Abstract
Healing large bone defects and non-unions remains a significant clinical problem. Current treatments, consisting of auto- and allografts, are limited by donor supply and morbidity, insufficient bioactivity and risk of infection. Biotherapeutics, including cells, genes and proteins, represent promising alternative therapies, but these strategies are limited by technical roadblocks to biotherapeutic delivery, cell sourcing, high cost, and regulatory hurdles. In the present study, the collagen-mimetic peptide, GFOGER, was used to coat synthetic PCL scaffolds to promote bone formation in critically-sized segmental defects in rats. GFOGER is a synthetic triple helical peptide that binds to the α2β1 integrin receptor involved in osteogenesis. GFOGER coatings passively-adsorbed onto polymeric scaffolds, in the absence of exogenous cells or growth factors, significantly accelerated and increased bone formation in non-healing femoral defects compared to uncoated scaffolds and empty defects. Despite differences in bone volume, no differences in torsional strength were detected after 12 weeks, indicating that bone mass but not bone quality was improved in this model. This work demonstrates a simple, cell/growth factor-free strategy to promote bone formation in challenging, non-healing bone defects. This biomaterial coating strategy represents a cost effective and facile approach translatable into a robust clinical therapy for musculoskeletal applications.
Keywords: biomimetic material, bone regeneration, ECM (extracellular matrix), integrin, peptide, scaffold
Introduction
Bone tissue deficiencies, including non-union fractures and bone loss associated with trauma, osteo-degenerative diseases, and tumors, have tremendous socioeconomic impact in terms of personal and occupational disability and related health care costs. For example, approximately $5,000,000,000 is spent annually in the United States on more than 1,000,000 procedures involving bone grafting, bone excision and fracture repair [1]. Although fracture repair of long bones is typically successful with little to no surgical intervention, non-unions, in which a large portion of the bone is missing or must be resected, will remain as open defects until treated. For clinical treatment of large non-unions, autografts remain the gold standard of care, followed closely in number of treatments by allografts [2]. Although these biological grafts exhibit high healing rates, approximately 20-30% of patients who receive autograft treatment experience chronic pain and morbidity at the donor site, while >30% of allograft procedures are complicated by fracture, non-union and infection [3-5]. These complications underscore the need for alternative treatments for clinical bone repair.
Protein therapeutics, including bone morphogenetic proteins (BMPs), have become promising alternatives to auto- and allografts. In particular, BMP-2 and BMP-7 have been FDA approved for clinical use in the United States since 2002 [6-8]. BMPs have demonstrated success rates equivalent to that of autografts with the added benefit of eliminating the need for donor tissue. However, as a soluble factor, diffusion of BMP away from the delivery site leads to rapid clearance and an insufficient residence time in the defect site. Accordingly, the effective dosage of BMP required to stimulate bone formation in humans is supraphysiological, raising concern about unregulated signaling. Furthermore, production of recombinant human BMP is a very costly procedure, which is inflated by the need for such high doses in clinical treatment [7-9].
Cell-based regenerative medicine strategies are also being pursued to improve existing treatment options. Many of these strategies implement a biomaterial carrier to deliver cells and bioactive factors to the defect site to enhance bone formation and repair [8, 10, 11]. While these strategies have demonstrated some success in preclinical and early clinical trials, considerable limitations remain. For example, cell-based bone regeneration therapies often rely on the expansion of isolated cells to produce sufficient cell numbers for therapy. This in vitro manipulation is limited by the growth potential of the primary cells in culture and can affect cell function and subsequent efficacy of the therapy [12]. Genetic engineering of implanted cells or local delivery of genetic material may overcome this limitation but may also lead to unregulated signaling, an undesired immune response, and tumorogenesis [13]. Therefore, a cost effective strategy that eliminates the need for cells or growth factors while providing the proper biologic cues to stimulate bone repair would provide a significant benefit to clinical orthopaedic repair.
Biomimetic material-based approaches, which strive to recapitulate the extracellular matrix (ECM) environment, incorporate short immobilized peptide sequences derived from ECM proteins into biomaterial scaffolds to promote cell adhesion, proliferation and differentiation [14, 15]. Many of these strategies take advantage of the specific interactions between ECM protein ligands and integrin cell surface receptors. Integrin receptors play a crucial role in cell attachment and ECM-mediated cell signaling [16]. Integrin dimers, consisting of one α and one β subunit, bind to specific sites contained within ECM proteins, thereby promoting cell attachment, migration, mechanotransduction, differentiation, and numerous other cell functions [17, 18].
We have previously demonstrated that a triple helical peptide containing the hexapeptide sequence GFOGER (Figure 1A), derived from type I collagen, promotes osteoblastic differentiation via specific binding of the α2β1 integrin receptor [19, 20]. This receptor is highly expressed on the surface of osteoblastic and osteoprogenitor cells [21], and it binds specifically to collagen I via the GFOGER sequence [22]. The interaction between type I collagen and α2β1 via GFOGER promotes osteoblastic differentiation in vitro [23, 24], and we have further demonstrated that GFOGER-mediated signaling can be used to enhance osteoblastic activity in vivo. When used as a coating for titanium implants, GFOGER promotes osseointegration and implant fixation in vivo to levels greater than that of uncoated implants or implants coated with full length collagen I [25].
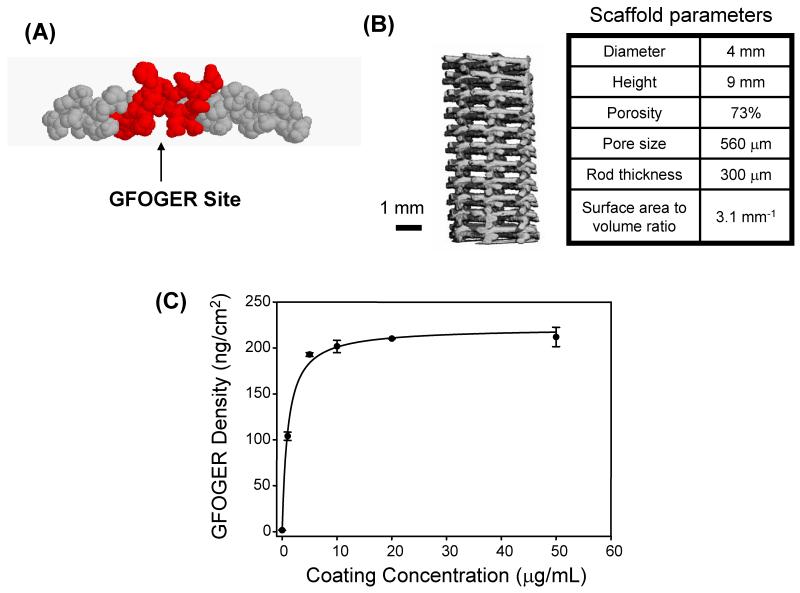
Figure 1.
The synthetic peptide GFOGER passively adsorbs to PCL scaffolds. (A) Space filling model of the GFOGER molecule shows its triple helical, collagen mimetic conformation. (B) MicroCT is used to characterize the structure of PCL scaffolds. Representative scaffold parameters are listed. (C) GFOGER adsorption profile as a function of coating concentration. Error bars represent standard deviation, n=3.
In the current work, GFOGER-coated polycaprolactone (PCL) scaffolds were implanted into critically-sized femoral defects in rats to examine the effectiveness of GFOGER in promoting bone repair in a challenging, non-healing site. GFOGER-coated scaffolds were compared to uncoated PCL scaffolds, and empty defects were included as controls. We hypothesized that GFOGER will stimulate osteoblastic differentiation and subsequent bone repair without the aid of implanted cells, genetic material or growth factors.
Materials and Methods
GFOGER peptide and PCL scaffolds
The peptide GGYGGGPC(GPP)5GFOGER(GPP)5GPC, where O is hydroxyproline, was synthesized by the Emory University Microchemical Facility using stepwise solid-phase procedures, as previously described [19]. This peptide adopts a triple helical conformation (Figure 1A), which mimics the structure of collagen I and is essential for peptide bioactivity [22]. The purified peptide was stored as a TFA salt at −20°C. Prior to use, the peptide was reconstituted to 10 mg/mL in 0.1% TFA and 0.01% sodium azide and stored at 4°C.
Polycaprolactone (PCL) scaffolds, produced by fused deposition modeling, were used as biomaterial scaffolds with well-defined architectures [26]. Sheets (9 mm thick) were cut into scaffolds using a 4 mm diameter dermal biopsy punch (Miltex). Micro-computed tomography (microCT) analysis was used to characterize the structural parameters of the scaffolds (Figure 1B). Prior to implantation, scaffolds were rinsed in 70% ethanol for 4 days on a shaker plate with daily ethanol replacement to remove endotoxin contaminants. Samples contained 10-fold lower levels of endotoxin than the United States Food and Drug Administration’s recommended 0.5 EU/mL, as determined by the LAL chromogenic assay (Cambrex). Subsequently, scaffolds were cleaned in fresh 70% ethanol for 30 minutes, rinsed in sterile ddH2O, then soaked in PBS for 10 minutes. GFOGER was passively adsorbed onto PCL scaffolds by incubating the scaffolds in a dilute solution of GFOGER in PBS for 2 hours at room temperature. Uncoated scaffolds remained in PBS. Immediately prior to implantation, scaffolds were briefly rinsed in PBS to remove unbound peptide.
For in vitro detection of GFOGER on PCL scaffolds, the carboxyl end of the GFOGER peptide was biotinylated using an EZ-Link® Amine-PEG3-Biotin kit (Pierce Biotechnology), according to manufacturer’s instructions. Unreacted biotin was removed via overnight dialysis against PBS using a Slide-A-Lyzer Dialysis Cassette with a molecular weight cut-off of 3500 (Thermo Scientific). After dialysis, protein concentration was measured using a BCA Protein Assay kit (Pierce Biotechnology). PCL scaffolds were coated with various concentrations of biotinylated GFOGER, then blocked in 0.25% heat denatured serum albumin with 0.0005% Tween-20, 1 mM EDTA, and 0.025% NaN3 in PBS for 1 hour at 37°C. After blocking, scaffolds were incubated with an anti-biotin antibody conjugated to alkaline phosphatase (clone BN-34, Sigma, 1:2000) for 1 hour at 37°C, then incubated with 60 μg/mL of 4-methylumbelliferyl phosphate substrate in diethanolamine buffer (pH 9.5) for 1 hour at 37°C. Fluorescent signal was detected on a HTS 7000 Plus Bio Assay Reader (Perkin Elmer) at 360/465 nm (ex/em) by transferring 100 μL of reaction solution from each scaffold to a microtiter plate. Uncoated PCL scaffolds and a substrate-only group (excluding the antibody) served as negative controls.
Segmental Defect Surgery
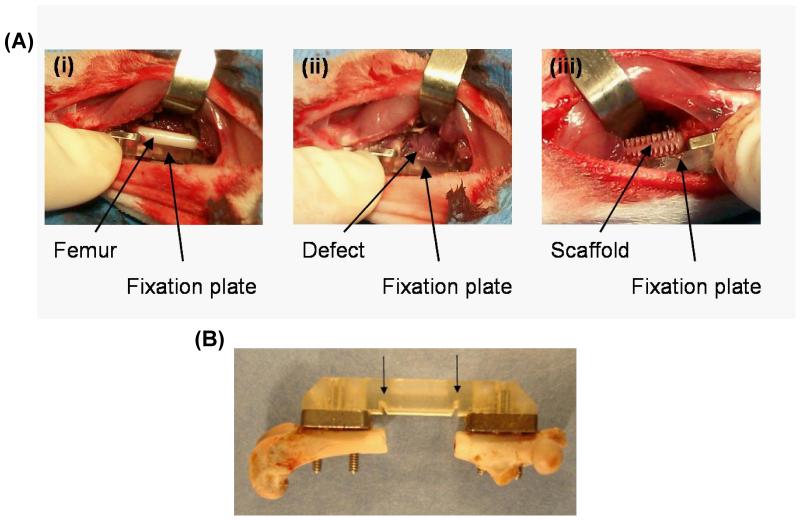
Femoral defects were created bilaterally in rats in compliance with IACUC-approved procedures as previously described [27]. Briefly, 13 to 15 week old female Lewis rats were anesthetized using isoflurane, and the hind limbs were shaved and swabbed with cycloheximide and alcohol. An anterolateral incision from hip to knee allowed blunt separation of the quadriceps muscles, exposing the femur (Figure 2A). For mechanical support, the bone was stabilized with a modular fixation device, consisting of two stainless steel plates affixed directly to the bone via screws and a polysulfone plate, which spanned the defect and was attached to the stainless steel plates. Use of this modular system provided for postmortem mechanical testing and non-invasive in vivo X-ray and microCT analyses. Furthermore, notches in the polysulfone plate, spaced 8.0 mm apart, ensured each defect was consistently created the same length (Figure 2B). To create the defect, an 8.0 mm segment of bone was removed via bone saw with irrigation, and a scaffold was press fit into the defect (Figure 2C). Muscle and skin was closed using Vicryl sutures and wound clips.
Figure 2.
Surgical procedure for critically-sized segmental defects in rat femurs. (A) Each defect is stabilized by a fixation plate. (i) Blunt dissection of the quadriceps exposes the femur and enables placement of the fixation plate. (ii) An 8.0 mm segment is removed from the femur via bone saw, and (iii) scaffolds are press fit into the defect. (B) An explanted femur shows the modular fixation plate attached to the bone via stainless steel screws. Notches in the polysulfone plate (marked with arrows) are spaced 8.0 mm apart ensuring each defect is created at the same size.
The following 3 groups were tested: (i) uncoated PCL scaffolds, (ii) GFOGER-coated PCL scaffolds and (iii) empty defect control. A coating concentration of 50 μg/mL was used for all implanted GFOGER-coated scaffolds. Uncoated scaffolds underwent the same coating procedures as the coated scaffolds, except they were incubated in PBS instead of GFOGER. For the empty defect group, the surgical procedure remained the same, but no scaffold was placed in the defect.
Following surgery, animals were given 3 doses of buprenorphine daily for 3 consecutive days to control pain. Animals were monitored daily for signs of pain and distress, maintenance of wound closure, regular eating habits and normal ambulation. A small percentage (< 8%) of rats developed infections in or around the surgery site, or experienced mechanical failure of the fixation device. These animals were removed from the study and euthanized, and any data collected from these animals was excluded. Two weeks post-surgery, skin wounds were completely healed, and wound clips were removed. At 4, 8 and 12 weeks post-surgery, animals were anesthetized with isoflurane and the hind legs were scanned via radiography and microCT as described below. Animals were euthanized by CO2 inhalation at 12 weeks post-implantation, and the femurs, along with surrounding muscle tissue, were harvested for postmortem microCT evaluation, histology and mechanical testing.
Radiography and MicroCT Analyses
X-ray images of each femur were non-invasively obtained using an MX-20 Specimen Radiography System (Faxitron X-ray Corporation) to make gross morphological observations of tissue growth in each defect. Animals were anesthetized with isoflurane, and each hind leg was scanned for 15 s at an X-ray beam energy of 23 kV.
In addition to radiographic imaging, samples were non-invasively analyzed via microCT using a vivaCT 40 scanner (Scanco Medical) at 4 and 12 weeks to quantify bone volume in each defect site. Animals were anesthetized with isoflurane and placed in a rodent holder with one leg outstretched for scanning. The area between the stainless steel plates of the fixation device was imaged with an X-ray beam energy of 55 kVp, beam intensity of 109 μA, integration time of 200 ms, and resolution of 38 μm. Noise was reduced from 3-dimensional reconstructions by applying a Gaussian filter (sigma=1.2, support=2) via the Scanco Medical μCT Evaluation Program. Images were thresholded at 270 mg HA/ccm to isolate mature bone, and bone volume was quantified using directly computed values.
Histological and Mechanical Testing Analyses
Immediately following euthanasia, samples for histological analysis were fixed in 10% neutral buffered formalin. Prior to embedding, fixed tissues were scanned in formalin via microCT as described above to allow matching of histological sections with microCT slices. After scanning, specimens were dehydrated in a series of alcohols, cleared in xylene, and embedded in methyl methacrylate (MMA). Ground sections, 50-80 μm thick, were prepared by Wasatch Histo Consultants, Inc. (Winnemucca, NV) and stained using Sanderson’s Rapid Bone Stain™ and a van Gieson counterstain. Stained histological sections were then compared to thresholded microCT scans from the same sample to confirm that microCT analysis was representative of mature bone.
For mechanical testing, explanted samples were wrapped in PBS-soaked gauze, and frozen at −20°C until use. Mechanical testing was performed as previously described [27]. Briefly, samples were thawed at room temperature in PBS and most of the soft tissue was removed, taking care not to mechanically disrupt tissue in the defect site. The proximal and distal ends of each bone were potted in Wood’s metal and secured with pins into potting blocks. Blocks were loaded onto an ElectroForce® mechanical testing machine (ELF 3200, Bose) and the polysulfone plate was removed just before testing. Samples were loaded in torsion at a displacement rate of 3°/s up to 360°. Maximum torque before failure was recorded for each sample. Stiffness and work to failure were calculated.
Statistical Analysis
MicroCT and mechanical testing data were analyzed using ANOVA in Systat v11. Bridging scores were analyzed using Kruskal Wallis non-parametric tests. Samples identified as statistical outliers that also met additional criteria for removal, such as improper plate placement, were removed from analysis. A p-value < 0.05 was considered significant.
Results
We examined the ability of a simple GFOGER peptide coating on PCL, a biomedically relevant polymer, to enhance bone repair in a non-healing segmental defect. We focused on passive adsorption of GFOGER onto PCL rather than covalent tethering because this approach is facile and we previously demonstrated that GFOGER adsorbs tightly onto synthetic materials [25]. The adsorption behavior of GFOGER onto PCL scaffolds was characterized via ELISA of biotinylated GFOGER on the scaffolds. GFOGER adsorption exhibited the expected hyperbolic profile in adsorbed density as a function of coating concentration reaching saturation values around 10 μg/mL (Figure 1C). Using titanium samples as a standard and previously obtained surface plasmon resonance measurements of GFOGER saturation on titanium, we estimate that PCL scaffolds saturated with GFOGER contain 162.4 +/− 7.5 ng GFOGER/cm2 scaffold surface area, which corresponds to a dose of approximately 260 ng GFOGER total per 9 mm scaffold. For all in vivo studies, GFOGER was used at a coating concentration of 50 μg/mL to ensure complete saturation of GFOGER on the scaffold surface.
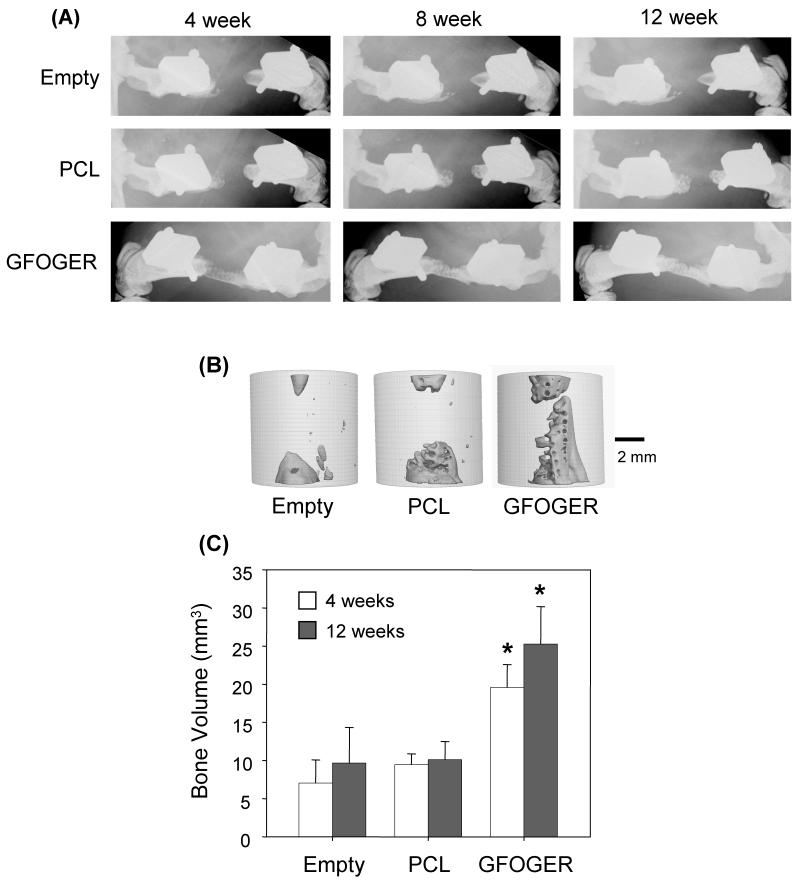
To examine the effect of GFOGER on bone formation in vivo, uncoated and GFOGER-coated PCL scaffolds were implanted in critically-sized defects in female Lewis rats, as shown in Figure 2. Empty defects, which received no scaffolds, served as negative controls. Within one week following surgery, animals displayed minimal signs of stress, regular eating habits and normal ambulation using both hind limbs. Bone formation was monitored via X-ray and microCT. As shown in representative X-ray images, radiodense tissue formation in defects treated with GFOGER-coated scaffolds was observed as early as 4 weeks, whereas negligible radiodense tissue ingrowth occurred in empty defects and uncoated PCL controls even after 12 weeks post-implantation (Figure 3A). Three-dimensional microCT reconstructions show that defects treated with GFOGER-coated scaffolds are nearly fully bridged after 12 weeks, while tissue formation in empty defects and uncoated PCL controls occurs only at the defect margins adjacent to the host bone (Figure 3B). Notably, bone formation in defects treated with GFOGER-coated scaffolds was significantly greater than that in PCL-treated and empty defects at both 4 and 12 weeks as measured by quantitative microCT (p < 0.05, Figure 3C). These results demonstrate that simple coating of PCL scaffolds with the integrin-specific, triple helical GFOGER peptide promotes significantly faster and greater bone formation compared to unmodified scaffolds in this demanding, orthotopic defect model.
Figure 3.
GFOGER-coated scaffolds significantly enhance bone formation in critically-sized defects compared to uncoated scaffolds and empty defect controls. (A) Faxitron images show that empty defects do not heal after 12 weeks, and negligible bone formation is present in uncoated PCL-treated defects. However, GFOGER-treated defects promote bone formation as early as 4 weeks. (B) MicroCT images for samples shown in (A) at 12 weeks. (C) Bone volume is significantly greater in GFOGER-treated samples at both 4 and 12 weeks compared to empty defects and uncoated PCL-treated samples. Error bars represent standard error of the mean (n=8 and n=9 for PCL and GFOGER at 4 weeks, respectively; n=7 and n=8 for PCL and GFOGER at 12 weeks, respectively). * Different from empty defect and PCL (p<0.05).
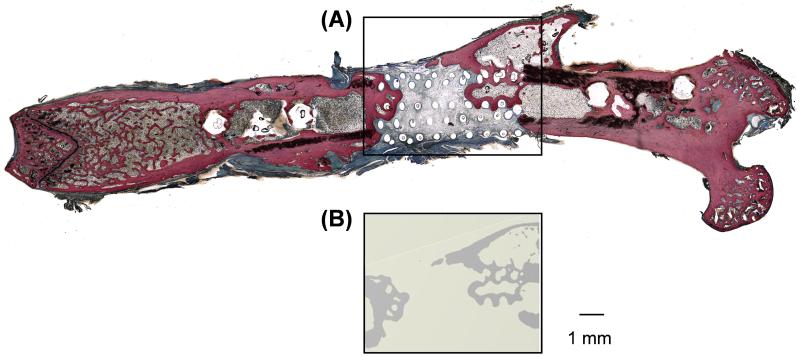
To further characterize the repair tissue, histological analysis was combined with microCT scanning. Sanderson’s Rapid Bone Stain™ was used to visually distinguish areas of mineralized bone (red/pink) from demineralized connective tissue and osteoid (blue/green) [28]. As demonstrated in Figure 4, regions of red/pink staining in histological sections, which correspond to mineralized bone tissue, directly match areas of high X-ray attenuation thresholded by microCT. This analysis demonstrates that the repair tissue detected by microCT can be histologically identified as mineralized bone tissue.
Figure 4.
Histological analysis confirms that areas of high attenuation revealed by microCT are bone tissue. (A) Sanderson’s rapid bone stain for a GFOGER-coated sample showing bone in red/orange and soft tissue in blue/green. (B) Two-dimensional microCT image from the same sample as (A) revealing bone formation in the same location.
To assess functionality of the tissue present in critically-sized defects, explanted femurs were subjected to torsion testing. Although microCT revealed differences in bone volume between defects treated with GFOGER-coated scaffolds and uncoated PCL, maximum torque sustained was not significantly different between these two groups (Figure 5A). Stiffness and work to failure were also evaluated and no significant differences were observed (data not shown). However, by calculating the Pearson product-moment correlation coefficient for bone volume and maximum torque for each sample, a positive correlation between these two variables was revealed, r = 0.744, p = 0.009 (Figure 5B). Based on the r-value obtained, we hypothesized that mechanical strength is not only dependent on bone volume but also on defect bridging. Whereas some samples contain a large amount of bone in the defect site, high levels of mechanical strength are only present when this bone is firmly attached to both the proximal and distal ends of the host bone. Without full attachment, or bridging of the defect, samples that have large bone volumes sustain low torque loads.
Figure 5.
Mechanical properties of repaired segmental defects. (A) Maximum torque is not significantly different despite differences in bone volume. (B) Bone volume correlates with maximum torque (p < 0.05). Samples were ranked by their extent of defect bridging (see C), and samples with higher bridging scores are shifted to the upper right of the correlation graph. (C) Criteria for bridging scores assigned to each sample, and representative X-ray images of samples from each bridging score category. (D) GFOGER-coated scaffolds display a greater number of fully bridged defects than uncoated scaffolds (p < 0.05).
To demonstrate that mechanical strength is dependent on defect bridging, X-ray images for all samples were blindly assessed for their extent of bridging and assigned a bridging score from 0-5 (0 corresponds to no bone in the defect, 5 represents a fully bridged defect). Scoring criteria representative X-ray images for each score are shown in Figure 5C. As shown in Figure 5B, samples with higher bridging scores are generally shifted towards the upper right of the bone volume/torsional strength correlation plot, indicating that fully bridged samples sustain greater torsional loading compared to unbridged samples. For this analysis, bridging scores were based solely on 2D X-ray Faxitron images, the most common method for assessment of bridging. However, rigorous assessment of bridging via inspection of microCT images reveals that samples scored as 5 by X-ray may have received a score of 4 if judged by microCT. This explains the difference in mechanical properties between the two samples with a score of 5 in Figure 5B, and these results make a case for more widespread use of microCT evaluation in the field. Finally, although there are no significant differences in torsional strength between uncoated PCL-treated defects and GFOGER-coated scaffold treated defects, a distribution of bridging scores shows that GFOGER scores are shifted towards fully bridged or nearly fully bridged (scores 4 and 5) compared to PCL scores (p < 0.05, Figure 5D).
Because GFOGER mediates osteoblastic differentiation by directly binding to integrin receptors, we hypothesized that the effects of GFOGER on bone formation are dependent on the area of GFOGER-coated scaffold in direct contact with host tissue. To test for surface area dependent effects of GFOGER-coated biomaterials, uncoated and GFOGER-coated PCL scaffolds with varying surface area to volume (SA/V) ratios were implanted in critically-sized segmental defects. Cross-sectional images of the scaffolds are shown in Figure 6A. MicroCT analysis revealed that GFOGER-coated scaffolds significantly enhance bone formation compared to uncoated PCL scaffolds when the SA/V ratio of the scaffolds is at least 3.1 mm−1 (Figure 6B). However, GFOGER-coated scaffolds with SA/V ratios of 2.4 or 1.7 mm−1 produced no significant effect on bone formation compared to uncoated scaffolds of the same SA/V ratio. This surface area-dependent effect on bone formation most likely results from the amount of scaffold surface available for host tissue interaction and/or a reduction in the effective dose of GFOGER delivered to the defect site.
Figure 6.
Effects of GFOGER are dependent on scaffold surface area to volume ratio. (A) Cross-sectional microCT images of scaffolds with different surface area to volume ratios. (B) Bone volume (quantified by microCT at 12 weeks post-op) is a function of surface to volume ratio. * Different from uncoated scaffolds in the same SA:Vol group, p < 0.05.
Discussion
Healing of large defects and non-unions in bone remains a significant clinical problem due to complications with current treatments, such as donor site morbidity and pain due to autografts, the high rate of allograft failure, and the high cost of treatment with BMPs. The present study is the first to demonstrate significant bone formation in critically-sized defects using a simple cell- and growth factor-free biomaterial strategy. The synthetic, collagen-mimetic, triple helical peptide, GFOGER, was used as a biomaterial coating to promote bone formation in vivo via specific targeting of the α2β1 integrin. We found that GFOGER, passively adsorbed to the surface of PCL scaffolds, promotes significantly more bone formation in critically-sized defects in rats compared to treatment with uncoated PCL scaffolds. This biomaterial-based approach represents a facile, cost-effective alternative to regenerative medicine strategies that focus on the delivery of cells, genes, and protein therapeutics. Because dried coatings of GFOGER retain biological activity, this peptide-based approach could be implemented in “off the shelf” and point of care applications. In addition, GFOGER readily adsorbs onto various synthetic materials, making this coating strategy potentially translatable to various devices for different musculoskeletal applications.
The present study examined the use of the collagen-mimetic peptide GFOGER in a critically-sized orthotopic segmental defect. Although the GFOGER sequence is present in native full length collagen, collagen alone does not enhance bone formation in critically-sized defects [29-31]. Activation of the α2β1 integrin is presently known to occur via binding of distinct adhesive sites contained within collagen I, namely DGEA and GFOGER [22, 32]. Although several studies have characterized the effect of DGEA on other cell types, the role of DGEA in osteoblast signaling and differentiation is presently unclear [33]. On the other hand, our group has previously reported increased osteoblastic differentiation on GFOGER-coated surfaces in vitro, and this effect translated to increased osseointegration and implant fixation on GFOGER-coated titanium implants in vivo [19, 20, 25]. In these studies, the effects of GFOGER on osteoblastic differentiation and mineralization were greater than that for native collagen I. This effect is most likely due to the elimination of other binding sites present on native collagen I. In the present study, we demonstrate a significant enhancement in bone formation, with complete defect bridging occurring in some cases, in response to GFOGER coating alone, without the delivery of cells or growth factors. It is likely that the suboptimal results obtained from uncoated scaffolds is due to non-specific adsorption of serum proteins to the scaffold surface, leading to non-specific signaling and unregulated host cell responses. We posit that GFOGER-coated scaffolds promote specific binding of α2β1 integrin, thereby upregulating osteogenesis in surrounding host cells. The enhanced healing response may occur via preferential binding and recruitment of osteoprogenitor cells present in or around the defect site to GFOGER, or via increased differentiation of uncommitted cells bound to GFOGER on the scaffold, or some combination thereof. We further demonstrated that GFOGER-mediated bone formation is surface area dependent, suggesting that the area of GFOGER in direct contact with host cells directs the host reparative response.
In the late 1990s, a 15 amino acid peptide sequence, termed P-15, was isolated from collagen I, and shown to increase cell adhesion when used as a surface coating for anorganic bone matrix (ABM) [34]. Although the signaling mechanism of P-15 has not been identified, use of P-15 as a coating on ABM promotes osteoblastic differentiation and mineralization in vitro [28]. However, ABM is porous particulate bone mineral derived from bovine sources, which is likely to be a naturally osteoconductive material, and preclinical studies showing significant bone growth due to P-15/ABM implants in orthotopic defects have not included uncoated ABM controls, making the relative contributions of the ABM scaffold and P-15 to bone healing unclear [35, 36]. In the present study, uncoated synthetic polymeric scaffolds, which contained a well characterized and consistent porous microstructure, did not produce significant healing in orthotopic defects. This choice of scaffold, which does not heal the defect alone, enabled evaluation of the effects of GFOGER on defect healing without contributions from the scaffold.
Interestingly, a recent study comparing DGEA, P-15, GFOGER, and RGD as cell adhesive coatings on hydroxyapatite showed that GFOGER produced no cell adhesion in this in vitro model [37]. The authors attribute this surprising result to the hydroxyapatite substrate employed. In contrast, we have observed GFOGER adsorption and cell adhesion onto GFOGER-coated hydroxyapatite surfaces as well as several other substrates (data not shown). The reasons for these conflicting results are not clear, although there are differences in the primary sequence of the peptides used in the Bellis study and the present work [37].
The majority of studies that report successful healing of bone defects rely solely on a combination of X-ray analysis and histological evaluation as a measure of defect healing. However, this approach does not provide functional information, such as mechanical properties, which is necessary to fully evaluate the success of a given treatment strategy for bone healing [38]. When mechanical testing of bone defects is performed, the most common testing method is torsion [27, 39-43]. We used torsional testing to evaluate the functional strength of the defects. Despite the significant differences in bone formation for GFOGER-coated scaffolds, the average torsional strength of defects treated with GFOGER-coated scaffolds was not significantly different from that of defects treated with uncoated scaffolds. This lack of differences in mechanical properties is most likely due to the variance in defect bridging for these treatments. Because GFOGER coating accelerated bone formation compared to the control biomaterials, it is possible that longer implantation times will result in higher bridging frequencies and torsional strengths.
Conclusions
Simple surface modification of PCL scaffolds with the collagen mimetic synthetic peptide GFOGER enhances bone repair in critically-sized defects without the use of exogeneous cells or growth factors. This strategy relies on the specific binding of the α2β1 integrin receptor, expressed on host cells, to the synthetic GFOGER peptide, which contains the α2β1 binding sequence found within collagen I. By using GFOGER as a biomaterial scaffold coating, host cell adhesion via targeted binding of α2β1 is promoted, which upregulates osteoblastic differentiation and in turn leads to an increase in bone formation. In the present study, passive adsorption of GFOGER onto PCL scaffolds significantly enhanced bone formation in critically-sized femoral defects compared to uncoated scaffolds without the delivery of cells or growth factors. Overall, this strategy imparts biologic functionality to synthetic biomaterials via passive adsorption of GFOGER to the scaffold surface, creating a facile, translatable and cost-effective alternative to regenerative medicine strategies that rely on the delivery of cells, genes, and protein therapeutics.
Acknowledgments
This work was by funded by the National Institutes of Health (R01 EB-004496) and Georgia Tech/Emory National Science Foundation ERC on the Engineering of Living Tissues (EEC-9731643). A.M.W. was supported by the Cell and Tissue Engineering NIH Biotechnology Training Grant (T32 GM-008433). The authors gratefully acknowledge Dr. Laura O’Farrell for veterinary consults, Angela Lin for technical assistance with microCT, and Joel Boerckel, Joseph Charest, Sean Coyer, Bryan Dierckman, David Dumbauld, Nduka Enemchukwu, Timothy Petrie, Ed Phelps, Jennifer Phillips, and Rachel Whitmire for assistance with surgical procedures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kretlow JD, Mikos AG. Review: mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007;13(5):927–38. doi: 10.1089/ten.2006.0394. [DOI] [PubMed] [Google Scholar]
- [2].De Long WG, Jr., Einhorn TA, Koval K, McKee M, Smith W, Sanders R, et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am. 2007;89(3):649–58. doi: 10.2106/JBJS.F.00465. [DOI] [PubMed] [Google Scholar]
- [3].Sorger JI, Hornicek FJ, Zavatta M, Menzner JP, Gebhardt MC, Tomford WW, et al. Allograft fractures revisited. Clin Orthop Relat Res. 2001;(382):66–74. doi: 10.1097/00003086-200101000-00011. [DOI] [PubMed] [Google Scholar]
- [4].Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;(432):210–6. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- [5].Schwartz CE, Martha JF, Kowalski P, Wang DA, Bode R, Li L, et al. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcomes. 2009;7(49) doi: 10.1186/1477-7525-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Boden SD. The ABCs of BMPs. Orthop Nurs. 2005;24(1):49–52. doi: 10.1097/00006416-200501000-00014. [DOI] [PubMed] [Google Scholar]
- [7].Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31(6):721–7. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28(29):4240–50. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- [9].Alt V, Heissel A. Economic considerations for the use of recombinant human bone morphogenetic protein-2 in open tibial fractures in Europe: the German model. Curr Med Res Opin. 2006;22(Suppl 1):S19–22. doi: 10.1185/030079906X80602. [DOI] [PubMed] [Google Scholar]
- [10].Awad HA, Zhang X, Reynolds DG, Guldberg RE, O’Keefe RJ, Schwarz EM. Recent advances in gene delivery for structural bone allografts. Tissue Eng. 2007;13(8):1973–85. doi: 10.1089/ten.2006.0107. [DOI] [PubMed] [Google Scholar]
- [11].Hutmacher DW, García AJ. Scaffold-based bone engineering by using genetically modified cells. Gene. 2005;347:1–10. doi: 10.1016/j.gene.2004.12.040. [DOI] [PubMed] [Google Scholar]
- [12].Guillot PV, Cui W, Fisk NM, Polak DJ. Stem cell differentiation and expansion for clinical applications of tissue engineering. J Cell Mol Med. 2007;11(5):935–44. doi: 10.1111/j.1582-4934.2007.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gottfried ON, Dailey AT. Mesenchymal stem cell and gene therapies for spinal fusion. Neurosurgery. 2008;63(3):380–91. doi: 10.1227/01.NEU.0000324990.04818.13. [DOI] [PubMed] [Google Scholar]
- [14].Hubbell JA. Materials as morphogenetic guides in tissue engineering. Curr Opin Biotechnol. 2003;14(5):551–8. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- [15].Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199(2):174–80. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- [16].Garcia AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005;26(36):7525–9. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- [17].Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268(5208):233–9. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- [18].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [19].Reyes CD, García AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J Biomed Mater Res A. 2003;65(4):511–23. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- [20].Reyes CD, García AJ. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research Part A. 2004;69A(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- [21].Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12(8):1189–97. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- [22].Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- [23].Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [24].Jikko A, Harris SE, Chen D, Mendrick DL, Damsky CH. Collagen integrin receptors regulate early osteoblast differentiation induced by BMP-2. J Bone Miner Res. 1999;14(7):1075–83. doi: 10.1359/jbmr.1999.14.7.1075. [DOI] [PubMed] [Google Scholar]
- [25].Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–35. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zein I, Hutmacher DW, Tan KC, Teoh SH. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23(4):1169–85. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- [27].Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25(7):941–50. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- [28].Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10(78):1148–59. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- [29].Azad V, Breitbart E, Al-Zube L, Yeh S, O’Connor JP, Lin SS. rhBMP-2 enhances the bone healing response in a diabetic rat segmental defect model. J Orthop Trauma. 2009;23(4):267–76. doi: 10.1097/BOT.0b013e31819f290e. [DOI] [PubMed] [Google Scholar]
- [30].Chen X, Schmidt AH, Mahjouri S, Polly DW, Jr., Lew WD. Union of a chronically infected internally stabilized segmental defect in the rat femur after debridement and application of rhBMP-2 and systemic antibiotic. J Orthop Trauma. 2007;21(10):693–700. doi: 10.1097/BOT.0b013e31815a7e91. [DOI] [PubMed] [Google Scholar]
- [31].Pekkarinen T, Jamsa T, Maatta M, Hietala O, Jalovaara P. Reindeer BMP extract in the healing of critical-size bone defects in the radius of the rabbit. Acta Orthop. 2006;77(6):952–9. doi: 10.1080/17453670610013286. [DOI] [PubMed] [Google Scholar]
- [32].Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273(49):32988–94. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- [33].Marquis ME, Lord E, Bergeron E, Bourgoin L, Faucheux N. Short-term effects of adhesion peptides on the responses of preosteoblasts to pBMP-9. Biomaterials. 2008;29(8):1005–16. doi: 10.1016/j.biomaterials.2007.10.047. [DOI] [PubMed] [Google Scholar]
- [34].Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5(1):53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- [35].Scarano A, Iezzi G, Petrone G, Orsini G, Degidi M, Strocchi R, et al. Cortical bone regeneration with a synthetic cell-binding peptide: a histologic and histomorphometric pilot study. Implant Dent. 2003;12(4):318–24. doi: 10.1097/01.id.0000095467.48241.68. [DOI] [PubMed] [Google Scholar]
- [36].Cakmak G, Bolukbasi S, Simsek A, Erdem O, Yilmaz G, Senkoylu A. Effect of synthetic cell-binding peptide on the healing of cortical segmental bone defects. Saudi Med J. 2006;27(6):777–80. [PubMed] [Google Scholar]
- [37].Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, et al. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30(10):1898–909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liebschner MA. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials. 2004;25(9):1697–714. doi: 10.1016/s0142-9612(03)00515-5. [DOI] [PubMed] [Google Scholar]
- [39].Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC. Recombinant human bone morphogenetic protein-7 induces healing in a canine long-bone segmental defect model. Clin Orthop Relat Res. 1994;(301):302–12. [PubMed] [Google Scholar]
- [40].Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC, Whitecloud TS., 3rd The effect of recombinant human osteogenic protein-1 on healing of large segmental bone defects. J Bone Joint Surg Am. 1994;76(6):827–38. doi: 10.2106/00004623-199406000-00006. [DOI] [PubMed] [Google Scholar]
- [41].Cook SD, Wolfe MW, Salkeld SL, Rueger DC. Effect of recombinant human osteogenic protein-1 on healing of segmental defects in non-human primates. J Bone Joint Surg Am. 1995;77(5):734–50. doi: 10.2106/00004623-199505000-00010. [DOI] [PubMed] [Google Scholar]
- [42].Hsu WK, Sugiyama O, Park SH, Conduah A, Feeley BT, Liu NQ, et al. Lentiviral-mediated BMP-2 gene transfer enhances healing of segmental femoral defects in rats. Bone. 2007;40(4):931–8. doi: 10.1016/j.bone.2006.10.030. [DOI] [PubMed] [Google Scholar]
- [43].Rai B, Oest ME, Dupont KM, Ho KH, Teoh SH, Guldberg RE. Combination of platelet-rich plasma with polycaprolactone-tricalcium phosphate scaffolds for segmental bone defect repair. J Biomed Mater Res A. 2007;81(4):888–99. doi: 10.1002/jbm.a.31142. [DOI] [PubMed] [Google Scholar]