Abstract
Contact activation of blood factor XII (FXII, Hageman factor) in neat-buffer solution exhibits a parabolic profile when scaled as a function of silanized-glass-particle activator surface energy (measured as advancing water adhesion tension in dyne/cm, where is water interfacial tension in dyne/cm and θ is the advancing contact angle). Nearly equal activation is observed at the extremes of activator water-wetting properties dyne/cm (0° ≤ θ < 120°), falling sharply through a broad minimum within the dyne/cm (55° < θ < 75°) range over which activation yield (putatively FXIIa) rises just above detection limits. Activation is very rapid upon contact with all activators tested and did not significantly vary over 30 minutes of continuous FXII-procoagulant contact. Results suggest that materials falling within the dyne/cm surface-energy range should exhibit minimal activation of blood-plasma coagulation through the intrinsic pathway. Surface chemistries falling within this range are, however, a perplexingly difficult target for surface engineering because of the critical balance that must be struck between hydrophobicity and hydrophilicity. Results are interpreted within the context of blood plasma coagulation and the role of water and proteins at procoagulant surfaces.
Keywords: Autoactivation, FXII, coagulation
1. Introduction
Contact activation of blood-plasma coagulation through the intrinsic pathway has been implicated as an important cause of the poor hemocompatibility of cardiovascular biomaterials (see ref. [1] for a recent review and citations therein that generally support this section). The initiating step of the intrinsic pathway is widely thought to be surface contact activation of the blood zymogen FXII (Hageman factor) into an active-enzyme form FXIIa; a reaction that is sometimes referred to as autoactivation in the hematology literature. Surface biophysics of autoactivation is poorly understood, as is the biochemistry that leads to enzyme-like specificity upon surface contact. It is proposed that autoactivation involves cleavage of the Arg353-Val354 bond in FXII, generating a two-chain molecule (αFXIIa) comprised of a heavy chain (353 residues) and a light chain (243 residues) held together by a disulfide bond [2]. Other FXII fragments with procoagulant activity such as FXIIf have also been identified [3, 4] (see further Section 4.4). As a consequence, amidolytic activity arising from contact activation of FXII may, in fact, be due to one-or-more enzymes.
Experimental evidence clearly shows that plasma coagulation is most efficiently activated by contact with anionic hydrophilic materials such as kaolin clay, glass, or generally any material with an oxidized surface chemistry. In fact, studies carried out with this latter group of activator materials (a.k.a. procoagulants) aimed at elucidating quantitative relationships among surface chemistry, energy, and the propensity to activate the intact plasma coagulation cascade show that contact activation is all-but-specific for fully-water-wettable surfaces (water contact angle θ = 0°). Activation by less-water-wettable surfaces (θ > 0°) is sharply reduced, leading to one of the most profound examples of hydrophilic/hydrophobic contrast in the biological response to materials. These observations, together with an evolving understanding of autoactivation biochemistry, naturally led to the conclusion that contact activation of FXII was also specific for (or at least caused most efficiently by contact with) hydrophilic surfaces.
However, recent work demonstrates that autoactivation is not specific for anionic-hydrophilic activators. Instead, it is found that hydrophobic and hydrophilic activators have nearly equal autoactivation properties in neat-buffer solutions of FXII, as measured by the solution yield of enzymes with coagulation activity (putatively FXIIa). The observed contact-activation specificity for hydrophilic procoagulants in plasma actually arises from an “adsorption-dilution” effect that causes hydrophobic procoagulants only to appear nearly inert. Adsorption of a plethora of blood proteins to hydrophobic surfaces significantly blocks adsorption of FXII, leading to a sharp reduction in FXII/surface contacts with commensurate reduction in autoactivation rate and yield. Nevertheless, hydrophobic procoagulants retain small-but-measurable plasma-activation properties, explaining the sluggish coagulation of blood in contact with hydrophobic materials such as plastic tubes [5].
Herein we report measurement of FXII autoactivation in neat-buffer solution with procoagulants sampling the full range of observable water-wettability. Results corroborate our previous findings that FXII activation is not specific to anionic-hydrophilic procoagulants and resolves a surface-energy dependence that leads to a distinct minimum in FXII activation. These findings suggest a route to the surface engineering of cardiovascular biomaterials with improved hemocompatibility.
2. Methods and Materials
2.1 Preparation and Characterization of Particulate Activators (Procoagulants)
Test procoagulants applied in this work were 425–600 µm diameter glass particles (Sigma Aldrich) in either cleaned or silanized form. The nominal specific area used in this work was 5×10−3 m2/g (based on 512.5 µm mean diameter and 168 µg/particle). The actual surface area measured by the Brunauer-Emmett- Teller (BET) method was 0.25 ± 0.09 m2/g (Micromeritics ASAP 2000 using liquid nitrogen as the probe gas) was 50X larger than the nominal value, reflecting the large dispersity in particle size and porosity. Nominal surface area was used herein for the sake of consistency with earlier work [6–9]. Choice of surface area had no impact on conclusions drawn herein because qualitative and quantitative comparisons were made among silanized procoagulants prepared from the same lot of glass particles with the same specific area.
Silanes applied in this work (used as received from Gelest Inc., Morrisville, PA) were octadecyltricholorosilane (OTS), 3-aminopropyltriethoxysilane (APTES), n-propyltriethoxysilane (PTES), and vinyltriethoxysilane (VTES). OTS-treated glass particles were optionally coated in a 0.2% solution of 1,1-pentadecafluorooctylmethacrylate in tricholorotrifloroethane (“Nyebar”, Nye Lubricants, Fairhaven, MA) by immersion followed by air drying. Glass coverslips (Fisher 22 × 30 × 0.1mm) were carried through all surface treatments with particles, providing a substrate suitable for measuring buffer contact angles. Glass particles and cover slips were first cleaned and activated by 30 min. immersion in heated piranha solution (30% H2O2 in concentrated H2SO4 at approximately 80 °C) followed by 3X sequential washes in each of 18 MΩ de-ionized water and ethanol. Piranha-solution-oxidized glass was air dried and subsequently oxidized by air-plasma treatment of a single layer of particles (or coverslips) held in a 15 mm Pyrex glass petri dish (10 min at 100 W plasma; Herrick, Whippany, NY) directly before use in silanization procedures or adsorption measurements. Glass surfaces treated in this manner were found to be fully-water-wettable and designated “clean glass”. Clean-glass particles and coverslip samples were silanized by 1.5 hr reaction with 5% v/v OTS in chloroform. Silanized samples were 3X rinsed with chloroform before curing in a vacuum oven at 110 °C for 12 hr. Cured OTS samples were optionally immersed in Nyebar solution for 10 min. and air dried to produce surface slightly more hydrophobic than rendered by OTS treatment alone (see Table 1). APTES silanizations were carried out in 95:5 v/v ethanol-water solutions by 20 min. reaction of clean glass with 5% APTES solution that had been hydrolyzed overnight in the ethanol-water mixture before use. APTES-treated glass was washed with ethanol and cured overnight in a vacuum oven at 110 °C. Silanization with PTES and VTES followed the APTES procedure except that 90:10 ethanol-water containing 0.5 % glacial acetic acid was used.
Table 1.
Water Wettability of Treated Glass-Particle Procoagulants
| Procoagulant Designation # (n) |
Procoagulant Surface Chemistry | Advancing Contact Angle Range (Degrees) |
|---|---|---|
| 1 | Nyebar | 109–118 |
| 2 | OTS | 106–109 |
| 3 | PTES | 93–97 |
| 4 | VTES | 85–90 |
| 5 | APTES | 42–58 |
| 6 | Clean Glass | 0 |
Notes: 425–600µm diameter glass particles in either cleaned or silanized/coated form. See Section 2.1 for details.
Buffer (PBS, see below) contact angles on glass cover-slip witness samples were measured using an automated contact-angle goniometer (First Ten Angstroms Inc., Portsmouth, VA) that employed the captive-drop method of measuring advancing/receding contact angles (see refs. [10, 11] for a comparison of goniometric techniques and discussion of experimental errors). Contact angles could not be read directly on glass particles but optical microscopy of the shape of the liquid meniscus of particles partly immersed in water on a microscope slide qualitatively confirmed that treated particles were not different from coverslip witness samples. Water wettability of the nth surface type was expressed as (advancing) water adhesion tension (where is the product of pure-buffer interfacial tension dyne/cm at 25 °C and cosine of the advancing contact angle θn observed on the nth sample). Results obtained for six surface treatments are collected in column 3 of Table 1. Surface chemistry of glass-particle surfaces was assayed using Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFT) collected on a Bruker IFS-66/S spectrometer using a diffuse-reflectance accessory (Collector II, Thermo Spectra-Tech). The instrument detector constantly purged with CO2-free dry air while 400 scans at 6 cm−1 resolution were acquired for both samples and references. Silica particles were loaded into a macro sampling cup without dilution and leveled with a spatula prior to analysis. Spectra were normalized to arbitrary reflection units relative to an Infragold (Labsphere) reference sample.
Physical mixtures of hydrophilic (n = 6) and hydrophobic (n = 1) procoagulant surfaces were prepared by gravimetry incrementally sampling 0.0 to 1.0 weight fractions. Particles were weighed directly into test tubes mixed in the dry state by shaking. Particles were further mixed in suspension with PBS before use in activation experiments described below. A hypothetical “equivalent wetting” parameter was calculated using a linear combination combining rule as described in Appendix B.
2.2 Plasma and Coagulation Proteins
Citrated human platelet-poor plasma (PPP) was prepared from outdated (within 2 days of expiration) lots obtained from the M.S. Hershey Medical Center Blood Bank. This work was performed with a two different lots (I and II) of pooled plasma aliquoted into 15 ml polypropylene tubes (Falcon, Becton Dickinson) and frozen at - 20 °C until use. We have observed consistent results with plasma prepared and stored in this manner over about one year of experimentation. Experience has shown that different lots of plasma yield quantitatively different but qualitatively similar results. Correction for differences between plasma lots I and II was made by measuring a FXIIa-titration calibration curve for each lot (see below).
Two lots of human FXII were used; Lot 1 from Haematologic technologies, Inc (Essex Junction, VT) and Lot 2 from Enzyme Research Laboratories(South Bend, IN) respectively. The single source of FXIIa was Enzyme Research Laboratories (South Bend, IN). All zymogens and enzymes were used as received. Activity of both FXII and FXIIa was specified by the vendor in traditional units of plasma-equivalent-units-per-mL (PEU/mL) [12]. FXIIa concentrations in mg/mL were converted from PEU/mL for activation yield calculations (Appendix A) using the vendor-supplied conversion factor of 73.5 PEU/mg. Neat-buffer solutions of FXII and FXIIa solutions were prepared in phosphate buffer saline (PBS; Sigma; 0.14M NaCl, 3mM KCL prepared from powder in 18MΩ de-ionized water at pH = 7.2).
2.3 FXIIa Assay
Plasma coagulation time (CT) was used as the traditional hematology method to quantify FXIIa by appealing to a “FXIIa titration” calibration curve that related CT to FXIIa concentration (expressed either in PEU/mL or mg/mL) [12, 13]. Protocol for the FXIIa assay applied in this work has been described in detail elsewhere [7–9, 14, 15]. Briefly, FXIIa titrations were carried out by equilibrating 500 µL of thawed PPP in 15 × 75 mm polystyrene tubes (VWR), mixing with increasing volumes of FXIIa solution in PBS, and diluting with sufficient additional PBS to bring total volume to 900 µL. Coagulation was induced by recalcification with 100 µL of 0.1 M CaCl2 and tube contents were mixed on a slowly-turning hematology mixer (Roto-shake Genie, Scientific Industries, Inc.). CT after recalcification was noted by a distinct change in fluid-like rheology to gel formation, allowing determination of a coagulation endpoint to within 10 sec. or so [14–16]. So-measured CT was observed to be exquisitely sensitive to FXIIa with a minimum quantifiable concentration of ~ 5 × 10−4 PEU/mL. FXIIa titration curves in PPP were linear when scaled on a logarithmic concentration axis. Calibration curves were fit to y = mlog10 x + c by linear least squares regression; where x is FXIIa concentration in PEU/mL and both m and c are adjustable parameters (m = −10.96±0.44, c = 6.76±0.80 with R2 = 94.6% from triplicate determinations using plasma Lot I and m = −8.68±0.25, c = 5.31±0.48 with R2 = 96.9% from triplicate determinations using plasma Lot II). Strictly speaking, this FXIIa assay measured net plasma-coagulation-inducing activity of the product(s) resulting from contact activation of FXII in buffer solution, reported herein in terms of FXIIa activity deduced from the calibration curve. As such, the coagulation assay did not discriminate among various activated fragments of FXII that might be produced by contact activation of FXII in neat-buffer solution (see further Sections 1 and 4.4). Possible presence of activated forms other than FXIIa did not alter the basic conclusion of this work that FXII activation in neat-buffer solution was a strong function of procoagulant surface energy.
2.4 Autoactivation of FXII in Neat-Buffer Solution
FXII activation in PBS solution was carried out as previously described [8, 9]. Briefly, test solutions of FXII in PBS were prepared at nominal physiological concentration (30 µg/mL [1]). Putative FXIIa activity produced by timed contact (2 to 30 min.) of 200 µL of FXII solution at 37 °C with 100 mg of procoagulant surfaces (see Table 1) was quantified using the FXIIa assay described above. FXIIa assay was performed by equilibrating 500 µl of thawed PPP in 15×75 mm polystyrene tubes (VWR), mixed with 100µl of supernate obtained following the aforementioned activation protocol and diluting with sufficient additional PBS to bring total volume to 900 µl. Coagulation was induced by recalcification with 100µl of 0.1 M CaCl2 and tube contents were mixed on a slowly-turning hematology mixer (Rotoshake Genie, Scientific Industries Inc.).
2.5 Contact Activation of Blood Plasma
Procoagulant catalytic potential to activate whole plasma coagulation was assayed using the surface-area titration method reported previously [6, 14–20]. Briefly, 0.5 mL of thawed plasma equilibrated with ambient temperature was transferred into 15×75 mm polystyrene tubes (VWR) containing sufficient weight of procoagulants selected from Table 1 to deliver surface area within the (0 ≤ A ≤ 15)×10−4 m2 range (based on a nominal specific surface area 5×10−3 m2/g) and diluted with sufficient PBS to bring the final total liquid volume to 0.9 mL. Plasma was recalcified with 0.1mL of 0.1 M CaCl2 and tubes were immediately capped with parafilm and mounted on the table of a slowly-turning hematology mixer (Roto-shake Genie, Scientific Industries, Inc.). Coagulation time after recalcification was noted by a distinct change in fluid-like rheology to gel formation, allowing determination of the end point of the coagulation process to within 10 s or so [14]. These simple, yet highly sensitive, recalcification-time assays eliminated extraneous contributions to coagulation associated with many modern instrumented tests (e.g. activating surfaces of stirrers and tubing) and yielded smooth dose-response curves that varied with procoagulant properties (surface chemistry and energy).
Surface area titration data was fit to theory described in ref. [14] designed to extract a parameter Kact that measured procoagulant catalytic potential measured relative to an internal standard (Kact has units of mL/m2 [6], not m−2 as reported in [14]). The standard of reference used herein was clean glass (n =6, Table 1). A rate constant required by theory (designated as either kp in ref. [6] or k2 in ref. [14]) that measured the rate of fibrin polymerization (for a particular lot of plasma) was determined by a two-parameter fit (kp and Kact) to the glass standard as described in refs. [6, 14], yielding kp = 0.37 ± 0.04 min−1 (best fit ± standard error of the fit, R2 = 99.7%; compare to 0.54 ± 0.10 min−1 for human plasma from ref. [6] and 0.70 ± 0.09 min−1 for porcine plasma from ref. [14]). Thus,Kact measured activation potential relative to the glass standard and should not to be interpreted as a quantitative characteristic of procoagulants studied herein. Likewise kp should not be regarded as representative of human PPP in general (see ref. [14] for more discussion). Fitted values obtained for plasma Lot II are collected in Table 4.
Table 4.
Procoagulant Catalytic Potential to Induce Plasma Coagulation
| Procoagulant Designation # (n) |
Advancing Contact Angle (θ, degrees) |
τ0 (dyne/cm) |
Kact (mL/m2) |
R2 (%) |
|---|---|---|---|---|
| 1 | 110.5±2.76 | −25.2± 3.06 | 0.009±0.002 | 86.1 |
| 2 | 102.3±2.56 | −15.33±3.14 | 0.13±0.02 | 97.0 |
| 3 | 93.41±1.51 | −4.28±1.89 | 0.05±0.01 | 97.2 |
| 4 | 85.12±1.70 | 6.12 ±2.12 | 0.19±0.04 | 93.4 |
| 5 | 54.6±0.42 | 41.69±2.63 | 0.01±0.01 | 58.2 |
| 6 | 0 | 72 | 19.2±1.9 | 99.4 |
Notes: Procoagulant number n corresponds to listing in Table 1. Error in θ is standard deviation of the mean of N = 3 measurements. Error in calculated by propagation of error. Results obtained with plasma lot II.
3.0 Results
3.1 Surface Chemistry of Glass-Particle Procoagulants
Evidence of silanization efficacy was obtained by diffuse reflection IR (DRIFT) as shown in Fig. 1. Presence of the –CH stretching vibration bands (2850 < ν < 3000 cm−1) introduced by silanization were clearly detected. Bands at 2850 and 2919 cm−1 were identified as C–H symmetric and anti-symmetric stretching, respectively.
Figure 1.
Diffuse-reflection IR (DRIFT) spectra of silane-modified glass-particle procoagulants (low frequency range 700 > ν < 1400 cm−1 not shown): (A) VTES, vinyltriethoxysilane, n = 4 of Table 1; (B) PTES, n-propyltriethoxysilane, n = 3 of Table 1 ; (C) APTES, 3-aminopropyltriethoxysilane, n = 5 of Table 1; (D) OTS, octadecyltricholorosilane, n = 2 of Table 1.
3.2 Autoactivation Kinetics in Neat-buffer Solution
Total procoagulant activity of products produced by contact activation of FXII with glass particles studied herein (Table 1) was quantified in terms of FXIIa concentration (PEU/mL) using the FXIIa assay described in Sections 2.3–2.4. This assay appealed to an exogenous FXIIa titration of plasma that generated a calibration curve like that shown in Fig. 2. It is stressed that this assay measured net plasma-coagulation-inducing activity of the product(s) resulting from contact activation of FXII in buffer solution and did not discriminate among various activated fragments of FXII that might be produced by contact activation of FXII in neat-buffer solution (see further Sections 1 and 4.4). Using this assay, it was observed that FXII activation in buffer solution was effectively instantaneous within the minimum elapsed-time resolution of experiment (about 2 min.) and was statistically constant over 30 min. of testing, as illustrated in Fig. 3. Occasionally, activation appeared to increase with time (as in OTS and clean glass cases shown in Fig. 3) but repeated measurements failed to confirm a trend at the 2σ confidence interval. Overall, results corroborate previous work [8, 9] in that FXIIa yield in continuous contact with activator particles was observed to depend on procoagulant surface energy but not contact time. We estimated that less than 10% of the total FXII available in solution was activated in all cases (see further below and Appendix A).
Figure 2.
FXIIa titration of human plasma (Lot I, see Section 2) relating coagulation time to concentration of exogenous FXIIa concentration expressed in plasma-equivalent-units-per-milliliter (PEU/ml). Dashed line drawn through data is a guide to the eye showing that plasma coagulation time is exquisitely sensitive to FXIIa. Inset plots data as a lin-log calibration curve used to deduce FXIIa concentrations in neat-buffer solutions used in autoactivation experiments. Solid line through the inset data results from linear-least-squares regression through the data interval shown.
Figure 3.
Autoactivation kinetics in 30 µg/ml neat-buffer solutions of FXII contacting (i) clean glass (n = 6), (ii) octadecyltricholorosilane treated glass (OTS, n = 2), or (iii) 3-aminopropyltriethoxysilane treated glass (APTES, n = 5) activators (see Table 1). Putative FXIIa solution concentration (left-hand ordinate) is expressed in plasma-equivalent-units-per-milliliter (PEU/ml). Solution yield is the estimated percent conversion of the initial FXII in solution to FXIIa (see Appendix B). Error bars represent uncertainty in FXIIa concentration arising from FXIIa titration calibration curves (see Fig. 2). Dashed-line annotations represent average yield over the incubation period.
3.3 Autoactivation as a Function of Procoagulant Surface Energy
Table 2 compiles results of FXII activation experiments conducted with glass-particle procoagulants with different surface chemistry/energy (obtained by silanization, see Section 2.1 and Table 1). Columns 5 and 6 report mean and standard deviation of N = 7 FXIIa determinations made over 2–30 min. activation experiments. FXIIa in % yield was calculated as described in Appendix A. Fig. 4 compares results of FXIIa activation experiments using two separate lots of plasma and FXII. Quantitatively-different results obtained were attributed to unknown differences in FXII preparations, not plasma lots, because the assay calibration curve corrected for lot-to-lot differences in plasma (see Sections 2.2 and 2.3). In both cases, however, a parabolic profile in autoactivation yield was obtained as a function of procoagulant surface energy. Nearly equal autoactivation was observed at the hydrophobic (left-hand-side of Fig. 4) and hydrophilic (right-hand-side) extremes of procoagulant water-wetting properties, falling sharply through a broad minimum within the dyne/cm (55° < θ < 75°) range over which FXIIa yield was just above detection limits.
Table 2.
Contact Activation of FXII by 30 minute Incubation with Hydrophilic and Hydrophobic procoagulants
| FXII Lot |
Procoagulant Designation # (n) |
Advancing Contact Angle (θ, degrees) |
(dyne/cm) |
Average FXIIa Solution Concentration (PEU/ml × 10−2, N = 7) |
Average FXIIa Yield (%, N = 7) |
|---|---|---|---|---|---|
| 1 | 1 | 109.42 ± 1.63 | −23.92 ± 1.93 | 2.68 ± 0.60 | 1.22 ± 0.27 |
| 2 | 106.30 ± 1.89 | −20.19 ± 2.27 | 4.21 ± 0.89 | 1.91 ± 0.40 | |
| 3 | 96.73 ± 1.67 | −8.4 ± 2.08 | 0.35 ± 0.10 | 0.16 ± 0.05 | |
| 4 | 85.13 ± 2.01 | 6.11 ± 2.51 | 0.24 ± 0.07 | 0.10 ± 0.03 | |
| 5 | 52.30 ± 1.57 | 44.01 ± 1.56 | 0.12 ± 0.04 | 0.06 ± 0.02 | |
| 5 | 42.30 ± 2.10 | 53.23 ± 1.77 | 0.29 ± 0.08 | 0.13 ± 0.04 | |
| 6 | 0.00 | 72 | 2.47 ± 0.55 | 1.12 ± 0.25 | |
| 2 | 1 | 118.13 ± 1.75 | −33.93 ± 1.94 | 1.91 ± 0.33 | 0.86 ± 0.15 |
| 2 | 108.33 ± 2.50 | −22.64 ± 2.99 | 0.84 ± 0.16 | 0.38 ± 0.07 | |
| 3 | 93.41 ± 1.55 | −4.27 ± 1.94 | 0.49 ± 0.10 | 0.22 ± 0.04 | |
| 4 | 89.54 ± 2.55 | 0.58 ± 3.20 | 0.43 ± 0.09 | 0.20 ± 0.04 | |
| 5 | 58.01 ± 3.77 | 38.12 ± 4.01 | 0.25 ± 0.05 | 0.11 ± 0.02 | |
| 6 | 0.00 | 72 | 0.83 ± 0.16 | 0.37 ± 0.07 |
Notes: Procoagulant number n corresponds to listing in Table 1. Error in θ is standard deviation of the mean of N = 3 measurements. Error in calculated by propagation of error. Error in FXIIa concentration is standard deviation of N = 7 measurements with error in percent yield computed by propagation of error. Results obtained with plasma lots I and II.
Figure 4.
Autoactivation yield obtained by contacting neat-buffer solutions of FXII (30 µg/ml; Panel A, FXII Lot 1; Panel B, FXII Lot 2) with glass-particle procoagulants exhibiting different surface energy expressed as water adhesion tension in dyne/cm (where is water interfacial tension in dyne/cm and θ is the advancing contact angle). Data and error bars represent mean and standard deviation of N = 7 measurements over 30 min. of continuous procoagulants-solution contact (see Fig. 3). Note the broad minimum over the approximate procoagulant wettability range dyne/cm (55° ≤ θ < 75°).
3.4 Contact Activation of FXII by Physical Mixtures of Hydrophilic and Hydrophobic Procoagulants
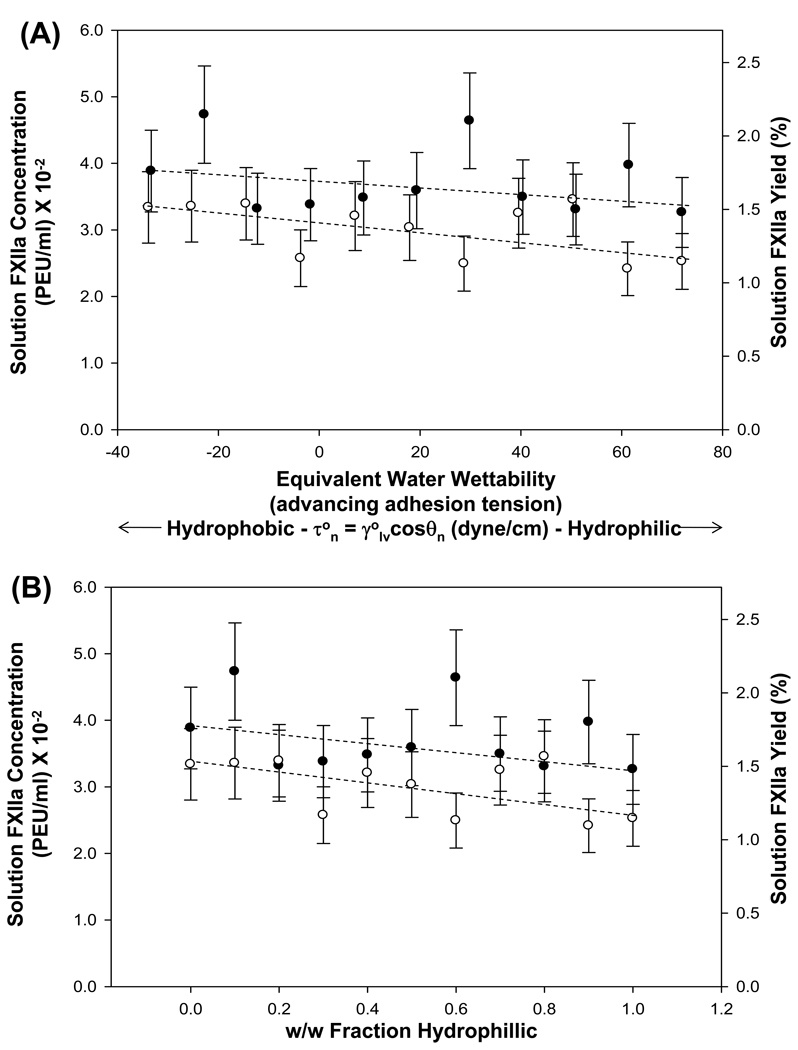
Physical mixtures of hydrophilic (clean glass, n = 6, Table 1, τo = 72 dyne/cm) and hydrophobic (Nyebar surface, n = 1 Table 1, τo = −34 dyne/cm) particles were prepared by gravimetry in proportions that simulate different “equivalent adhesion tensions” (see further Section 4.3.3 and Appendix B). The intent was to compare activation by particulates with a molecularly-dispersed coverage of silane functional groups to a mixture of segregated hydrophilic and hydrophobic chemistries. Table 3 summarizes activation yield (columns 5 and 6) obtained with physical mixtures representing the full range of hypothetical wetting properties (columns 2–4) for two different lots of FXII (1 and 2). As shown in Fig. 5, activation yield deceased in a linear-like trend with equivalent adhesion tension (Panel A) or w/w fraction (Panel B) and did not reproduce the parabolic profile observed with silanized procoagulant particles (Fig. 4).
Table 3.
Contact Activation of FXII by Incubation with Physical Mixtures of Hydrophilic and Hydrophobic Procoagulants
| FXII Lot |
w/w Fraction Hydrophilic |
Hypothetical Contact Angle (θ) (Degrees) |
Hypothetical (dyne/cm) |
Average FXIIa Solution Concentration ((PEU/ml) × 10−2, N=2) |
Average FXIIa Yield (%, N=2) |
|---|---|---|---|---|---|
| 1 | 0.0 | 117.57 | −33.31 | 3.89 ± 0.61 | 1.76 ± 0.28 |
| 0.1 | 108.45 | −22.78 | 4.73 ± 0.73 | 2.14 ± 0.33 | |
| 0.2 | 99.80 | −12.25 | 3.32 ± 0.53 | 1.50 ± 0.24 | |
| 0.3 | 91.37 | −1.72 | 3.38 ± 0.54 | 1.53 ± 0.25 | |
| 0.4 | 82.97 | 8.80 | 3.48 ± 0.56 | 1.57 ± 0.25 | |
| 0.5 | 74.41 | 19.32 | 3.59 ± 0.57 | 1.62 ± 0.26 | |
| 0.6 | 65.48 | 29.85 | 4.64 ± 0.72 | 2.10 ± 0.33 | |
| 0.7 | 55.86 | 40.38 | 3.49 ± 0.56 | 1.58 ± 0.25 | |
| 0.8 | 44.97 | 50.91 | 3.31 ± 0.53 | 1.50 ± 0.24 | |
| 0.9 | 31.38 | 61.44 | 3.97 ± 0.63 | 1.80 ± 0.28 | |
| 1.0 | 0.00 | 71.97 | 3.26 ± 0.53 | 1.48 ± 0.24 | |
| 2 | 0.0 | 118.08 | −33.87 | 3.34 ± 0.53 | 1.51 ± 0.24 |
| 0.1 | 110.59 | −25.31 | 3.35 ± 0.54 | 1.52 ± 0.24 | |
| 0.2 | 101.62 | −14.50 | 3.39 ± 0.54 | 1.54 ± 0.25 | |
| 0.3 | 92.94 | −3.69 | 2.57 ± 0.43 | 1.16 ± 0.19 | |
| 0.4 | 84.32 | 7.11 | 3.21 ± 0.52 | 1.45 ± 0.23 | |
| 0.5 | 75.58 | 17.92 | 3.03 ± 0.49 | 1.37 ± 0.22 | |
| 0.6 | 66.47 | 28.73 | 2.49 ± 0.41 | 1.13 ± 0.19 | |
| 0.7 | 56.67 | 39.54 | 3.25 ± 0.52 | 1.47 ± 0.24 | |
| 0.8 | 45.60 | 50.35 | 3.46 ± 0.55 | 1.57 ± 0.25 | |
| 0.9 | 31.80 | 61.16 | 2.42 ± 0.40 | 1.10 ± 0.18 | |
| 1.0 | 0.00 | 71.97 | 2.52 ± 0.42 | 1.15 ± 0.19 |
Notes: Hydrophilic glass particles (n = 6) mixed with hydrophobic glass particles (n = 12) listed in Table 1. Total procoagulant mass = 100 mg. Hypothetical θ and computed from a linear combination rule (Appendix B). Error in FXIIa concentration is standard deviation of N = 3 measurements with error in percent yield computed by propagation of error. Results obtained with plasma lot II.
Figure 5.
Autoactivation yield obtained with physical mixtures of clean-glass (n = 6 of Table 1, dyne/cm, θa = 0°) and Nyebar (n = 1 of Table 1, dyne/cm, θa = 118°) procoagulants in contact with neat-buffer solutions of FXII (30 µg/ml). Panel A scales autoactivation in terms of the equivalent water wettability of a hypothetical composite surface bearing molecularly-dispersed n = 6 and n = 2 chemistries at areal surface coverage equal to the weight-fraction of the physical mixtures (see Section 4.3.3 and Appendix B). Panel B scales the same data in terms of weight fraction n = 6 in the physical mixture. Data points and error bars represent mean and standard deviation of N = 2 measurements (closed symbols = FXII Lot 1; open symbols = FXII Lot 2). Dashed lines are guides to the eye suggesting a slight (but statistically insignificant) decreasing autoactivation trend as a function of physical-mixture composition.
3.5 Contact Activation of Blood Plasma
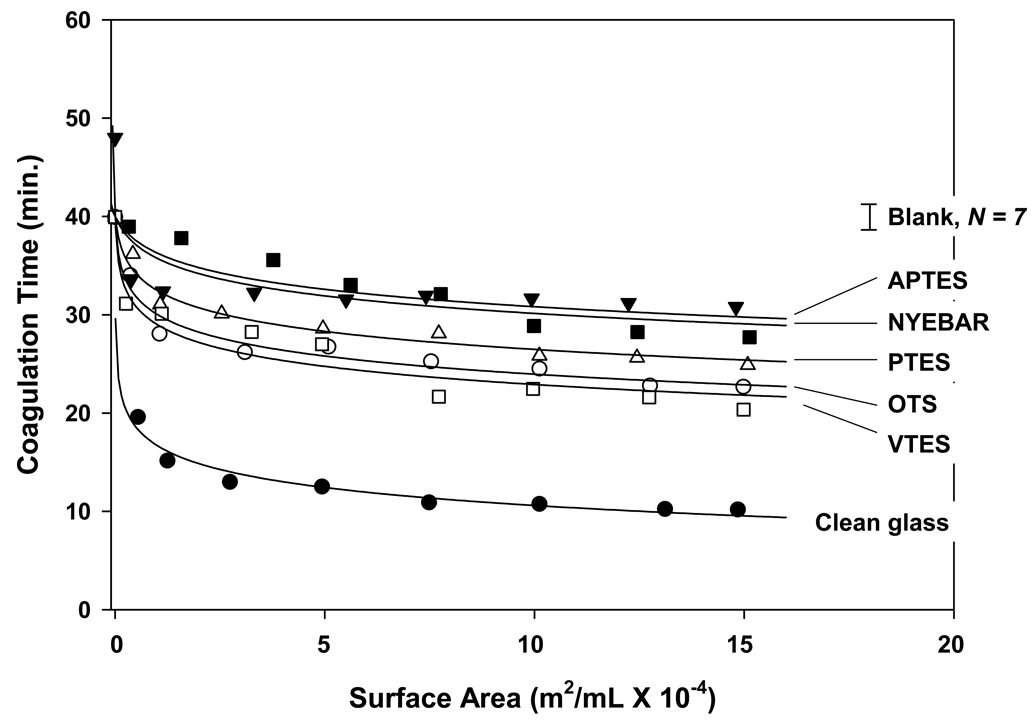
Efficiency of contact activation of whole human plasma induced by contact with glass procoagulants studied herein (Table 1) was observed to depend on surface energy in the order: clean glass ≫ VTES > OTS > PTES > APTES > Nyebar as shown in Figs. 6. This activation efficiency was quantified by the Kact parameter in Table 4 (see Section 2.5) that measured activator catalytic potential to induce plasma coagulation. Kact (mL/m2) scaled with procoagulant water wettability in a sharp exponential-like way as shown in Fig. 7 (right-hand axis), generally consistent with our previous reports [6, 14]. However, this previous work indicated that OTS was a less efficient procoagulant than APTES [6]. The cause of this relatively minor difference between current and past results was possibly due to differences in surface treatment but was not further pursued because it did not alter basic conclusions drawn from this work.
Figure 6.
Surface-area titration of human plasma using activators listed in Table 1. Error bar annotated “Blank” represents mean and standard deviation of N = 7 measurements of plasma coagulation time in polystyrene tubes used in the coagulation assay containing no activator particles. Smooth curves through the data represent best fit of data to theory described in Sections 2.5, 3.5 and 4.6) from which the Kact parameter that measures activator catalytic potential is deduced (Table 4).
Figure 7.
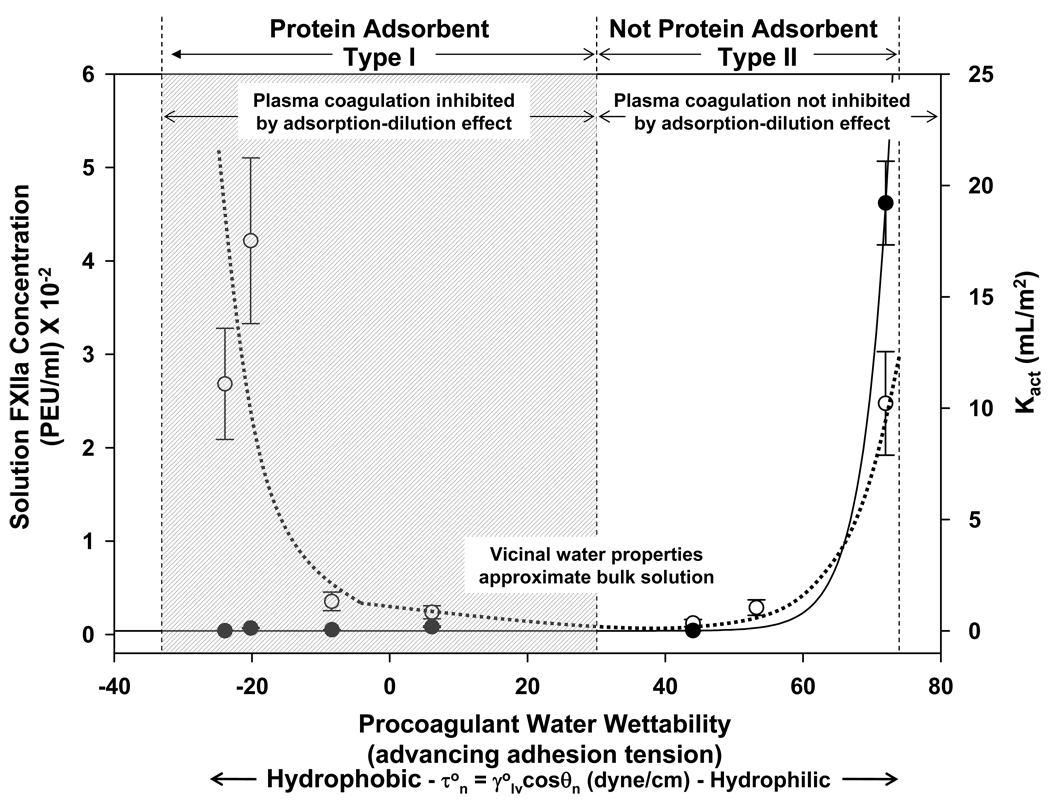
Comparison of FXII activation in neat-buffer solution (left-hand ordinate, dashed line) to catalytic potential in plasma Kact (right-hand ordinate, solid line) for activator (procoagulant) particles exhibiting different surface energy (abscissa) expressed as water adhesion tension in dyne/cm (where is water interfacial tension in dyne/cm and θ is the advancing contact angle). Data for FXIIa taken from Fig. 4. Annotations along the top of the graphic identify protein-adsorbent Type I surfaces and non-adsorbent Type II surfaces. Grayed panel represents the region in which the adsorption-dilution effect renders Type I surfaces all-but-inert to plasma coagulation (baseline Kact). Both FXII activation in neat-buffer solution and Kact rise sharply in the Type II region. In the activator surface-energy region falling roughly within dyne/cm (55° < θ < 75°), it is proposed that vicinal (interfacial) water properties approximate that of bulk solution, rendering these surfaces inert to activation of FXII in either neat-buffer solution or plasma. Water properties at either hydrophobic or hydrophilic extremes cause activation of FXII in either neat-buffer solution or plasma through unknown biochemistry, but the adsorption-dilution effect renders hydrophobic activators immersed in plasma (or concentrated protein cocktails) nearly inert.
4.0 Discussion
4.1 Glass-Particle Activators
We applied conventional silane chemistry to prepare glass-particle activators with different surface chemistry and water-wetting characteristics. Water contact angles and diffuse-reflectance IR (DRIFT) confirm the anticipated result that well-established silanization procedures yield uniform surface chemistries on glass particles (Table 1 and Fig. 1). As-prepared silanized-glass surfaces certainly do not meet the standard of rigorous surface engineering employing, for example, Self Assembled Monolayers (SAMs, see refs. [21–25] for a few reviews selected from many). However, this limitation is a strength in the sense that measured FXII autoactivation is assuredly not an outcome peculiar to an exotic surface chemistry prepared using special methods, but rather an effect that might be caused by contact with ordinary mixed and/or undefined surface chemistries possibly encountered in clinical application of cardiovascular biomaterials.
Anyone attempting to prepare surfaces incrementally sampling the full range of water wettability will discover the difficulty of obtaining specimens falling within dyne/cm (55° < θ < 75° ) over which FXII autoactivation is herein found to be minimal (see Sections 2.1 and 3.2). Apparently, surfaces within this “sweet spot” bear a critical number of Lewis acid/base functional groups capable of hydrogen bonding to water such that, in combination with ubiquitous dispersion-force interactions, wetting falls just between hydrophobic and hydrophilic [26]. In our hands, obtaining surfaces within the sweet spot has been entirely an accident of persistent trial-and-error using relatively crude surface-oxidation methods described in prior work [14, 15, 27–30] or by application of the particular silanes discussed herein that just happen to yield surface chemistries falling within this region. Indeed, investigations using sophisticated mixed-component SAMs (e.g. monolayers prepared from mixtures of –CH3 and –OH terminated thiols) shows that contact angle dependence on hydrophilic/hydrophobic surface composition is highly non-linear [31–33]. Full understanding of the relationships among surface-functional-group chemistry, surface density, and water wetting remains among the more important unresolved problems of materials science. The biological response to these materials and its correlation with protein adsorption is an important open issue in biomaterials surface science [26].
4.2 Discontinuous FXII Activation in the Continuous Presence of Activator Particles
We find that autoactivation yield (putatively FXIIa) produced by contact with activator surface chemistries incrementally sampling the full range of observable water wettability is statistically invariant over 30 min. of continuous FXII solution contact, as illustrated in Fig. 3 for hydrophilic (clean glass) and silanized activators. This observation corroborates and extends our previous work showing that activation kinetics in neat-buffer solution are too rapid to follow using methods outlined in Section 2 [1, 8, 9]. Furthermore, we find that the maximum autoactivation yield converts less than 3.5% of the total FXII available in buffer solution under experimental conditions applied herein (Fig. 3 and Table 2; see also Appendix A for calculation of autoactivation yield and correction for FXIIa adsorption to activator particles).
The cause(s) underlying what appears to be discontinuous autoactivation in the continuous presence of activator particles suspended in dilute FXII solution are unclear. Prior work indicates that autoactivation in buffer increases in proportion to activator surface area [8] and confirms that hydrophilic activator surfaces are not “poisoned” in the process of contact activation [6, 9, 14, 15]. Thus, it seems that autoactivation rapidly self-terminates at low conversion even though conditions favorable to continued autoactivation prevail. We have speculated that discontinuous activation is due to putative “autoinhibition” reaction(s) in which FXIIa itself inhibits autoactivation (see ref. [1] and citations therein). But autoinhibition alone does not explain the fact that autohydrolysis (FXII+FXIIa → 2FXIIa) is found to be a facile reaction in buffer solutions of FXII and FXIIa in the absence of activator particles (little-or-no autohydrolysis is observed in plasma [7]). Apparently, presence of a procoagulant surface in FXII/FXIIa buffer solutions somehow blocks or inhibits autohydrolysis through as-yet unknown surface biochemistry. Or perhaps autohydrolysis is not a significant reaction when FXIIa is endogenously produced from FXII by surface contact (as compared to mixture of exogenous FXIIa with FXII). Clear understanding of the mechanisms of the presumptive reactions termed “auto…” remains an important fundamental problem in biomaterial surface science and a practical issue in the prospective design of advanced cardiovascular biomaterials [1].
4.3 Autoactivation of FXII in Neat-Buffer Solution is a Sharp Function of Activator Surface Energy
Autoactivation yield produced by contact of a buffer solution at fixed FXII concentration with equal activator surface area exhibiting different surface energy (water wettability) exhibits a distinctive parabolic profile when scaled as a function of activator water wettability (Fig. 4). Nearly equal autoactivation is observed at the extremes of procoagulant water-wetting properties ( dyne/cm, 0° ≤ θ < 120°), falling sharply through a broad minimum within the dyne/cm (55° < θ < 75° ) range over which FXIIa yield rises just above detection limits. Data at hand cannot absolutely rule out the possibility of an activation spike within this minimum range (producing a “W” rather than “U” pattern), but this would require a seemingly improbable sharp response to changing surface chemistry that underlies water wettability. Even so, such an eventuality would not alter the primary conclusion of this work that autoactivation of FXII in neat-buffer solution is a strong function of procoagulant surface energy.
4.3.1 FXII Activation and Vicinal Water Properties
The parabolic activation pattern of Fig. 4 is evocative of the structure and reactivity of water at surfaces inferred from diverse literature sources (see refs. [34, 35] and citations therein). This latter evidence has suggested to us that there are two basic kinds of interfacial or “vicinal” water at the hydrophilic/hydrophobic extremes of surface wetting. Between these extremes, in a region of the wetting continuum near dyne/cm, it is proposed that vicinal water retains bulk-water-like properties. We have speculated that biology in contact with materials with different surface energy responds to the properties of vicinal water - not the surface directly - giving rise to different acute biological responses to materials with different water wettability [26, 35]. We have further proposed that this effect manifests itself as dominantly “Type I” biological responses to surfaces more hydrophobic than the τo = 30 dyne/cm pivot point and dominantly “Type II” biological responses to surfaces more hydrophilic than the τo = 30 dyne/cm pivot point [35]. Type I biological responses are both mediated and moderated by protein adsorption to hydrophobic materials whereas Type II biological responses are neither mediated nor moderated by protein adsorption because protein does not adsorb to hydrophilic surfaces [27, 28, 36, 37]. This distinction arises primarily because the concentration of protein within the interphase surrounding hydrophobic particles is higher than bulk solution due to adsorption whereas the concentration of protein within the interphase surrounding hydrophilic particles is equal, or nearly equal, to bulk solution because no adsorption (concentration above bulk solution) occurs.
Based on the above proposition, we are led to interpret the parabolic pattern in FXII activation in neat-buffer solution as Type I and Type II activation (see annotations top of Fig. 4). FXII adsorbs from neat-buffer solution to (concentrates within the interphase of) hydrophobic surfaces, increasing frequency of FXII autoactivation (Type I). But FXII does not formally adsorb to (concentrate within the interphase of) hydrophilic surfaces, only encountering the hydrophilic interphase region as FXII molecules pass through solution by random diffusion/thermal motion (Type II). Thus we speculate (but have not proven) from the limited data at hand that Type I autoactivation is fundamentally different than Type II in as-yet unrealized ways, possibly related to the relative proportions of different fragments created by autoactivation reactions.
4.4 Activation of FXII in Neat-Buffer Solution by Physical Mixtures of Type I and Type II Activators
Wetting properties of materials are related to the type, surface-area coverage, and distribution of surface-functional groups; with increasing hydrophilicity associated with increasing capacity to hydrogen bond to water [26]. Clean-glass particles used herein are fully wettable, presumably because of the high area coverage of water-reactive –SiOH and –Si-O-Si– type moieties. Reduced wetting can be achieved by capping these end groups with silanes that decrease the net hydrogen-bond capacity of treated surfaces. Surface chemistry of silanized glass can be quite complex [21, 38–45], especially for APTES [38], but it seems reasonable to presume that silane surface chemistry is more-or-less uniformly (randomly) distributed on procoagulant particle surfaces studied herein, as opposed to segregated into microscopic/macroscopic islands in a recognizable repeat pattern over the whole activator surface.
It is of interest in this regard that FXII activation by silanized surfaces was completely different than activation by physical mixtures of hydrophobic (n = 1, Nyebar-treated glass) and hydrophilic (n = 6 clean glass) particles (Fig. 6, Table 3). For convenience of comparison of silanized glass to physical mixtures, we computed a purely hypothetical “equivalent wetting parameter” for physical mixtures. This equivalent wetting parameter is based on a linear combining rule that estimates the hydrophilicity of an imaginary surface populated by the surface-area fraction hydrophilic and hydrophobic components represented by the w/w fraction of the physical mixture (Appendix B). It is emphasized that this notion of equivalent wetting has no physical basis in reality and is a purely conceptual tool that allows activation by physical mixtures of Type I and Type II procoagulants to be compared to activation by silanized activators on the same water-wetting basis (Fig. 5A).
Not surprisingly perhaps, activation by physical mixtures was essentially invariant across the full range of equivalent wetting. Mixing Type I and Type II activators, each with nearly equal FXII activation efficiency, simply results in a composite activation efficiency that cannot be clearly differentiated from either of the components. We are thus led to conclude that activation by uniformly-distributed surface chemistry (silanized activators) is substantially different from activation by macroscopically-distributed chemistry (physical mixtures). This outcome is consistent with our previous work showing that contact activation of blood plasma by nanoscopically-patterned surface chemistry was measurably different from activation by uniform surface chemistries of either component in the pattern [18]. An alternative explanation suggesting that FXII activation is specific for the different surface chemistries used to create particulate activators with different wettability cannot be rejected on the basis of data at hand. But this alternative is inconsistent with our observation that contact activation of blood plasma coagulation follows a “homologous series in procoagulant surface energy” with little discernable correlation with SAM chemistry used to prepare various test activators [14, 15].
4.5 FXII Fragments Exhibiting Plasma Coagulation Activity
Dunn et al. [46] reported in 1982 the formation and structure of Hageman factor fragments as follows: “FXII activates to form FXIIa (αFXIIa) by cleaving the bond connecting Arg353-Val354 and generating a two-chain molecule composed of a heavy chain Mr = 52,000 and a light chain Mr = 28,000, held together by a disulfide bond. Proteolytic cleavage further proceeds on FXIIa to yield a major active product at a molecular weight of 40,000 dalton as well as Hageman factor fragment (FXIIf), which appear as two closely related molecular species of Mr=28,000 and Mr=30,000. A minor active product of Mr=70,000 is also seen. Upon reduction of each of the active forms, a chain with Mr=28,000 is released which contains the active site. FXII digestion by kallikrein results in formation of αFXIIa, followed by further breakdown to FXIIf and degradation of the heavy chain region to an inactive fragment at 40,000 daltons, which is then degraded to an end product of Mr=36,000. Production of the active species with Mr=40,000 and Mr=70,000 is significantly diminished when kallikrein is the FXII activator, and these active forms are shown to be primarily formed by autodigestion.” An alternative mechanistic opinion has it that FXII activates into either FXIIf or FXIIa, refuting the idea of sequential formation of FXIIf [47]. However interpreted, these and other literature reports [4, 48] clearly show that FXII autoactivation leads to a mixture of fragments with amidolytic activity. Furthermore, there is evidence that different fragments (variously referred to as HFf, βFXIIa, or factor XIILMW, HFa, αFXIIa, or XIIHMW [4]) have different procoagulant activity. For example, Revak et al. reported that FXII fragments produced by contact activation of plasma activate prekallikrein and factor XI with different efficiency [3]. FXIIf activates prekallikrein, but at only 2–4% of the coagulant activity of αFXIIa [49].
All of the aforementioned studies have been performed in plasma. We are unaware of any systematic studies of αFXIIa and FXIIf production following contact activation of FXII in neat-buffer solution of the kind reported herein. Thus, we cannot exclude the possibility that Type I and Type II activation produces different relative amounts of αFXIIa and FXIIf (and possibly other FXII fragments). If so, measured yield of FXIIa reported herein may actually be a composite of activated FXII forms that varies in composition across the procoagulant surface energy range explored herein. In any event, the minimum observed in Fig. 4 represents a minimum in production of FXII fragments with plasma-coagulation activity because we employed a plasma-coagulation assay (rather than a chromogenic assay for example [7]) to measure autoactivation yield. Clearly, much more work is required to fully resolve the molecular details of autoactivation.
4.6 Contact Activation of Blood Plasma
Contact activation of blood plasma exhibits dependence on both surface area and surface energy, as illustrated in Fig. 6 for the glass-particle procoagulants studied in this work (Table 1). Surface area trends shown in Fig. 6 are interesting because coagulation time asymptotically reaches a surface-energy-dependent minimum whereupon continuous contact with increasing surface area of a given activator does not lead to significant reduction in coagulation time. This surface-area dependence has been the subject of mathematical models that allow extraction of a Kact parameter that measures procoagulant catalytic potential [6, 14, 20] from surface-area titration curves like those shown in Fig. 6 (smooth curves drawn through the data represent the best fit of the model with Kact as the single adjustable parameter). Consistent with this previous work, we find that Kact (Table 4) scales in a sharp exponential-like way with water wetting as shown in Fig. 7 (riht-hand axis). Historically, the observation that contact activation of whole plasma is nearly specific for hydrophilic surfaces gave rise to the idea that FXII activation is also nearly specific for hydrophilic surfaces [1]. As reported herein, however, FXII activation exhibits a parabolic dependence on procoagulant surface energy. The discrepancy between FXII activation and whole plasma activation dependence on procoagulant surface energy is rationalized on the basis of the adsorption-dilution effect briefly introduced in Section 1 more thoroughly described in the following section.
4.7 Autoactivation of FXII in Neat-Buffer Solution and Plasma
The nearly equal autoactivation observed at the extremes of procoagulant water-wetting properties (maximal yields of Type I and Type II activation) has been explained as a serendipitous outcome of competing autoactivation and autoinhibition reactions at hydrophobic surfaces [1, 8, 9]. Although adsorption concentrates FXII within the surface region (interphase) surrounding hydrophobic activators and presumably enhances autoactivation yield by increasing frequency of direct FXII-surface contacts, increased autoinhibition efficiency due to increased FXIIa concentrations within the interphase nearly counterbalances this effect. Accordingly, FXII activation at hydrophobic surfaces would be much higher than at hydrophilic surfaces were it not for this latter compensating effect.
Activation of endogenous FXII in plasma is much more complex than activation in neat-buffer at Type I surfaces because adsorption of a plethora of other blood proteins (about 1000 proteins at concentrations varying over six decades [50, 51]) competes with FXII adsorption. FXII adsorption from plasma to hydrophobic procoagulant surfaces is thus significantly blocked by competing proteins, efficiency of FXII contacts are sharply diminished, and FXIIa production is commensurately reduced; a phenomenon we have referred to as an “adsorption-dilution effect [1, 5]. Type I surfaces thus only appear to be inefficient activators of blood plasma coagulation even though Type I surfaces exhibit increasing autoactivation properties with increasing hydrophobicity [1]. By contrast, Type II surfaces retain FXII activation properties in plasma because proteins do not adsorb to hydrophilic surfaces [27–30, 34–36, 52]. If the latter were not true and proteins did indeed adsorb to hydrophilic surfaces, then plasma activation properties of Type I procoagulants would resemble that of Type II, leading to nearly inert procoagulation across the full range of procoagulant water wettability.
Fig. 7 illustrates the above-described interplay among FXII activation in buffer, plasma coagulation, and activator hydrophilicity by comparing FXII autoactivation yield in buffer (left-hand ordinate, data from Fig. 4A) to procoagulant “catalytic potential” measured in plasma (Kact , right-hand ordinate, deduced from Fig. 6; see Table 4 and Section 3.5). As discussed in Section 4.3, FXII autoactivation yield in neat-buffer solution exhibits a parabolic profile, rising from a minimum near τo = 30 dyne/cm at both ends of the water-wettability scale. By contrast, procoagulant catalytic potential in plasma sharply increases from very low levels observed for hydrophobic Type I surfaces to maximal levels for hydrophilic Type II procoagulants (see also refs. [14, 15]). Ordinarily, one might anticipate that Type I procoagulants would efficiently activate plasma coagulation because these surfaces efficiently catalyze autoactivation FXII in neat-buffer solution. However, Type I surfaces are also efficient protein adsorbents, with the adsorbent capacity increasing from immeasurably small at surfaces exhibiting τo > 30 dyne/cm to maximal levels at the extreme hydrophobic (left-hand) end of the water-wetting continuum (not shown in Fig. 4) [27–30, 34–36, 52]. According to the adsorption-dilution effect interpretation, protein adsorption competition renders Type I procoagulants nearly inert in plasma, leading to the observed exponential-like rise in procoagulant catalytic potential Kact with increasing hydrophilicity of Type II surfaces, rather than a parabolic profile that mirrors FXII autoactivation in neat-buffer solution.
It is interesting that Kact trends with Type II surface energy (right-hand axis of Fig. 7) in a manner similar to autoactivation yield in buffer (left-hand axis of Fig. 7), suggesting that plasma coagulation scales in proportion to the extent of endogenous FXII activation. Although such an inference is not proven by this work, it is consistent with the work of Chatterjee et al. [17] who find that autoactivation and reciprocal (kallikrein-mediated) activation increase in the same proportion with activator surface energy. Thus it appears that endogenous FXII autoactivates in proportion to activator surface energy which, in turn, is amplified by about 4X through reciprocal amplification (according to Chatterjee) for all activator surface chemistries. The product of the activation complex is a bolus of FXIIa (and/or other products of activation) that potentiates subsequent steps of the intrinsic pathway, penultimately producing a bolus of thrombin in proportion to autoactivation stimulus [1, 6, 16]. This thrombin bolus catalyzes rapid production of fibrin from fibrinogen that leads to rapid plasma coagulation [6, 14, 20]. Thrombin activity in plasma rapidly decays after coagulation by unknown regulation mechanisms [1]. Although the actual biochemistry of plasma coagulation is likely more concerted than the step-wise process suggested above, this basic mechanistic outline seems to adequately correlate FXII activation observed in neat-buffer solutions with endogenous activation in plasma (or protein cocktails [1, 8]) and explain proportionalities among autoactivation yield, reciprocal amplification, activator surface energy, and plasma coagulation time. Key features that remain unexplained by this broad rationalization of experimental evidence are the discontinuous FXII autoactivation in the continuous presence of activator surfaces and the actual biochemistry of FXII activation/deactivation.
5.0 Conclusion
Contact activation of human Factor XII in neat-buffer solution is a sharp function of activator surface energy. Nearly equal activation is observed at the extremes of activator water-wetting properties dyne/cm (0° ≤ θ < 120°), falling sharply through a broad minimum within the dyne/cm (55° < θ < 75°) range over which FXIIa yield rises just above detection limits. We propose that interfacial (vicinal) water properties drive a “Type I” autoactivation at surfaces more hydrophobic than dyne/cm and “Type II” autoactivation to surfaces more hydrophilic than dyne/cm [35]. Type I autoactivation is strongly moderated by protein adsorption whereas Type II autoactivation is due to “collisions” of FXII molecules with procoagulant surfaces passing through solution by random thermal motion. We also conclude that activation by uniformly-distributed surface chemistry is substantially different from activation by macroscopically-distributed chemistry. These findings refine our understanding of FXII autoactivation mechanism and suggest a route to the surface engineering of cardiovascular biomaterials with improved hemocompatibility.
Acknowledgments
This work was supported by the National Institute of Health grant PHS 2R01HL069965. Authors appreciate support of the Materials Research Institute and departments of Materials Science and Engineering and Bioengineering, Penn State University.
Appendices
Appendix A: Percent Autoactivation Yield and Correction for FXIIa Adsorption to Hydrophobic Activator Surfaces
Autoactivation yield Y in terms of percent activation of available FXII can be calculated from the simple relationship , where WBF12a is the solution concentration (mg/mL) of FXIIa and is the initial FXII solution concentration (mg/mL). This relationship can be implemented if solution concentrations of FXII and FXIIa can be determined and assuming that: (i) FXIIa is the only FXII product that causes coagulation of the plasma used in the FXIIa assay applied herein (see Section 2.3), (ii) autoactivation proceeds according to the one-for-one stoichiometry suggested by the chemical formula , and (iii) all FXIIa produced by autoactivation is released into solution. We calculated from vendor-supplied stock-solution concentrations in mg/mL. The calibration curve shown in Fig. 2 was used to estimate unknown solution concentrations of FXIIa in PEU/mL which was subsequently converted to WBF12a in mg/mL using a vendor-supplied conversion factor. There is strong evidence that assumption (i) is not entirely correct (see Section 4.4) and assumption (ii) remains unverified [1]. Consequently, autoactivation yield reported herein should be regarded only as an approximation. Veracity of assumption (iii) depends on the absorbency of activators used herein which varies depending on activator surface chemistry. However, the following analysis suggests that the amount of FXIIa adsorbed to activator surfaces is negligible compared to experimental error.
The amount of FXIIa adsorbed to an activator surface is related to the bulk solution concentration WBF12a by a partition coefficient , where WIF12a is the concentration of FXIIa at the solution-activator interface (interphase). We estimate from prior work that PF12a ≈ 100 for a protein with FXIIa dimensions adsorbing to a hydrophobic surface such as OTS-treated glass particles [53], with a decreasing value for surfaces with increasing hydrophilicity. Thus, at most, WIF12a can be as much as 100X greater than the solution from which FXIIa is adsorbed. However, the total adsorbed mass mIF12a ≡ WIF12aVI = PF12aWBF12aVI) is not large compared to the mass dissolved in solution (mBF12a ≡ WBF12aVB) because the total interface volume VI turns out to be small compared to bulk solution volume (200 µL). Globular blood proteins are oblate spheroids with core protein radius following rv = (6.72×10−8)(MW)1/3 to a very good approximation (packing-volume radius in cm for molecular weight MW expressed in kDa; see refs. [54–59] for basic information on spherical dimensions of proteins). Thus, the thickness Ω of an adsorbed monolayer of molecules with FXII (or FXIIa) dimensions [36] would be approximately Ω = 2rv = 2(6.72×10−8)(80)1/3 = 5.7×10−7 cm = 5.7 nm. It follows then that VI = ΩA = (5.7×10−7)(250) = 1.43×10−4 cm3 = 143 nL based on total procoagulant surface area of 250 cm2 (see Section 2). Consequently, it can be estimated that , meaning that 7.2% of the total autoactivation yield calculated on the basis of Y above could conceivably be adsorbed to hydrophobic procoagulants (that is to say, reported Y values for the most hydrophobic procoagulants might be underestimated by as much as 7.2%). This potential underestimation is a very conservative upper bound for all other more hydrophilic procoagulants because adsorption decreases as a function of increasing hydrophilicity. Thus, autoactivation results reported herein have not been corrected for adsorption. Basic conclusions drawn from the data would not be altered by making such corrections.
Appendix B: Equivalent Wetting Parameter for Physical Mixtures of Activator Particles
A linear combining rule was used to calculate an “equivalent contact angle” of water on a hypothetical composite surface comprised of hydrophilic and hydrophobic components at the various weight fractions listed in Table 3. Accordingly, the equivalent wetting of such a hypothetical composite surface is given by cosθequivalent = f cosθ1 + (1 − f)cosθ2, where f is the area fraction of component 1 exhibiting advancing contact angle θ1 and (1 − f) is the area fraction of component 2 exhibiting advancing contact angle θ2. Area fractions are identical to activator weight fractions in the physical mixture since component 1 (clean class, n = 6 of Table 1, dyne/cm, θa= 0°) and component 2 (OTS, n = 2 of Table 1, dyne/cm, θa = 118°) were derived from the same stock of glass particles with a single specific surface area. As an illustrative computational example, a 90:10 mixture of components 1 and 2 were prepared by gravimetry. The equivalent contact angle is thus given by cosθequivalent = 0.9×cos(0) + 0.1×cos(118) = 0.85 θequivalent = 32°).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Impact Statement: This work shows how contact activation of FXII depends on activator surface chemistry/energy and suggests a critical role of water in the control of hemocompatibility.
Citations
- 1.Vogler EA, Siedlecki CA. Contact Activation of Blood Plasma Coagulation. Biomaterials. 2009;30:1857–1869. doi: 10.1016/j.biomaterials.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmaier AH. The Elusive Physiologic Role of Factor XII. J Clin Invest. 2008;118(9):3006–3009. doi: 10.1172/JCI36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revak SD, Cochrane CG, Bouma BN, Griffin JH. Surface and Fluid Phase Activities of 2 Forms of Activated Hageman-Factor Produced During Contact Activation of Plasma. J Expt. Med. 1978;147(3):719–729. doi: 10.1084/jem.147.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderkamp K, Vanoeveren W. Factor XII Fragment and Kallikrein Generation in Plasma During Incubation with Biomaterials. J Biomed Mat Res. 1994;28(3):349–352. doi: 10.1002/jbm.820280309. [DOI] [PubMed] [Google Scholar]
- 5.Barnthip N, Parhi P, Golas A, Vogler EA. Volumetric Interpretation of Protein Adsorption: Kinetics of Protein-Adsorption Competition from Binary Solution. Biomaterials. 2009;30:6495–6513. doi: 10.1016/j.biomaterials.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant Stimulus Processing by the Intrinsic Pathway of Blood Plasma Coagulation. Biomaterials. 2005;26:2965–2973. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Zhuo R, Vogler EA. Practical Application of a Chromogenic FXIIa Assay. Biomaterials. 2006;27:4840–4845. doi: 10.1016/j.biomaterials.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhuo R, Siedlecki CA, Vogler EA. Competitive-Protein Adsorption in Contact Activation of Blood Factor XII. Biomaterials. 2007;28:4355–4369. doi: 10.1016/j.biomaterials.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of Blood Factor XII at Hydrophilic and Hydrophobic Surfaces. Biomaterials. 2006;27:4325–4332. doi: 10.1016/j.biomaterials.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan A, Liu Y-H, Cha P, Allara DL, Vogler EA. An Evaluation of Goniometric Methods. J Colloid and Interf Sci. 2005;43:95–98. doi: 10.1016/j.colsurfb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Lander LM, Siewierski LM, Brittain WJ, Vogler EA. A Systematic Comparison of Contact Angle Methods. Langmuir. 1993;9:2237–2239. [Google Scholar]
- 12.Friberger P. Synthetic Peptide Substrate Assays and Fibrinolysis and Their Application on Automates. Seminars in Thrombosis and Haemostasis. 1983;9(4):281–300. [PubMed] [Google Scholar]
- 13.Brown B. Hematology: Principles and Procedures. 3 ed. Philadelphia: Lea and Febiger; 1980. [Google Scholar]
- 14.Vogler EA, Graper JC, Harper GR, Lander LM, Brittain WJ. Contact Activation of the Plasma Coagulation Cascade.1. Procoagulant Surface Energy and Chemistry. J Biomed Mat Res. 1995;29:1005–1016. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]
- 15.Vogler EA, Graper JC, Sugg HW, Lander LM, Brittain WJ. Contact Activation of the Plasma Coagulation Cascade.2. Protein Adsorption on Procoagulant Surfaces. J Biomed Mat Res. 1995;29:1017–1028. doi: 10.1002/jbm.820290814. [DOI] [PubMed] [Google Scholar]
- 16.Vogler EA, Nadeau JG, Graper JC. Contact Activation of the Plasma Coagulation Cascade. 3. Biophysical Aspects of Thrombin Binding Anticoagulants. J Biomed Mat Res. 1997;40(1):92–103. doi: 10.1002/(sici)1097-4636(199804)40:1<92::aid-jbm11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee K, Guo Z, Vogler EA, Siedlecki CA. Contributions of Contact Activation Pathways of Coagulation Factor XII in Plasma. J Biomed, Mat Res Part A. 2009;90A:27–34. doi: 10.1002/jbm.a.32076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller R, Guo Z, Vogler EA, Siedlecki CA. Plasma Coagulation Response to Surfaces with Nanoscale Heterogeneity. Biomaterials. 2006;27:208–215. doi: 10.1016/j.biomaterials.2005.05.087. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee K, Vogler EA, Siedlecki CA. Procoagulant Activity of Surface-immobilized Hageman Factor. Biomaterials. 2006;27(33):5643–5650. doi: 10.1016/j.biomaterials.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Guo Z, Bussard KM, Chatterjee K, Miller R, Vogler EA, Siedlecki CA. Mathematical modeling of material-induced blood plasma coagulation. Biomaterials. 2006;27:796–806. doi: 10.1016/j.biomaterials.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Ulman A. Self-Assembled Monolayers of Alkyltrichlorosilanes: Building Blocks for Future Organic Materials. Adv Materials. 1990;2(12):573–582. [Google Scholar]
- 22.Ulman A. An introduction to ultrathin organic films : from Langmuir-Blodgett to self-assembly. Boston: Academic Press; 1991. [Google Scholar]
- 23.Ulman A. Formation and Structure of Self-Assembled Monolayers. Chem Rev. 1996;96(4):1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 24.Whitesides GM. Polymer Surfaces and Interfaces - Key to High Performance Materials. Chimia. 1990;44(10):310–311. [Google Scholar]
- 25.Sagiv J, Gun J, Maoz R, Netzer L. Self-Assembling Monolayers: A Study of Their Formation, Composition, and Structure. In: Mittal KL, Bothorel P, editors. Surfactants in Solution. New York: Plenum Press; 1986. pp. 965–978. [Google Scholar]
- 26.Vogler EA. How Water Wets Biomaterials. In: Morra M, editor. Water in Biomaterials Surface Science. New York: John Wiley and Sons; 2001. pp. 269–290. [Google Scholar]
- 27.Noh H, Vogler EA. Volumetric Interpretation of Protein Adsorption: Mass and Energy Balance for Albumin Adsorption to Particulate Adsorbents with Incrementally-Increasing Hydrophilicity. Biomaterials. 2006;27:5801–5812. doi: 10.1016/j.biomaterials.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Cha P, Krishnan A, Fiore VF, Vogler EA. Interfacial Energetics of Protein Adsorption from Aqueous Buffer to Surfaces with Varying Hydrophilicity. Langmuir. 2008;24:2553–2563. doi: 10.1021/la703310k. [DOI] [PubMed] [Google Scholar]
- 29.Vogler EA. Practical Use of Concentration-Dependent Contact Angles as a Measure of Solid-Liquid Adsorption II: Experimental Aspects. Langmuir. 1992;8:2013–2020. [Google Scholar]
- 30.Vogler EA, Martin DA, Montgomery DB, Graper JC, Sugg HW. A Graphical Method for Predicting Protein and Surfactant Adsorption Properties. Langmuir. 1993;9:497–507. [Google Scholar]
- 31.Ulman A, Evans SD, Shnidman Y, Sharma R, Eilers JE, Chang JC. Concentration-Driven Surface Transition in the Wetting of Mixed Alkanethiol Monolayers on Gold. J Am Chem Soc. 1991;113:1499–1506. [Google Scholar]
- 32.Ulman A. Wetting Studies of Molecularly Engineered Surfaces. Thin Solid Films. 1996;273:48–53. [Google Scholar]
- 33.Horr T, Ralston J, Smart R. The Use of Contact Angle Measurements to Quantify Adsorption Density and Thickness of Organic Molecules on Hydrophilic Surfaces. Colloids and Surfaces A: Physicochem Eng Aspects. 1995;97:183–196. [Google Scholar]
- 34.Vogler EA. Structure and Reactivity of Water at Biomaterial Surfaces. Adv Colloid and Interface Sci. 1998;74(1–3):69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 35.Vogler EA. Water and the Acute Biological Response to Surfaces. J Biomat Sci Polym Edn. 1999;10(10):1015–1045. doi: 10.1163/156856299x00667. [DOI] [PubMed] [Google Scholar]
- 36.Parhi P, Golas A, Barnthip N, Vogler EA. Volumetric Interpretation of Protein Adsorption: Capacity Scaling with Adsorbate Molecular Weight and Adsorbent Surface Energy Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.09.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parhi P, Golas A, Vogler EA. Role of Water and Proteins in the Attachment of Mammalian Cells to Surfaces: A Review. J Adhesion Sci and Tech. 2009 in press. [Google Scholar]
- 38.Howarter JA, Youngblood JP. Optimization of Silica Silanization by 3-Aminopropyltriethoxysilane. Langmuir. 2006;22:11142–11147. doi: 10.1021/la061240g. [DOI] [PubMed] [Google Scholar]
- 39.Parker JL, Claesson PM. Forces Between Hydrophobic Silanized Glass Surfaces. Langmuir. 1994;10:635–639. [Google Scholar]
- 40.DePalma V, Tillman N. Friction and Wear of Self-Assembled Trichlorosilane Monolayer Films on Silicon. Langmuir. 1989;5:868–872. [Google Scholar]
- 41.Tripp CP, Hair ML. Chemical Attachment of Chlorosilanes to Silica: A Two-Step Amine Promoted Reaction. J Phys Chem. 1993;97:5693–5698. [Google Scholar]
- 42.Tripp CP, Hair ML. Reaction of Methylsilanols with Hydrated Silica Surfaces: The Hydrolysis of Trichloro-, Dichloro-, and Monochlormethylsilanes and the Effects of Curing. Langmuir. 1995;11:149–155. [Google Scholar]
- 43.Wasserman SR, Tao Y-T, Whitesides GM. Structure and Reactivity of Alkylsiloxane Monolayers Formed by Reaction of Alkyltrichlorosilanes on Silicon Substrates. Langmuir. 1989;5:1074–1087. [Google Scholar]
- 44.Trau M, Murray BS, Grant K, Grieser F. An Ellipsometric Study of Thin Films on Silica Plates formed by Alkylchlorosilylation Reagents. J Colloid and Interface Sci. 1992;148(1):182–189. [Google Scholar]
- 45.Arkles B. Tailoring Surfaces with Silanes. Chemtech. 1977 December;7:766–778. [Google Scholar]
- 46.Dunn JT, Silverberg M, Kaplan AP. The Cleavage and Formation of Activated Human Hageman Factor by Autodigestion and by Kallikrein. J Biol Chem. 1982;257:1779–1784. [PubMed] [Google Scholar]
- 47.Smith D, Gilbert M, Owen WG. Tissue Plasminogen Activator Release In Vivo in Response to Vasoactive Agents. Blood. 1985;66(4):835–839. [PubMed] [Google Scholar]
- 48.Vanderkamp K, Hauch KD, Feijen J, Horbett TA. Contact Activation During Incubation of 5 Different Polyurethanes or Glass in Plasma. J Biomed Mat Res. 1995;29(10):1303–1306. doi: 10.1002/jbm.820291018. [DOI] [PubMed] [Google Scholar]
- 49.Hong SL. Effect of Bradykinin and Thrombin on Prostacyclin Synthesis in Endothelial Cells from Calf and Pig Aorta and Human Umbilical-Cord Vein. Thrombosis Research. 1980;18(6):787–795. doi: 10.1016/0049-3848(80)90201-7. [DOI] [PubMed] [Google Scholar]
- 50.Putnam FW. Alpha, Beta, Gamma, Omega - The Roster of the Plasma Proteins. In: Putnam FW, editor. The Plasma Proteins: Structure, Function, and Genetic Control. New York: Academic Press; 1975. pp. 58–131. [Google Scholar]
- 51.Anderson NL, Anderson NG. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Molecular and Cellular Proteomics. 2002;1(11):845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 52.Vogler EA. Practical Use of Concentration-Dependent Contact Angles as a Measure of Solid-Liquid Adsorption I: Theoretical Aspects. Langmuir. 1992;8:2005–2012. [Google Scholar]
- 53.Noh H, Vogler EA. Volumetric Interpretation of Protein Adsorption: Partition Coefficients, Interphase Volumes, and Free Energies of Adsorption to Hydrophobic Surfaces. Biomaterials. 2006;27:5780–5793. doi: 10.1016/j.biomaterials.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 54.Richards FM. Areas, Volumes, Packing and Protein Structure. Ann Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- 55.Chothia C. Structural Invariants in Protein Folding. Nature. 1975;254:304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- 56.Miller S, Lesk A, Janins J, Chothia C. The Accessible Surface Area and Stability of Oligomeric Proteins. Nature. 1987;328(27):834–836. doi: 10.1038/328834a0. [DOI] [PubMed] [Google Scholar]
- 57.Miller S, Janin J, Lesk A, Chothia C. Interior and Surface of Monomeric Proteins. J Mol Biol. 1987;196:641–656. doi: 10.1016/0022-2836(87)90038-6. [DOI] [PubMed] [Google Scholar]
- 58.Tsai J, Taylor R, Chothia C, Gerstin M. The Packing Density in Proteins: Standard Radii and Volumes. J Mol Bio. 1999;290:253–266. doi: 10.1006/jmbi.1999.2829. [DOI] [PubMed] [Google Scholar]
- 59.Gerstein M, Chothia C. Packing at the Protein-Water Interface. Proc Natl Acad Sci. 1996;93:10167–10172. doi: 10.1073/pnas.93.19.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]