Abstract
Rats learn to prefer a flavored solution (CS+) paired with a gastrointestinal glucose infusion over an alternate flavor (CS−) paired with a non-caloric infusion. Prior work implicates a post-gastric site of glucose action, which is the focus of this study. In Exp. 1, male rats (8–10/group) were infused in the duodenum (ID), mid-jejunum (IJ), or distal ileum (II) with 8% glucose or water as they drank saccharin-sweetened CS+ and CS− solutions, respectively, in one-bottle 30-min sessions. Two-bottle tests (no infusions) were followed by a second train-test cycle. By the second test, the ID and IJ groups preferred the CS+ (69%, 67%) to the CS− but the II group did not (48%). Satiation tests showed that ID and IJ infusions of glucose reduced intake of a palatable solution similarly, while II infusions were ineffective. In Exp. 2, rats (10/group) drank CS solutions in one-bottle, 30-min sessions and were given 2-h ID or hepatic portal vein (HP) infusions. The CS+ and CS− were paired with 10 ml infusions of 10% glucose and 0.9% saline, respectively. Following 8 training sessions, the ID group preferred the CS+ (67%) to the CS− but the HP g roup did not (47%) in a two-bottle test. The similar CS+ preferences displayed by ID and IJ, but not II groups implicate the jejunum as a critical site for glucose-conditioned preferences. A preabsorptive glucose action is indicated by the CS+ preference displayed by ID but not HP rats in Exp. 2. Our data were obtained with non-nutritive CS solutions. HP glucose infusions are reported to condition preferences for a flavored food that itself has pre- and postabsorptive actions. Thus, there may be multiple sites for glucose conditioning with the upper or mid-intestines being the first site of action.
Keywords: intestine, duodenum, jejunum, ileum, hepatic portal vein, flavor conditioning
Introduction
Preference for the flavors (primarily tastes and odors) of foods reflects both an unlearned attraction to particular taste qualities (e.g., sweetness) and learned preferences for flavors. The acquisition of these preferences is a Pavlovian conditioning process, with the flavor serving as an orosensory conditioned stimulus (CS) that is associated with a reinforcing unconditioned stimulus (US). The US may be another flavor presented contiguously with the CS (flavor-flavor learning) or nutrient effects in or beyond the gut (flavor-nutrient learning). In an oral pairing of a flavor and a sugar, both processes can occur: the sweet taste of a glucose solution is associated with the added cherry flavor, and the post-oral nutrient effects of the sugar also associate with cherry flavor. To understand the separate contribution of a food’s nutrient effects to a conditioned flavor preference, the nutrient can be administered post-orally. The training procedure we have used in many rodent studies involves pairing post-oral (stomach or duodenum) infusion of a nutrient with the concurrent intake of a flavored CS+ solution, and infusion of water with intake of an alternate flavor CS−. In a two-bottle test conducted without infusions, when the animals display preferences for the CS+ relative to the CS−, it indicates that the nutrient US is reinforcing.
We have examined some aspects of the orosensory flavor qualities that contribute to the effectiveness of learning [3,4,17], and begun to identify the brain areas that are important for flavor preference conditioning [66]. We have also found that nutrients vary in their ability to reinforce preferences, which may be due to how rapidly they are detected by the gut or to differences in nutrient-specific sensors or pathways. We have also tested some of the putative sensors and signal carriers involved in mediating the learning. The present experiments continue our attempts to determine the nature of the post-oral signal generated by glucose, which appears to be the most effective unconditioned stimulus [5].
Much work demonstrates that glucose is rewarding, with both oral (sweet taste) and post-oral components that mediate the acquisition of preferences for associated flavors. To study post-oral glucose reinforcement, many studies have used intragastric (IG) infusion of glucose and related carbohydrates to condition preferences for a concurrently consumed flavor [2,8,40,41,45,51,53]. We know that the detection of the critical stimulus for post-oral glucose reinforcement lies beyond the stomach: confining infused glucose to a rat’s stomach does not result in a conditioned preference for the paired flavor, and infusions that bypass the stomach and put the glucose directly into the duodenum support flavor preference learning [16]. The duodenum could thus be the critical site for generation of the reinforcing signal, or that site might be more distal, as glucose travels further along the intestine. A recent proposal that the duodenum is more important as a sensor for control of sugar absorption than as an absorption site per se [29] suggests the possibility that sensors for glucose reinforcement effects might also reside there. To test this we compared duodenal and more distal intestinal glucose infusions for their potency in flavor preference conditioning.
Another possibility is that glucose reinforcement for flavor preference learning is sensed at a post-absorptive site. Several studies have examined the ability of systemic glucose administration to condition flavor preferences but with mixed results. In the earliest study, rats trained with different flavored chows paired with subcutaneous injections of 30% glucose or saline avoided the glucose-paired chow in a subsequent two-choice test [31]; this outcome may have been due to aversive effects of the hypertonic injection [7]. Other studies have used intravenous (IV) infusion of glucose paired with flavored solutions or chows. Food-restricted rats given IV infusions of 10% glucose 10–20 min after consuming a flavored saccharin mixture did not acquire a preference for this solution over unflavored saccharin [48]. In another study food-restricted rats trained to sham-feed flavored 36% sucrose solutions paired with IV infusions of 10% glucose or saline did not prefer the glucose-paired flavor in a two-bottle test conducted after 10 training trials [24]. Two studies trained non-deprived rats to eat different flavored chows that were paired with jugular infusions of glucose or saline. In one study, rats trained for 3 h at the start of the dark period failed to learn a preference for a flavored chow that had been paired with infusions of 25% glucose after a total of four glucose and four saline training trials [62]. In the other study, rats trained 24 h/day with spontaneous meals of flavored chow paired with concurrent infusions of 30% glucose or saline displayed a ~73% preference for the glucose-paired flavored chow after a total of 20 glucose and saline training days [37]. Together these studies indicate that only extensive training with systemic administration of glucose can condition a flavor preference in rats.
Detection of glucose in or near the liver has also been examined as a potential post-absorptive sensor area. The hepatic portal (HP) system collects absorbed glucose from the intestines and brings it to the liver. Rats given 3-h access to flavored chows paired with HP infusions of 25% glucose and saline subsequently preferred the glucose-paired chow in a 4-h two-choice test [62]. As noted above, other rats trained with jugular glucose infusions failed to acquire a flavor preference, which indicated the HP glucose was acting in or near the liver. An unpublished dissertation study [25] observed that HP glucose infusions conditioned a preference for a flavored 18% glucose solution that was orally consumed, but no preference was observed when the HP glucose was paired with a flavored non-caloric saccharin solution. Thus, in the only cases in which IV or HP glucose infusions have conditioned flavor preferences, the CS+ flavor was mixed into a nutritive substance (chow or glucose solution), which contrasts with the many reports of IG and ID glucose conditioning preferences for non-nutritive flavored solutions. This pattern of results suggests that the presence of nutrients in the gut may be required for effective conditioning with IV or HP infusions, which in turn would indicate a critical role for intestinal nutrient detection in flavor conditioning.
The goal of the present study was to examine multiple infusion sites for glucose-based flavor preference learning. The first experiment compared duodenal, jejunal, and ileal intestinal sites for flavor learning and satiation of ongoing feeding. The second experiment compared the flavor conditioning effects of slow HP and ID glucose infusions to clarify the effectiveness of pre-and post -absorptive glucose to condition preferences for non-nutritive flavored solutions.
Experiment 1
Intraduodenal (ID) infusions of glucose or glucose polymers have been shown to condition a preference for a CS+ flavored saccharin solution [16,32,46,54]. These studies all used catheter placements 1–2 cm distal to the pylorus. Experiment 1 evaluated the effect of shifting the infusion site further down the intestine, using sites previously used to study the satiating effects of intestinal nutrients. An intrajejunal (IJ) catheter was placed 19 cm beyond the stomach [12], and an intraileal (II) catheter was placed 14 cm proximal to the cecum [71]. These were compared to an ID catheter placed 1 cm beyond the stomach. If the animals with lower-gut infusions acquire a flavor preference, then duodenal glucose stimulation, even though it occurs in the course of normal gut passage of ingested foods, may not be critical to the learning process.
Intestinal glucose infusions, in addition to their potential for reinforcing flavor preference, can also engage satiation mechanisms that suppress feeding. The systems serving these mechanisms differ, because reinforcing and satiating effects can be separated [55]. Furthermore, increasing the satiating actions of a nutrient solution, e.g., increasing its concentration, may decrease its post-oral reinforcing effects [33,34]. In this experiment we tested for differences in glucose-induced satiation at the three intestinal sites because this could influence the flavor conditioning effects of the infusions. To determine the satiating potency of the glucose infusions, the rats were trained to consume a palatable fluid accompanied by water infusion, and then glucose infusion was substituted. Reduced oral intake during nutrient relative to water infusion is evidence for a satiating effect [35,46,53,54].
Method
Animals
Male rats born in our laboratory from Sprague -Dawley stock obtained from Charles River Laboratories (Wilmington, MA) were studied. The animals were individually housed in stainless steel hanging cages with ad lib access to water in rooms maintained on a 12:12 h light:dark cycle (lights on 0800 h) at 21 degrees C. The maintenance diet was powdered chow (No. 5001, PMI Nutrition International, Brentwood, MO; 3.3 kcal/g) and tap water was always available ad libitum in the home cage. The rats were 13–15 weeks old at surgery. Ad lib body weights after surgical recovery were 413–566 g (mean 496 g).
Surgery
The rats were anesthetized with isoflurane and fitted with an ID (n = 8), IJ (n = 8), or II (n = 10) catheter according to a technique adapted from Savastano et al. [50]. The abdominal cavity was opened with a midline incision and the appropriate section of intestine was located. A 20-g needle was used to make an incision into the duodenum 1 cm beyond the pylorus, or into the jejunum 19 cm beyond the pylorus, or into the ileum 14 cm proximal to the cecum. A pursestring suture was tied loosely around the incision. A silastic catheter (0.025-in. i.d., 0.047-in. o.d.) 22 cm long with an 1 cm x 0.5 cm piece of dacron mesh attached 1 cm from its end was inserted caudally into the incision until the mesh was adjacent to the incision. The pursestring suture was tied around the catheter and the mesh was sutured to the adjacent intestinal wall. The intestines were returned to the abdominal cavity. The catheter was routed through an incision in the abdominal muscle wall subcutaneously to the animal’s head. The end of the catheter was connected to a Luer-lock assembly which was then fixed onto the skull by stainless steel screws and dental cement. The abdominal musculature and skin were closed with sutures. Thereafter, 0.2 ml of water was infused daily to maintain catheter patency.
Apparatus
The rats were trained and tested in plastic infusion cages (23 × 24 × 31.5 cm) with stainless steel mesh flooring. Above the cage, plastic tubing from a syringe pump (A-99, Razel Scientific, Stamford, CT) was connected to the input port of a swivel on a counterbalanced lever. Plastic tubing, protected by a stainless-steel spring, connected the swivel’s output port to the rat’s Luer-lock assembly. Stainless steel drinking spouts were available through holes in the front wall of the cage, centered 32 mm apart. The spouts were attached to drinking tubes mounted on motorized holders (ENV-252M, Med Associates, Georgia, VT) that positioned the spouts at the front of the cage at the start of the session and retracted them at the end of the session. Fluid spillage, which was minimal, was collected in trays below the spouts; spillage amounts were recorded and used to correct the intake data. Licking behavior was monitored by an electronic lickometer (ENV-250B, Med Associates) and a microcomputer. On training trials, the rat’s licking responses activated the syringe pump. The infusion rate was set at 0.54 ml/min and the oral intake/infusion ratio was maintained at approximately 1:1 by the computer software until the limit was reached. Infusion limits were set at 10 ml in the first cycle and 15 in the second cycle (see Conditioning).
Test solutions
All solutions were prepared on a weight/weight basis using tap water. The rats were pretrained to drink unflavored 0.2% sodium saccharin (Sigma, St. Louis, MO) solution. The conditioned stimulus (CS) solutions consisted of 0.2% sodium saccharin flavored with 0.05% cherry or grape unsweetened Kool-Aid (Kraft Foods, White Plains, NY). The Kool-Aid flavor mixes contain citric acid; the CS solutions thus had a common base of sweet and sour tastes, and differed primarily in their odors. The rats were infused with tap water and 8% glucose (BioServ, Frenchtown, NJ). In satiation tests, the rats were given a palatable mixture of 2% maltodextrin (Maltrin QD M580, Grain Processing Corp., Muscatine, IA) and 0.2% saccharin (M+S), paired with infusions of water, 8% and 16% glucose solutions. Solution intakes were recorded by weighing the drinking tubes to the nearest 0.1 g, and amounts infused were recorded by reading the infusion syringe to the nearest 0.5 ml.
Pretraining
The rats were given 0.2% saccharin solution and water in an overnight two bottle test in their home cages, to familiarize them with saccharin and identify any animals that avoided saccharin. All animals consumed the saccharin solution. The rats were next adapted to the infusion cages and the bottle retractors by housing them in the test cages overnight with saccharin and water available for 30 min/h; chow was available ad libitum. They were then placed on a food restriction schedule that maintained them at ~85% of their free-feeding body weight. This target body weight was maintained throughout the study (except for the surgical and recovery periods) to ensure that the animals would be motivated to drink in the daily sessions. During the next 5 days they were given 30-min sessions with 0.2% saccharin available in the test cages, returned to their home cages and given their chow rations 1 h later. Then they were given ad libitum chow prior to surgery.
The surgical groups were matched for ad libitum body weight and 30-min saccharin intake. They were allowed to recover from surgery for 10–14 days with ad libitum chow and water. Over the next 7 days they were food restricted and given daily 30-min sessions with 0.2% saccharin in the test cages, On the fourth day they were connected to the infusion system with the pumps inactivated, and on the remaining pretraining days they were infused with a maximum of 10 ml water as they drank. On these and all subsequent days, chow rations were provided 1 h after the session to maintain the rats at 85% of ad libitum weight.
Conditioning
For each animal, one flavored saccharin solution (the CS+) was paired with infusion of 8% glucose, and the other flavor was paired with water infusion. Assignment of flavors was counterbalanced across rats. In cycle 1, the rats were given eight sessions of one-bottle training: the CS+ and glucose infusion were offered on day 1, 3, 5 and 7, and the CS− and water infusion on days 2, 4, 6 and 8. Left-right placement of the bottle followed an ABBA pattern. The infusion was set to a maximum of 10 ml, while the oral intake was unlimited. This was done because the infusion rate was slower than the intake rate, and as in prior work we did not want the infusions to become aversive by introducing excessive volume when summed with oral intakes. Following this training, four preference tests were given, with the left-right positions of the flavored solutions counterbalanced to control for side preferences. No infusions were given during these tests. A second cycle of training and testing was similar to the first, except that the infusion limit was increased to 15 ml and only 6 days of one-bottle training were conducted.
Satiation testing
Following the test of cycle 2, the rats were given daily sessions with the M+S solution. On days 1, 2, 3, 5 and 6 they were co-infused with water; no limit was placed on the infusion. On day 4, 8% glucose was substituted for the water infusion, and on day 7 a 16% glucose solution was infused. No flavor cues were added to the orally-consumed M+S solution so that changes in solution intake reflected the unconditioned response to the glucose infusions.
Statistical analysis
The intake data were summed over 4 (cycle 1) or 3 (cycle 2) days of training, and 2-day averages were used to compare the first and second two-bottle preference tests in each cycle. The percent preference for the CS+ flavor was also used to compare the groups. For the satiation tests, total fluid (oral plus infused) was compared on water and glucose infusion days. The data were analyzed by repeated measures analysis of variance.
Results
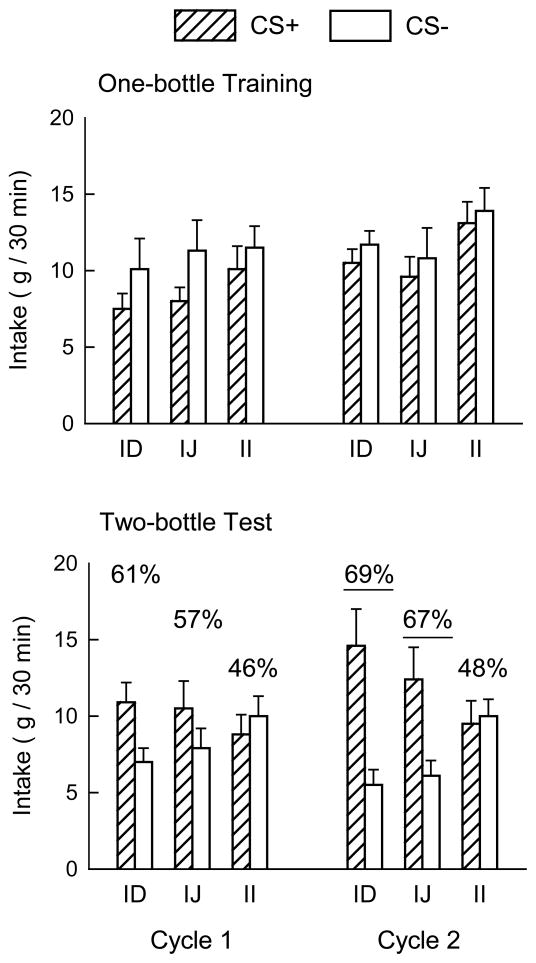
Total training intakes of the CS+ and CS− solutions in cycles 1 and 2 were compared among groups, revealing a three-way interaction (F(2,23) = 5.22, p < 0.05). Separate analyses by cycle showed that the differences occurred only in Cycle 1, with greater intake of CS− than CS+ (F(1,23) = 9.39, p < 0.01). The significant group x flavor interaction (F(2,23) = 4.80, p < 0.05) in Cycle 1 occurred because only the IJ group reliably consumed more CS− than CS+. Total tr aining intakes did not differ by group in Cycle 2. Analysis of total glucose infusion during training showed no differences among the groups (28.4, 28.5 and 33.1 ml/cycle for ID, IJ and II groups). Fewer than half the animals were infused with the maximum volume per session in each training cycle. The training intakes are shown in Figure 1 as daily averages, to provide a scale similar to that of test intakes.
Figure 1.
Mean (+SEM) intakes of CS+ and CS− solutions by the intraduodenal (ID), intrajejunal (IJ) and intraileal (II) groups during one-bottle training (top panel) and two-bottle testing (bottom panel) in cycles 1 and 2 of Experiment 1. Numbers atop the columns in the bottom panel indicate the percentage preference for the CS+; underlined values denote significantly greater intake of CS+ than CS− in the two-bottle tests.
Overall analysis of test intakes in the two cycles showed that CS+ intake exceeded CS− intake (F(1,23) = 11.56, p < 0.01), and CS solution interacted with group (F(2,23) = 5.26, p = 0.01) and cycle (F(1,23) = 5.36, p < 0.05). In the first cycle, preference test intakes of CS+ and CS− did not differ, although the effect approached significance (p = 0.08). There were no group differences in solution intakes in Cycle 1, and average oral intake was close to the 10 ml infusion limit. Based on our prior work, which commonly finds increases in intake across cycles even with matched infusions, we expected the animals to drink more in the second cycle and therefore the oral limit was increased to 15 g. By the second cycle, the groups differed (F(1,23) = 12.29, p < 0.01), with the ID and IJ groups drinking more CS+ than CS− while the II group’s solution intakes were similar (interaction F(2,23) = 4.29, p < 0.05; Fig. 1). The groups differed in 4-day mean percent preferences for the CS+ in cycle 1, (F(2,23) = 4.09, p < 0.05) and cycle 2, (F(2,23) = 5.03, p < 0.05). In cycle 1 the ID group’s 61% preference exceeded the 46% preference of the II group, with the IJ group intermediate at 57%. By cycle 2 both the ID and IJ groups’ preferences exceeded that of the II group (69% and 67% vs. 48%; Fig. 1).
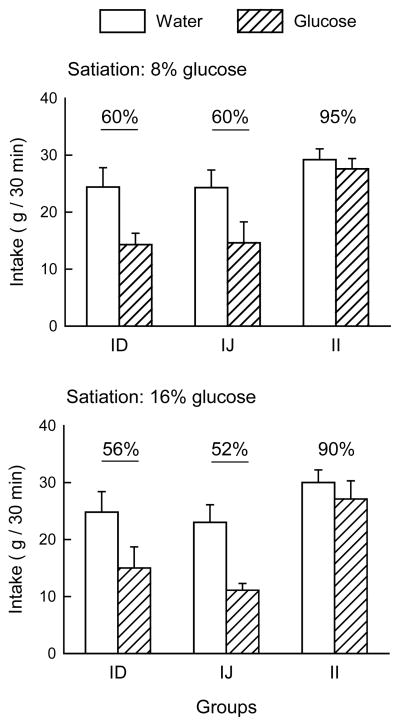
In the satiation test phase (Fig. 2), intakes of the M+S solution were greater when water was infused than when glucose was infused, F(1,23) = 59.51, p < 0.001; the glucose concentrations of 8% and 16% did not differ in their suppressive effects. The groups did not differ in baseline intake (mean 26.2 g) when infused with water, but responded differently when glucose was infused. Simple main effects showed that the ID and IJ groups reduced their intake similarly in glucose infusion sessions, whereas the II group consumed the same amount during water and glucose infusions. Expressed as a percent of water baseline, the ID and IJ groups behaved similarly when infused with glucose, consuming less than the II group (Fig. 2), F(2,23) = 18.44, p < 0.001.
Figure 2.
Mean (+SEM) intakes of 2% maltodextrin + 0.2% saccharin solution by the intraduodenal (ID), intrajejunal (IJ) and intraileal (II) groups during the satiation tests in Experiment 1. Responses to 8% glucose are shown in the top panel and to 16% glucose in the bottom panel. Numbers atop the columns indicate the percentage reduction in intake during glucose infusion relative to water infusion; significant reductions are underlined.
Discussion
The primary result of Experiment 1 is that glucose infused at different points along the intestinal tract differed in potency as the unconditioned stimulus for flavor preference learning. The rats were able to associate the CS+ flavor with glucose reinforcement equally well when it was delivered by duodenal or jejunal infusions, but infusing glucose more distally, in the lower ileum, was ineffective. The similarity of ID and IJ groups, and their difference from the II group, extended to the satiation tests. This suggests that glucose detection relevant to reinforcing the preference for paired flavors and to satiating effects may not extend throughout the intestine, but rather may be confined to more proximal sections.
In prior studies of flavor conditioning with ID infusions, the rate of acquisition was faster. For example, in a previous study we obtained a significant 79% CS+ preference for the glucose-paired flavor after only two training sessions each of CS+ and CS−, and the preference increased to 85% in a second cycle [16]. Those animals consumed somewhat more CS+ than CS− solution during training, in contrast to the present ID group, but overall the volumes consumed were similar in the two studies. Those rats were female, whereas the present study used males, but similar strong and rapid conditioning was seen in males [54] and females [32] trained with 8% maltodextrin infusions. The latter studies did not impose limits on intake, but even when rats were constrained to 7 ml/session, they showed a 68% preference for a maltodextrin-paired flavor in the first test [46]. Maltodextrin is rapidly hydrolyzed to glucose in the intestine; preventing its hydrolysis abolishes post-oral reinforcement by maltodextrins [17]. Thus maltodextrin reinforcement value should be comparable to that of glucose, and there are no obvious differences from previous work to explain why acquisition was slower for the present ID rats.
There is one prior study of flavor conditioning with jejunal infusions. After finding that prolonged jejunal infusion of a liquid diet produced greater suppression of chow intake than a duodenal infusion, Canbeyli and Koopmans [12] paired different flavored solutions with liquid diet and control saline infusions. In contrast to the present results, their animals were indifferent in a preference test between the flavors. This is probably due to the lengthy infusion sessions and concurrent access to chow, factors which would make the association of flavor and infusion more difficult. The liquid diet may also be a less potent stimulus for flavor conditioning than a simple glucose infusion.
The satiating similarity of ID and IJ carbohydrate infusion has been observed previously [39]. Efficient uptake of glucose by these more proximal sections of intestine means that ileal glucose concentrations are generally low compared to those of upper intestine [18]. Studies of nutrient introduction to the ileum generally report the ileal brake effect: reductions in gut motility and secretion, increased satiety and reduced food intake [36]. We did not observe the ileal brake on intake: when glucose was infused to the ileum, the rats did not reduce intake in the satiation test, even at a 16% glucose concentration. The previous study using this infusion locus [71] used a longer glucose infusion period (2 h) and measured chow intake in ad libitum fed animals. In their rats, which had both ileal and duodenal catheters, ileal infusion was more effective than duodenal infusion at suppressing food intake; however, their shortest reported measure is at 2 h, whereas our measures only lasted during the 30-min session. Thus it remains possible that our ileal glucose infusions would have reduced intake, and that 16% glucose would have suppressed intake more than 8% glucose, if we had conducted longer sessions, used a chow diet rather than palatable solution, and/or studied ad libitum fed animals. The importance of our satiation results is that the failure of the ileal glucose infusions to condition a flavor preference was not due to a strong inhibition of intake by the infusions. Implications of the parallels in flavor preference and satiation results are further considered in the General Discussion.
Experiment 2
Experiment 1 established that glucose reinforcement can be generated with duodenal and jejunal infusions. The next line of inquiry asked whether the signal for flavor preference learning requires intestinal stimulation, or is based on post-absorptive events. In Experiment 2 we looked at the effect of glucose introduced beyond its normal absorption site, as an overall test for the involvement of the intestines in generating the reinforcing signal.
The hepatic portal system is the first postabsorptive locus for nutrient detection. There are glucose sensors in the hepatic portal vein that contribute to glucose homeostasis [61]. Hepatic portal venous infusions of glucose reduce food intake in rats in most experiments, [e.g., 9,11,30,62]. Most studies are concerned with the control of ongoing feeding rather than flavor conditioning, so there are few measures of learning with the glucose infusion as the unconditioned stimulus. As part of a demonstration that HP-infusion induced food intake reductions were not due to aversive effects, Tordoff and Friedman [62] showed that HP glucose enhanced preference for a flavored chow consumed during the infusion, relative to another flavored chow given during a saline infusion. They infused 540 mg glucose (1.5 M solution, 4.5 mg glucose/min for 2 h) and iso-osmotic 0.75 M saline as a control in alternate sessions. During training, intakes were similar in the first pair of trials, with lower intakes during glucose infusions in the next three pairs of sessions. In the single 4-h preference test, the glucose-paired chow flavor was preferred over the saline-paired chow flavor. In contrast, rats treated identically, except that the infusions were jugular, consumed the two flavored chows similarly in training and in testing sessions.
Although the Tordoff and Friedman study [62] suggests a hepatic site for the detection of glucose reinforcement in flavor preference conditioning, an unresolved question is the importance of intestinal stimulation by nutrients during training. Because the flavors were delivered in the chow consumed during the HP infusions, the ingested nutrients may have played a role in the resulting preference. In an unpublished dissertation study, Gowans [25] asked whether HP infusions could condition preferences for a flavor delivered in a non-nutritive saccharin solution, thus eliminating nutrient stimulation of the gut. Two experiments with saccharin-sweetened flavors found no preference for the HP-glucose paired flavor. In a final experiment, she infused 10% glucose at 8.3 mg glucose/min) to deliver 1 g of glucose over a 2-h period; the infusions began at the onset of 30-min access to the flavored sweet solution during training. Separate groups of rats were trained with flavored 0.15% saccharin or flavored 18% glucose; for each group, one flavor was paired with HP glucose and the other with HP saline for 6 sessions each. The final test was a 4-h access period to the two flavored solutions: the saccharin group showed no preference, whereas the glucose group showed a significant 71% preference for the HP glucose-paired flavor.
Together, these data suggest that intestinal nutrient stimulation may be required for the acquisition of preferences based on HP infusion of glucose. This in turn raises the question of the requisite site for the detection of glucose reinforcement. If it does not occur when nutrient is absent from the gut, this would suggest an important role for a gut sensor in the process. Both the Tordoff [62] and Gowans [25] experiments used ad libitum fed animals, which would have some food present in the gut during training. The present experiment studied food-restricted animals as a further means of controlling nutrient stimulation during training. We compared hepatic portal glucose infusions using Gowans’ infusion parameters to ID glucose infused with the same parameters. Conceivably, the failure of an HP glucose infusion to condition a preference for a flavored saccharin solution may be related to the slow rate of post-oral glucose delivery. In this case, a 2-h ID glucose infusion, unlike the ~30-min ID infusions used in Experiment 1, would also fail to support flavor conditioning of a flavored saccharin solution. Anticipating this possible outcome, additional rats were tested with the same amount of glucose infused ID over a 1-h period to provide an intermediate delivery rate for comparison.
Experiment 2A
To examine the hepatic portal route without the confound of nutrient stimulation in the gut, we paired hepatic portal infusion of glucose with intake of a flavored saccharin solution in food-restricted rats. We used the Gowans [25] parameters (1 g glucose delivered in 2 h).
Method
Animals
Ten male rats of the same description and housing as Experiment 1 were studied. The animals were 14–18 weeks old at surgery. Ad libitum body weights after surgical recovery were 370–532 g (mean 445 g).
Surgery
The HP catheter consisted 12 cm of m icro-renathane tubing (Braintree Scientific Incorporation; 0.014 in. i.d. × 0.033 in. o.d.) inserted 4 cm into 16 cm of silastic tubing (0.025 in. i.d. × 0.047 in. o.d.). A 1 cm × 1 cm piece of polypropylene mesh (Bard) was attached with silicone adhesive at the point where the micro-renathane emerged from the silastic tubing. A 2 mm long piece of silastic tubing (0.025 in. i.d. × 0.047 in. o.d.) was secured 2.5 cm from the beveled end of the micro-renathane tubing portion of the catheter as a cuff. Catheters were prepared at least 1 day prior to implantation. Prior to surgery the catheter was sterilized by activated dialdehyde solution (Cidex®, Advanced Sterilization Products, Irvine, CA) for 20–30 min.
Rats were anesthetized with an intraperitoneal injection of a ketamine HCl (63 mg/kg) and xylazine (9.4 mg/kg) mixture. The abdominal cavity was opened with a midline incision. A 1-cm section of the ileocolic vein was isolated and its caudal end was tied off with a suture knot. The micro-renathane portion of the catheter was inserted into a small incision in the vein and advanced toward the liver until the cuff reached the incision. A suture next to the cuff fixed the catheter in the vein and Nexaband glue (Closure Medical Corporation, Raleigh, NC) was applied to secure the catheter insertion site.
The silastic portion of the catheter was routed through a small incision in the abdominal muscle wall subcutaneously to the animal’s head. The mesh on the silastic tube was sutured to the inner abdominal wall before closing the cavity. The silastic end of the catheter was connected to a Luer-lock assembly which was then fixed onto the skull by stainless steel screws and dental cement. Before closing the catheter with a cap, 0.15 ml of polyvinylpyrrolidone (Sigma-Aldrich, St. Louis) in heparin (500 IU/ml) was infused into the catheter [58]. For catheter maintenance, 0.1 ml of isotonic sterile saline-heparin solution (100 IU/ml) was infused into the catheter for the first two recovery days. Thereafter, the saline-heparin solution was replaced by isotonic sterile saline solution.
Catheter patency was examined three times: prior to the one-bottle training, prior to the two-bottle choice test, and after the completion of the two-bottle choice test. Animals were infused with 0.1 ml of a 10:7 ketamine:xylazine mixture (providing 5.9 mg ketamine + 0.8 mg xylazine) followed by 0.3 ml of isotonic sterile saline [30]. In this challenge, if the catheter remains in place, the effect of anesthesia is expected to appear within 1 min. Data from the ten animals that passed the anesthesia test were included in the statistical analysis. Autopsy was performed to further examine the position of the catheter. A small amount of green dye (0.1 – 0.2 ml) was infused into the catheter to test for leakage from the catheter.
Apparatus
The rats were trained and tested in the same plastic infusion cages as in Experiment 1. The infusion rate was set at 0.083 ml/min. On training trials, the syringe pumps were activated by the rat’s licking responses and delivered a 10 ml infusion over a 2-h period. These infusion parameters were adopted from Gowans [25], and the rate of 8.3 mg glucose/min is within the physiological range for glucose absorption from the gut [58,63].
Test solutions
The rats w ere pretrained to drink unflavored 0.2% sodium saccharin. The CS solutions were the same Kool-Aid flavored saccharin solutions as in Experiment 1. The infusion paired with CS+ was 10% (w/v) sterile glucose. The infusion paired with CS− was sterile 0.9% saline.
Pretraining
The rats were trained to drink saccharin in the test cages as in Experiment 1. The food restriction schedule maintained the rats at ~95% of their free-feeding body weight. Prior to surgery they were given ad libitum chow.
Animals were given at least five days for recovery after surgery with ad libitum chow and water, and were then food restricted to maintain them at ~94% of their free-feeding body weight. Over the next 3 days they were again food restricted and given daily 30-min sessions with 0.2% saccharin in the test cages. On the second and third days they were connected to the infusion system with the pumps inactivated. On these and all subsequent days, chow rations were provided immediately after the session.
Conditioning
Assig nment of CS+ and CS− flavors was counterbalanced as in Experiment 1. The flavor preference training was conducted for 8 consecutive 135 min/day sessions (15 min were added to the 2-h period to allow for animals that did not drink immediately at the beginning of the session, which in turn would delay the onset of the infusion). On days 1, 3, 5 and 7 animals were given 30-min access to the CS− solution paired with 10 ml HP saline infusion. On days 2, 4, 6 and 8 the animals were given 30-min access to the CS+ solution paired with 10 ml HP glucose infusion. Left-right placement of the bottle followed an ABBA pattern across days. Once each rat made 20 licks on the CS+ or CS− sipper tube, its syringe pump was activated and delivered the paired infusion at a rate of 0.083 ml/min continuously until the volume reached 10 ml.
A two-bottle choice test was conducted on 2 consecutive days. On each day animals had concurrent access to the CS+ and CS− solutions for 30 min without infusions. The left -right presentation of the CS solutions was counterbalanced across sessions.
Statistical analysis
The intake data were averaged over 4 days of training and 2 days of testing, and evaluated with paired t-tests.
Results and discussion
During one-bottle training, the animals consumed similar amounts of the CS+ and CS− solutions (5.9 and 6.1 ml, respectively). In the two-bottle choice test, the rats showed an average of 46% preference for the CS+ flavor (3.5 ml) relative to the CS− flavor (4.3 ml). There were no statistical differences between the CS+ and CS− intakes during one -bottle training or the two-bottle choice test.
These data are consistent with those of Gowans [25]: rats trained with hepatic portal infusion of glucose did not learn to prefer a flavored saccharin solution that was paired with the infusion. By studying food-restricted animals, we have shown that the failure to learn in the Gowans study is not attributable to the use of ad libitum feeding. In a second experiment Gowans also failed to condition a preference for a flavored saccharin solution in rats trained with a 2-h HP infusion of 5% glucose, which represented a rate of glucose infusion (4.2 mg/min) similar to that used in the Tordoff study. This supports the idea that Gowans’ and our HP infusions were not excessive compared to Tordoff’s.
Experiment 2B
The hepatic portal infusion in Experiment 2A delivered 1 g of glucose over a 2-h period whereas in Experiment 1 the ID infusions delivered ~0.8 g of glucose over a ~30 min period. Thus, it is possible that the HP infusions failed to condition a preference because the glucose delivery was too dispersed in time. Another difference between the two experiments is that the ID rats received a total of eight glucose infusions (two cycles of four infusions each) compared to the HP rats (one cycle of four glucose infusions). Accordingly, we conducted Experiment 2B with rats given glucose infusions delivered to the duodenum at the same rate and for the same number of training sessions as in Experiment 2A. Additional rats were given the same amount of glucose infused at twice the rate to determine if ID infusion rate affects the strength of flavor conditioning.
Method
Animals and Surgery
The rats were 17–21 weeks old at the time of ID catheter surgery, which was performed as in Experiment 1, except that the anesthetic was ketamine and xylazine as in Experiment 2A. Ad lib body weights after surgical recovery were 404–510 g (mean 448 g). Animals were given at least five days to recover, and were then food restricted to maintain them at ~92% of their free-feeding body weight.
Procedure
Pretraining, conditioning, and testing procedures were identical to those of Experiment 2A with two exceptions. The infusion site was ID rather than HP, and the rats were divided intotwo groups. The ID -2H group (n=10) was infused for 2 h at 0.083 ml/min to match the HP group of Experiment 2A; the ID-1H group (n=9) was infused for 1 h at 0.18 ml/min. As in Experiment 2A, both groups received 10 ml of 10% glucose or 0.9% saline on training days.
Results and discussion
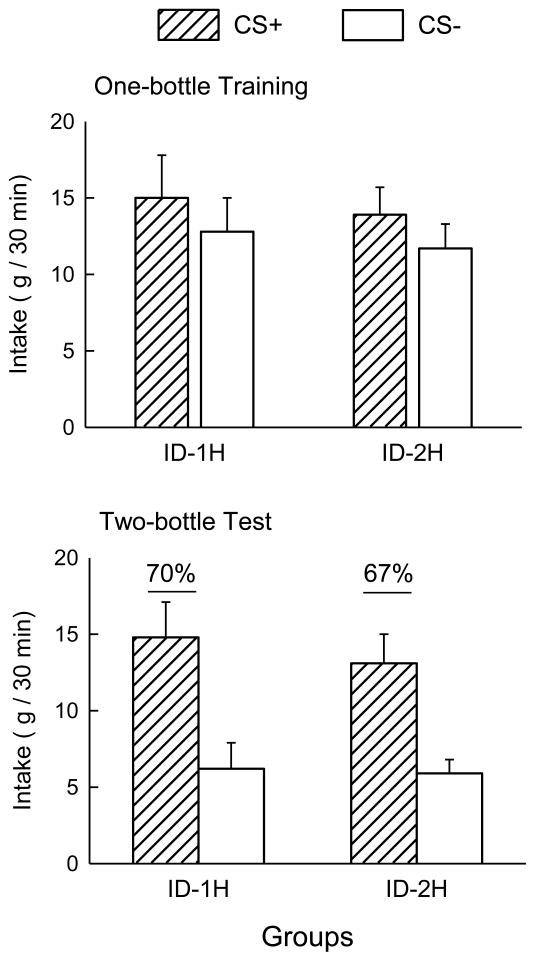
The one-bottle training and two-bottle test data are presented in Figure 3. During the one-bottle training, both groups consumed somewhat more of the CS+ than of the CS− (F(1, 17) = 22.65, p < 0.01). In the two-bottle choice test, both the ID-1H and ID-2H groups consumed more of the CS+ than the CS− (F(1, 17) = 22.05, p < 0.01). There were no differences between the groups in training or testing. The percent preferences for the CS+ solution during two-bottle tests for the ID-1H and ID-2H groups were 70% and 67%, respectively, and did not differ significantly.
Figure 3.
Mean (+SEM) intakes of CS+ and CS− solutions by the one -hour (ID-1H) and two-hour (ID-2H) groups during training (top panel) and testing (bottom panel) in Experiment 2B. Numbers atop the columns in the bottom panel indicate the percentage preference for the CS+; underlined values denote significantly greater intake of CS+ than CS− in the two -bottle tests.
Intraduodenal infusion of glucose at the same concentration, volume and rate as the hepatic portal infusions of Experiment 2A was clearly an adequate unconditioned stimulus: the ID-2H rats acquired a preference for the CS+ flavor paired with the ID glucose infusion. Similar CS+ preferences were observed in the ID-2H and ID-1H rats, which were infused with glucose at rates (8.3 and 18 mg/min) that are slightly lower and higher, respectively, than the expected glucose absorption rate (about 12 mg/min) for animals of this size [42]. In addition to expressing a preference for the CS+ in two-bottle tests, the rats in both groups also consumed more CS+ than CS− during train ing. This stimulation of CS+ intake during training was not observed in the first experiment, perhaps because of the higher rate of ID infusion.
The CS+ preference displayed by the ID-2H rats is in marked contrast with the failure of the HP rats to acquire a CS+ preference in Experiment 2A. A potential drawback to the comparison between the two experiments is the disparity in training intakes: the ID-2H rats consumed more than twice as much of the CS+ (and CS−) solutions during training than did the HP rats, although they were infused with identical amounts of glucose. CS training intakes, however, do not appear to be a critical factor in preference conditioning. Note first, that the training intakes of the rats in the Gowans experiment were higher than those of the HP rats in Experiment 2A (11 ml vs. 6 ml), yet they also did not learn a CS+ preference. Second, rats limited to CS intakes as little as 3 g per 30-min training session develop strong preferences (>80%) for the CS+ flavor when it is paired with afixed IG glucose infusion (8 ml of 8%)[64].
Although similar infusion parameters were used in the HP and ID experiments, the 2-h ID infusion may not have delivered glucose to the hepatic portal system at the same rate, pattern, and concentration as the HP infusion. It is possible that other HP glucose infusion parameters than those used here and in Gowans’ study might support flavor preference conditioning in the absence of intestinal nutrient stimulation. Thus far, though, the data point to a requirement for intestinal involvement in the glucose signal for flavor preference learning.
General Discussion
The present study provides new information on the possible sites of action for glucose-conditioned flavor preferences in rats. Glucose infusions into the duodenum and jejunum supported flavor conditioning whereas infusions into the distal ileum failed to do so. Bypassing the intestine, to mimic a post-absorptive glucose increase, was ineffective for conditioning a preference for a flavored non-nutritive solution. In contrast, the same infusion parameters were sufficient when the glucose was delivered to the duodenum, even though the infusion was quite slow compared to prior successful ID conditioning procedures. The slow ID glucose infusion, which would be efficiently absorbed as it entered the intestine, as well as the lack of flavor preference with ileal infusions, indicate that the proximal intestine is the critical site of action for glucose conditioning of flavor preferences.
Although post-oral glucose is probably detected rapidly, the stimulus might also persist for some time as sugar proceeds through the intestine. Experiment 1 shows that bypassing the duodenum by infusing glucose to proximal jejunum was just as effective as infusing into the duodenum. From this comparison, the boundaries of the effective conditioning area are not clear; it may extend from duodenum to jejunum, or the duodenum need not be involved at all, since some glucose presumably makes its way past this segment during normal digestion. However, given the generally efficient uptake of glucose in the intestine, the proximal parts of the tract are best positioned to capture information about nutrient content for this associative process. The 2-h infusion rate in Experiment 2B probably allowed complete glucose absorption in proximal intestine, but we did not attempt to measure the extent of intestinal stimulation. Consequently, it is not known whether glucose confined to the duodenum would support flavor learning. One possible method of accomplishing this would be to infuse glucose into a surgically isolated duodenal section that drained into the ileum.
While HP glucose infusions failed to support flavor preference conditioning in the present study and two experiments conducted by Gowans, they were effective in two other experiments in which HP glucose infusions were paired with flavored foods (chow or glucose) [25,62]. In addition, IV glucose infusions were reported to condition a preference for flavored chow in a long-term conditioning study, whereas IV glucose did not condition preferences for non-nutritive solutions in other studies (flavored saccharin, or sham-fed flavored glucose [24,48]). Collectively, these studies suggest that HP or IV glucose infusions support flavor conditioning only when combined with pre-absorptive nutrient stimulation. The site of the pre-absorptive stimulation has not been investigated, but is likely to be the upper intestines. Note, for example, the ineffectiveness of IG glucose to condition a preference for a flavored sucrose solution that was sham-fed and thus stimulated only the mouth and stomach [24]. It may be, then, that preference conditioning by IG, ID, and IJ glucose infusions is due to the successive stimulation of intestinal and post-absorptive nutrient sensors. This need not be the case, however, and it remains to be determined if post-absorptive glucose stimulation is essential for flavor preference learning to occur.
While the present and previous findings [25] indicate a critical role for intestinal nutrient sensing in glucose-based preference conditioning, the identity of the nutrient sensors remains to be established. The recent discovery of T1R2/T1R3 sweet taste receptors and related signaling elements in intestinal cells [e.g., 10] suggest the intriguing possibility that the same receptors that initiate sugar reward in the mouth mediate sugar reinforcement in the gut. However, IG glucose or sucrose infusions can condition flavor preferences in knockout mice missing the T1R3 receptor or other taste signaling elements (gustducin, Trpm5) [23,57], which indicates that these gut sweet detectors are not required. Furthermore, the ineffectiveness of some sweet receptor ligands (fructose, galactose, sucralose) to condition flavor preferences when infused IG suggests that the nutrient sensor involved is specific to glucose rather than sweeteners in general [53,56]. One potential candidate is SGLT-3, which is thought to be a glucose sensor that does not respond to fructose or galactose [47,73]. However, preliminary findings indicate that the SGLT blocker phlorizin does not prevent preference conditioning by ID glucose infusions (Yiin and Sclafani, unpublished). The role of SGLT-3 and other glucose sensors requires further study.
Transmission of post-oral reinforcement signals generated by intestinal and post-absorptive glucose sensors to brain circuits that process food preference learning could involve neural and/or humoral pathways. Lesioning vagal and nonvagal neural afferents by complete abdominal vagotomy, selective afferent abdominal vagotomy, celiac-superior mesenteric ganglionectomy, or capsaicin treatment did not block flavor preference conditioning by glucose polymers, however [32,52,54]. Together, these findings suggest that neural transmission of the post-oral signal is not necessary for glucose-conditioned flavor preferences. Consistent with this interpretation, recent fMRI findings [67] indicate that vagotomy does not prevent the rapid activation by IG glucose infusions of brain reward sites, including the nucleus accumbens and amygdala, implicated in flavor preference learning [13,64,65]. Note that while not required for glucose conditioning, capsaicin-sensitive visceral afferents may contribute to preference conditioning by other nutrients and training procedures [72].
Humoral signaling is the alternate method for getting the glucose message to the brain. Early work suggested a role for cholecystokinin in flavor preference learning [38]. However, a subsequent study revealed that blockade of CCKA receptors with devazepide, which reduced the satiating effect of duodenal carbohydrate infusion, did not block the acquisition of a preference for the flavor paired with the infusion [46]. Insulin is implicated as a humoral signal for post -oral glucose [67]. However, we observed that post-oral glucose-based flavor preference learning is not dependent on an intact insulin secretory response to glucose [1]. Insulin injections were found to produce aversions and preferences, respectively, for a flavored sugar-milk diet that was real-fed and sham-fed [68]. Other findings indicate that insulin may act in the brain to reduce sweet taste signaling and sugar activation of brain reward systems [19–22]. Together, these data do not suggest a primary rolefor insulin in glucose -based flavor learning.
Glucagon-like-peptide 1 (GLP-1) is another potential source of glucose reinforcement signal, but it is not a strong candidate. Although GLP-1 is secreted in response to intestinal nutrients, and GLP-1 receptors are present in a number of brain regions important in feeding behavior, GLP-1 is rapidly inactivated [14] and has little chance of transmitting a signal directly to the brain. Its peripheral role in glucose homeostasis [61] and satiation [49] via hepatic portal GLP-1 sensors relies on vagal transmission, whereas glucose flavor conditioning does not require an intact vagus as discussed above. Secretion of GLP-1 has been linked to the gut T1R3 receptor and gustducin; KO mice did not secrete GLP-1 in response to gastric or duodenal glucose infusion [26]. However, T1R3 KO mice can learn to prefer flavors paired with IG sucrose [57], and gustducin KO mice learned to prefer a flavor paired with IG maltodextrin (Sclafani, unpublished data). Together these data argue against GLP-1 as a primary signal in flavor preference conditioning. GLP-1 secretion could be a component of an integrated signal from the gut, carried by another humoral messenger.
A gut hormone with some features compatible with a role in flavor conditioning is ghrelin. Unlike other gut hormones that suppress food intake, ghrelin is an orexigenic peptide that promotes eating. Ghrelin levels are high prior to a meal and decline with feeding. The magnitude of this response is nutrient specific, being stronger after glucose and amino acids than after isocaloric lipid [44] or fructose [60]. There are other characteristics that parallel features of flavor preference conditioning. Glucose-induced suppression of ghrelin requires post-gastric stimulation [69] and, congruent with the present study, can be elicited by infusions confined to the duodenum or jejunum [44]. An intact vagus is not required for the ghrelin response to nutrient infusion [70] or the stimulation of feeding by exogenous ghrelin [6]. Together, these finding suggest the possibility that the decline in circulating ghrelin produced by intestinal glucose may be a signal to the brain that mediates flavor preference learning. In fact, ghrelin actions have been linked to brain reward and memory systems [15,43] as well as glucose homeostasis [59]. However, increases, not decreases in ghrelin signaling are associated with reward and activation of the nucleus accumbens dopamine system that has been implicated in flavor preference conditioning [43,64]. For example, peripheral administration of ghrelin in mice, at a dose that elevated accumbens dopamine, was effective in conditioning a place preference [27]. Ghrelin receptor antagonism, on the other hand, blocked ethanol-induced place preference conditioning and dopamine release [28]. These findings would appear inconsistent with the idea that a glucose-triggered decline in ghrelin would promote flavor conditioning. However, ghrelin actions are complex and incompletely understood and its role in food preference learning requires study.
In summary, the present study showed that flavor preference learning based on post-oral glucose relies on glucose stimulation of the proximal intestine. Given the differential strength of nutrients as unconditioned stimuli, the specific mechanisms mediating flavor learning based on other nutrients may differ. Identification of the upper-intestinal humoral signal(s) remains an important task in the study of glucose-based flavor preference conditioning.
Acknowledgments
This work was supported by grant NIH/NIDDK 31135 from the National Institute of Diabetes and Digestive and Kidney Diseases. Experiment 2 is part of Yeh-Min Yiin’s doctoral dissertation. The expert technical assistance of Kwame K. McCartney and Martin S. Zartarian is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A, Axen KV. Diabetic rats prefer glucose-paired flavors over fructose-paired flavors. Appetite. 1997;28:73–83. doi: 10.1006/appe.1996.0058. [DOI] [PubMed] [Google Scholar]
- 2.Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- 3.Ackroff K, Sclafani A. Flavor quality and ethanol concentration affect ethanol-conditioned flavor preferences. Pharmacol Biochem Behav. 2002;74:229–40. doi: 10.1016/s0091-3057(02)00987-5. [DOI] [PubMed] [Google Scholar]
- 4.Ackroff K, Sclafani A. Fructose conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–97. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Ackroff K. Learned flavor preferences: the variable potency of post-oral nutrient reinforcers. Appetite. 2008;51:743–6. doi: 10.1016/j.appet.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arzuffi R, Racotta IS, Angeles TP, Racotta R. The role of conditioned taste aversion in the hypophagia induced by intraperitoneal epinephrine and glucose. Horm Behav. 1995;29:1–11. doi: 10.1006/hbeh.1995.1001. [DOI] [PubMed] [Google Scholar]
- 8.Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric sugar infusions in rats: Maltose is more reinforcing than sucrose. Physiol Behav. 1998;64:535–41. doi: 10.1016/s0031-9384(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 9.Baird JP, Grill HJ, Kaplan JM. Effect of hepatic glucose infusion on glucose intake and licking microstructure in deprived and nondeprived rats. Am J Physiol. 1999;277:R1136–43. doi: 10.1152/ajpregu.1999.277.4.R1136. [DOI] [PubMed] [Google Scholar]
- 10.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–9. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 11.Campbell CS, Davis JD. Licking rate of rats is reduced by intraduodenal and intraportal glucose infusion. Physiol Behav. 1974;12:357–65. doi: 10.1016/0031-9384(74)90110-3. [DOI] [PubMed] [Google Scholar]
- 12.Canbeyli RS, Koopmans HS. Comparison of gastric, duodenal and jejunal contributions to the inhibition of food intake in the rat. Physiol Behav. 1984;33:951–7. doi: 10.1016/0031-9384(84)90235-x. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept. 2005;128:117–24. doi: 10.1016/j.regpep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Depoortere I. Targeting the ghrelin receptor to regulate food intake. Regul Pept. 2009;156:13–23. doi: 10.1016/j.regpep.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Drucker DB, Sclafani A. The role of gastric and post-gastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav. 1997;61:351–8. doi: 10.1016/s0031-9384(96)00414-3. [DOI] [PubMed] [Google Scholar]
- 17.Elizalde G, Sclafani A. Starch -based conditioned flavor preferences in rats: Influence of taste, calories, and CS− US delay. Appetite. 1988;11:179–200. doi: 10.1016/s0195-6663(88)80002-3. [DOI] [PubMed] [Google Scholar]
- 18.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259:G822–37. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 19.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–6. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol. 2008;295:R388–94. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giza BK, Scott TR. Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars. Am J Physiol. 1987;252:R994–1002. doi: 10.1152/ajpregu.1987.252.5.R994. [DOI] [PubMed] [Google Scholar]
- 22.Giza BK, Scott TR, Vanderweele DA. Administration of satiety factors and gustatory responsiveness in the nucleus tractus solitarius of the rat. Brain Res Bull. 1992;28:637–9. doi: 10.1016/0361-9230(92)90116-f. [DOI] [PubMed] [Google Scholar]
- 23.Glass DS, Margolskee RF, Sclafani A. Glucose-conditioned preferences in taste-impaired TRPM5 knockout mice. Appetite. 2009;52:833. [Google Scholar]
- 24.Gowans SE, Weingarten HP. Elevations of plasma glucose do not support taste-to-postingestive consequence learning. Am J Physiol. 1991;261:R1409–R17. doi: 10.1152/ajpregu.1991.261.6.R1409. [DOI] [PubMed] [Google Scholar]
- 25.Gowans SE. PhD dissertation. Hamilton, ON, Canada: McMaster University; 1992. Role of portal and plasma glucose elevations in taste-to-postingestive consequence learning. [Google Scholar]
- 26.Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerlhag E. Systemic administration of ghrelin induces conditioned place preference and stimulates accumbal dopamine. Addict Biol. 2008;13:358–63. doi: 10.1111/j.1369-1600.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 28.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 2009;106:11318–23. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008:28. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 30.Langhans W, Grossmann F, Geary N. Intrameal hepatic-portal infusion of glucose reduces spontaneous meal size in rats. Physiol Behav. 2001;73:499–507. doi: 10.1016/s0031-9384(01)00479-6. [DOI] [PubMed] [Google Scholar]
- 31.Le Magnen J. Effects of postprandial administration of glucose on the acquisition of appetites (first published in French in 1959) Appetite. 1999;33:14–6. doi: 10.1006/appe.1999.0252. [DOI] [PubMed] [Google Scholar]
- 32.Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. Am J Physiol. 1996;270:R1059–R64. doi: 10.1152/ajpregu.1996.270.5.R1059. [DOI] [PubMed] [Google Scholar]
- 33.Lucas F, Ackroff K, Sclafani A. High-fat diet preference and overeating mediated by postingestive factors in rats. Am J Physiol. 1998;275:R1511–R22. doi: 10.1152/ajpregu.1998.275.5.R1511. [DOI] [PubMed] [Google Scholar]
- 34.Lucas F, Azzara AV, Sclafani A. Flavor preferences conditioned by intragastric Polycose in rats: More concentrated Polycose is not always more reinforcing. Physiol Behav. 1998;63:7–14. doi: 10.1016/s0031-9384(97)00364-8. [DOI] [PubMed] [Google Scholar]
- 35.Lucas F, Sclafani A. Differential reinforcing and satiating effects of intragastric fat and carbohydrate infusions in rats. Physiol Behav. 1999;66:381–8. doi: 10.1016/s0031-9384(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 36.Maljaars PWJ, Peters HPF, Mela DJ, Masclee AAM. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95:271–81. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Mather P, Nicolaidis S, Booth DA. Compensatory and conditioned feeding responses to scheduled glucose infusions in the rat. Nature. 1978;273:461–3. doi: 10.1038/273461a0. [DOI] [PubMed] [Google Scholar]
- 38.Mehiel R. Hedonic-shift conditioning with calories. In: Bolles RC, editor. The hedonics of taste. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 107–26. [Google Scholar]
- 39.Meyer JH, Hlinka M, Tabrizi Y, Dimaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol. 1998;275:R1293–R307. doi: 10.1152/ajpregu.1998.275.4.R1293. [DOI] [PubMed] [Google Scholar]
- 40.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: II. Taste reactivity analysis. Physiol Behav. 2001;74:495–505. doi: 10.1016/s0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- 41.Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: I. Intake acceptance and preference analysis. Physiol Behav. 2001;74:481–93. doi: 10.1016/s0031-9384(01)00595-9. [DOI] [PubMed] [Google Scholar]
- 42.Niewoehner CB, Gilboe DP, Nuttall FQ. Metabolic effects of oral glucose in the liver of fasted rats. Am J Physiol. 1984;246:E89–E94. doi: 10.1152/ajpendo.1984.246.1.E89. [DOI] [PubMed] [Google Scholar]
- 43.Olszewski PK, Schioth HB, Levine AS. Ghrelin in the CNS: from hunger to a rewarding and memorable meal? Brain Res Rev. 2008;58:160–70. doi: 10.1016/j.brainresrev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146:845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 45.Pérez C, Lucas F, Sclafani A. Increased flavor acceptance and preference conditioned by the postingestive actions of glucose. Physiol Behav. 1998;64:483–92. doi: 10.1016/s0031-9384(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 46.Pérez C, Lucas F, Sclafani A. Devazepide, a CCKA antagonist, attenuates the satiating but not the preference conditioning effects of intestinal carbohydrate infusions in rats. Pharmacol Biochem Behav. 1998;59:451–7. doi: 10.1016/s0091-3057(97)00439-5. [DOI] [PubMed] [Google Scholar]
- 47.Raybould HE. Sensing of glucose in the gastrointestinal tract. Auton Neurosci. 2007;133:86–90. doi: 10.1016/j.autneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Revusky SH, Smith MH, Jr, Chalmers DV. Flavor preference: Effects of ingestion-contingent intravenous saline or glucose. Physiol Behav. 1971;6:341–3. doi: 10.1016/0031-9384(71)90165-x. [DOI] [PubMed] [Google Scholar]
- 49.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–81. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Savastano DM, Carelle M, Covasa M. Serotonin-type 3 receptors mediate intestinal Polycose- and glucose-induced suppression of intake. Am J Physiol. 2005;288:R1499–508. doi: 10.1152/ajpregu.00745.2004. [DOI] [PubMed] [Google Scholar]
- 51.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 1993;265:R320–R5. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 52.Sclafani A, Lucas F. Abdominal vagotomy does not block carbohydrate-conditioned flavor preferences in rats. Physiol Behav. 1996;60:447–53. doi: 10.1016/s0031-9384(96)80018-7. [DOI] [PubMed] [Google Scholar]
- 53.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusion of galactose, glucose, and fructose in rats. Physiol Behav. 1999;67:227–34. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 54.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav. 2003;78:285–94. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- 55.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 56.Sclafani A, Glendinning JI. Flavor conditioning by oral and post-oral actions of sucrose and sucralose in mice. Appetite. 2006;46:381. [Google Scholar]
- 57.Sclafani A, Glass D, Glendinning JI, Margolskee RF. T1R3 knockout mice learn to prefer flavors paired with intragastric sucrose infusions. International Symposium on Olfaction and Taste/Association for the Chemoreception Sciences; San Francisco. 2008. [Google Scholar]
- 58.Strubbe JH, Bruggink JE, Steffens AB. Hepatic portal vein cannulation for infusion and blood sampling in freely moving rats. Physiol Behav. 1999;6x:243–50. doi: 10.1016/s0031-9384(98)00248-0. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Asnicar M, Smith RG. Central and peripheral roles of ghrelin on glucose homeostasis. Neuroendocrinology. 2007;86:215–28. doi: 10.1159/000109094. [DOI] [PubMed] [Google Scholar]
- 60.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 61.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Opin Clin Nutr Metab Care. 2004;7:471–8. doi: 10.1097/01.mco.0000134368.91900.84. [DOI] [PubMed] [Google Scholar]
- 62.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–R6. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 63.Tordoff MG, Tluczek JP, Friedman MI. Effect of hepatic portal glucose concentration on foodintake and metabolism. Am J Physiol. 1989;257:R1474–80. doi: 10.1152/ajpregu.1989.257.6.R1474. [DOI] [PubMed] [Google Scholar]
- 64.Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27:1525–33. doi: 10.1111/j.1460-9568.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- 65.Touzani K, Bodnar RJ, Sclafani A. Dopamine D1-like receptor antagonism in amygdala impairs the acquisition of glucose-conditioned flavor preference in rats. Eur J Neurosci. 2009;30:289–98. doi: 10.1111/j.1460-9568.2009.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Touzani K, Sclafani A. Learned flavor aversions and preferences. In: Squire LR, editor. Encyclopedia of neuroscience. Oxford: Academic Press; 2009. pp. 395–9. [Google Scholar]
- 67.Tsurugizawa T, Uematsu A, Nakamura E, Hasumura M, Hirota M, Kondoh T, Uneyama H, Torii K. Mechanisms of neural response to gastrointestinal nutritive stimuli: the gut-brain axis. Gastroenterology. 2009;137:262–73. doi: 10.1053/j.gastro.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 68.Vanderweele DA, Deems RO, Kanarek RB. Insulin modifies flavor aversions and preferences in real-and sham -feeding rats. Am J Physiol. 1990;259:R823–R8. doi: 10.1152/ajpregu.1990.259.4.R823. [DOI] [PubMed] [Google Scholar]
- 69.Williams DL, Cummings DE, Grill HJ, Kaplan JM. Meal-related ghrelin suppression requires postgastric feedback. Endocrinology. 2003;144:2765–7. doi: 10.1210/en.2003-0381. [DOI] [PubMed] [Google Scholar]
- 70.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–7. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 71.Woltman T, Reidelberger R. Effects of duodenal and distal ileal infusions of glucose and oleic acid on meal patterns in rats. Am J Physiol. 1995;269:R7–14. doi: 10.1152/ajpregu.1995.269.1.R7. [DOI] [PubMed] [Google Scholar]
- 72.Zafra MA, Molina F, Puerto A. Learned flavor preferences induced by intragastric administration of rewarding nutrients: role of capsaicin-sensitive vagal afferent fibers. Am J Physiol. 2007;293:R635–R41. doi: 10.1152/ajpregu.00136.2007. [DOI] [PubMed] [Google Scholar]
- 73.Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics. 2007;8:113–28. doi: 10.2174/138920207780368187. [DOI] [PMC free article] [PubMed] [Google Scholar]