Abstract
This work describes observed changes in the proton T1 relaxation time of both water and lipid when they are in relatively homogeneous mixtures. Results obtained from vegetable oil–water emulsions, pork kidney and lard mixtures, and excised samples of white and brown adipose tissues are presented to demonstrate this change in T1 as a function of mixture fat fraction. As an initial proof of concept, a simpler acetone-water experiment was performed to take advantage of complete miscibility between acetone and water and both components’ single chemical shift peaks. Single-voxel MR spectroscopy was used to measure the T1 of predominant methylene spins in fat and the T1 of water spins in each setup. In the vegetable oil–water emulsions, the T1 of fat varied by as much as 3-fold when water was the dominant mixture component. The T1 of pure lard increased by 170 msec (+37%) when it was blended with lean kidney tissue in a 16% fatty mixture. The fat T1 of lipid-rich white adipose tissue was 312 msec. In contrast, the fat T1 of leaner brown adipose tissue (fat fraction 53%) was 460 msec. A change in the water T1 from that of pure water was also observed in the experiments.
Keywords: fat, water, T1 relaxation, T1 bias, T1 relaxation in mixture
Several reports have recently described robust chemical-shift fat-water separation methods in MRI (1–4). Fat quantification studies utilizing some of these techniques have been described in assessing hepatic steatosis (5), epicardial fat (6), and total body fat composition in obesity research (7). With methods such as the iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL) approach (2), a fat-signal fraction is typically computed on a voxel-by-voxel basis as F/(F +W), where F and W denote the decomposed fat and water signals, respectively. In order for the fat-signal fraction to accurately represent the underlying fat content, several works have shown that it is important to consider a multipeak rather than a single-peak spectral model for fat (8,9), T2* weighting (8,10), and T1 and noise bias between F and W signals (8,11). To specifically minimize T1-bias between fat and water spins, the use of small flip angles (≈5°) in IDEAL spoiled-gradient-echo imaging has been suggested (11). In addition to the fat-signal fraction, a fat-only signal fraction (F/FPURE) has also been reported, where FPURE is the signal from a separate reference voxel containing pure fat (12). This work specifically investigates deviation in the proton T1 spin-lattice relaxation rate of fat from its pure natural T1 value when fat is present in relatively homogeneous mixtures. Thus, it is hypothesized that the F/FPURE ratio may also be susceptible to T1-bias. Results from several phantoms constructed of acetone-water mixtures, oil-water emulsions, kidney-lard mixtures, and excised white and brown adipose tissue samples from mice are presented to corroborate this hypothesis. Although the change in the T1 of the methylene fat moiety is the primary focus in this article, we also demonstrate the change in the proton T1 of water (and acetone).
MATERIALS AND METHODS
T1 Measurements
All experiments were performed at room temperature on a General Electric 3-T scanner (Signa HD 12M5; GE Healthcare, Waukesha, WI). We used single-voxel proton MR spectroscopy (point-resolved MRS) (13) for both accurate spectral separation of chemical moieties and T1 measurements. Data were acquired with a wrist coil (BC-10; Mayo Clinic, Rochester, MN). After Fourier transformation of the acquired free-induction-decay signal, baseline and phase correction were performed. Subsequently, the area under each spectral peak of interest was computed. Analysis was performed with the Java-based magnetic resonance user interface (MRUI) software (http:/sermn02.uab.cat/mrui/) (14,15), where the user can specify the number of spectral peaks to be fitted. Signal integrals were quantified for the water peak (near 4.7 ppm) and only the primary methylene (—CH2—)n fat peak (near 1.3 ppm). For the acetone-water setup, the acetone peak was quantified (near 2.4 ppm downfield from water) in place of fat. Scan parameters for MRS were echo time = 23 msec, 20 × 20 × 20mm3 voxel, 2048 data points, 2.5- kHz bandwidth, no water suppression, and at least eight signal averages. In each experiment, the pulse repetition time (TR) was varied while all other parameters were held constant. The specific TR values used in each experiment are listed in Table 1. We used different TR values to ensure adequate sensitivity in measuring the anticipated T1 values. After spectral peak quantification, the T1 relaxation rates of acetone/water/methylene fat were estimated with least-squares curve-fitting routines in Matlab (The MathWorks, Natick, MA). The computed areas under each spectral peak were plotted vs TR, and a monoexponential saturation-recovery equation STR = SO(1 − exp(−TR/T1)) was used for fitting. STR is the integrated peak spectral area for a given TR, and SO is the equilibrium value (weighted by constant T2 relaxation). No numerical constraint was placed on the estimated T1 values during data fitting. The procedures were similar to those used by Sharma et al. (16).
Table 1.
List of TR Values Used in Each Spectroscopy Experiment
| Experiment | Figure number | TR (ms) |
|---|---|---|
| Acetone-water | 1 | 1070, 1570, 2070, 2570, 3070, 3570, 4070, 5070, 6070, 7070 |
| Oil-water | 2, 3 | Emulsions |
| 1070, 1570, 2070, 2570, 3070, 3570, 4070, 4570, 5070, 5570, 6070, 6570 | ||
| Pure oil | ||
| 550, 620, 720, 820, 920, 1020, 1120, 1220, 1320 | ||
| Kidney-lard | 4 | Mixtures |
| 525, 625, 725, 825, 1025, 1525, 2025, 2525, 3025, 3525, 4025, 4525 | ||
| Pure lard | ||
| 525, 625, 725, 825, 925, 1025, 1125, 1225, 1325, | ||
| Brown and white adipose tissue | 5 | Brown |
| 525, 625, 725, 825, 925, 1025, 1125, 1225, 1325, 1425, 1525, 1625, 2025, 2525, 3025, | ||
| 3525, 4025, 5025 | ||
| White | ||
| 525, 575, 625, 675, 725, 775, 825, 875, 925, 975, 1075, 1275, 1375, 1475, 1775, 2075 |
Acetone-Water Mixtures
As an initial proof of concept, we substituted fat with acetone. Mixtures of acetone (Alfa Aesar, Ward Hill, MA) and un-doped distilled water were prepared in 20% increments by volume in 50-mL vials. Acetone was used for convenience due to its complete miscibility with water. Furthermore, unlike fat, acetone is characterized by a true single chemical shift peak (2.3 ppm), as shown in Fig. 1. MRS measurements were acquired separately for each mixture of acetone and water. We hypothesized that the component T1 values of acetone and water would change as a function of mixture composition.
FIG. 1.
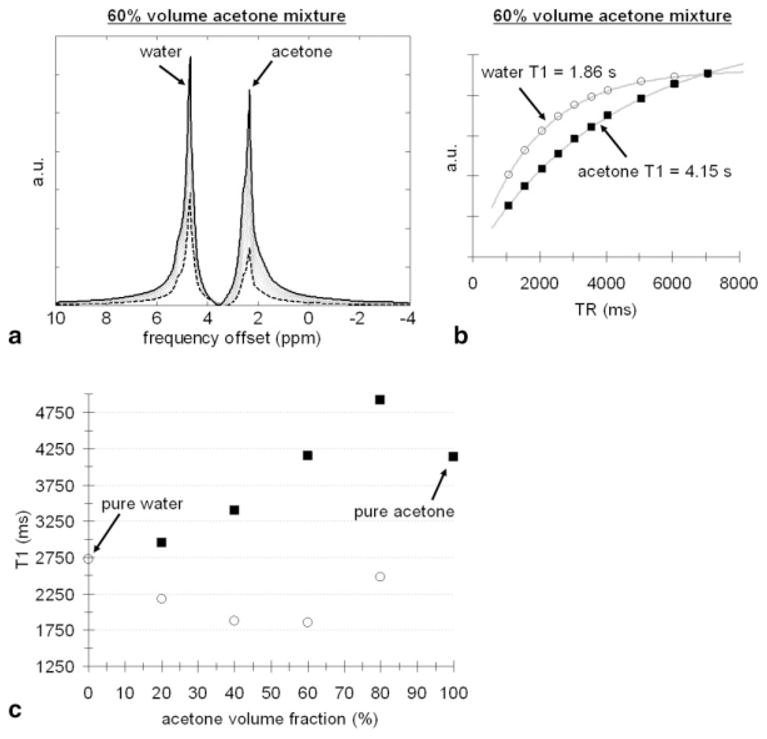
Results from acetone-water mixtures. a: Raw, unprocessed MRS spectra from the 60% acetone mixture. A spectrum obtained with a TR of 7070 msec is shown in solid black. A spectrum obtained with a TR of 1070 msec is shown in dashed black. b: Plot of spectral signal as a function of TR for acetone (black square) and water (open circle) components with saturation-recovery fitted curves. c: Plot of acetone and water T1 as a function of mixture composition, demonstrating evident changes of T1 in mixture from pure values.
Oil-Water Emulsions
Homogeneous emulsions consisting of vegetable (corn) oil and distilled water were prepared (Fig. 2a) in 60-mL bottles, similar to the setup described previously by Bernard et al. (17). Agar gel (2% by weight) and dioctyl sulfosuccinate sodium salt (Alfa Aesar) were used to stabilize the emulsions. No contrast agent or additional chemicals were added to the water. The emulsions were prepared slowly over a heat-stir plate and subsequently cooled to allow the mixture to stay intact. As the photograph in Fig. 2a shows, the oil and water suspensions are homogeneous and stable. MRS measurements were collected on a subset of the mixtures (10%, 30%, 60%, and 80% fat by volume). We similarly hypothesized that the component T1 values of fat and water would change as a function of mixture composition.
FIG. 2.
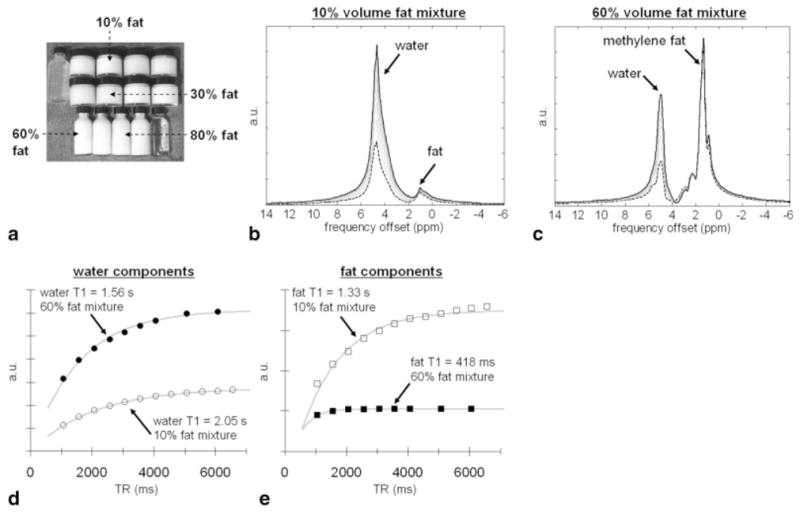
Results from oil-water emulsions. a: Photograph of the emulsions. Clear white liquid on the upper left is water and agar. Clear liquid on the lower right is vegetable (corn oil). b,c: Raw, unprocessed MRS spectra from 10 and 60% fat mixtures for various TR values (long TR, solid black; short TR, dashed black, respectively). d,e: Plots of spectral signal as a function of TR for water (circles) and fat (squares) components, respectively, along with fitted curves. Note evident differences in T1 values.
Pork Kidney–Lard Mixtures
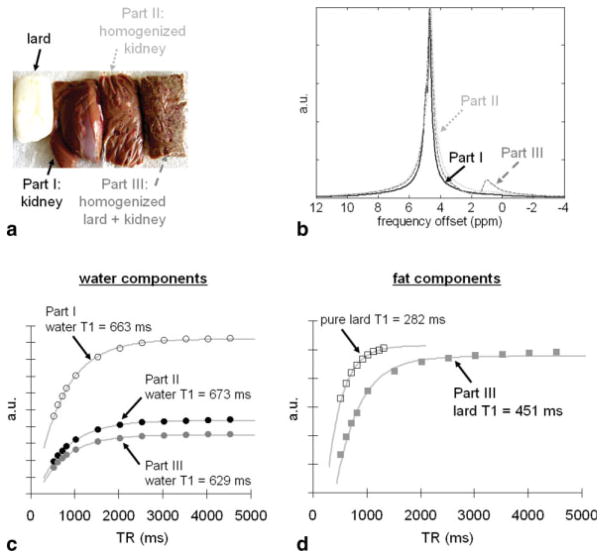
Fresh pork kidney and lard (Farmer John, Los Angeles, CA) were purchased from a local market. The kidney was cut into three portions and was initially devoid of any fat tissue (Fig. 4a). In part I, the tissue was left unaltered. In part II, the tissue was thoroughly homogenized in a blender. In part III, the tissue was additionally mixed with melted lard and further homogenized in a blender. The resulting purée-like mixture had a fat fraction of about 16%, as subsequently determined by MRS. MRS measurements were collected separately for each of the three portions and for pure lard. We hypothesized that the T1 of water (kidney) would not change between parts I and II as a result of homogenization. We additionally hypothesized that the T1 of lard would change in part III from that of pure lard.
FIG. 4.
Results from kidney-lard mixtures. a: Photograph of lard (white) and the three-part samples. b: Corresponding MRS spectra. Part III has a fat fraction of approximately 16%. c: Spectral signal plots as a function of TR for the water component in parts I-III. Homogenizing the tissues (part II) does not appear to affect the T1 of the water component in comparison to reference intact kidney tissue (part I). d: Spectral signal plots for the lard component in part III and in pure lard, demonstrating a near 170-msec (37%) difference in the measured lard T1.
White vs Brown Adipose Tissue
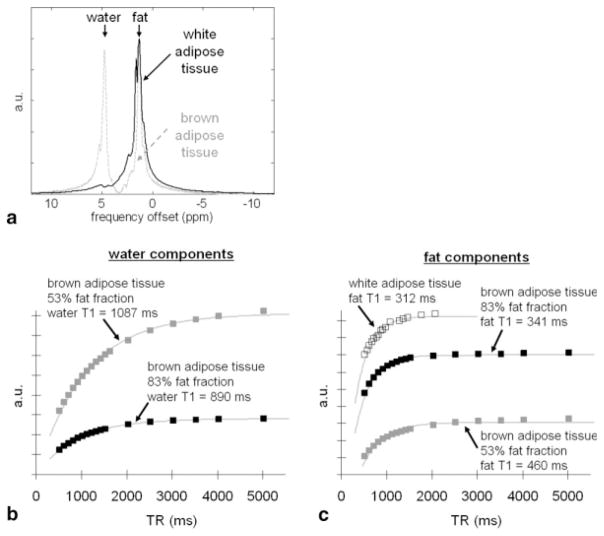
Excised samples of white and brown adipose tissues were obtained from mice (Fig. 5a). Whereas white adipose tissue is primarily a lipid storage reservoir, brown adipose tissue is involved in energy expenditure and thermogenesis (18). White and brown adipocytes represent two naturally occurring physiologic tissues that have distinct differences in intracellular fat content. Due to its higher metabolic activity, the fat fraction of brown adipose tissue has been shown to be lower than that of white adipose tissue (19). Under light microscopy, brown adipocytes are characterized by numerous small intracellular lipid droplets that are surrounded by an abundance of intracellular water. In contrast, white adipocytes contain only a single large lipid micelle and have very little additional intracellular space. The chemical composition of triglycerides in brown and white adipose tissue is not significantly different (20). We performed MRS measurements on one sample of white adipose tissue and two samples of brown adipose tissue with different fat fractions. We hypothesized that the T1 of fat and water in white and brown adipose tissue would differ.
FIG. 5.
Results from white and brown adipose tissue. a: MRS spectra of the two tissue types, demonstrating that white adipose tissue (solid black) is composed nearly entirely of lipids (1.3 ppm) while brown adipose tissue (dashed gray) contains appreciable water signals (4.7 ppm). b: Spectral signal plots of the water component as a function of TR, showing slight differences in water T1 for two samples of brown adipose tissue with different fat content. c: Corresponding plots of the fat component, showing more pronounced differences in the fat T1 from that of pure white adipose tissue.
RESULTS
Acetone-Water Mixtures
Figure 1 summarizes results from the acetone-water mixtures. Raw, unprocessed MRS spectra obtained from the 60% acetone mixture are shown in Fig. 1a in units of ppm. Spectra obtained with the shortest (dashed black) and longest TRs (solid black) are highlighted. The effect of saturation recovery as a function of TR is clearly evident. Figure 1b plots the integrated spectral signals as a function of TR for acetone and water. The measured data points and fitted curves suggest excellent agreement with the theoretical T1 saturation-recovery signal model. The estimated component T1s of acetone and water are indicated. Figure 1c illustrates the evident change in both the T1 of acetone and water as a function of acetone volume fraction. Pure water (0%) had a T1 of 2.7 sec, whereas pure acetone (100%) had a longer T1 of 4.1 sec. Across the mixtures, water T1 changed by approximately 32%, while acetone T1 varied by approximately 28%. Note that the component water T1 approaches the pure T1 of acetone as the acetone volume fraction nears 100% (e.g., mixture is acetone dominant). Similarly, the component acetone T1 approaches the pure T1 of water as the acetone volume fraction nears 0% (e.g., mixture is water dominant).
Oil-Water Emulsions
Figures 2 and 3 summarize results from the oil-water emulsions. A photograph of the homogenized suspensions is shown in Fig. 2a. Data are presented in a similar format to Fig. 1. Raw, unprocessed MRS spectra from 10% and 60% fat emulsions are shown in Fig. 2b and c, respectively. In Fig. 2d, fitted data curves for the water components are shown. Similarly, in Fig. 2e, fitted data curves for the fat components are plotted. The data curves in Fig. 2e have been scaled individually to facilitate plotting in the same figure. Figure 3 illustrates the change in both the T1 of fat and water as a function of mixture fat fraction. The largest observed change in fat T1 from its pure value is more than 3-fold, while the largest variation in water T1 is approximately 59% of its pure value. A similar trend is observed in comparison to Fig. 1c. The component water T1 approaches the pure T1 of fat as the fat volume fraction nears 100% (e.g., mixture is fat dominant). Likewise, the component fat T1 approaches the pure T1 of water as the fat volume fraction nears 0% (e.g., mixture is water dominant). Whereas acetone was the longer T1 moiety in the previous experimental setup, water molecules were the longer T1 species in the oil-water emulsions. Interestingly, note that the shape of the acetone data points in Fig. 1c (squares) and those of water (open circles) in Fig. 3 behave similarly as a function of mixture composition, albeit in a mirrored manner.
FIG. 3.
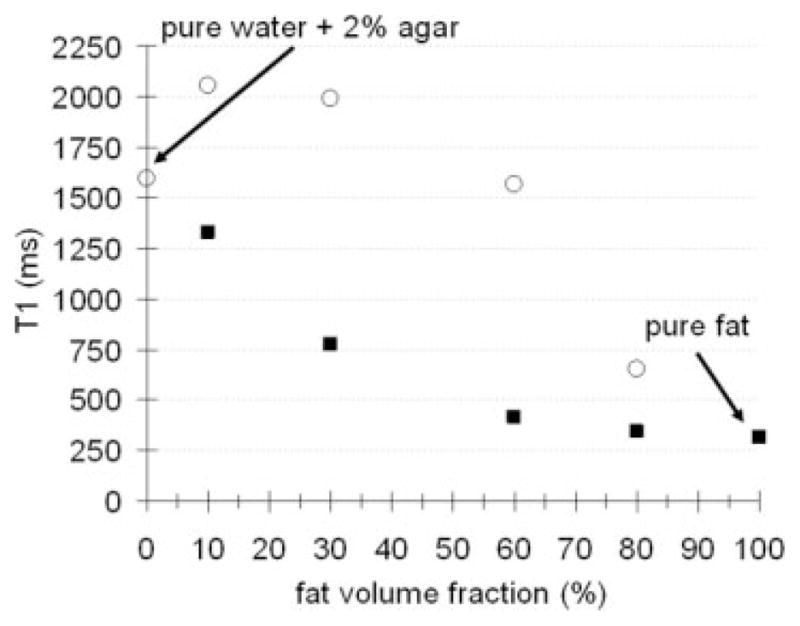
Overall result from oil-water emulsions. Plot of fat (black squares) and water (open circles) T1 as a function of fat fraction. The change in the T1 of both moieties from their pure values when in relatively homogeneous emulsions is evident.
Pork Kidney–Lard Mixtures
Figure 4a shows a photograph of the three different kidney/lard preparations. Figure 4b shows the corresponding MRS spectra. The fat peak (at 1.3 ppm) and the 16% fat fraction in part III from melted lard are clearly noticeable in the spectrum. The spectra also show that parts I and II are devoid of any fat species. Figure 4c plots the integrated signals for the water component in parts I, II, and III. As anticipated, homogenizing the kidney (part II) did not affect the T1 of the water component relative to intact kidney tissues (part I). There is a slight decrease in the water T1 of about 34 msec (5% change) when kidney tissues were homogenized with lard (part III). This change is insignificant in comparison to the variation observed in the associated lard T1. Figure 4d plots similar curves for the lard component between pure lard and mixed lard in part III. The measured T1 of lard in part III (black squares) represents a significant 37% increase (282 to 451 msec) over the T1 of pure lard (open squares). The data curve for pure lard in Fig. 4d has been scaled down 5-fold to facilitate plotting in the same figure.
White vs Brown Adipose Tissue
Figure 5 summarizes results from the experiment using excised white and brown adipose tissue from mice. Figure 5a shows representative MRS spectra of the two tissues. Note that white adipose tissue is predominantly composed of lipids, whereas brown adipose tissue contains an appreciable amount of water signal at around 4.7 ppm. Figure 5b plots the integrated signals for the water components of two samples of brown adipose tissue with different fat fractions. The leaner sample with a 53% fat fraction had a water T1 of 1087 msec (gray squares), while the fattier sample with a fat fraction of 83% had a shorter water T1 of 890 msec (black squares). Figure 5c shows corresponding plots for the fat components. Note that white adipose tissue had a fat T1 of 312 msec, consistent with previous literature findings in vivo (21,22). However, the fat T1 increased as the fat fraction decreased. The fat T1 of the 83% brown adipose tissue was 341 msec, while the fat T1 of the 53% sample was 460 msec.
DISCUSSION AND CONCLUSION
We have demonstrated the phenomenon of change in the T1 of both fat and water moieties from their pure T1 values when they are present in relatively homogeneous mixtures. Fat and water are naturally immiscible and a mixture of the two can only be treated as a suspension. Acetone and water are, however, completely miscible, and results from the proof-of-concept acetone-water experiment provided compelling evidence of the T1 effect investigated in this work. For the three fat-water experiments, we observed in general an increase in the T1 of fat from its pure T1 value as the fat fraction decreased. Conversely, the T1 of water decreased from its pure T1 value when the mixture composition became less water dominant. In the oil-water emulsion experiment, the variation in the fat and water T1 values was significant when the mixture composition became less than 30% fat (water dominant) and 30% water (fat dominant), respectively. The use of MRS provided good separation of the different chemical moieties in each experimental setup, as well as excellent data fits for the saturation-recovery signal equation. Based on present results, all of our hypotheses are corroborated.
The present findings reinforce the issue of T1-bias when using fat fraction indices from chemical-shift-based methods such as IDEAL. The T1-bias between fat (F) and water (W) spins is intuitive (11). However, the variation in component fat and water T1 as a function of fat fraction is not obvious and further complicates the issue of fat quantification. Nonetheless, low-flip-angle approaches (8,11) that minimize T1-bias or schemes that explicitly measure T1 (11) to correct for the signal bias remain important in accurate fat fraction quantification. A particularly interesting conclusion can be drawn from the current results. At low fat fractions—often encountered in liver fat quantification—the component T1 of water and fat is potentially very close in value (Fig. 3). This similarity in T1 values actually lessens the degree of T1 bias between fat and water. Consequently, the need for T1-bias correction is less of an issue, and an Ernst angle approach could potentially be favorable. A similar argument can be made for high-fat-fraction scenarios. Figure 3 further suggests that the greatest T1 bias between fat and water in mixture likely occurs at intermediate fat fractions.
In this work, we focused only on the T1 of methylene fat protons. One limitation of this is that the observed change in water T1 may be partly influenced by the olenific fat peak (5.2–5.4 ppm), which is in close proximity to the water peak. However, the acetone-water setup was not susceptible to this limitation. Previous literature has reported that other fat components (e.g., olenific, methyl, diallyic) have individual and different natural T1 values (23,24). It can be implied that the T1 of these minor fat peaks may also change when in mixture. Quantification of the degree of T1 change in these minor fat peaks will require a higher magnetic field strength for greater spectral resolution.
The findings of fat T1 variation will unlikely have any implications on conventional T1-based fat suppression methods (short T1/tau inversion recovery). Based on the oil-water phantom experiment (Fig. 3), pure fat had a T1 of 314 msec. The fat T1 did not begin to deviate substantially from its natural T1 value until the fat fraction was less than 30%. Let MO_F denote the fully relaxed longitudinal magnetization of pure fat. An inversion recovery (IR) sequence using inversion time = 218 msec that is set to null pure fat will more than adequately suppress not only pure fat but also a majority of voxels with fat-signal fractions between 30% and 100%. Consider a voxel with a 30% fat fraction, whose fully relaxed longitudinal magnetization can be denoted as 0.3·MO_F. According to Fig. 3, the fat T1 is 779 msec. During an IR experiment, MZ will relax at most from −0.3·MO_F to +0.3·MO_F. At inversion time =218 msec, |MZ| will have recovered to approximately 50% of (0.3·MO_F), which results in a net detectable magnetization of only 15% of MO_F. For another voxel with a 10% fat fraction (fat T1 1332 msec), the net magnetization at inversion time =218 msec is less than 7% of MO_F. In terms of short T1/tau inversion recovery fat suppression performance in clinical applications, these residual fat signals will not likely raise concerns.
We have also performed experiments using IDEAL with inversion-recovery fast spin echo and driven equilibrium single pulse observation of T1 (25) imaging approaches in lieu of MRS to measure fat and water T1 values in mixture (26). Comparable results and trends in T1 were observed in phantoms and in vivo. With IDEAL-inversion-recovery fast spin echo, data were acquired as a function of inversion time. With driven equilibrium single pulse observation of T1, data were acquired as a function of flip angle, similar to the approach used by Liu et al. (11). IDEAL-reconstructed fat and water signals were fitted to either inversion-recovery or spoiled-gradient-echo signal equations to determine component T1 values. Confounding factors such as , T1 bias, multifat-peak modeling, and amplitude of radiofrequency field flip-angle nonuniformity were collectively considered for accurate IDEAL fat-water decomposition (8–11) and signal fitting. For both inversion-recovery fast spin echo and driven equilibrium single pulse observation of T1, a high number of signal averages (>5) were needed to ensure ample signal-to-noise ratio in the decomposed fat and water images. Physiologic constraints were also set for the T1 estimates (11) from driven equilibrium single pulse observation of T1 and inversion-recovery fast spin echo. These constraints were necessary based on our previous experience in estimating T1 values from noisy signal curves at low fat and water fractions acquired with only one to two signal averages.
The oil-water phantom results from this work bear resemblance to a recent study by Sharma et al. (16), which also used MRS to measure the water and fat T1 values, but at 1.5 T. In emulsions of 10 and 30% fat fractions, the authors found both the water and fat T1 to decrease with increasing concentrations of oil. For the 10% mixture, the T1 of water was measured as 821.7 msec, while in the 30% mixture, it was 591.7 msec. The T1 of fat in the 10% mixture was 680.3 msec, while in the 30% mixture, it was a significantly lower 185.2 msec. The emulsions also contained varying concentrations of iron additives, which were used to generate differences in T2 relaxation. Sharma et al. (16) further reported the hepatic fat T1 in a subject with a 13% fatty liver to be 485 msec, which anecdotally seems greater than reported literature values of pure fat at 1.5 T (280–340 msec) (21,22). In another article, by Poon et al. (27), the authors performed a study at 1.5 T in thigh muscle in a patient with myositis (skeletal muscle inflammation), which is often accompanied by fatty infiltration. It was found that the fat T1 increased from 187 to 396 msec and the water T1 decreased from 1257 to 993 msec when the fat-signal fraction increased from 24 to 71% in several regions of interest. In constructing several homogeneous fat-water emulsions of different fat fractions with matched fat and water T1 values at 3 T, Dyke et al. (28) reported that it was necessary to dope the water and agarose gel ingredients with varying amounts of gadolinium contrast agent to decrease the water T1 and match it to the shorter fat T1.
A detailed description behind the change in T1 is beyond the scope and intent of this article, but insights can be gleaned from literature on general relaxation theory (29), relaxation behaviors in binary liquid mixtures (30), and some intuition. It is known in chemistry that when two components (A and B) are combined to form a mixture, the critical points of the solution (e.g., freezing, boiling) will consequently vary nonlinearly as a function of the concentrations of A and B present. This principle can be extended to T1 spin-lattice relaxation. Fundamentally, T1 depends on a match between the amplitude of static field–dependent Larmor frequency (fLarmor) of protons in a molecule and the tumbling rate (inverse molecule correlation time) of the local molecular lattice (flattice) surrounding the molecule of interest. When the two are equal, T1 relaxation is the most energy efficient, leading to a short T1 value. This is the primary reason underlying fat’s characteristic short T1 and free water’s long T1 in physiologic MRI. The tumbling rate of a molecular lattice composed of large fat molecules is closely matched the proton Larmor frequency at 1.5 and 3 T (e.g., fLarmor ≠ flattice, therefore, short T1). In contrast, the tumbling rate of a lattice composed of small, free-water molecules is significantly greater than the Larmor value (e.g., fLarmor = flattice, therefore, long T1). Therefore, it is plausible that any structural change in the lattice will lead to variations in the lattice tumbling rate and thus affect T1.
In oil-water suspensions, it is conceivable that the structure of the molecular lattice will change as a function of the concentration of fat and water present in the lattice. At very low or high fat fractions, the dominant lattice is likely determined by the majority species. Large fatty acid moieties may aggregate into small micelles at low fat fractions when water is the dominant lattice. Conversely, they may link together to form large molecular sheets at high fat fractions where fat is the dominant lattice. Consequently, the observed T1 spin-lattice relaxation rates of water and fat species will likely depend on the dominant lattice. This potentially explains why the T1 values of water and fat are very close at low and high fat fractions where the dominant lattice is clearly defined by the majority species. At intermediate fat fractions where both water and fat are present in comparable amounts, an intricate and complex lattice will likely form, giving rise to distinct water and fat T1 values.
Consider the extreme case where a large fat molecule that is surrounded in a solvent consisting primarily of smaller water molecules (low fat fraction). Also consider in contrast the opposite extreme case where the fat molecule is surrounded in a solvent composed mostly of similar fat molecules (high fat fraction). In the low-fat-fraction environment, the increase in the number of local smaller water molecules with shorter correlation times and faster tumbling rates will lead to a flattice that is greater than the proton fLarmor of the fat molecule. As a result of this mismatch, the fat molecule is energetically less efficient at interacting with the water-dominant lattice. Consequently, an increase in fat T1 will occur. In contrast, fLarmor and flattice are more closely matched for the high-fat-fraction environment (fat-dominant lattice), thereby promoting faster T1 relaxation. The same argument can be applied from the perspective of a water molecule. An increasing presence of larger, slow-tumbling fat molecules in the solvent within the immediate vicinity of a water molecule will decrease the local flattice in comparison to that of a water-rich lattice. This will effectively bring the local flattice surrounding the water molecule of interest closer to the fLarmor of the water spins, thereby lowering the water T1.
Applications involving fat infiltration of skeletal muscles and organs can potentially benefit from the present findings. Relationships between T1 and muscle fiber composition have been reported (31), where it was speculated that the proportion of slow- to fast-twitch fibers, along with their relative fat contents, plays a determining role. Assessment of muscle and organ triglyceride content remains important in studies of Duchenne muscular dystrophy (32) and Gaucher’s disease (33) and in metabolic disorders and the etiology of obesity (34,35). Furthermore, differences in triglyceride composition (fatty acid chain length, degree of saturation) may also influence T1 due to molecular size and geometry.
In conclusion, this work has described the variation in T1 of fat and water as a function mixture composition and has provided supporting evidence from MRS. It is an additional factor that falls under the complex framework of accurate fat fraction quantification in MRI and reinforces the notion that T1 bias is a required consideration.
Acknowledgments
The authors thank Daniel L. Smith, Jr. and Timothy R. Nagy (University of Alabama at Birmingham) for providing the samples of white and brown adipose tissues, Gavin Hamilton (University of California San Diego) for assistance with MRUI software, and Charles A. McKenzie (University of Western Ontario) and Robert Lenkinski (Beth Israel Deaconess Medical Center) for helpful suggestions and discussions on T1 relaxation.
Grant sponsor: NCI Centers for Transdisciplinary Research on Energetics and Cancer; Grant number: U54 CA 116848.
Grant sponsor: NIDDK; Grant number: R21 DK 081173.
References
- 1.Ma J. Breath-hold water and fat imaging using a dual-echo two-point Dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med. 2004;52:415–419. doi: 10.1002/mrm.20146. [DOI] [PubMed] [Google Scholar]
- 2.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636–644. doi: 10.1002/mrm.20624. [DOI] [PubMed] [Google Scholar]
- 3.Xiang QS. Two-point water-fat imaging with partially-opposed-phase (POP) acquisition: an asymmetric Dixon method. Magn Reson Med. 2006;56:572–584. doi: 10.1002/mrm.20984. [DOI] [PubMed] [Google Scholar]
- 4.Hernando D, Haldar JP, Sutton BP, Ma J, Kellman P, Liang ZP. Joint estimation of water/fat images and field inhomogeneity map. Magn Reson Med. 2008;59:571–580. doi: 10.1002/mrm.21522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, Shulman GI, Caprio S, Constable RT. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point Dixon and three-point IDEAL. Magn Reson Med. 2008;59:521–527. doi: 10.1002/mrm.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kellman P, Hernando D, Shah S, Zuehlsdorff S, Jerecic R, Mancini C, Liang ZP, Arai AE. Multiecho Dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn Reson Med. 2009;61:215–221. doi: 10.1002/mrm.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornert P, Keupp J, Eggers H, Aldefeld B. Whole-body 3D water/fat resolved continuously moving table imaging. J Magn Reson Imaging. 2007;25:660–665. doi: 10.1002/jmri.20861. [DOI] [PubMed] [Google Scholar]
- 8.Bydder M, Yokoo T, Hamilton G, Middleton MS, Chavez AD, Schwimmer JB, Lavine JE, Sirlin CB. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60:1122–1134. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, McKenzie CA, Shimakawa A, Vu AT, Brau AC, Beatty PJ, Pineda AR, Brittain JH, Reeder SB. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 11.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T1 and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 12.Hu HH, Nayak KS. Quantification of absolute fat mass using an adipose tissue reference signal model. J Magn Reson Imaging. 2008;28:1483–1491. doi: 10.1002/jmri.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 14.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 15.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–286. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Martin DR, Pineda N, Xu Q, Vos M, Anania F, Hu X. Quantitative analysis of T2-correction in single-voxel magnetic resonance spectroscopy of hepatic lipid fraction. J Magn Reson Imaging. 2009;29:629–635. doi: 10.1002/jmri.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard CP, Liney GP, Manton DJ, Turnbull LW, Langton CM. Comparison of fat quantification methods: a phantom study at 3.0T. J Magn Reson Imaging. 2008;27:192–197. doi: 10.1002/jmri.21201. [DOI] [PubMed] [Google Scholar]
- 18.Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006;16:569–574. doi: 10.1016/j.numecd.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Hu HH, Smith DL, Nagy TR, Goran MI, Nayak KS. Identification of brown adipose tissue in mice using IDEAL fat-water MRI. 17th Meeting of the International Society of Magnetic Resonance in Medicine; Honolulu, Hawaii. 2009. p. 210. [Google Scholar]
- 20.Zancanaro C, Nano R, Marchioro C, Sbarbati A, Boicelli A, Osculati F. Magnetic resonance spectroscopy investigations of brown adipose tissue and isolated brown adipocytes. J Lipid Res. 1994;35:2191–2199. [PubMed] [Google Scholar]
- 21.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 22.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. AJR Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 23.Brix G, Heiland S, Bellemann ME, Koch T, Lorenz WJ. MR imaging of fat-containing tissues: valuation of two quantitative imaging techniques in comparison with localized proton spectroscopy. Magn Reson Imaging. 1993;11:977–991. doi: 10.1016/0730-725x(93)90217-2. [DOI] [PubMed] [Google Scholar]
- 24.Ren J, Dimitrov I, Sherry AD, Malloy CR. Composition of adipose tissue and marrow fat in humans by H NMR at 7 tesla. J Lipid Res. 2008;49:2055–2062. doi: 10.1194/jlr.D800010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003;49:515–526. doi: 10.1002/mrm.10407. [DOI] [PubMed] [Google Scholar]
- 26.Hu HH, Sung K, Nayak KS. Apparent change in the T1 of lipids in mixture. 17th Meeting of the International Society of Magnetic Resonance in Medicine; Honolulu, Hawaii. 2009. p. 4448. [Google Scholar]
- 27.Poon CS, Szumowski J, Plewes DB, Ashby P, Henkelman RM. Fat/water quantitation and differential relaxation time measurement using chemical shift imaging technique. Magn Reson Imaging. 1989;7:369–382. doi: 10.1016/0730-725x(89)90486-4. [DOI] [PubMed] [Google Scholar]
- 28.Dyke JP, Lauto A, Schneider E, Matei C, Borja J, Mao X, Shungu DC, Jakubowski A, Lis E, Ballon D. Homogeneous water-lipid phantoms with matched T1 and T2 relaxation times for quantitative magnetic resonance imaging of tissue composition at 3.0 tesla. 12th Meeting of the International Society of Magnetic Resonance in Medicine; Kyoto, Japan. 2004. p. 2212. [Google Scholar]
- 29.Cowan B. Nuclear magnetic resonance and relaxation. Cambridge: Cambridge University Press; 1997. p. 458. [Google Scholar]
- 30.Ragozzino E, Bortone C. Proton spin-lattice relaxation in binary liquid mixtures: determination of the mixed translational contributions. Mol Phys. 1971;22:525–533. [Google Scholar]
- 31.Houmard JA, Smith R, Jendrasiak GL. Relationship between MRI relaxation time and muscle fiber composition. J Appl Physiol. 1995;78:807–809. doi: 10.1152/jappl.1995.78.3.807. [DOI] [PubMed] [Google Scholar]
- 32.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]
- 33.Terk MR, Dardashti S, Liebman HA. Bone marrow response in treated patients with Gaucher disease: evaluation by T1-weighted magnetic resonance images and correlation with reduction in liver and spleen volume. Skeletal Radiol. 2000;29:563–571. doi: 10.1007/s002560000276. [DOI] [PubMed] [Google Scholar]
- 34.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes. 2004;5:219–226. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]