Abstract
Targeted gene therapy can potentially minimize undesirable off-target toxicity due to specific delivery. Neuron-specific gene delivery in the central nervous system is challenging because neurons are non-dividing and also outnumbered by glial cells. One approach is to transfect dividing neural stem and progenitor cells (NSCs and NPCs, respectively). In this work, we demonstrate cell-specific gene delivery to NPCs in the brains of adult mice using a peptide-modified polymeric vector. Tet1, a 12-amino acid peptide which has been shown to bind specifically to neuronal cells, was utilized as a neuronal targeting ligand. The cationic polymer polyethylenimine (PEI) was covalently modified with polyethylene glycol (PEG) for in vivo salt stability and Tet1 for neuron targeting to yield a Tet1-PEG-PEI conjugate. When plasmid DNA encoding the reporter gene luciferase was complexed with Tet1-PEG-PEI and delivered in vivo via an injection into the lateral ventricle, Tet1-PEG-PEI complexes mediated increased luciferase expression levels in brain tissue when compared to unmodified PEI-PEG complexes. In addition, cells transfected by Tet1-PEG-PEI complexes were found to be exclusively adult NPCs whereas untargeted PEG-PEI complexes were found to transfect a heterogenous population of cells. Thus, we have demonstrated targeted, nonviral delivery of nucleic acids to adult NPCs using the Tet1 targeting ligand. These materials could potentially be used to deliver therapeutic genes for the treatment of neurodegenerative diseases.
INTRODUCTION
Gene therapy to the central nervous system (CNS) has the potential to benefit both acute injuries, such as stroke, as well as neurodegenerative diseases, such as Parkinson’s, Alzheimer’s, and Huntington’s disease. A major germinal center for new neurons in the adult mammalian brain is the subventricular zone (SVZ), which lies in the walls of the lateral ventricle [1]. Many neurodegenerative diseases are associated with abnormal levels of neural stem and progenitor cell (NSC and NPC, respectively) proliferation in the SVZ, which designates these cells as potential therapeutic targets [2]. Potential applications for CNS gene therapy include the delivery of genes that encode mitogenic factors to stimulate proliferation of NSCs and NPCs for neurogenesis [3] or transcription factors to alter NPC fates [4]. The first requirement for gene therapy is a safe and efficient delivery vehicle.
Recombinant viruses have been predominantly used as gene carriers; however, currently they face safety limitations including immunogenicity and insertional mutagenesis. In response to the safety concerns of viral-based carriers, a multitude of synthetic carriers have been developed as alternatives [5]. There have been several applications of polymer-based carriers to the CNS [6–9]. However, existing nonviral gene carrier technology is still in nascent stages and limited by low in vivo efficiency after CNS delivery [10]. In order to address this, one strategy that has been employed is the incorporation of peptides to improve intracellular trafficking [11], including peptides for targeting [12] and vesicular escape [13, 14].
Cell-specific targeting is important to minimize off-target delivery and has been shown to increase the specificity and efficiency of gene delivery vehicles [15]. Neuron targeting has been achieved with polypeptide materials, from peptides to full-length proteins [10]. One prevalent example is tetanus toxin, a protein derived from bacteria that binds specifically to neuronal cells through the triasialoganglioside receptor, GT1b [16]. Although little is known about ganglioside function and expression in NSCs and NPCs, GT1b expression has been suggested to occur during early stages of neuronal differentiation [17]. When synthetic particles were modified with a non-toxic fragment of tetanus toxin, particles were able to efficiently bind and be internalized by neuronal cells in vitro [18], indicating that mediating uptake via the tetanus receptor is a viable strategy for neuron targeted gene delivery. Recently, phage display against GT1b has identified a 12-mer peptide, referred to as Tet1, that exhibits high affinity and specificity for GT1b [19]. The use of small peptides over full-length polypeptides is advantageous for nanoparticulate delivery systems because they can be easily synthesized and incorporated while having minimal effects on the physicochemical properties of the particle. The Tet1 peptide has been successfully used in our group to target polyethylenimine (PEI)-based gene delivery vehicles in vitro to cultured neuronal cells [20, 21].
The goal of this work is to evaluate Tet1-modified vehicles for in vivo targeting to neuronal populations in the CNS. Because cationic polymer-based nanoparticle delivery vehicles aggregate in physiological levels of salt, they may cause toxicity after in vivo delivery [22, 23]. Polyethylene glycol (PEG) modification has been widely used for stabilization of delivery vehicles in biological fluids [23]. It has been shown that targeting ligands should be tethered at the distal ends of PEG to prevent steric interference to receptor binding [24]. Herein we describe the synthesis, characterization, and application of a Tet1-targeted vehicle system stabilized with PEG for in vivo CNS delivery. Tet1-targeted vehicles and untargeted controls were administered by injection into the lateral ventricle of adult mice and the populations of transfected cells were identified by immunohistochemistry.
MATERIALS AND METHODS
Synthesis of Tet1-PEG-PEI and PEI-PEG
Tet1 (HLNILSTLWKYRC) was synthesized using solid phase peptide synthesis and HPLC purified. 25,000 Mw branched PEI (Sigma, St. Louis, MO) was dissolved in DMF at 20 mg/mL and 2 mole equivalents of 5,000 Mw SPA-PEG-OPSS (Nektar Therapeutics, Huntsville, AL) in DMF was added. After stirring overnight at room temperature, 2.3 mole equivalents of Tet1 peptide in DMF were added and the reaction was stirred for an additional day. The resulting Tet1-PEG-PEI conjugate was acidified to pH 4 and purified by dialysis against water in a 3,500 MWCO membrane. A control polymer without Tet1 modification was synthesized by reacting PEI with 2 mole equivalents of 5,000 Mw SPA-PEG (Nektar) for 24 hours. Extent of PEG modification was determined using 1H NMR.
Complex formation and characterization
Complexes were formed by adding equal volumes of polymer to gWiz Luciferase plasmid DNA (Aldevron, Fargo, ND) and rapidly mixing. Polymer amine to DNA phosphate (N/P) ratio was calculated based on subunits of 43 g/mol and 330 g/mol for PEI and DNA, respectively. Particles were incubated for 10 minutes after mixing to allow for complete complexation. For measuring hydrodynamic size, polyplexes were formulated as described above using 1 μg of DNA in 10 μL. 50 μL of water was added and hydrodynamic size was measured using dynamic light scattering on a ZetaPlus Zeta Potential Analyzer (Brookhaven Instruments Corporation, Holtsville, NY). To measure the stability of particles in physiological salt concentrations, an equal volume of 300 mM phosphate buffered saline (PBS) was added and hydrodynamic size was measured again. Water was added for a final concentration of 10 mM PBS and zeta potential was measured. All samples were measured in triplicate and are reported as the mean diameter ± SD.
In vivo evaluation
Lateral ventricle delivery
All animal procedures were done after approval by the Institutional Animal Care and Use Committee at the University of Washington. Polyplexes were prepared as described above in 5% glucose using 2.5 μg of DNA in 10 μL at N/P 15. 7–9 week old female C57/Bl6 mice from Jackson Laboratories were housed for 1 week prior to experimentation. Mice were anesthetized by an intraperitoneal injection of Avertin. A 1 mm diameter craniotomy was made on the left side of the skull using a dental drill and 10 μL polyplex, DNA or 5% glucose solution was stereotaxically injected at 1 mm lateral, 0.5 mm caudal to bregma, and 1.75 mm deep from the dura using a 33 gauge 10 μL Hamilton syringe. The injection was made over 10 minutes and the syringe was kept in place for 2 minutes after injection to prevent backflow.
Lysate preparation
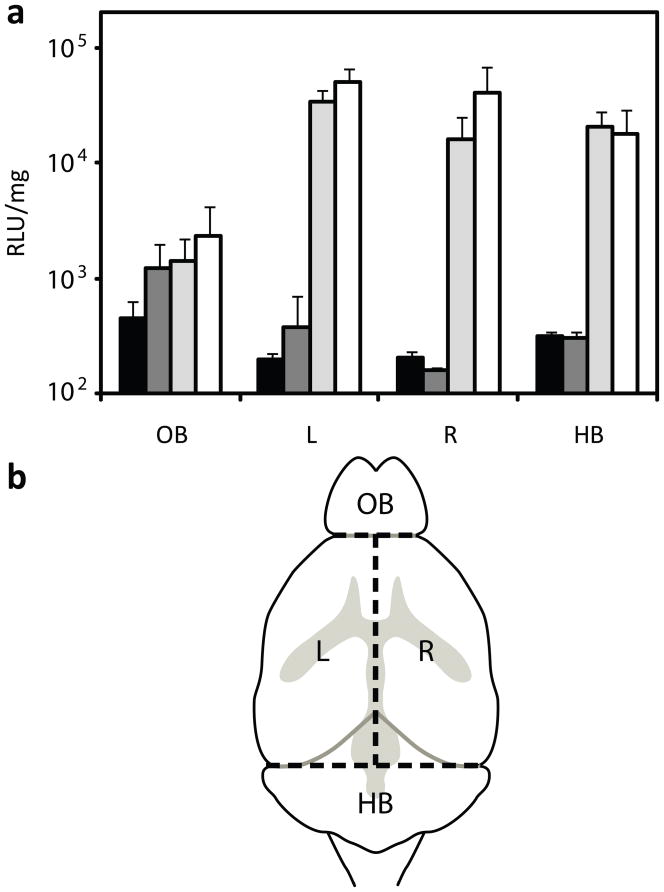
Brains were harvested from mice at the designated day post injection and separated into four sections: olfactory bulb, hindbrain, and left and right hemispheres (Fig. 2b). Tissues were collected in lysis buffer supplemented by protease inhibitors (Roche, Nutley, NJ) and three freeze-thaw cycles were performed in liquid nitrogen. Tissues were mechanically homogenized and lysate was cleared by spinning at 21,000 g for 15 minutes at 4 °C. 20 μL of lysate was assayed for luminescence with 100 μL of luciferase substrate. Luminescence was measured for 10 seconds in a Berthold LB 953 tube luminometer. Luminescence measurements were normalized by protein content, determined using a BCA Protein Assay Kit (Pierce), and reported as relative light units (RLU) per mg protein.
Figure 2.
(a) Bulk luciferase activity in mouse brains 3 days after injection of glucose control (black bars), DNA (dark grey bars), PEI-PEG complexes (light grey bars), and Tet1-PEG-PEI complexes (white bars) (* p<0.05). (b) Brains were sectioned into the olfactory bulb (OB), left (L) and right (R) hemispheres, and the hindbrain (HB).
Immunofluorescent labeling
Injections were done as described above using polyplexes formulated with gWiz Beta-gal plasmid (Aldevron). Three days post injection, mice were euthanized with sodium pentobarbital and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB) after which brains were excised and cryoprotected in 30% sucrose in PB. Brains were embedded in OCT and 20 μm-thick coronal tissue sections were cut onto glass slides.
For immunofluorescent labeling, slides were rinsed with PBS and blocked in 0.5% TritonX-100, 5% donkey serum for 1 hour. Primary antibodies were applied to tissue sections in PBS, 0.5% TritonX-100, 5% donkey serum overnight at 4 °C. Rabbit anti-β-galactosidase (Cappel Labs; Cochranville, PA) was used at 1:2000 or mouse anti-β-galactosidase (Cappel) was used at 1:200. Phenotype markers included: NPC markers goat anti-nestin (Santa Cruz Biotechnology, Santa Cruz, CA; 1:100) and goat anti-sox2 (Santa Cruz Biotechnology; 1:250), astrocyte markers rabbit anti-S100β (S. Want, Bellinzona, Switzerland; 1:400) and guinea pig anti-GFAP (glial fibrillary acidic protein; Advanced ImmunoChemical Inc. Long Beach, CA; 1:2000), glial progenitor markers rabbit anti-Olig2 (oligodendrocyte transcription factor 2; Immuno-Biological Laboratories Co., Ltd. Gunma, Japan; 1:400) and rabbit anti-NG2 (chondroitin sulphate proteoglycan; a generous gift from W. Stallcup, Burnham Institute, La Jolla, CA; 1:500), immature neuronal markers mouse anti-Map2ab (microtubule associated protein, forms a and b; Sigma-Aldrich, St. Louis, MO; 1:500), goat anti-doublecortin (Santa Cruz Biotechnology; 1:100) and mouse anti-TUJ1 (Covance, Denver, PA; 1:500), microglial markers rabbit anit-Iba1 (ionized calcium binding adaptor molecule 1; Wako Chemicals USA, Inc., Richmond, VA; 1:500), mouse anti-OX-42 (CD11b, AbD Serotec, Raleigh, NC; 1:200) and rat anti-F4/80 (AbD Serotec; 1:20), radial glial markers chicken anti-vimentin (Chemicon/Millipore, Billerica, MA; 1:1000), mouse anti-RC2 (a generous gift of P. Leprince at the University of Liège, Liège, Belgium; 1:50), and mouse anti-GT1b (Seikagaku Corporation, Tokyo, Japan; 1:50). Slides were rinsed 3 times for 10 minutes in PBS and species appropriate secondary antibodies conjugated with fluorophore were incubated in PBS, 0.5% TritonX-100 with 5% donkey serum overnight at 4 °C. Slides were rinsed 4 times for 10 minutes in PBS, with the last rinse containing the nuclear marker, 4′,6-diamidino-2-phenylindole (DAPI; 1:1000). Slides were then coverslipped with gelvatol and imaged using confocal microscopy.
RESULTS
Synthesis of Tet1-PEG-PEI and PEI-PEG
Liu et al. used phage display to identify Tet1, a 12-amino acid peptide that binds to the neuronal ganglioside GT1b [19]. Tet1-PEG-PEI was synthesized using a heterobifunctional PEG to tether between a primary amine of PEI and cysteine-terminated Tet1. Control conjugate PEI-PEG was synthesized using a monofunctional PEG that was capped with a methoxy group. 1H NMR was used to determine extent of PEG substitution by comparing the ratio of PEI protons (δ = 2.5–3 ppm) to PEG protons (δ = 3.6 ppm). Substitution for both conjugates was found to be ~2 PEG molecules per PEI.
Polyplex characterization
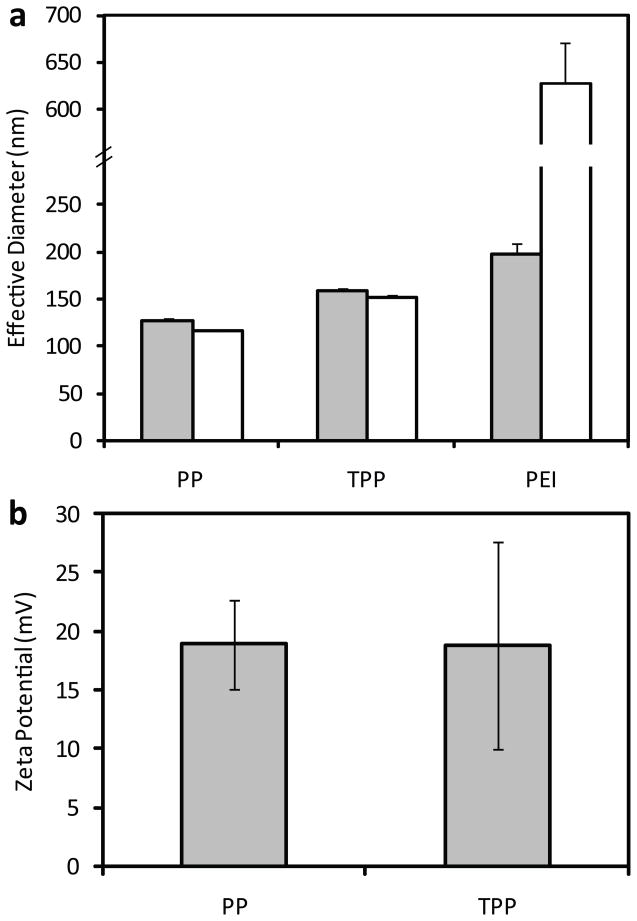
Complexes of plasmid PEI and DNA, termed polyplexes, were formed by mixing equal volumes of each component followed by incubation at room temperature for 10 minutes. Hydrodynamic sizes of triplicate samples were measured using dynamic light scattering (DLS) (Fig. 1a). PEI-PEG polyplexes were 127 ± 3 nm in water and 116 ± 1 nm after addition of saline whereas Tet1-PEG-PEI polyplexes were 158 ± 3 nm in water and 152 ± 3 nm after addition of saline. Sizes were found to be similar before and after the addition of physiological levels of salt and Tet1-PEG-PEI polyplexes were slightly larger than untargeted polyplexes. When polyplexes were formulated without PEG modification, polyplexes aggregated in physiological levels of salt (Fig. 1a) as reported previously in the literature [23]. The zeta potential of PEI-PEG polyplexes (19 ± 4 mV) was similar to Tet1-PEG-PEI polyplexes (19 ± 9 mV) (Fig. 1b).
Figure 1.
(a) Hydrodynamic diameters of PEI-PEG (PP), Tet1-PEG-PEI (TPP) and PEI complexes formulated at N/P ratio 15. Diameters were measured in water (grey bars) and after the addition of physiological levels of salt (white bars). (b) Surface charge PP and TPP complexes formulated at N/P ratio 15. Triplicate formulations were characterized at each charge ratio. Results are reported as mean diameter ± SD.
Bulk analysis of brain tissue after in vivo delivery
In order to compare the transfection efficiency of PEI-PEG and Tet1-PEG-PEI in vivo, polyplexes were injected into the left lateral ventricle of mice at an N/P ratio of 15. Three days post injection, mice brains were excised and dissected into olfactory bulb (OB), hindbrain (HB), and left (L) and right (R) hemispheres for bulk tissue analysis (Fig. 2). Normalized luciferase activity in OB, HB, L, and R lysate, respectively, was 1426 ± 779 RLU/mg, 33756 ± 9052 RLU/mg, 15859 ± 9089 RLU/mg, and 20386 ± 7104 RLU/mg in PEI-PEG polyplex delivered mice and 2270 ± 1912 RLU/mg, 50464 ± 15074 RLU/mg, 40156 ± 28710 RLU/mg, and 17798 ± 10748 RLU/mg in Tet1-PEG-PEI polyplex delivered mice. In glucose injected control mice, expression was less than 500 RLU/mg in all sections. Expression after delivery of Tet1-PEG-PEI polyplexes compared to PEI-PEG polyplexes was 1.5-fold higher in the left hemisphere and 2.5-fold higher in the right hemisphere (p-value < 0.05). The results shown are representative of three separate experiments. In all experiments, transfection efficiencies were higher in brains delivered Tet1-PEG-PEI polyplexes over PEI-PEG polyplexes.
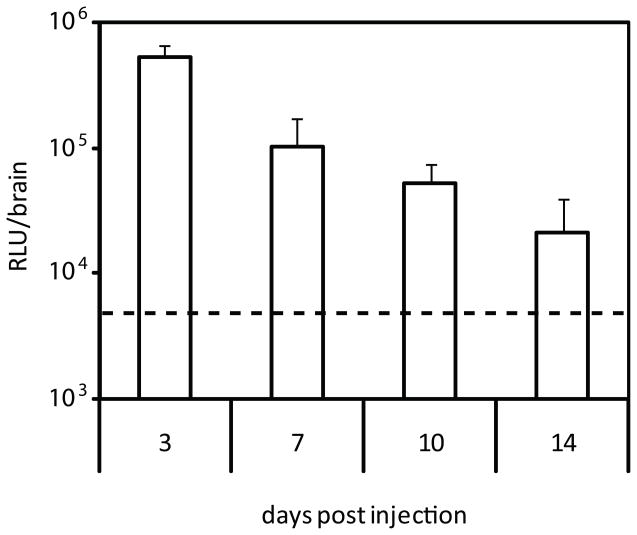
To evaluate the duration of transgene expression, Tet1-PEG-PEI polyplexes were delivered and whole brain luciferase expression was quantified 3, 7, 10, and 14 days post injection and compared to glucose control (Fig. 3). Expression declined exponentially, but was sustained above glucose control at 10 days (p<0.05) but not at 14 days.
Figure 3.
Bulk luciferase activity in mouse brains 3, 7, 10, and 14 days post injection of Tet1-PEG-PEI polyplexes. Whole brain expression is reported and is compared to glucose control (dashed line).
Immunofluorescent staining of brain tissue sections
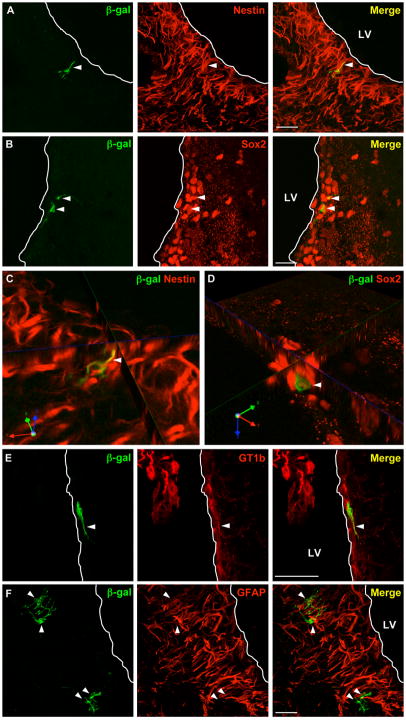
In order to identify the distribution and cell phenotypes of transfected cells, 20 μm brain tissue sections were taken from mice injected with Tet1-targeted and untargeted polyplexes 3 days post injection. Tissue sections were stained with antibodies against β-galactosidase, GT1b, and cell-specific markers for neural progenitors (sox2 and nestin), microglia (Iba1, F4/80, OX-42), astrocytes (GFAP and S100β), radial glia (vimentin and RC2), and glial progenitors (olig2 and NG2). The results of staining are summarized in Table 1. β-galactosidase+ cells were found in the SVZ, just below the ependymal surface of the lateral ventricles. The increase in bulk luciferase expression observed in Tet-PEG-PEI polyplex treated mice over PEI-PEG polyplex treated mice (Fig. 2) is supported in the staining of over 100 tissue sections for each group, with an average of 1.03 β-galactosidase+ cells/tissue section in Tet1-PEG-PEI polyplex treated mice and 0.34 β-galactosidase+ cells/tissue section in PEI-PEG polyplex treated mice. In tissue sections from mice injected with Tet1-PEG-PEI polyplexes, all β-galactosidase+ cells were sox2+/nestin+ across over 30 stained sections and 18% of β-galactosidase+ cells were vimentin+, indicating transfection of neural progenitors (Fig. 4, Table 1). In addition, β-galactosidase+ cells were negative for all other markers listed above. In contrast, in tissue sections from mice treated with PEI-PEG polyplexes, the population of β-galactosidase+ cells comprised of nestin+/sox2+ (33%), vimentin+ (30%), S100β+ (25%), and vimentin/S100β+ (18%) subpopulations (Fig. 5, Table 1). 58% of transfected cells were positive for GT1b when mice were treated with Tet1-targeted vehicles, compared with 13% when mice were treated with untargeted vehicles.
Table 1.
Summary of positive marker staining of β-galactosidase+ cells after Tet1-PEG-PEI or PEG-PEI delivery.
| Marker | Tet1-PEG-PEI | PEG-PEI |
|---|---|---|
| nestin/sox2 | 100% | 33% |
| GT1b | 58% | 13% |
| vimentin | 18% | 30% |
| S100β | 0% | 25% |
| olig2 | 0% | 0% |
| NG2 | 0% | 0% |
Figure 4.
Merged confocal images of immunofluorescently labeled 20 μm brain sections collected from mice 3 days after injection with Tet1-PEG-PEI complexes shows β-galactosidase+ cells (green) are located in the SVZ and are immunopositive for nestin (red; A) and sox2 (red; B). C and D are rotated, zoomed single z-plane views to demonstrate marker co-localization (yellow). Figure E shows a representative β-galactosidase+/GT1b+ positive cell and F shows representative β-galactosidase+/GFAP− cells. Scale bars = 50 μm; white line indicates edge of lateral ventricle (LV).
Figure 5.
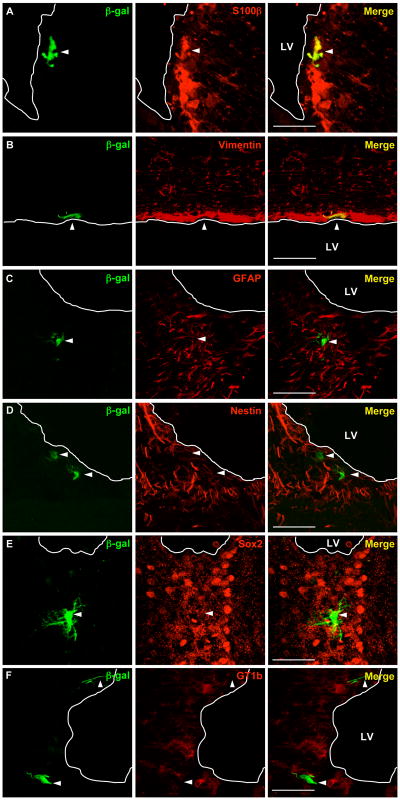
Merged confocal images of immunofluorescently labeled 20 μm brain sections collected from mice 3 days after injection with PEG-PEI complexes shows β-galactosidase+ cells (green) are located in the SVZ and that some cells are immunopositive for S100 (red; A) and vimentin (red; B). Images C-D show examples of β-galactosidase+ cells that are negative for GFAP (red; C), nestin (red; D), sox2 (red; E), and GT1b (red; F). Scale bars = 50 μm; white line indicates edge of lateral ventricle (LV).
DISCUSSION
There is an unmet need for treatment of neurodegenerative diseases. Neural progenitor cells (NPCs) of the subventricular zone (SVZ) are an attractive target for gene therapy to increase neurogenesis in the diseased brain [3]. Using a nonintegrating platform to deliver genes that encode proteins to increase division, improve survival, and control fate of NPCs is particularly suitable since expression of these proteins should be transient.
In this work, we describe the application of a PEG-stabilized, targeted gene delivery vehicle that exclusively transfects NPCs when administered in vivo. We demonstrated that particles formed were between 120–160 nm in size with positive surface charge and were stable in physiological levels of salt (Fig. 1). The physicochemical properties of targeted and untargeted complexes were similar and thus unlikely the cause of differences in cell specificity and transfection efficiency observed. When these vehicles were delivered to the brains of adult mice via the lateral ventricle, bulk analysis of tissue showed that targeted material was able to mediate a ~2.5-fold increase in the ipsilateral hemisphere and a ~1.5-fold increase in the contralateral hemisphere compared to untargeted material (Fig. 2). The increase in bulk expression achieved using targeted over untargeted materials is comparable to previously reported studies that deliver plasmid in vivo to the CNS [25–27]. Bulk expression level after targeted delivery was sustained above glucose control for up to 10 days, which is a time length appropriate for stimulating cell proliferation as demonstrated by the delivery of growth factors via osmotic pumps [28].
In order to investigate the distribution and phenotype of cells expressing the transgene β-galactosidase, tissue sections were immunofluorescently labeled using antibodies against β-galactosidase and cell-specific markers, the results of which are summarized in Table 1. Transfected cells were found to be in the SVZ, just below the ependymal cell layer (Figs. 4 and 5). Besides mediating increased transgene expression (Fig. 3), β-galactosidase+ cells in brains delivered with Tet1-targeted complexes are positively stained by antibodies to NPC-associated proteins nestin and sox2 (Fig. 4) and negatively stained for all other cell-specific markers tested. The proximity of transfected cells to the lateral ventricle wall together with the positive staining for both nestin/sox2 and negative staining for GFAP is highly indicative of NPCs [29]. In brains injected with untargeted complexes, transfected cells included several subpopulations that were positive for nestin, sox2, vimentin, and S100β (Fig. 5). These results suggest untargeted complexes transfect a heterogeneous population of cells, including NPCs, radial glia, and immature astrocytes. Based on results from bulk luciferase measurements and immunolabeling, Tet1-targeted complexes transfect cells more efficiently when compared to untargeted complexes and are specific for NPCs.
We hypothesize that expression was limited to NPCs that reside in the SVZ due to several reasons. First, expression was likely limited to NPCs in the SVZ and not other germinal niches due to the proximity of the SVZ to the lateral ventricles. Second, it has been shown that the success of PEI-based vehicles depends on cell division [30], allowing for passive targeting of dividing NSC and NPC populations. Lastly, preferential transfection of NPCs by Tet1-modified vehicles compared with unmodified vehicles (Figs. 4 and 5) indicates active targeting via GT1b and is reflected by the number of transfected cells positively stained for GT1b found after Tet1-mediated delivery (58%) as compared to untargeted delivery (13%). Although a complete study of ganglioside expression in early neural cells has yet to be established, GT1b has been observed to be expressed on the surface of neural progenitor cells [17]. Incomplete GT1b staining of transfected cells may be due to below detectable GT1b expression or loss of GT1b expression. Previous literature has reported that expression from ventricular delivery of unPEGylated polyplexes prepared from linear PEI was largely limited to subependymal GFAP+ neural stem cells and their progeny [9], although others have observed that expression occurred in both ependymal and subependymal layers even with a nestin specific promoter [31]. In our system prepared from PEGylated branched PEI, we found that expression after delivery of untargeted vehicles was generally nonspecific, whereas targeted vehicles showed exclusive transfection of sox2+/nestin+ cells.
Cultured NPCs were explored as an in vitro model to screen and optimize formulations since cultured monolayers were shown to be positive for sox2 and nestin (Supplemental Information, Figure S1). However, in vitro results did not corroborate with in vivo results when transgene expression and particle association were studied in NPCs isolated from the SVZ of adult mouse brains (Supplementary Information) as well as NGF-differentiated PC-12 neuron-like cells (data not shown). These differences may be because the requirements for successful in vivo delivery include cell-specific delivery in the presence of multiple cell-types as well as optimization for three-dimensional cellular architecture. The discordance between the relative efficiencies of delivery vehicles in vitro and in vivo has been observed previously [14, 32].
Before selecting direct injection into the lateral ventricle, several administration routes for polyplex delivery were surveyed. Intrathecal delivery has been successfully used to delivery PEI polyplexes [6]. In our hands, significant transfection was observed after intrathecal delivery, but expression levels were highly variable between mice. Variability may have been due to the technical difficulties associated with injecting into the small confines of the intrathecal space. It has been reported that co-delivery of mannitol can increase transgene expression, presumably by providing an osmotic shock that would allow for a transient increase in particle penetration [33]. Mannitol was delivered by co-injection with polyplex or intravenously 20 minutes prior to polyplex injection. Although mannitol increased transfection efficiency in some mice, observed increases were also variable. Lateral ventricular delivery was chosen as the route of administration since it consistently yielded expression levels above those observed in control glucose injections.
It was previously shown that PEGylation can adversely affect the intracellular trafficking of gene delivery vehicles [34, 35]. The negative effects of PEG on transfection efficiency are also reflected in our studies in vitro, with unPEGylated PEI showing increased transgene expression over PEGylated PEI (Supplementary Information, S2A). It may be that PEG decreases cell-association (Supplementary Information, S2B). However, PEG is necessary for in vivo delivery due to particle stabilization [23]. In initial studies, significant mouse morbidity (50%) was observed when unPEGylated polyplexes were delivered, likely due to complex aggregation.
CONCLUSIONS
In this work, we described a targeted and salt-stabilized nonviral gene delivery system that exclusively transfects neural progenitor cells. In addition, targeted vehicles mediated transgene expression that was increased compared to untargeted vehicles. Despite Tet1-mediated increases in transgene expression, the observed efficiency remains relatively low. Incorporation of peptides to improve intracellular trafficking of delivery vehicles, such as the use of membrane-active peptides for enhanced vesicular escape of vehicles or peptides to mediate the nuclear delivery of vehicles may further increase efficiency of synthetic gene delivery systems.
Supplementary Material
Acknowledgments
The authors thank Fernando Wong for his assistance with preparing brain tissue sections. This research was funded by NIH/NINDS (Grant Numbers 1R01NS064404 and 1R21NS052030). EJK was supported by the University of Washington’s Engineered Biomaterials Training Program (Grant Number T32GM06098) and NIH/NINDS (Grant Number 1F31NS064805). JL and PJH were supported by the International Foundation for Research on Paraplegia. BEJ was supported by the University of Washington Training Grant in Neurobiology (NIH GM007108) and by ARCS. We thank W. Stallcup (Burnham Institute for Medical Research, La Jolla, CA) for a generous gift of NG2 antibody. We thank P. Leprince at the University of Liège, Liège, Belgium for a generous gift of RC2 antibody. PJH is a member of the University of Washington Institute for Stem Cell and Regenerative Medicine and the Center on Human Development and Disability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 2.Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007;8(9):712–723. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- 3.Jandial R, Singec I, Ames CP, Snyder EY. Genetic modification of neural stem cells. Mol Ther. 2008;16(3):450–457. doi: 10.1038/sj.mt.6300402. [DOI] [PubMed] [Google Scholar]
- 4.Shim JW, Park CH, Bae YC, Bae JY, Chung S, Chang MY, et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007;25(5):1252–1262. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 5.Li SD, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13(18):1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- 6.Tang GP, Zeng JM, Gao SJ, Ma YX, Shi L, Li Y, et al. Polyethylene glycol modified polyethylenimine for improved CNS gene transfer: effects of PEGylation extent. Biomaterials. 2003;24(13):2351–2362. doi: 10.1016/s0142-9612(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wang J, Lee CG, Wang CY, Gao SJ, Tang GP, et al. CNS gene transfer mediated by a novel controlled release system based on DNA complexes of degradable polycation PPE-EA: a comparison with polyethylenimine/DNA complexes. Gene Ther. 2004;11(1):109–114. doi: 10.1038/sj.gt.3302135. [DOI] [PubMed] [Google Scholar]
- 8.Yurek DM, Fletcher AM, Smith GM, Seroogy KB, Ziady AG, Molter J, et al. Long-term transgene expression in the central nervous system using DNA nanoparticles. Mol Ther. 2009;17(4):641–650. doi: 10.1038/mt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemkine GF, Mantero S, Migne C, Raji A, Goula D, Normandie P, et al. Preferential transfection of adult mouse neural stem cells and their immediate progeny in vivo with polyethylenimine. Mol Cell Neurosci. 2002;19(2):165–174. doi: 10.1006/mcne.2001.1084. [DOI] [PubMed] [Google Scholar]
- 10.Bergen JM, Park IK, Horner PJ, Pun SH. Nonviral approaches for neuronal delivery of nucleic acids. Pharm Res. 2008;25(5):983–998. doi: 10.1007/s11095-007-9439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin ME, Rice KG. Peptide-guided gene delivery. Aaps J. 2007;9(1):E18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng W, Jackson DC, Murray J, Rose K, Brown LE. Totally synthetic lipid-containing polyoxime peptide constructs are potent immunogens. Vaccine. 2000;18(11–12):1031–1039. doi: 10.1016/s0264-410x(99)00346-1. [DOI] [PubMed] [Google Scholar]
- 13.Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci U S A. 1992;89(17):7934–7938. doi: 10.1073/pnas.89.17.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon EJ, Bergen JM, Pun SH. Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem. 2008;19(4):920–927. doi: 10.1021/bc700448h. [DOI] [PubMed] [Google Scholar]
- 15.Schaffer DV, Lauffenburger DA. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J Biol Chem. 1998;273(43):28004–28009. doi: 10.1074/jbc.273.43.28004. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro RE, Specht CD, Collins BE, Woods AS, Cotter RJ, Schnaar RL. Identification of a ganglioside recognition domain of tetanus toxin using a novel ganglioside photoaffinity ligand. J Biol Chem. 1997;272(48):30380–30386. doi: 10.1074/jbc.272.48.30380. [DOI] [PubMed] [Google Scholar]
- 17.Yanagisawa M, Taga T, Nakamura K, Ariga T, Yu RK. Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J Neurochem. 2005;95(5):1311–1320. doi: 10.1111/j.1471-4159.2005.03452.x. [DOI] [PubMed] [Google Scholar]
- 18.Townsend SA, Evrony GD, Gu FX, Schulz MP, Brown RH, Langer R. Tetanus toxin C fragment-conjugated nanoparticles for targeted drug delivery to neurons. Biomaterials. 2007;28(34):5176–5184. doi: 10.1016/j.biomaterials.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu JK, Tenga QS, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, et al. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol Dis. 2005;19(3):407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Park IK, Lasiene J, Chou SH, Horner PJ, Pun SH. Neuron-specific delivery of nucleic acids mediated by Tet(1)-modified poly(ethylenimine) J Gene Med. 2007;9(8):691–702. doi: 10.1002/jgm.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon EJ, Bergen JM, Park IK, Pun SH. Peptide-modified vectors for nucleic acid delivery to neurons. J Control Release. 2008;132(3):230–235. doi: 10.1016/j.jconrel.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goula D, Remy JS, Erbacher P, Wasowicz M, Levi G, Abdallah B, et al. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Therapy. 1998;5(5):712–717. doi: 10.1038/sj.gt.3300635. [DOI] [PubMed] [Google Scholar]
- 23.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Therapy. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 24.Kichler A. Gene transfer with modified polyethylenimines. J Gene Med. 2004;6:S3–S10. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- 25.Huang RQ, Qu YH, Ke WL, Zhu JH, Pei YY, Jiang C. Efficient gene delivery targeted to the brain using a transferrin-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Faseb J. 2007;21(4):1117–1125. doi: 10.1096/fj.06-7380com. [DOI] [PubMed] [Google Scholar]
- 26.Ma N, Wu SS, Ma YX, Wang X, Zeng J, Tong G, et al. Nerve growth factor receptor-mediated gene transfer. Mol Ther. 2004;9(2):270–281. doi: 10.1016/j.ymthe.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Huang R, Han L, Ke W, Shao K, Ye L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30(25):4195–4202. doi: 10.1016/j.biomaterials.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? (vol 10, pg 153, 2009) Nature Reviews Neuroscience. 2009;10(4):312–312. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- 30.Brunner S, Sauer T, Carotta S, Cotten M, Saltik M, Wagner E. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 2000;7(5):401–407. doi: 10.1038/sj.gt.3301102. [DOI] [PubMed] [Google Scholar]
- 31.Falk A, Holmstrom N, Carlen M, Cassidy R, Lundberg C, Frisen J. Gene delivery to adult neural stem cells. Exp Cell Res. 2002;279(1):34–39. doi: 10.1006/excr.2002.5569. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen J, Xie XL, Neu M, Dumitrascu R, Reul R, Sitterberg J, et al. Effects of cell-penetrating peptides and pegylation on transfection efficiency of polyethylenimine in mouse lungs. J Gene Med. 2008;10(11):1236–1246. doi: 10.1002/jgm.1255. [DOI] [PubMed] [Google Scholar]
- 33.Bourgoin C, Emiliani C, Kremer EJ, Gelot A, Tancini B, Gravel RA, et al. Widespread distribution of beta-hexosaminidase activity in the brain of a Sandhoff mouse model after coinjection of adenoviral vector and mannitol. Gene Therapy. 2003;10(21):1841–1849. doi: 10.1038/sj.gt.3302081. [DOI] [PubMed] [Google Scholar]
- 34.Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, et al. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Molecular Therapy. 2005;11(3):418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.