts CK. Determinants of specificity for aflatoxin B1-8,9-epoxide in alpha-class glutathione S-transferase. Biochem. J. 1999;339:95–101. [PMC free article] [PubMed] [Google Scholar]
- McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinine oxidoreductase 1, GSTs and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutrition. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Morin D, Lakritz J, Jones AD. Quantitative profiling of tissue and gender related expression of GST isoenzymes in the mouse. Biochem. J. 1997;325:207–216. doi: 10.1042/bj3250207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EJ, Neal GE, Judah DJ. The mercapturic acid pathway metabolites of a glutathione conjugate of aflatoxin B1. Chem. Biol. Interactions. 1985;55:139–155. doi: 10.1016/s0009-2797(85)80124-1. [DOI] [PubMed] [Google Scholar]
- O’Brien K, Moss E, Judah D, Nea,l G. Metabolic basis of species difference to aflatoxin B1 induced hepatotoxicity. Biochem. Biophys. Res. Comm. 1983;114:813–821. doi: 10.1016/0006-291x(83)90854-9. [DOI] [PubMed] [Google Scholar]
- Sell S, Hunt JM, Dunsford HA, Chisari FV. Synergy between hepatits B virus expression and chemical hepatocarcinogens in transgenic mice. Cancer Res. 1991;51:1278–1285. [PubMed] [Google Scholar]
- Sell S. Mouse models to study the interaction of risk factors for human liver cancer. Cancer Res. 2003;63:7553–7562. [PubMed] [Google Scholar]
- Shupe T, Sell S. Low hepatic glutathione-S-transferase and increased hepatic DNA adduction contribute to tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicology Lett. 2004;148:1–9. doi: 10.1016/j.toxlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Kobayashi T, Yamamura H, Kato T, Tashiro F, Nakmura K, Ohtsubo K. Effects of long term feeding of nivalenol on aflatoxin B1 initiated hepatocarcinogenesis in mice. IARC Sci. Publ. 1991;105:420–423. [PubMed] [Google Scholar]
- van Ness KP, McHugh TE, Bammler TK, Eaton DL. Identification of amino acid resides essential for high aflatoxin B1-8,9-epoxide conjugation activity in alpha class glutathione s transferases through site-directed mutagenesis. Toxicol, Appl. Pharmacol. 1998;152:166–174. doi: 10.1006/taap.1998.8493. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N, Wogan GN, Lombard LS, Rao KVN. Aflatoxin B1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- Wogan GN. Metabolism and biochemical effects of aflatoxins. In: Goldblatt LA, editor. Aflatoxin – Scientific background control and implications. New York: Academic Press; 1969. pp. 151–186. [Google Scholar]
- Wogan GN. Aflatoxins as risk factors for hepatocellular carcinoma in humans. Cancer Res. 1992;52 7 Suppl.:2114s–2118s. [PubMed] [Google Scholar]
- Wong ZA, Hsieh DPH. The comparative metabolism and toxicokinetics of aflatoxin B1 in the monkey, rat and mouse. Toxicol. Appl. Pharmacol. 1980;55:115–125. doi: 10.1016/0041-008x(80)90227-6. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sharma R, Zimniak P, Awasthi YC. Role of alpha class glutathione S-transferases as antioxidant enzymes in rodent tissues. Toxicol. Appl. Pharmacol. 2002;182:105–115. doi: 10.1006/taap.2002.9450. [DOI] [PubMed] [Google Scholar]